1. Introduction
The World Health Organization (WHO) classifies scrub
typhus as a neglected tropical disease and a major public health
issue in the Asia-Pacific region. Scrub typhus markeldy impacted
the Allied forces during World War II and in the Vietnam War. In
the Pacific theater, >18,000 troops contracted the disease,
while in Vietnam, it remained a prominent cause of severe,
undifferentiated febrile illness (1). Orientia tsutsugamushi is a
Gram-negative bacterium that depends on living cells for survival
and causes scrub typhus, a disease transmitted by mites (2) Fig.
1. The earliest known cases of scrub typhus were documented in
China in 313 A.D. The bacterium Orientia tsutsugamushi was
initially discovered in Guangzhou, China. It is estimated that
globally, more than a billion individuals are at risk of
contracting scrub typhus, with approximately one million cases
being reported annually (3,4).
This disease, prevalent in regions of Asia, the Pacific and the
Indian Ocean islands, is spread to humans through the bites of
larval trombiculid mites, commonly referred to as chiggers
(5,6). Scrub typhus has become a pressing
global health issue, characterized by increasing cases and
inadequate medical resources to address the problem. Some
countries, including China, Korea, Japan and Thailand, have
implemented preventive measures, such as rodent control,
insecticide use and surveillance systems. A system for monitoring
scrub typhus cases was introduced in 1952.
These mites primarily infest rodents and were first
identified as carriers of scrub typhus by Hashimoto in Japan in
1899 (7,8). Following a bite from an infected
mite, the incubation period for scrub typhus generally lasts
between 10 and 12 days, although it may vary from 6 to 21 days
(9). The symptoms resemble those
of the flu and may include fever, rash, a dark eschar at the bite
site, headache, muscle pain, cough, swollen lymph nodes, nausea,
vomiting and abdominal discomfort (10). In severe cases, the infection can
cause life-threatening complications affecting the lungs, heart,
liver, brain or kidneys (11,12).
Research from Assam has also shown that infection with Orientia
tsutsugamushi can cause acute encephalitis syndrome, a severe
condition that can be fatal (13).
The bacterium is primarily transmitted through the
bite of red-colored, microscopic chigger larvae, which are more
abundant in dense, vegetated areas during the rainy season, giving
rise to names, such as river fever or flood fever. These mites
typically lay their eggs between July and December. While mites and
rodents inhabit various ecosystems, ‘scrub’ refers to the
vegetation type that facilitates chigger-host interactions. The
hallmark of scrub typhus is the formation of an eschar at the bite
site, which develops into a black, crusty lesion resembling a
cigarette burn. While studies from Korea have identified a notable
number of cases with eschar, data from Thailand and Taiwan indicate
a markedly lower prevalence (14).
2. Scrub typhus in various regions in
Asia
Scrub typhus in India
Scrub typhus has been documented in India since at
least 1917 with notable outbreaks occurring during World War II and
the 1965 Indo-Pakistani War, primarily along the border with Burma.
The disease resurfaced in the 1990s near the Pakistan border
(15). Despite decades of
awareness, scrub typhus remains a frequently underdiagnosed health
concern in India The disease presents a serious health risk, with a
substantial number of cases reported from various parts of the
country (16). Recent data
indicate that scrub typhus is prevalent in various regions of
India, including the southern states, northern regions,
northeastern states, eastern areas and western states (17). The extensive distribution of this
disease is largely due to the vast geographic expanse and varied
ecological landscapes of India.
In Odisha in India, scrub typhus was identified in
four districts, with two experiencing the most significant impact
(18). One of these heavily
affected districts also faced a substantial number of dengue cases,
further straining local health services. However, no cases of
co-infection were reported in the region at that time. The
likelihood of simultaneous infections with diseases, such as
typhoid, dengue, or influenza in patients with scrub typhus can
obscure clinical presentations, complicating diagnosis and
treatment efforts (19). This
situation places additional pressure on healthcare systems,
particularly since vulnerable groups such as children, individuals
with weakened immune systems, and the elderly are at increased risk
(20). Studies conducted in
hospitals in Southern India have found that a number of children
with scrub typhus exhibit acute, nonspecific symptoms (21). Neurological complications
(meningitis, meningoencephalitis, encephalopathy and seizures) have
been shown to be associated with high mortality rates in these
patients (22-24).
Scrub typhus has drawn considerable attention after
thousands of confirmed cases and several fatalities were reported
in India during 2023(17). This
zoonotic infection affected multiple regions, leading to 17 deaths.
The Indian states of Himachal Pradesh, Odisha and Rajasthan were
among the hardest hit, with Himachal Pradesh reporting nine deaths
and Odisha recording eight. In Himachal Pradesh, all the fatalities
occurred in the Shimla district, while deaths in Odisha were
limited to two districts. Furthermore, Telangana State recorded
cases of scrub typhus co-occurring with dengue and influenza.
Scrub typhus in China (Yunnan
Province)
Scrub typhus is a common infectious disease in
China, with Yunnan Province being a hotspot. According to official
records, >41,000 cases were reported in Yunnan between 2010 and
2019, with a significant concentration in the western regions,
particularly Baoshan, Lincang and Dehong (25). The first recorded case of scrub
typhus in China appeared in 1948(26). A cluster of scrub typhus cases has
been reported in Yunnan Province, particularly in Longling City,
which has reported the highest number of cases over the past
decade. The diverse climate of Yunnan Province, particularly the
tropical and subtropical conditions in Baoshan City, creates
favourable environments for small mammals and mites, potential
hosts and vectors of scrub typhus. This region has consistently
reported the highest number of cases in the province. Recent
studies have identified additional mite species, beyond
Leptotrombidium deliense, as potential vectors in Yunnan. A
total of 182,991 cases and 186 reated deaths were documented over a
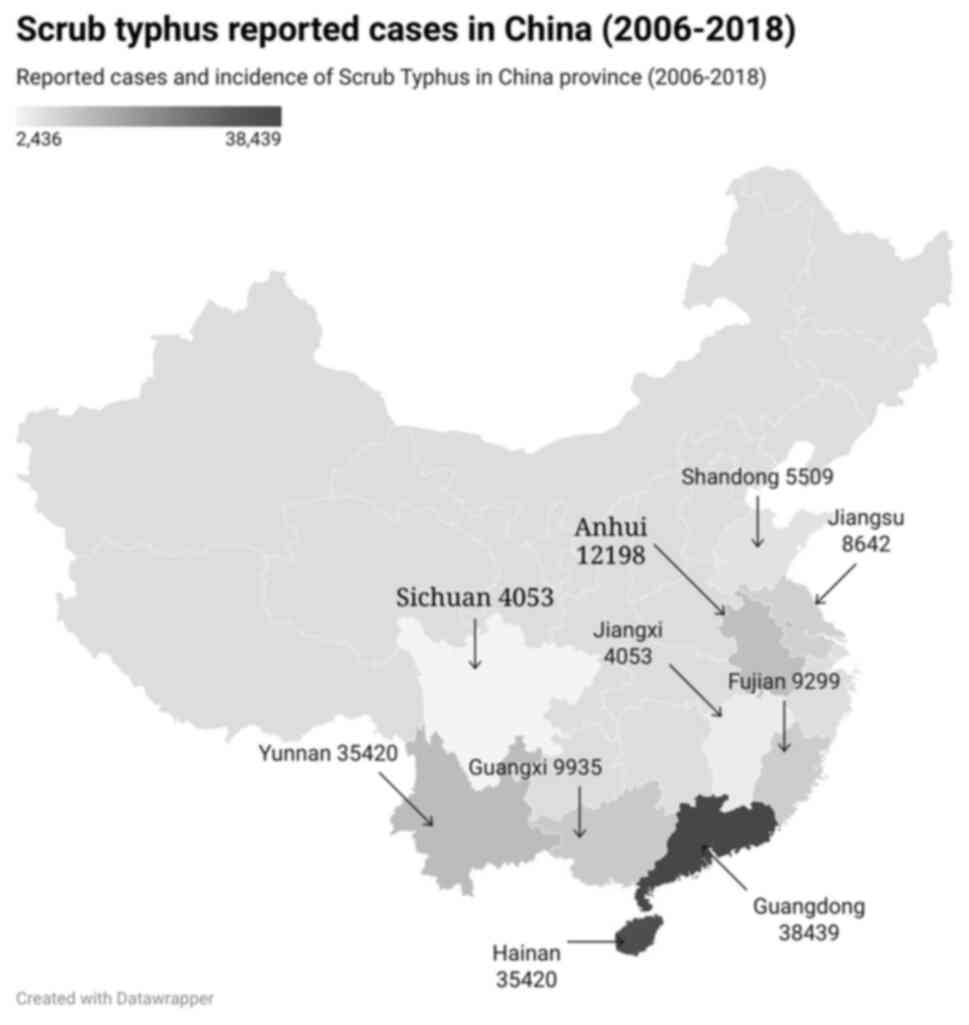
period of 51 years in China (27).
The geographical location of scrub typhus cases in China is
illustrated in the map presented in Fig. 2.
Scrub typhus in Thailand
Data from the national surveillance system in
Thailand reveal a marked increase in reported scrub typhus cases
between 2003 and 2018(28). Males
were more commonly affected than females, and agricultural workers
accounted for the majority of cases, primarily among adults. The
disease is seasonal, peaking during the rainy season. Northern
provinces, particularly Chiangrai, experienced the highest number
of cases. Factors, such as agricultural activity, elevation,
rainfall, temperature and land cover contribute to the disease
burden in Chiangrai. These findings enhance the current
understanding of scrub typhus in Thailand and help identify
potential risk factors.
Scrub typhus in the Chungcheong region
of Korea
To understand the trends in scrub typhus cases
within the Chungcheong region, epidemiological data were collected
from 14,379 cases reported to the Korea Centers for Disease Control
and Prevention between 2012 and 2022(29). Geographical analysis revealed an
association between areas with a high scrub typhus incidence and a
larger elderly population. The elderly population and changes in
agricultural practices were significantly linked to the occurrence
of scrub typhus. Although the overall number of cases and
agricultural activity in the Chungcheong region has declined since
2012-2013, recent data indicate a possible increase in the number
of cases due to more individuals spending time outdoors (30,31).
Older individuals are at a greater risk of infection and may also
experience repeated infections or infections with other illnesses
that cause fever. Scrub typhus cases were documented in Korea as
early as the Korean War in 1951. Studies conducted between 2001 and
2006 revealed a higher incidence of the disease among females
compared to males (~65 vs. 35%). Researchers attributed this
disparity to differences in agricultural practices, suggesting that
the more crouched working posture of females may increase their
exposure to the mites that transmit the disease (32). These findings underscore the need
for updated prevention and promotion strategies tailored to the
changing demographics and risk factors for scrub typhus in the
region.
3. Seasonal variations and factors
controlling the spread of scrub typhus
Scrub typhus cases exhibit seasonal variations
across different regions. In Korea, outbreaks typically occur in
the fall (October to November) (33), while in India, they peak during the
monsoon and post-monsoon seasons (July to February) (21). Nepal and Thailand report peak cases
in the summer and early fall (34). In China, particularly in the
southern regions, scrub typhus cases often peak in the summer, with
a recent trend of additional peaks in September and October in
Yunnan Province (35).
Environmental factors, such as temperature, humidity, and
precipitation play crucial roles in the transmission of scrub
typhus in China. Research suggests that higher temperatures and
rainfall can increase mite populations, leading to a greater risk
of transmission (36). However,
the specific environmental conditions conducive to mite abundance
and transmission vary across different regions. Additionally,
humidity levels must be within a suitable range to support both
mite and small mammal populations, which are essential for the
transmission cycle of the disease.
The Tsutsugamushi Triangle
The term ‘Tsutsugamushi’ comes from two Japanese
words: ‘tsutsuga’, meaning illness, and ‘mushi’, meaning insect
(37). The primary endemic region
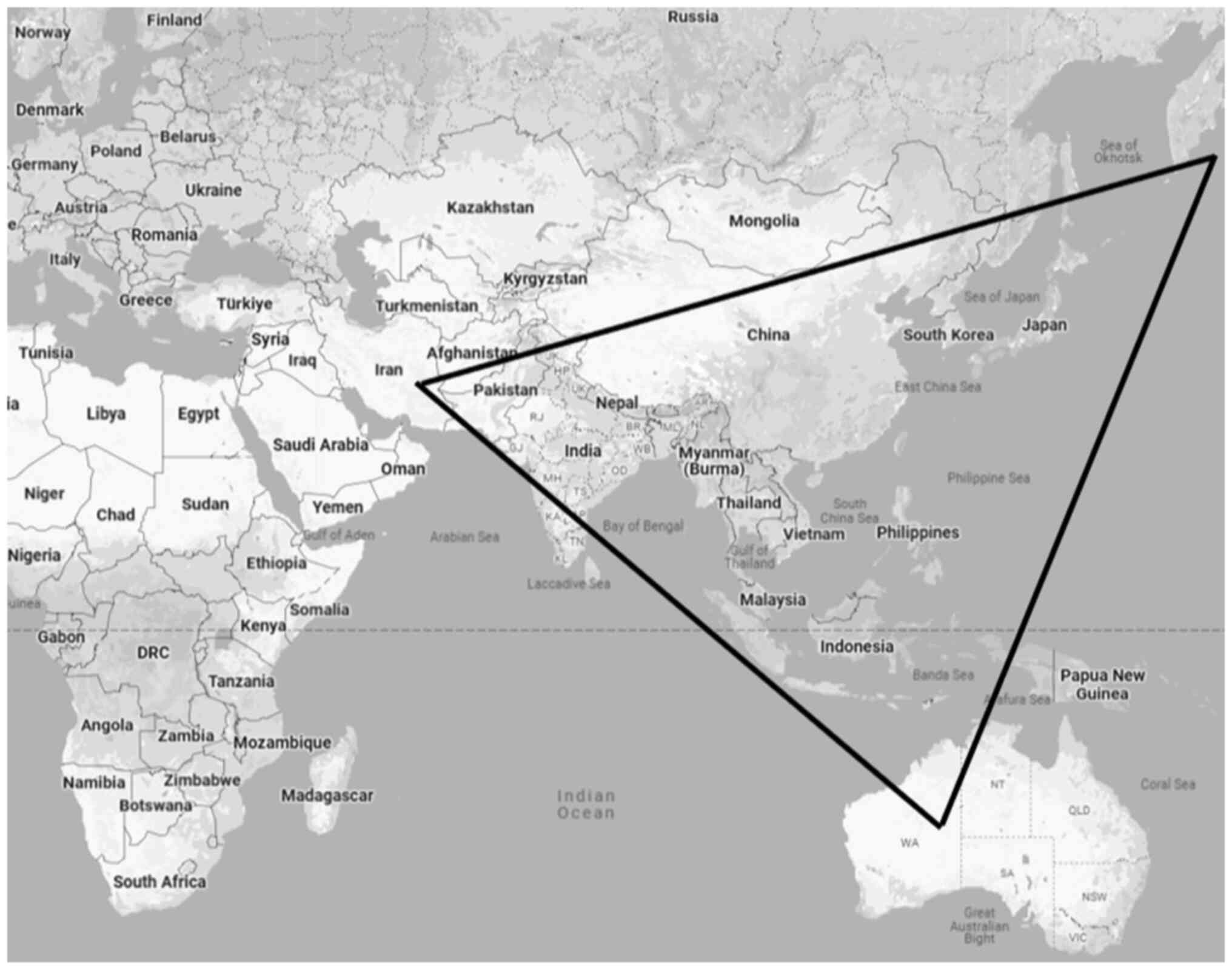
for scrub typhus is referred to as the ‘Tsutsugamushi Triangle’,
covering over eight million square kilometers (6). This region stretches from the Russian
Far East in the north to Pakistan in the west, Australia in the
south and Japan in the east (Fig.
3). Approximately one billion individuals are at risk of
infection in the highly populated area. The Tsutsugamushi Triangle
encompasses over half of the global population, placing more than
one billion individuals at risk (16).
Globalization and increased travel have contributed
to the spreading of infected individuals to areas outside the
endemic regions, heightening concerns about the broader reach of
sscrub typhus (38). The
diverse antigenic and genetic properties of Orientia
tsutsugamushi strains, coupled with the uncertain link between
these differences and the severity of human illness, hinder our
epidemiological understanding of scrub typhus. A more in-depth
understanding of its epidemiology is essential for creating
effective prevention and control measures (39). This section of the review explores
the geographic distribution of scrub typhus and the associated risk
factors, both in endemic areas and among travelers from non-endemic
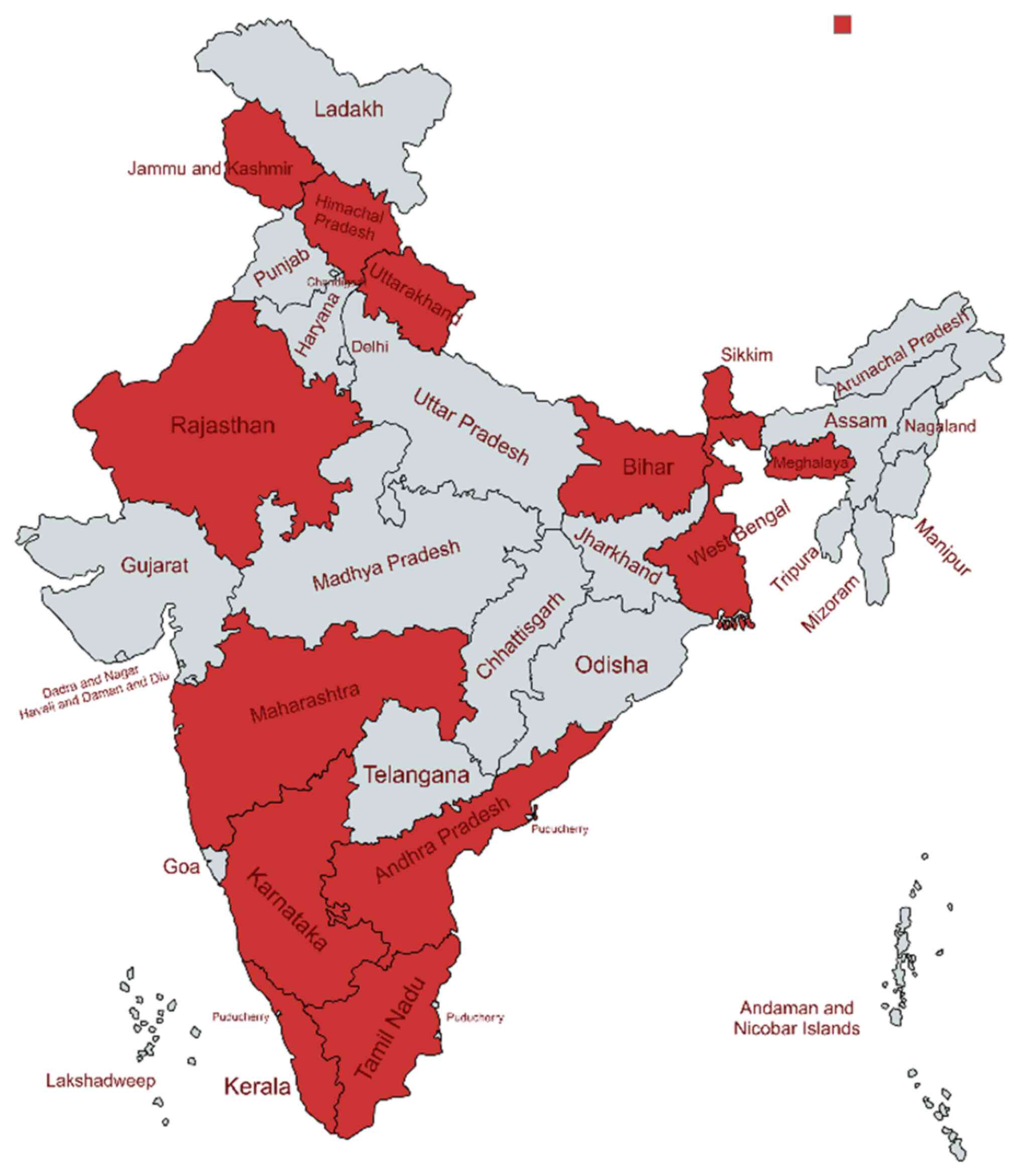
regions. In India, scrub typhus has been reported across a wide
range of geographical regions as shown in Fig. 4 and as previously demonstrated
(13,40-43).
Previous studies, primarily conducted in rural areas
of India, have indicated that scrub typhus is widespread and
disproportionately affects vulnerable populations, including
impoverished farmers and children (44,45).
In some regions, the increasing number of cases has led to scrub
typhus being recognized as a re-emerging infectious disease,
raising serious public health concerns. In certain areas, ~20 to
24% of patients with unexplained fever have been diagnosed with
scrub typhus, with 53% of these cases exhibiting signs of acute
kidney injury (44).
4. Life cycle and transmission of
Orientia tsutsugamushi
Orientia tsutsugamushi targets a
variety of cells throughout its life cycle
Initially, it infects dermal dendritic cells and
activates monocytes at the bite site, using these cells to spread
to lymph nodes. In mice, the bacteria prefer macrophages, while in
humans, it primarily infects endothelial cells in multiple organs,
including the skin, heart, lungs, kidneys and brain. Other target
cells include mouse fibroblasts, neutrophils and polymorphonuclear
leukocytes in various infection models (16,46).
Cell invasion and the intracellular life cycle of Orientia
involve complex interactions with host cells. The bacteria attach
to extracellular matrix components, particularly fibronectin, which
interacts with Orientia proteins to facilitate entry into
nonphagocytic cells. Integrins and heparan sulfate proteoglycans
also play roles in this process (47).
Once it has gained entry into the host,
Orientia utilizes clathrin-mediated endocytosis to enter
host cells and rapidly escapes the phagosome to avoid the defense
mechanisms of the host. The bacteria replicate in the cytoplasm and
may accumulate in high densities before being released from the
host cell, potentially via membrane budding. Despite notable
advancements being made, several aspects of intracellular life
cycle and escape mechanisms of Orientia remain unclear
(37).
The larvae of trombiculid mites, also known as
chiggers, are primary vectors for rickettsial pathogens,
particularly Orientia spp., which cause the zoonotic disease
scrub typhus. Chiggers are the only known vectors for
Orientia bacteria, which cause scrub typhus. Chiggers are
primarily parasitic on wild vertebrates, including small mammals,
reptiles and birds. While humans are not their preferred hosts,
they can be incidentally parasitized by chiggers. Chiggers of the
genus Leptotrombidium are the primary vectors for
transmitting Orientia tsutsugamushi to humans; a recent
study in Chile identified Herpetacarus antarctica as another
potential vector for scrub typhus caused by Candidatus Orientia
chiloensis (48). Chiggers do
not suck blood from the host, but produce a specialized feeding
organ known as a stylostome, which is comprised of glycoprotein and
forms after attaching to the host (49). The digestive enzyme secreted from
the mouth is released into the deepest layer of the skin, known as
the dermis and digests dermis tissue protein (50). Chiggers are known to feed only once
on their host before detaching to molt through three nymphal
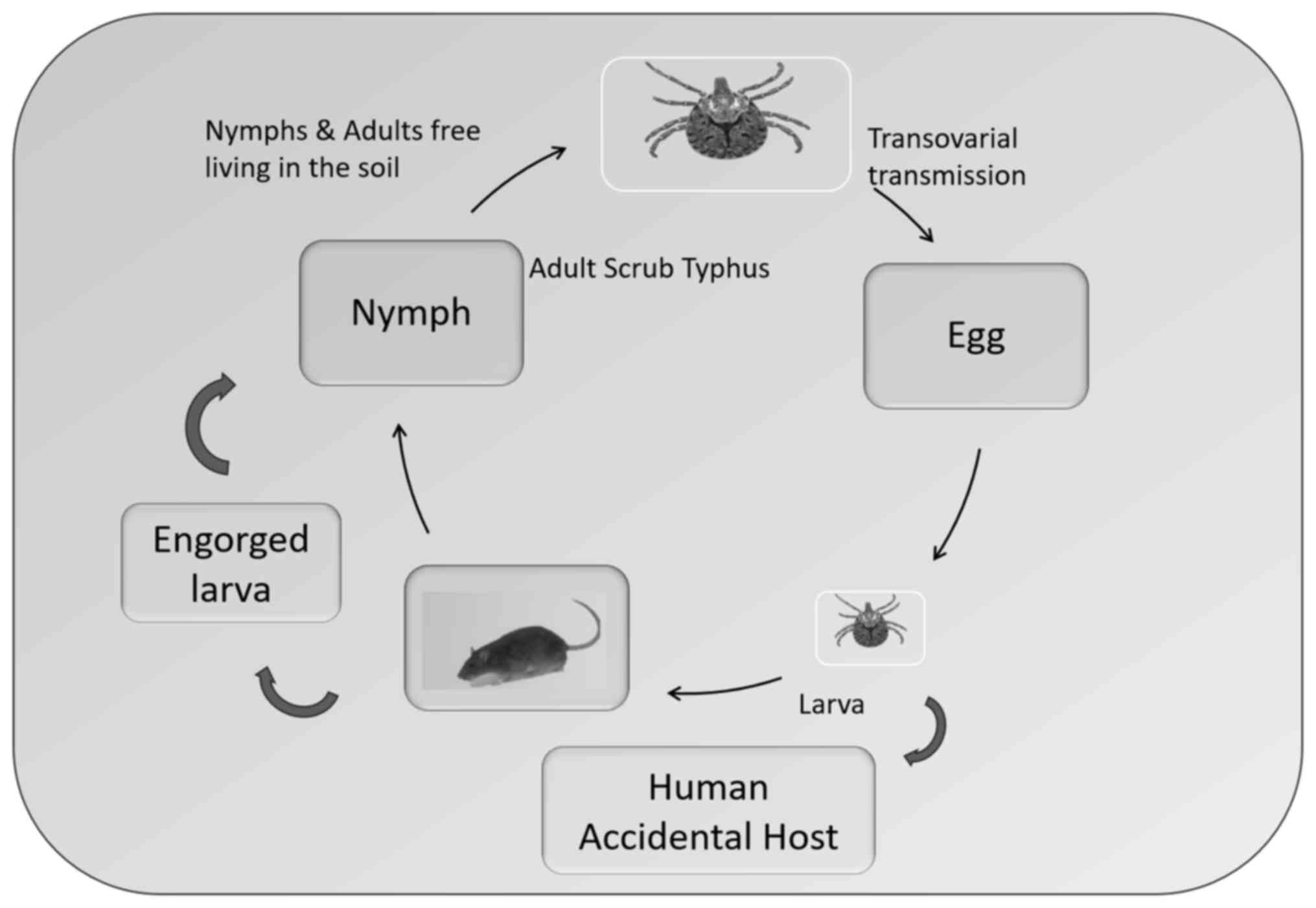
stages: Protonymph, deutonymph and tritonymph (51). Trombiculid mites undergo a complex
life cycle with several stages: Egg, prelarva, larva (chigger),
protonymph, deutonymph, tritonymph and adult. After 6 days of
laying the egg, when the shell breaks down, eggs hatch into
inactive prelarvae, which then develop into active, six-legged
larvae (12 days). These larvae feed on a host for several days
before dropping off to become inactive protonymphs. Protonymphs
transform into active, eight-legged deutonymphs, which then enter a
quiescent tritonymph stage. Finally, tritonymphs develop into
eight-legged adults. Mites in the prelarval, protonymphal and
tritonymphal stages are inactive and do not feed. By contrast,
mites in the deutonymphal and adult stages are non-parasitic and
typically feed on arthropod eggs or small arthropods (52). After reaching adulthood, these
mites become free-living predators in the soil, primarily feeding
on the eggs of other arthropods. The life cycle of chiggers (genus
Leptotrombidium) is illustrated in Fig. 5.
5. Clinical manifestations of scrub
typhus
The symptoms of scrub typhus can vary from mild
fever to severe illness, life-threatening cases involving
multi-organ dysfunction syndrome (MODS) (53). Common symptoms include fever,
gastrointestinal issues, fatigue, cough, muscle pain and headache.
A maculopapular rash typically develops by the end of the first
week, beginning on the trunk and spreading to the limbs. Regional
lymph node swelling is often observed, and an eschar at the bite
site is considered highly indicative of scrub typhus. The reported
prevalence of eschars in patients varies from 7 to 80%, which may
be attributed to the difficulty in identifying small eschars in
individuals with darker skin, differences in the eschar-forming
ability of various Orientia tsutsugamushi strains, and
atypical eschar appearance in moist or damp areas of the skin
(21).
Eschars typically begin as small papules that
gradually enlarge and develop central necrosis, forming a
distinctive black lesion. Common areas for eschar development
include the groin, armpits, waist and other exposed body parts
(54). Eschars were commonly found
on the chest and within 30 cm of the navel in both males and
females. However, males more commonly had eschars on their lower
limbs, while females often had them on their backs. These patterns
may differ across populations due to variations in gender-related
clothing and outdoor activities (24).
Systemic symptoms of scrub typhus may emerge within
the second week of infection, particularly in untreated cases.
Multiple organ systems can be involved, including the heart,
kidney, lungs and brain.
6. Association of various body systems with
scrub typhus
Central nervous system and scrub
typhus
Scrub typhus, a rickettsial disease, can cause
neurological symptoms such as tremors, nervousness and delirium
(55). The central nervous system
is frequently affected in patients with Rocky Mountain spotted
fever and other rickettsial illnesses. A small number of patients
also exhibit symptoms of encephalitis and meningitis. In a previous
study on 1,650 febrile American servicemen in Vietnam, direct
fluorescent antibody testing identified scrub typhus in 109 cases;
Berman and Kund (56) found
mononuclear cells in cerebrospinal fluid samples from several
patients with scrub typhus, who also exhibited generalized
lymphadenopathy and lymphocytosis. Meningitis or
meningoencephalitis has been observed in 14 to 83% of individuals
with scrub typhus (57). Acute
disseminated encephalomyelitis was also reported in patients with
Scrub typhus (58). An elderly
individual with scrub typhus exhibited ptosis (drooping of the
upper eyelid) and ophthalmoplegia (ophthalmoplegia) (59). Ptosis can be present from birth
(congenital) or later in life (acquired) suggesting involvement of
multiple cranial nerves in this disease. Hearing loss accompanied
by fever is a common symptom in up to one-third of individuals with
scrub typhus (60). Research has
shown that cerebellitis can occur in individuals with scrub typhus
involving the nervous system; Rana et al (61) reported cerebellitis in 11% of such
cases, either in isolation or alongside generalized neurological
symptoms.
Cardiovascular system
Potential issues include rhythm disturbances,
myocardial involvement leading to congestive heart failure and
vasculitis. Scrub typhus has been linked to acute myocardial
infarction (55). However, heart
failure due to this infection is less common. In another study, Ray
et al (62) documented a
case of acute heart failure in a female patient. The patient
exhibited elevated NT-proBNP levels, right heart enlargement and
bilateral pulmonary edema. A Weil-Felix test and IgM antibodies for
scrub typhus both yielded positive results, confirmed by the
diagnosis. Previous literature may have underestimated the
prevalence of heart failure syndrome in acute scrub typhus
infections (63). Of note, two
prospective cohort studies, employing echocardiography and cardiac
biomarker analysis, found that a significant proportion of patients
(30.9-42.8%) exhibited a reduced ejection fraction and elevated
levels of troponin T or creatine kinase-muscle/brain isoenzyme
(61.7-72.8%) (64-65). Furthermore, the severity of
systolic dysfunction was associated with elevated cardiac biomarker
levels (64-65). These findings emphasize the
significance of recognizing heart failure as a possible
complication of scrub typhus.
Scrub typhus can increase the risk of developing
atrial fibrillation, a common heart rhythm condition. This is
likely due to the inflammation it causes. The inflammation can
damage heart muscle cells, leading to cell death and scarring. It
can also disrupt the electrical signals in the heart, causing
irregular rhythms (66,67).
Renal system
The link between scrub typhus and acute kidney
failure has also been observed. In India, Between September, 2011
and November, 2012, 49 of 201 patients tested were diagnosed with
scrub typhus through nested PCR (68). The average age of these patients
was 34 years (range, 11-65 years). The majority of the patients
were male, and the majority of the cases were found in the rainy
season. In addition, 82% of the patients experienced renal
complications, including acute kidney infection (53% of patients).
Urinalysis abnormalities included albuminuria (55%) and microscopic
hematuria (16%) (68). Jaundice
was associated with acute kidney infection. A total of 8 patients
succumbed, including 3 patients requiring dialysis. Oliguria, acute
respiratory distress syndrome (ARDS) and acute kidney injury were
linked to mortality (68).
Scrub typhus can lead to acute kidney injury through
several mechanisms (69-72).
In another study, the new biomarker link with acute kidney injury
was shown with 138 patients being included (73). Among these 138 patients, 25
patients developed scrub typhus-associated acute kidney injury.
Several novel biomarkers for acute kidney injury were evaluated,
including neutrophil gelatinase-associated lipocalin and kidney
injury molecule 1. These results suggest that these biomarkers may
be valuable tools for diagnosing and monitoring acute kidney injury
in individuals with scrub typhus (73).
Respiratory system
Complications can include interstitial pneumonia and
ARDS. The case study of a 52-year-old female patient with ARDS was
published in 2015 in the ‘Journal of Clinical and Diagnostic
Research’ (74). The patient was
admitted to the intenstive care unit with clinical symptoms of high
fever (39˚C), chills, cough and shortness of breath, a heart rate
of 134/min and a respiratory rate of 36/min. The patient presented
with decreased breath sounds and crackles in both lungs, indicative
of respiratory distress. Blood gas analysis confirmed type I
respiratory failure. Chest imaging revealed bilateral alveolar
shadows consistent with ARDS. A physical examination identified a
characteristic eschar on the right thigh, a key diagnostic sign for
scrub typhus. A positive Weil-Felix test, along with the exclusion
of other febrile illnesses, confirmed the diagnosis of scrub typhus
complicated by ARDS. Doxycycline treatment was initiated (74).
Gastrointestinal system
Gastrointestinal manifestations of scrub typhus are
less commonly described in the literature. Typical symptoms include
abdominal pain, the vomiting of blood, and diarrhea. Of note, 2
cases were reported of peritonitis associated with scrub typhus,
which highlights the importance of considering scrub typhus in the
differential diagnosis of peritonitis in regions where Orientia
tsutsugamushi is endemic. Until 2009, no case was reported of
scrub typhus associated with peritonitis. In 2010, A 71-year-old
male patient complained of abdominal pain with a high temperature
(39˚C), pulse rate, 110/min; respiration rate, 26/min. Indirect
immunofluorescence assay and clinical diagnostic test and eschar in
the left axillar confirmed the diagnosis of scrub typhus. The
patient was treated with doxycycline for scrub typhus and
antibiotics for peritonitis (75).
In severe cases, MODS may occur. Due to the wide
range of possible symptoms, diagnosing scrub typhus can be
difficult, often leading to delays or missed diagnoses.
7. Laboratory methods for the detection of
scrub typhus
Historically, the diagnosis of scrub typhus relied
on serological methods; however, the integration of DNA-based
pathogen detection, particularly during the early stages of
infection, has led to the combined use of PCR and serology for the
diagnosis of the majority of rickettsial diseases. The outdated
Weil-Felix OX-K agglutination test, which was developed in 1916 was
replaced by the indirect immunofluorescence assay (IFA) in the
1960s (76-78).
Over time, the IFA was refined with new positivity criteria during
the 1970s and 1980s, and it remained the gold standard for decades
(79). However, inconsistencies in
standardizing antigens and criteria led to the introduction of
dynamic titer increase (a fourfold rise in IgM or IgG levels) as a
more reliable marker for diagnosis. ELISAs using either
culture-derived Orientia tsutsugamushi antigens or
recombinant proteins have improved the diagnostic accuracy and
allow for higher throughput compared to the more labor-intensive
IFA and immunoperoxidase tests (80). Rapid diagnostic tests (RDTs)
targeting anti-Orientia IgM and IgG antibodies are currently under
evaluation, with hopes that future advancements will include
antigen-capture-based RDTs for early detection at the point of care
(81). However, pre-existing IgM
and IgG antibodies in populations residing in endemic areas can
complicate serological diagnoses during the acute phase by causing
false-positive results. This is particularly problematic when a
convalescent serum sample is unavailable to confirm a dynamic rise
in antibody titers (82).
The introduction of PCR testing has revolutionized
the diagnostic landscape for rickettsial diseases. PCR provides
greater diagnostic accuracy and has expanded the diagnostic window
to earlier stages of infection (83). Commonly targeted genes in PCR
assays include the 56-kDa, 47-kDa, 16S rRNA, and groEL genes. This
combined diagnostic approach has led to improved treatment outcomes
by ensuring the timely administration of anti-rickettsial therapy,
reducing both morbidity and mortality rates. It has also provided
stronger evidence for diagnostic and vaccine research. The time
from symptom onset to medical presentation, often referred to as
‘days of fever before admission’, is a critical indicator of
disease progression and informs the choice of diagnostic methods.
Bacteraemia in scrub typhus can persist for up to 10 days after the
onset of fever, while the antibody response in individuals with no
prior exposure typically takes 7 to 10 days to develop. For optimal
diagnosis, both PCR and serological tests (such as RDT or ELISA)
are recommended (84).
When accessible, eschar swabs or crust samples serve
as valuable non-invasive diagnostic specimens for scrub typhus.
These samples typically contain high bacterial loads, which are
less influenced by the bloodstream, allowing for accurate molecular
detection, even in the later stages of the disease and after
treatment has begun. Culturing Orientia species from blood
is challenging due to the fastidious growth requirements of the
bacteria, often taking several weeks to yield results. This process
necessitates the use of specialized cell culture techniques and
biosafety level 3 laboratories for safety and accuracy (85).
Loop-mediated isothermal amplification (LAMP) is a
DNA amplification technique that employs three specially designed
primer pairs and Bst DNA polymerase (86). Unlike PCR, LAMP does not require
complex DNA extraction or temperature cycling. The entire reaction
occurs at a constant temperature, thus rendering it suitable for
simple equipment such as a water bath or heating block. The LAMP
results can be visually assessed, with a positive reaction
indicated by a white pellet, eliminating the need for specialized
equipment. A small preliminary study involving 9 patients
demonstrated the sensitivity of LAMP in detecting DNA levels as low
as 14 copies/µl, compared to 3 copies/µl for real-time PCR
(84). However, further validation
through prospective clinical trials is necessary to establish the
reliability of LAMP in a clinical setting.
Multi-locus sequence typing (MLST) is a technique
used to analyze multiple genetic loci, providing a more
comprehensive understanding of evolutionary associations between
isolates compared to single-gene genotyping methods like the 56-kDa
genotype analysis. MLST employs ‘housekeeping’ or conserved genes
to minimize the impact of selective pressure and recombination on
phylogenetic relationships. Notably, MLST analyses were not
performed on the genomes of Orientia tsutsugamushi Boryong
and Ikeda, despite their sequencing (87,88).
While MLST is commonly used to classify other microorganisms, its
application to Orientia tsutsugamushi has been relatively
limited (51).
8. Immune response in scrub typhus
The immune defense against Orientia involves
both humoral and cell-mediated responses. While the humoral
response mainly offers protection against the same strain with
minimal cross-protection against others, the cell-mediated response
provides short-term protection against different strains; however,
this protection diminishes over time. The spreading of the pathogen
throughout the body is a process likened to a ‘Trojan horse’ effect
(89). This bacteremia phase
aligns with the onset of fever and typically lasts ~10 days.
However, the immune response to scrub typhus is short-lived and
offers limited cross-protection between different strains. Immunity
acquired after infection can diminish within months, rendering
individuals vulnerable to symptomatic illness upon re-exposure to
different strains, although immunity to the same strain may last
for >1 year (90).
Understanding the balance between these immune
mechanisms and how they influence bacterial spread is essential for
the development of an effective vaccine (91). With timely treatment, the majority
of patients with scrub typhus experience fever resolution within 48
h. However, regions such as northern Thailand and southern India
have reported delayed fever clearance and high fatality rates of
12-13%. Ongoing research is investigating the potential
contribution of antibiotic resistance in Orientia species to
adverse clinical outcomes in affected regions (92).
Orientia tsutsugamushi, the causative agent
of scrub typhus, initiates infection by binding to the heparin
sulfate proteoglycan receptor, specifically syndican-4, expressed
on dermal cells (93). Upon entry
into the dermis, the bacteria are phagocytosed by dendritic cells
(macrophages), leading to the upregulation of MHC class II and
co-stimulatory markers (CD40, CD80, CD86 and CD83) (94). This triggers mononuclear cell
infiltration in the dermis.
Once in the bloodstream, Orientia
tsutsugamushi activates the type-1 interferon pathway and MAPK
signaling, resulting in the production of IFN-β. Additionally,
NF-κB nuclear translocation suggests its involvement in inducing
chemokine production. MAPK pathways also contribute to the
upregulation of TNF-α. Notably, Orientia tsutsugamushi can
manipulate host immune responses by increasing the production of
immunosuppressive molecules, which inhibit TNF-α production and
enhance bacterial survival within phagocytic cells (95). Dendritic cells and monocytes act as
vehicles for spreading Orientia tsutsugamushi, while
circulating to the lymph nodes (89).
The bacteria subsequently spread to various organs,
including the kidneys and liver, heart and lungs, where they enter
endothelial cells. The recognition of Orientia tsutsugamushi
by nucleotide-binding oligomerization domain-like receptors in
kidney endothelial cells triggers a cascade of events. The
upregulation of adhesion molecules, such as ICAM-1, VCAM-1 and
ALCAM, facilitates leukocyte adhesion and trafficking, contributing
to disease severity (96).
Furthermore, the increased expression of angiopoietin (Ang)-2 and
the elevated Ang-2/Ang-1 ratio, along with the production of
endothelin-1 and endothelial nitric oxide synthase, promote
endothelial activation (97). The
upregulation of IL-33 and its receptor in the liver and kidneys
further exacerbates endothelial cell activation and dysfunction,
leading to significant tissue damage and organ impairment (98). The proposed mechanisms of
Orientia tsutsugamushi infection in humans and the
subsequent immune response to renal cell damage are depicted in
Fig. 6.
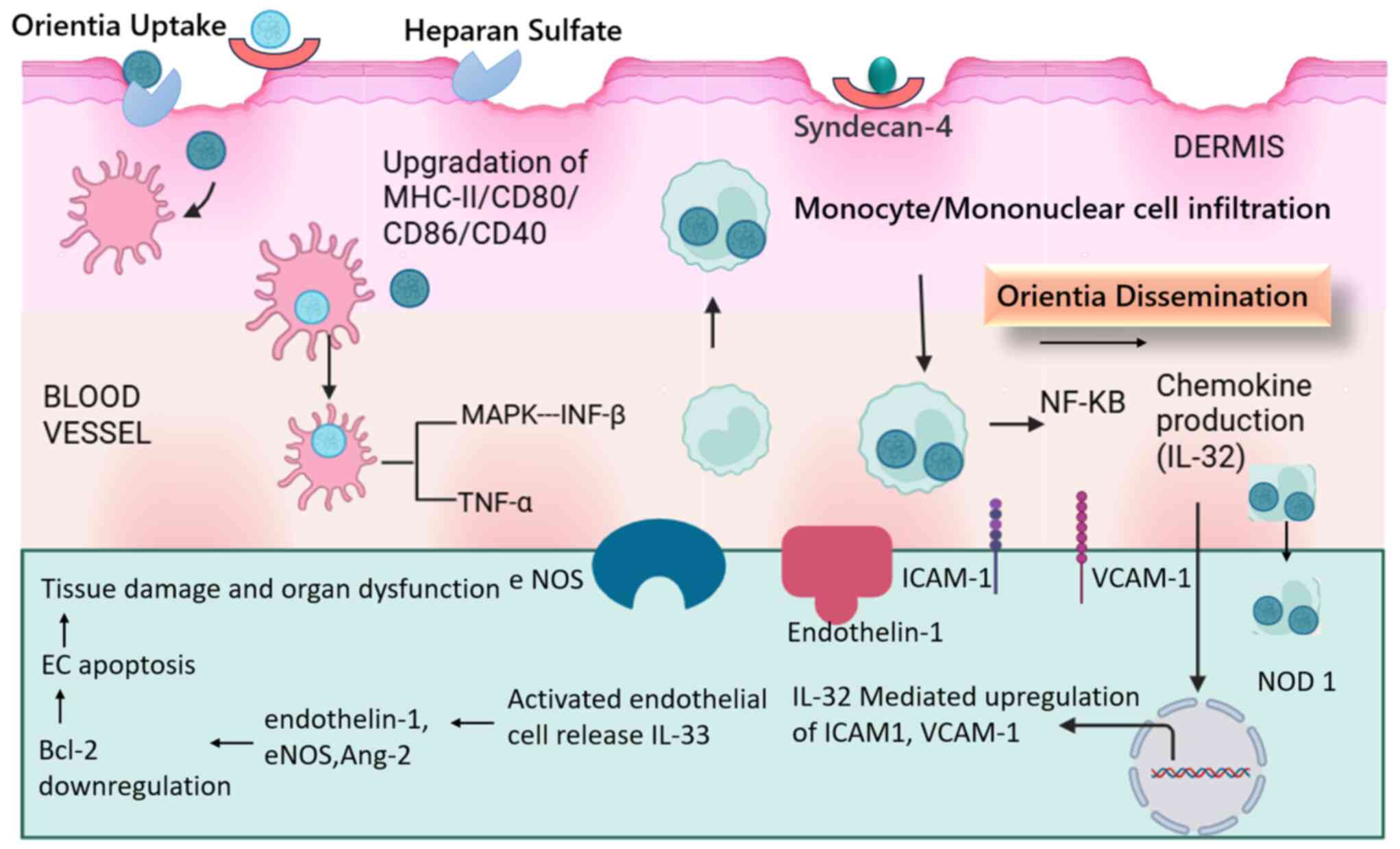 | Figure 6The proposed mechanisms of
Orientia tsutsugamushi infection in human and organ
dysfunction. Orientia tsutsugamushi infection of endothelial
cells triggers intracellular recognition by NOD1. This activation
stimulates the infected endothelium, leading to IL-32-mediated
upregulation of inflammatory and adhesion molecules, such as
ICAM-1, VCAM-1, and E-selectin. The enhanced expression of adhesion
molecules and endothelial cell activation alters the balance of
angiogenic factors, increasing the Ang2/Ang1 ratio. Additionally,
endothelial nitric oxide synthase and endothelin-1 levels are
dysregulated. This complex interplay is further influenced by the
intranuclear expression and release of IL-33, which contributes to
increased endothelin-1 and Ang-2 levels. Ultimately, these cellular
events culminate in the downregulation of the anti-apoptotic
protein Bcl-2, inducing apoptosis of renal endothelial cells and
subsequent renal tissue damage. EC, endothelial cell; eNOS,
endothelial nitric oxide synthase. |
9. Therapeutic and prevention strategies for
scrub typhus
Tetracycline, doxycycline, azithromycin and
rifampicin are effective treatments for scrub typhus with low rates
of treatment failure. Limited evidence suggests a minimal to no
difference between tetracycline, doxycycline and azithromycin. Due
to low-certainty evidence and the risk of inducing resistance in
undiagnosed tuberculosis, rifampicin should not be a first-line
treatment. It may be considered a second-line option after ruling
out active tuberculosis (99).
Scrub typhus is effectively treated with
antibiotics, such as doxycycline (preferred), azithromycin or
chloramphenicol. Early empirical therapy is recommended based on
clinical suspicion, as fever reduction is often observed within 48
h of initiating doxycycline. The standard treatment regimen
consists of doxycycline 100 mg taken orally twice a day for 7 days.
Tetracycline, although less commonly used, may be an alternative
treatment at a dose of 500 mg every 6 h for 7 days (99).
Azithromycin, which has comparable effectiveness to
doxycycline, is commonly prescribed as a 3-day regimen. This
typically involves an initial dose of 1,000 mg or 500 mg, followed
by 500 mg or 250 mg for the following 2 days. Azithromycin is the
preferred option for pregnant women and children, due to concerns
over the side-effects associated with doxycycline and
tetracyclines. Chloramphenicol, another effective alternative to
doxycycline, is administered at a dose of 500 mg every 6 h for
adults, or 50 to 75 mg/kg/day in children for 7 days. However, its
use has declined due to its potential for hematological
side-effects (1 in 21,600 cases) and the rare occurrence of gray
baby syndrome in premature infants, which can cause circulatory
collapse (100).
For delayed responses to treatment, the combined use
of doxycycline and either azithromycin or chloramphenicol may be
effective, as it can shorten the fever clearance time and reduce
complications, particularly in pregnant women. There have been no
reported negative interactions between these drug combinations.
When combining treatments with rifampicin, dosage adjustments
should be made to prevent reduced drug levels.
For patients with suspected typhoid fever or typhus,
high-dose azithromycin (in the typhoid treatment range) or
chloramphenicol can be considered, as it covers both infections.
For uncomplicated cases of scrub typhus, shorter treatment regimens
have been explored; 3-day courses of doxycycline have shown similar
effectiveness to the traditional 7-day regimens, with comparable
fever resolution and no treatment failures. In areas such as
northern Thailand, where doxycycline resistance has been reported,
azithromycin remains an effective alternative, despite slower fever
clearance times.
Antibiotic resistance
Scrub typhus has historically received limited
clinical attention due to the availability of effective treatments
(99). While chloramphenicol and
tetracyclines, particularly doxycycline, remain effective, concerns
about potential side-effects in specific populations, such as
pregnant women and children, have prompted the exploration of
alternative therapies (101,102). Macrolides, quinolones and
rifampicin have emerged as promising options, although their
efficacy may vary. A study in 1996 reported that scrub typhus
patients from Chiangrai, Thailand, responded poorly to standard
doxycycline treatment compared to those from western Thailand and
Vietnam (92). This slower
response was not attributed to poor drug absorption, but was
hypothesized to be due to either more virulent or resistant strains
of Orientia tsutsugamushi. Subsequent analysis identified a
doxycycline-resistant isolate (C3) and a partially resistant
isolate (C27) from Chiangrai. While the resistant strains exhibited
reduced virulence in the absence of the drug, the strains
maintained their ability to invade cells even in the presence of
doxycycline (92).
Of note, two potential mechanisms have been proposed
for the emergence of resistant strains. The first, though less
likely, involves the persistence of dormant organisms in patients
undergoing prolonged antibiotic therapy. However, the transmission
of such resistant strains to chiggers, the primary reservoir of
infection, remains unclear. The second mechanism involves the
potential development of drug resistance in chiggers due to
antibiotic supplementation in poultry feed. Chiggers, feeding on
rodents that consume this contaminated grain, could inadvertently
ingest the antibiotics. If Orientia tsutsugamushi can be
transmitted transovarially, this could lead to the emergence of
drug-resistant strains in subsequent generations of chiggers,
ultimately infecting humans.
A second Orientia tsutsugamushi strain,
designated AFSC-4, was isolated from a patient in Kanchanaburi,
western Thailand, in 1990. This strain, geographically distant from
the Chiangrai isolates, exhibited reduced susceptibility to
doxycycline (103). A study in
1995 investigated azithromycin's efficacy against Orientia strains,
including a doxycycline-resistant isolate. It demonstrated that
AFSC-4 (O. tsutsugamushi strain showing reduced doxycycline
susceptibility) required a significantly higher concentration of
doxycycline to inhibit growth compared to the reference strain
Karp. The strain AFSC-4 required a 32-fold higher doxycycline
concentration (0.25 mg/ml) to achieve an equivalent level of growth
inhibition as observed in the control strain (Karp). This suggests
a significant difference in drug susceptibility between the two
strains (104). A study in 2000
conducted in northern Thailand investigated the efficacy of
rifampicin in treating scrub typhus patients who responded poorly
to standard doxycycline therapy (105). Previous research has demonstrated
the superior activity of rifampicin against northern Thai
Orientia tsutsugamushi strains compared to doxycycline
(92). The results indicated that
rifampicin was more effective than doxycycline, with more rapid
fever clearance times and a lower relapse rate. These findings
suggest that rifampicin may be a valuable alternative treatment
option for scrub typhus infections in regions with drug-resistant
strains.
Prevention of scrub typhus
Scrub typhus is one of the top 10 causes of acute,
potentially life-threatening illnesses among travelers returning
from tropical regions. With improved awareness among healthcare
professionals and the availability of more effective diagnostic
tools, there has been an increase in the identification of scrub
typhus cases, including severe and fatal outcomes, among travelers.
The majority of cases are contracted in Southeast Asia, where the
disease-transmitting chigger mites are commonly found in scrublands
and tall grasses of rural areas. This puts tourists, locals, and
military personnel at risk of exposure (106,107).
The following precaution can be taken to avoid the
bites of chiggers: Wearing clots having long sleeves, keeping
clothes off the grass during sitting on the grass, using insect
repellents applied to the skin, and clothing containing dibutyl
phthalate, benzyl benzoate, diethyl toluamide, clearing the
vegetation where rodents live, provide health education of the
people regarding the modes of transmission.
Preventive measures primarily involve the use of
insect repellents, such as N,N-diethyl-meta-toluamide, which offers
effective but short-term protection against mites and ticks during
travel in areas where the disease is endemic. Currently, no
licensed vaccines are available for scrub typhus, rendering
personal protective measures essential for reducing the risk of
infection (108).
Scrub typhus symptoms often mimic other illnesses,
rendering diagnosis challenging. In the case that an individual
recently traveled to an endemic region and has experienced fever
and an eschar (a small, dark scab), that individual should seek
immediate medical attention. Early diagnosis and treatment are
crucial for a full recovery. To prevent infection, travelers to
endemic areas should take preventive measures to avoid mite bites.
Some strategies to increase the awareness of scrub typhus and which
may help to prevent scrub typhus are the following: i) The creation
of informative brochures, posters and videos that explain the
symptoms, causes, prevention and treatment of scrub typhus; ii) the
organization of community events and workshops to educate the
population about the disease, particularly in rural areas and
regions with high incidence rates; iii) the use of social media
platforms to share information, raise awareness and dispel myths
about the disease; iv) the organization of CME programs to educate
healthcare providers about the clinical presentation, diagnosis and
management of scrub typhus; v) following the clinical guidelines
for the diagnosis and treatment of scrub typhus.
10. Strategies to mitigate the impact of
socioeconomic factors
The following strategies may be used to mitigate the
impact of socioeconomic factors: i) Enhanced healthcare
accessibility: This involves expanding healthcare infrastructure
and services in rural regions which can facilitate the timely
diagnosis and treatment of scrub typhus. ii) Public health
education and awareness: This includes raising public awareness
about the disease, its symptoms, preventive measures and treatment
options, which can empower individuals to take proactive steps to
reduce their risk of infection. iii) Vector control strategies:
These involve implementing effective vector control measures, such
as clearing vegetation and using appropriate insecticides, which
can greatly reduce chigger populations and consequently, the
transmission of scrub typhus. iv) Socioeconomic development and
disease control: This involves addressing poverty, improving
sanitation and promoting sustainable development, which can
indirectly contribute to reducing the burden of scrub typhus. By
addressing these underlying socioeconomic factors, a more resilient
environment can be created and the impact of the disease can be
minimized.
By comprehending the intricate association between
socioeconomic factors and the prevalence of scrub typhus,
comprehensive strategies can be formulated to prevent and control
this disease, especially among vulnerable populations.
11. Conclusion and future perspectives
The incidence of scrub typhus and Orientia
tsutsugamushi infection is continuously spreading globally.
Scrub typhus, a potentially life-threatening illness presents
several challenges in both diagnosis and treatment. The initial
symptoms of scrub typhus, such as fever, headache and muscle aches,
are often indistinguishable from other common illnesses such as flu
or dengue fever. This can lead to misdiagnosis and delayed
treatment. In a number of regions where scrub typhus is endemic,
healthcare providers may not be aware of the disease or may not
consider it in their differential diagnosis, further delaying
appropriate care. While serological tests and PCR-based assays can
confirm scrub typhus, these tests may not be readily available in
all healthcare settings, particularly in resource-limited areas.
There is growing concern about the emergence of drug-resistant
strains of Orientia tsutsugamushi, which could render
current antibiotics less effective. In some cases, scrub typhus can
lead to severe complications such as encephalitis, pneumonia and
multi-organ failure, which can be difficult to manage and may have
long-term consequences. As aforementioned, delayed diagnosis can
lead to delayed treatment, which can increase the risk of severe
complications and death. Furthermore, there are limited public
health resources. In a number of endemic regions, public health
resources may be limited, rendering it difficult to implement
effective prevention and control strategies. Addressing these
limitations requires a multi-pronged approach, including increased
awareness among healthcare providers and the public, improved
diagnostic tools, the development of new and effective treatments,
and strengthened vector control measures. By working together, the
burden of scrub typhus can be reduced, and improve patient recovery
outcomes.
Emerging evidence from Africa, the Middle East and
South America indicates that scrub typhus is more prevalent than
previously recognized. These regions, undergoing significant
demographic, economic and ecological shifts, are particularly
vulnerable. Recent events, such as the earthquake in Nepal,
underscore the potential for environmental disturbances to
exacerbate the risk of scrub typhus (109). To accurately assess the global
burden of this disease, comprehensive and up-to-date mapping of
human cases and chigger mite distribution is essential. Given the
lack of reliable data, increased attention and research are
urgently needed to inform effective health policies.
Acknowledgements
The authors would like to thank Sharda University
(Greater Noida, India) for providing valuable resources such as
central library and internet resources for the present study.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RY and AV were involved in the conceptualization of
the study and in the preparation of the original draft of the
manuscript. VK, GP and PS were involved in the reviewing and
editing of the manuscript. GK, SB and RY prepared the figures and
supervised the study. All authors have read and agreed to the
published version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walker DH: Scrub Typhus-scientific
neglect, Ever-widening impact. N Engl J Med. 375:913–915.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dasgupta S, Asish PR, Rachel G, Bagepally
BS and Chethrapilly Purushothaman GK: Global seroprevalence of
scrub typhus: A systematic review and Meta-analysis. Sci Rep.
14(10895)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang J, Deng K, Chen J and Zhang M:
Epidemiological and clinical characteristics of scrub typhus in
northern Fujian, China, from 2015 to 2019. BMC Infect Dis.
23(479)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Coinfection with severe fever with
thrombocytopenia syndrome and scrub typhus in Korea-PubMed.
|
|
5
|
Wang Q, Ma T, Ding F, Lim A, Takaya S,
Saraswati K, Sartorius B, Day NPJ and Maude RJ: Global and regional
seroprevalence, incidence, mortality of, and risk factors for scrub
typhus: A systematic review and meta-analysis. Int J Infect Dis.
146(107151)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jiang J and Richards AL: Scrub typhus: No
longer restricted to the tsutsugamushi triangle. Trop Med Infect
Dis. 3(11)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Watt G and Parola P: Scrub typhus and
tropical rickettsioses. Curr Opin Infect Dis. 16:429–436.
2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chakraborty S and Sarma N: Scrub typhus:
An emerging threat. Indian J Dermatol. 62(478)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Singh OB and Panda PK: Scrub Typhus. In:
StatPearls. StatPearls Publishing, Treasure Island, FL, 2024.
|
|
10
|
Paris DH, Shelite TR, Day NP and Walker
DH: Unresolved problems related to scrub typhus: A seriously
neglected Life-threatening disease. Am J Trop Med Hyg.
89(301)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bal M, Kar CR, Behera HK, Kar PC, Biswas
S, Dixit S, Khuntia HK, Pati S and Ranjit M: Scrub typhus
associated acute kidney injury: An emerging health problem in
Odisha, India. J Vector Borne Dis. 58:359–367. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shivalli S: Diagnostic evaluation of rapid
tests for scrub typhus in the Indian population is needed. Infect
Dis Poverty. 5(40)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Khan SA, Bora T, Laskar B, Khan AM and
Dutta P: Scrub typhus leading to acute encephalitis syndrome,
assam, india. Emerg Infect Dis. 23(148)2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kundavaram AP, Jonathan AJ, Nathaniel SD
and Varghese GM: Eschar in scrub typhus: A valuable clue to the
diagnosis. J Postgrad Med. 59:177–178. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Singh P: Scrub typhus, a case report :
Military and regional significance. Med J Armed Forces India.
60:89–90. 2004.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu G, Walker DH, Jupiter D, Melby PC and
Arcari CM: A review of the global epidemiology of scrub typhus.
PLoS Negl Trop Dis. 11(e0006062)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mohapatra RK, Al-Haideri M, Mishra S,
Mahal A, Sarangi AK, Khatib MN, Gaidhane S, Zahiruddin QS, Mohanty
A and Sah R: Linking the increasing epidemiology of scrub typhus
transmission in India and South Asia: Are the varying environment
and the reservoir animals the factors behind? Front Trop Dis.
5(1371905)2024.
|
|
18
|
Sahu S, Misra SR, Padhan P and Sahu S:
Scrub typhus in a tertiary care hospital in the eastern part of
Odisha. Apollo Medicine. 12:2–6. 2015.
|
|
19
|
Agrawal A, Parida P, Rup AR, Patnaik S and
Biswal S: Scrub typhus in paediatric age group at a tertiary care
Centre of eastern India: Clinical, Biochemical Profile and
Complications. J Family Med Prim Care. 11:2503–2506.
2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Seong SY, Choi MS and Kim IS: Orientia
tsutsugamushi infection: Overview and immune responses.
Microbes Infect. 3:11–21. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Devasagayam E, Dayanand D, Kundu D, Kamath
MS, Kirubakaran R and Varghese GM: The burden of scrub typhus in
India: A systematic review. PLoS Negl Trop Dis.
15(e0009619)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Damodar T, Singh B, Prabhu N, Marate S,
Gowda VK, Lalitha AV, Dsouza FS, Sajjan SV, Kariyappa M, Kinhal UV,
et al: Association of scrub typhus in children with acute
encephalitis syndrome and meningoencephalitis, southern India.
Emerg Infect Dis. 29(711)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Garg D and Manesh A: Neurological facets
of scrub typhus: A comprehensive narrative review. Ann Indian Acad
Neurol. 24(849)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Varghese GM, Trowbridge P, Janardhanan J,
Thomas K, Peter JV, Mathews P, Abraham OC and Kavitha ML: Clinical
profile and improving mortality trend of scrub typhus in South
India. Int J Infect Dis. 23:39–43. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Luo YY, Geater AF and Yin JX: The impact
of meteorological parameters on the scrub typhus incidence in
Baoshan City, western Yunnan, China. Front Public Health.
12(1384308)2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li Z, Xin H, Sun J, Lai S, Zeng L, Zheng
C, Ray SE, Weaver ND, Wang L, Yu J, et al: Epidemiologic Changes of
Scrub Typhus in China, 1952-2016. Emerg Infect Dis.
26(1091)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Peng PY, Duan HY, Xu L, Zhang LT, Sun JQ,
Zu Y, Ma LJ, Sun Y, Yan TL and Guo XG: Epidemiologic changes of a
longitudinal surveillance study spanning 51 years of scrub typhus
in mainland China. Sci Rep. 14(3138)2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wangrangsimakul T, Elliott I, Nedsuwan S,
Kumlert R, Hinjoy S, Chaisiri K, Day NPJ and Morand S: The
estimated burden of scrub typhus in Thailand from national
surveillance data (2003-2018). PLoS Negl Trop Dis.
14(e0008233)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang S, Park G and Kim Y: Incidence of
scrub typhus according to changes in geographic and demographic
characteristic in the chungcheong region of Korea. Trop Med Infect
Dis. 9(147)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lu J, Liu Y, Ma X, Li M and Yang Z: Yang.
Impact of meteorological factors and southern oscillation index on
scrub typhus incidence in guangzhou, southern China, 2006-2018.
Front Med (Lausanne). 8(667549)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pan K, Lin F, Xue H, Cai Q and Huang R:
Exploring the influencing factors of scrub typhus in Gannan region,
China, based on spatial regression modelling and geographical
detector. Infect Dis Model. 10:28–39. 2025.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kweon SS, Choi JS, Lim HS, Kim JR, Kim KY,
Ryu SY, Yoo HS and Park O: Rapid increase of scrub typhus, south
Korea, 2001-2006. Emerg Infect Dis. 15(1127)2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee IY, Lim JW, Seo JH, Kim HC, Lee KJ,
Yong TS, Lee WJ, Yu JR and Sim S: Geographical distribution and
epidemiologic factors of chigger mites on apodemus agrarius during
autumn in Korea. Korean J Parasitol. 59(473)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dhimal M, Dumre SP, Sharma GN, Khanal P,
Ranabhat K, Shah LP, Lal BK, Jha R, Upadhyaya BP and Acharya B: An
outbreak investigation of scrub typhus in Nepal: Confirmation of
local transmission. BMC Infect Dis. 21(193)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yao H, Wang Y, Mi X, Sun Y, Liu K, Li X,
Ren X, Geng M, Yang Y, Wang L, et al: The scrub typhus in mainland
China: Spatiotemporal expansion and risk prediction underpinned by
complex factors. Emerg Microbes Infect. 8:909–919. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ding F, Wang Q, Hao M, Maude RJ, John Day
NP, Lai S, Chen S, Fang L, Ma T, Zheng C and Jiang D: Climate
drives the spatiotemporal dynamics of scrub typhus in China. Glob
Chang Biol. 28(6618)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Banerjee A and Kulkarni S: Orientia
tsutsugamushi: The dangerous yet neglected foe from the East.
Int J Med Microbiol. 311(151467)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Phetsouvanh R, Sonthayanon P,
Pukrittayakamee S, Paris DH, Newton PN, Feil EJ and Day NPJ: The
diversity and geographical structure of Orientia
tsutsugamushi strains from scrub typhus patients in Laos. PLoS
Negl Trop Dis. 9(e0004024)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kumar A, Biswal M, Zaman K, Sharma N, Suri
V and Bhalla A: Genetic diversity of Orientia tsutsugamushi
strains from patients in north India. Int J Infect Dis. 84:131–135.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kumar D and Jakhar SD: Emerging trends of
scrub typhus disease in southern Rajasthan, India: A neglected
public health problem. J Vector Borne Dis. 59:303–311.
2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sadanandane C, Jambulingam P, Paily KP,
Kumar NP, Elango A, Mary KA, Agatheswaran S, Sankari T and Mishra
BB: Occurrence of Orientia tsutsugamushi, the etiological
agent of scrub typhus in animal hosts and mite vectors in areas
reporting human cases of acute encephalitis syndrome in the
Gorakhpur Region of Uttar Pradesh, India. Vector Borne Zoonotic
Dis. 18:539–547. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gurung S, Pradhan J and Bhutia PY:
Outbreak of scrub typhus in the North East Himalayan region-Sikkim:
An emerging threat. Indian J Med Microbiol. 31:72–74.
2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Viswanathan S, Muthu V, Iqbal N, Remalayam
B and George T: Scrub typhus meningitis in South India-a
retrospective study. PLoS One. 8(e66595)2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Varghese GM, Janardhanan J, Mahajan SK,
Tariang D, Trowbridge P, Prakash JA, David T, Sathendra S and
Abraham OC: Molecular epidemiology and genetic diversity of
Orientia tsutsugamushi from patients with scrub typhus in 3
regions of India. Emerg Infect Dis. 21:64–69. 2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jain HK, Das A, Dixit S, Kaur H, Pati S,
Ranjit M, Dutta A and Bal M: Development and implementation of a
strategy for early diagnosis and management of scrub typhus: An
emerging public health threat. Front. Public Health threat. Front
Public Health. 12(1347183)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sharma P, Kakkar R, Kaore S, Yadav V and
Sharma RK: Geographical distribution, effect of season & life
cycle of scrub typhus, 2010.
|
|
47
|
Lai CH, Huang CK, Chen YH, Chang LL, Weng
HC, Lin JN, Chung HC, Liang SH and Lin HH: Epidemiology of acute q
Fever, scrub typhus, and murine typhus, and identification of their
clinical characteristics compared to patients with acute febrile
illness in southern taiwan. J Formos Med Assoc. 108:367–376.
2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Acosta-Jamett G, Martínez-Valdebenito C,
Beltrami E, Silva-de La Fuente MC, Jiang J, Richards AL, Weitzel T
and Abarca K: Identification of trombiculid mites (Acari:
Trombiculidae) on rodents from Chiloé Island and molecular evidence
of infection with Orientia species. PLoS Negl Trop Dis.
14(e0007619)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chaisiri K, Linsuwanon P and Makepeace BL:
The chigger microbiome: Big questions in a tiny world. Trends
Parasitol. 39:696–707. 2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shatrov AB: Stylostome formation in
trombiculid mites (Acariformes: Trombiculidae). Exp Appl Acarol.
49:261–280. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Luce-Fedrow A, Lehman ML, Kelly DJ,
Mullins K, Maina AN, Stewart RL, Ge H, John HS, Jiang J and
Richards AL: A Review of Scrub Typhus (Orientia
tsutsugamushi and Related Organisms): Then, Now, and Tomorrow.
Trop Med Infect Dis. 3(8)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lipovsky PJ: Studies of the food habits of
postlarval stages of chiggers (Acarina, Trombiculidae). University
of Kansas, Lawrence, Kan, 1954.
|
|
53
|
Li W, Huang L and Zhang W: Scrub typhus
with multi-organ dysfunction syndrome and immune thrombocytopenia:
A case report and review of the literature. J Med Case Rep.
13(358)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sharma N, Biswal M, Kumar A, Zaman K, Jain
S and Bhalla A: Scrub Typhus in a Tertiary Care Hospital in North
India. Am J Trop Med Hyg. 95:447–451. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Mahajan SK and Mahajan SK:
Neuropsychiatric manifestations of scrub typhus. J Neurosci Rural
Pract. 8:421–426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Berman SJ and Kundin WD: Scrub typhus in
South Vietnam. A study of 87 cases. Ann Intern Med. 79:26–30.
1973.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Basu S and Chakravarty A: Neurological
manifestations of scrub typhus. Curr Neurol Neurosci Rep.
22:491–498. 2022.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chen PH, Hung KH, Cheng SJ and Hsu KN:
Scrub typhus-associated acute disseminated encephalomyelitis. Acta
Neurol Taiwan. 15:251–254. 2006.PubMed/NCBI
|
|
59
|
Kim J, Kim JS, Shin DI, Lee SH, Lee SS and
Choi SY: Ophthalmoplegia due to scrub typhus. J Neuroophthalmol.
35:284–286. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Mahajan SK and Bakshi D: Acute reversible
hearing loss in scrub typhus. J Assoc Physicians India. 55:512–514.
2007.PubMed/NCBI
|
|
61
|
Rana A, Mahajan SK and Sharma A, Sharma S,
Verma BS and Sharma A: Neurological manifestations of scrub typhus
in adults. Trop Doct. 47:22–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ray A, Nangia V, Chatterji RS and Dalal N:
Scrub typhus infection presenting as acute heart failure: A case
report and systematic review of literature of cardiopulmonary
involvement in scrub typhus infection. Lung India.
33(439)2016.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang M, Zhao ZT, Wang XJ, Li Z, Ding L
and Ding SJ: Scrub typhus: Surveillance, clinical profile and
diagnostic issues in Shandong, China. Am J Trop Med Hyg.
87:1099–1104. 2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Pannu AK, Debnath MK, Sharma N, Biswal M,
Vijayvergia R, Bhalla A, Kaur J and Kumar S: Circulating cardiac
biomarkers and echocardiographic abnormalities in patients with
scrub typhus: A prospective cohort study from a tertiary care
center in North India. J Vector Borne Dis. 58:193–198.
2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Karthik G, Sudarsan TI, Peter JV,
Sudarsanam T, Varghese GM, Kundavaram P, Sathyendra S, Iyyadurai R
and Pichamuthu K: Spectrum of cardiac manifestations and its
relationship to outcomes in patients admitted with scrub typhus
infection. World J Crit Care Med. 7:16–23. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Jang SY, Kang KW, Kim JH, Kim B, Chin JY,
Park SH, Choi YJ, Jung KT and Lee SK: New-onset atrial fibrillation
predicting for complicating cardiac adverse outcome in scrub typhus
infection. Clin Cardiol. 42:1210–1221. 2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Aviles RJ, Martin DO, Apperson-Hansen C,
Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR,
Psaty BM, Lauer MS and Chung MK: Inflammation as a risk factor for
atrial fibrillation. Circulation. 108:3006–3010. 2003.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kumar V, Kumar V, Yadav AK, Iyengar S,
Bhalla A, Sharma N, Aggarwal R, Jain S and Jha V: Scrub typhus is
an under-recognized cause of acute febrile illness with acute
kidney injury in India. PLoS Negl Trop Dis. 8(e2605)2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Yen TH, Chang CT, Lin JL, Jiang JR and Lee
KF: Scrub typhus: A frequently overlooked cause of acute renal
failure. Ren Fail. 25:397–410. 2003.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Thap LC, Supanaranond W, Treeprasertsuk S,
Kitvatanachai S, Chinprasatsak S and Phonrat B: Septic shock
secondary to scrub typhus: Characteristics and complications.
Southeast Asian J Trop Med Public Health. 33:780–786.
2002.PubMed/NCBI
|
|
71
|
Upadhyaya A, Alam MR, Raeen AA, Upadhyaya
S, Pathania M, Upadhyaya S and Sivanu K: Scrub Typhus
Meningoencephalitis: An Overlooked Entity. Cureus.
14(e28989)2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Young PC, Hae CC, Lee KH and Hoon CJ:
Tsutsugamushi infection-associated acute rhabdomyolysis and acute
renal failure. Korean J Intern Med. 18:248–250. 2003.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Sun IO, Shin SH, Cho AY, Yoon HJ, Chang MY
and Lee KY: Clinical significance of NGAL and KIM-1 for acute
kidney injury in patients with scrub typhus. PLoS One.
12(e0175890)2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Sankuratri S, Kalagara P, Samala KB,
Veledandi PK and Atiketi SB: Scrub typhus with acute respiratory
distress syndrome (ARDS) and its management in intensive care unit:
A case report. J Clin Diagn Res. 9:OD10–OD11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lee CH, Lee JH, Yoon KJ, Hwang JH and Lee
CS: Peritonitis in patients with scrub typhus. Am J Trop Med Hyg.
86:1046–1048. 2012.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bozeman FM and Elisberg BL: Serological
diagnosis of scrub typhus by indirect immunofluorescence. Proc Soc
Exp Biol Med. 112:568–573. 1963.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Oberhansli G: Contribution to the
serological diagnosis of classic and murine stain fever. Schweiz Z
Pathol Bakteriol. 10:545–564. 1947.PubMed/NCBI(In German).
|
|
78
|
Fletcher W: Typhus-like Fevers of Unknown
Ætiology, with special reference to the malay states. Proc R Soc
Med. 23:1021–1030. 1930.PubMed/NCBI
|
|
79
|
Brown GW, Shirai A, Rogers C and Groves
MG: Diagnostic criteria for scrub typhus: Probability values for
immunofluorescent antibody and Proteus OXK agglutinin titers. Am J
Trop Med Hyg. 32:1101–1107. 1983.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Robinson DM, Brown G, Gan E and Huxsoll
DL: Adaptation of a microimmunofluorescence test to the study of
human Rickettsia tsutsugamuskh antibody. Am J Trop Med Hyg.
25:900–905. 1976.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Blacksell SD, Bryant NJ, Paris DH, Doust
JA, Sakoda Y and Day NPJ: Scrub typhus serologic testing with the
indirect immunofluorescence method as a diagnostic gold standard: A
lack of consensus leads to a lot of confusion. Clin Infect Dis.
44:391–401. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
82
|
Blacksell SD, Tanganuchitcharnchai A,
Nawtaisong P, Kantipong P, Laongnualpanich A, Day NPJ and Paris DH:
Diagnostic accuracy of the InBios scrub typhus detect enzyme-linked
immunoassay for the detection of IgM antibodies in northern
Thailand. Clin Vaccine Immunol. 23:148–154. 2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Blacksell SD, Paris DH, Chierakul W,
Wuthiekanun V, Teeratakul A, Kantipong P and Day NPJ: Prospective
evaluation of commercial antibody-based rapid tests in combination
with a loop-mediated isothermal amplification PCR assay for
detection of Orientia tsutsugamushi during the acute phase
of scrub typhus infection. Clin Vaccine Immunol. 19:391–395.
2012.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Paris DH, Blacksell SD, Nawtaisong P,
Jenjaroen K, Teeraratkul A, Chierakul W, Wuthiekanun V, Kantipong P
and Day NP: Diagnostic accuracy of a loop-mediated isothermal PCR
assay for detection of Orientia tsutsugamushi during acute
Scrub Typhus infection. PLoS Negl Trop Dis. 5(e1307)2011.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Paris DH and Dumler JS: State of the art
of diagnosis of rickettsial diseases: The use of blood specimens
for diagnosis of scrub typhus, spotted fever group rickettsiosis,
and murine typhus. Curr Opin Infect Dis. 29:433–439.
2016.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Paris DH, Blacksell SD, Newton PN and Day
NPJ: Simple, rapid and sensitive detection of Orientia
tsutsugamushi by loop-isothermal DNA amplification. Trans R Soc
Trop Med Hyg. 102:1239–1246. 2008.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Nakayama K, Kurokawa K, Fukuhara M,
Urakami H, Yamamoto S, Yamazaki K, Ogura Y, Ooka T and Hayashi T:
Genome comparison and phylogenetic analysis of Orientia
tsutsugamushi strains. DNA Res. 17:281–291. 2010.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Wongprompitak P, Duong V, Anukool W,
Sreyrath L, Mai TT, Gavotte L, Moulia C, Cornillot E, Ekpo P,
Suputtamongkol Y, et al: Orientia tsutsugamushi, agent of
scrub typhus, displays a single metapopulation with maintenance of
ancestral haplotypes throughout continental South East Asia. Infect
Genet Evol. 31:1–8. 2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Paris DH, Phetsouvanh R,
Tanganuchitcharnchai A, Jones M, Jenjaroen K, Vongsouvath M,
Ferguson DP, Blacksell SD, Newton PN, Day NP and Turner GD:
Orientia tsutsugamushi in human scrub typhus eschars shows
tropism for dendritic cells and monocytes rather than endothelium.
PLoS Negl Trop Dis. 6(e1466)2012.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Valbuena G and Walker DH: Approaches to
vaccines against Orientia tsutsugamushi. Front Cell Infect
Microbiol. 2(170)2012.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Mathai E, Rolain JM, Verghese GM, Abraham
OC, Mathai D, Mathai M and Raoult D: Outbreak of scrub typhus in
southern India during the cooler months. Ann N Y Acad Sci.
990:359–364. 2003.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Watt G, Chouriyagune C, Ruangweerayud R,
Watcharapichat P, Phulsuksombati D, Jongsakul K, Teja-Isavadharm P,
Bhodhidatta D, Corcoran KD, Dasch GA and Strickman D: Scrub typhus
infections poorly responsive to antibiotics in northern Thailand.
Lancet. 348:86–89. 1996.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Longley RL, Woods A, Fleetwood A, Cowling
GJ, Gallagher JT and Couchman JR: Control of morphology,
cytoskeleton and migration by syndecan-4. J Cell Sci.
112:3421–3431. 1999.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Song JH, Kang HB, Park SH, Jeong JH, Park
J, You Y, Lee YH, Lee J, Kim E, Choi KC and Jun W: Extracts of
Porphyra tenera (Nori Seaweed) activate the immune response in
mouse RAW264.7 macrophages via NF-κB signaling. J Med Food.
20:1152–1159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Netea MG, Simon A, van de Veerdonk F,
Kullberg BJ, Van der Meer JWM and Joosten LAB: IL-1beta processing
in host defense: Beyond the inflammasomes. PLoS Pathog.
6(e1000661)2010.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Page AV and Liles WC: Biomarkers of
endothelial activation/dysfunction in infectious diseases.
Virulence. 4:507–516. 2013.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Chousterman BG, Swirski FK and Weber GF:
Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol.
39:517–528. 2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Shelite TR, Liang Y, Wang H, Mendell NL,
Trent BJ, Sun J, Gong B, Xu G, Hu H, Bouyer DH and Soong L:
IL-33-Dependent endothelial activation contributes to apoptosis and
renal injury in Orientia tsutsugamushi-Infected mice. PLoS
Negl Trop Dis. 10(e0004467)2016.PubMed/NCBI View Article : Google Scholar
|
|
99
|
El Sayed I, Liu Q, Wee I and Hine P:
Antibiotics for treating scrub typhus. Cochrane Database Syst Rev.
9(CD002150)2018.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Watt G, Kantipong P, Jongsakul K,
Watcharapichat P and Phulsuksombati D: Azithromycin activities
against Orientia tsutsugamushi strains isolated in cases of
scrub typhus in Northern Thailand. Antimicrob Agents Chemother.
43:2817–2818. 1999.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Song JH, Lee C, Chang WH, Choi SW, Choi
JE, Kim YS, Cho SR, Ryu J and Pai CH: Short-course doxycycline
treatment versus conventional tetracycline therapy for scrub
typhus: A multicenter randomized trial. Clin Infect Dis.
21:506–510. 1995.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Rajapakse S, Rodrigo C and Fernando SD:
Drug treatment of scrub typhus. Trop Doct. 41:1–4. 2011.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Ives TJ, Manzewitsch P, Regnery RL, Butts
JD and Kebede M: In vitro susceptibilities of Bartonella henselae,
B. quintana, B. elizabethae, Rickettsia rickettsii, R. conorii, R.
akari, and R. prowazekii to macrolide antibiotics as determined by
immunofluorescent-antibody analysis of infected Vero cell
monolayers. Antimicrob Agents Chemother. 41:578–582.
1997.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Strickman D, Sheer T, Salata K, Hershey J,
Dasch G, Kelly D and Kuschner R: In vitro effectiveness of
azithromycin against doxycycline-resistant and-susceptible strains
of Rickettsia tsutsugamushi, etiologic agent of scrub typhus.
Antimicrob Agents Chemother. 39:2406–2410. 1995.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Watt G, Kantipong P, Jongsakul K,
Watcharapichat P, Phulsuksombati D and Strickman D: Doxycycline and
rifampicin for mild scrub-typhus infections in northern Thailand: A
randomised trial. Lancet. 356:1057–1061. 2000.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Jensenius M, Han PV, Schlagenhauf P,
Schwartz E, Parola P, Castelli F, von Sonnenburg F, Loutan L, Leder
K and Freedman DO: GeoSentinel Surveillance Network. Acute and
potentially life-threatening tropical diseases in western
travelers-a GeoSentinel multicenter study, 1996-2011. Am J Trop Med
Hyg. 88:397–404. 2013.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Nachega JB, Bottieau E, Zech F and Van
Gompel A: Travel-acquired scrub typhus: Emphasis on the
differential diagnosis, treatment, and prevention strategies. J
Travel Med. 14:352–355. 2007.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Jensenius M, Fournier PE and Raoult D:
Rickettsioses and the international traveler. Clin Infect Dis.
39:1493–1499. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
109
|
Basnyat B: Typhoid versus typhus fever in
post-earthquake Nepal. Lancet Glob Health. 4:e516–517.
2016.PubMed/NCBI View Article : Google Scholar
|