Introduction
Among the continents, Asia recorded the highest
incidence and mortality rates of nasopharyngeal carcinoma (NPC).
GLOBOCAN 2022 (version 1.1) estimates that Malaysia is one of the
Asian countries with the highest age-standardized rate (ASR) of NPC
at 5.7 per 100,000 individuals (1). Moreover, according to ‘Cancer
Tomorrow’ (2), the estimated
number of new NPC cases in Malaysia will increase from 2,410 in
2022 to 3,660 in 2045. According to the National Cancer Registry,
between 2012 and 2016, NPC was the fifth most common type of cancer
among Malaysian males, with the incidence increasing rapidly after
the age of 25 years and peaking at the age of 65 years (3). There were 4,597 cases of NPC in
Malaysia between the years 2012 and 2016(3). Of note, ~69% of male and 65% of
female patients with NPC in Malaysia present with late stages of
the disease, at stages III and IV (3), leading to a 5-year relative survival
rate of only ~46%, with a median survival time of 40.6 months
(4). In Malaysia, NPC in childhood
(ages 0-19 years) was the 7th and 9th most common type of cancer
between the years 2007-2011 and 2012-2016, respectively (5).
NPC is the most common type of tumour that may
develop in the nasopharynx (6,7).
NPC, a type of cancer originating from the nasopharynx epithelium
(8), is prevalent in Southern
China, Southeast Asia, North Africa, and among the Eskimos of
Alaska and Greenland (9). The
Bidayuh, a native ethnic group of East Malaysia, has a
predisposition for NPC (10). The
ASR for Bidayuh was 24.6 per 100,000 males and 9.3 per 100,000
females (10). There are multiple
risk factors involved in the development of NPC, including
Epstein-Barr Virus (EBV) infection, genetic predisposition, diet,
environmental exposure and tobacco smoking. The probable
cancer-related risk factors contributing to the development of NPC
among the Bidayuh population have been previously reviewed
(11).
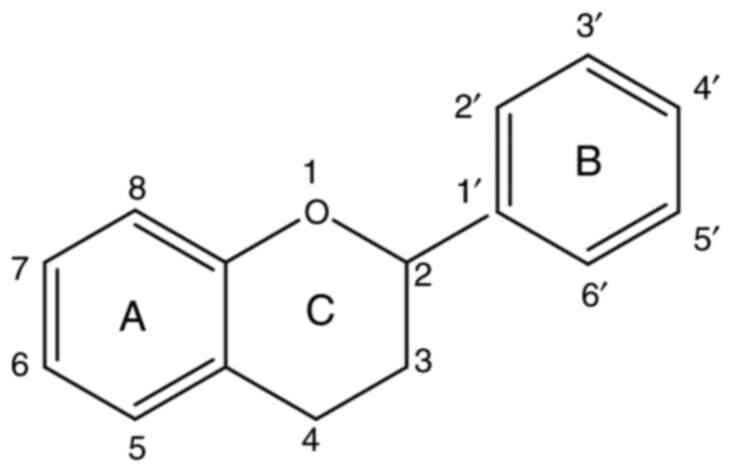
Flavonoids, low-molecular-weight polyphenolic
compounds, are classified based on the carbon atom of ring C
(closed pyran) to which ring B (benzene ring) is attached, the
degree of unsaturation and oxidation of ring C (Fig. 1) (12). Sub-classes of flavonoids include
anthocyanins, chalcones, flavones, isoflavones and flavonols.
Patients undergoing locoregional therapy for
advanced stages of NPC are treated with concurrent chemotherapy and
radiotherapy (13,14). Cytotoxic chemotherapeutic drugs
include cisplatin and 5-fluorouracil. However, the use of cisplatin
is frequently associated with renal toxicity. Due to toxicities
often associated with conventional cancer therapeutics,
alternative, less toxic treatments are essential. There is a surge
of interest in researching plant-derived flavonoids and their
glycosides in human nutrition due to their health-related benefits
for various chronic diseases, including cancer. Plant-derived
flavonoids have the potential to be developed as an alternative
therapy for the treatment of NPC with no side-effects. For example,
quercetin was previously shown to reduce cisplatin toxicity in the
LLC-PK1 pig kidney-derived cell line (15). Another study demonstrated that in
the kidneys of tumour-bearing rats, quercetin exhibited protective
activity from toxic damage inflicted by cisplatin (16). Furthermore, another study
demonstrated that in vivo, cynaroside
(luteolin-7-O-glucoside) decreased cisplatin-induced nephrotoxicity
in the kidneys of mice, whereas, in vitro, cynaroside
reduced the cisplatin-induced death in human kidney proximal tubule
HK-2 cells (17). Simultaneous
reports of the inhibitory effects of flavonoids in their free forms
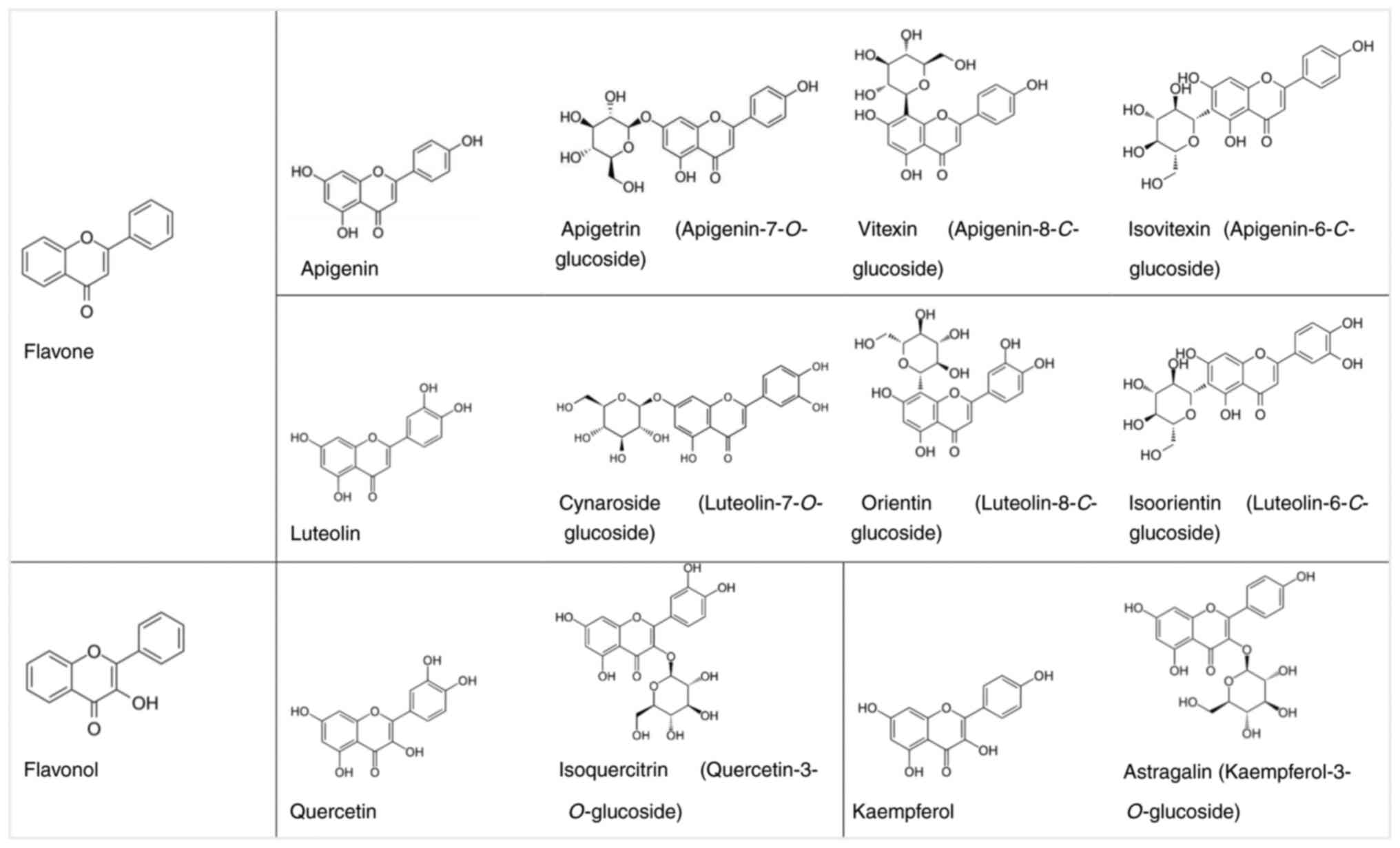
and glycosides are uncommon. The present study aimed to determine
the inhibitory effects of selected aglycones of flavones (apigenin
and luteolin) and flavonols (kaempferol and quercetin) and their
respective glycosides (apigetrin, vitexin, isovitexin, cynaroside,
orientin, isoorientin, astragalin and isoquercitrin) (Fig. 2) on C666-1 and HK1 cells, two cell
lines often used as cell models for NPC. Finally, molecular docking
was carried out against B-cell lymphoma-w (BCL-w) in an aim to
better understand the mechanisms of action of flavones, flavonols
and their glycosidic derivatives.
These flavones and flavonols were selected for the
present study based on numerous literature reports of their
inhibitory effects on various human cancer cell lines (18-24).
Numerous literature reports exist on the therapeutic properties of
flavonoids and their dietary applications (25-27).
Medicinal properties of flavonoids are ascribed to their ability to
prevent injuries caused by reactive oxygen and nitrogen species.
Hydroxyl groups of flavonoids react with and convert radicals into
stable forms (28). Flavonoids
exhibit potent antioxidant properties by neutralizing free radicals
and reducing oxidative stress, which plays a crucial role in cancer
progression (29). Additionally,
flavonoids modulate inflammatory responses, which may prove to be
beneficial in the prevention and treatment of cancer (30). Previous studies have demonstrated
that flavonoids induce the apoptosis (programmed cell death) of
cancer cells, suggesting their potential use as anticancer agents
(29,31). Moreover, flavonoids can target
multiple molecular pathways involved in cancer cell survival and
proliferation, demonstrating their effectiveness in inhibiting
tumour growth (31). Furthermore,
apigenin (32), luteolin (33,34)
and kaempferol (35) have been
found to inhibit EBV reactivation, a key factor in NPC, as NPC is
strongly associated with EBV (36).
Materials and methods
Chemicals
Apigenin, apigetrin, astragalin, cynaroside,
isoorientin, isoquercitrin, isovitexin, kaempferol, luteolin,
orientin, quercetin and vitexin were purchased from Merck KGaA. The
purity of all the flavonoids was ≥98%, determined by
high-performance liquid chromatography (data not shown). Cisplatin
was obtained from Cayman Chemical Co.
In vitro evaluation of NPC cell
viability inhibition. Cells and cell culture
HK1(37), an NPC
cell line, was maintained in Roswell Park Memorial Institute
(RPMI)-1640 (Gibco; Thermo Fisher Scientific, Inc.) medium
supplemented with 10% heat-inactivated foetal calf serum (FCS;
Gibco; Thermo Fisher Scientific, Inc.), 10 U/ml penicillin (Gibco;
Thermo Fisher Scientific, Inc.) and 10 µg/ml streptomycin (Gibco;
Thermo Fisher Scientific, Inc.) in a 5% carbon dioxide
(CO2) humidified atmosphere at 37˚C. The C666-1 cells
(38), another NPC cell line, was
maintained in a similar manner, apart from the addition of 1X
GlutaMAX-I Supplement (Gibco; Thermo Fisher Scientific, Inc.).
HaCaT (39) cells, an immortalised
human skin keratinocyte cell line, was maintained in Dulbecco's
Modified Eagle Medium (DMEM) (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FCS. The HaCaT cell line (cell cat. no.
300493) was procured from CLS Cell Lines Service GmbH. The HaCaT
cell line, as well as the immortalised nasopharyngeal epithelial
cell lines, NP460hTERT (40) and
NP69SV40T (41), represented
normal cells. NP69SV40T was maintained in 1X keratinocyte
serum-free medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 25 µg/ml bovine pituitary extract and 0.16 ng/ml
recombinant epidermal growth factor. The NP460hTERT cells were
maintained at a 1:1 ratio of 1X defined keratinocyte serum-free
medium (Gibco; Thermo Fisher Scientific, Inc.) with the addition of
growth supplement: EpiLife Medium (Gibco; Thermo Fisher Scientific,
Inc.) with EpiLife Defined Growth Supplement (Cascade Biologics,
Life Technologies; Thermo Fisher Scientific, Inc.). DNA
fingerprinting for HK1, C666-1, NP69SV40T and NP460hTERT cells was
performed using the AmpFISTRIdentifiler® polymerase
chain reaction amplification kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The routine detection of the mycoplasma
contamination was carried out using the e-Myco™ Mycoplasma
Polymerase chain reaction (PCR) Detection Kit (iNtRON
Biotechnology, Inc,), according to the protocol of the
manufacturer. Only mycoplasma-free cells were used throughout the
study.
Evaluation of cell viability inhibition using MTS
assay. A total of 5,000-30,000 C666-1, HK1, NP460hTERT,
NP69SV40T and HaCaT cells/100 µl medium/well were seeded in 96-well
microtiter plates and returned into the incubator at 37˚C in a 5%
CO2 atmosphere for 1 day. After 1 day, the culture
medium was aspirated and replaced with 100 µl freshly prepared
culture medium containing 0-300 µM flavonoids dissolved in dimethyl
sulfoxide (DMSO) (MilliporeSigma). The solvent control group was
cells in 0.5% DMSO. The microtiter plates were returned to the
incubator for 1-3 days. Using the CellTiter 96®
AQueous One Solution Cell Proliferation (MTS) assay
(Promega Corporation) as previously described (42), the percentage MTS signal was
obtained after 1-3 days to determine whether flavonoids could
inhibit the cells. Briefly, 20 µl MTS reagent were added to each
well and the microtiter plates were returned to the incubator for
1-4 h, according to the optimal incubation time for MTS reagent.
The absorbance at 490 nm and non-specific absorbance at 630 nm was
measured using an EnVision multilabel plate reader (PerkinElmer,
Inc.). Wells with medium but without cells served as the blank
control. The percentage of MTS signal was calculated and compared
to that of the solvent control group. Dose-response curves for 1-3
days of flavonoid exposure were plotted. IC50 values,
specifically the effective concentration that inhibited 50% MTS
signal relative to the solvent control group, were obtained by
using non-linear regression (curve fit) on GraphPad Prism version
6.07 for Windows (Dotmatics). At least three independent
experiments were performed, and the average IC50 value
was reported. Statistical analysis, as for example, using a t-test
or ANOVA, is not typically performed for reports of IC50
values. Rather, the IC50 values of the flavonoids were
compared to cisplatin, a conventional chemotherapeutic drug for
NPC, and used herein as the positive control. Cisplatin (0-300 µM)
was assayed for 1-3 days. Additionally, calculating the selectivity
index of the flavonoids and standard chemotherapeutic drugs is also
useful. The selectivity index (SI) was calculated as the ratio of
IC50 values in normal cells to cancer cells (43).
xCELLigence cell inhibition assay. The
C666-1, HK1, NP460hTERT, NP69SV40T and HaCaT cells were seeded at a
density of 5,000-3,0000 cells/100 µl medium/well into E-Plate 16
(ACEA Biosciences, Inc.) for 1 day. The culture medium was then
aspirated and replaced with 100 µl freshly prepared medium
containing apigenin, kaempferol, or luteolin, or their respective
glucoside to a final concentration of 25, 50, or 100 µM. Cells in
0.5% DMSO were the solvent control group. The growth pattern of the
cells was monitored dynamically on the impedance-based real-time
cell analyser xCELLigence RTCA DP (ACEA Biosciences, Inc.) for a
further 76 h at 37˚C in a 5% CO2 atmosphere to validate
the effect of flavonoids on cells. Throughout the 76-h period, the
culture medium was neither aspirated nor replaced. The data
analysis software used was RTCA version 2.0 (ACEA Biosciences,
Inc.). The cell index was normalised to the time immediately point
prior to the addition of the flavonoids. At least three independent
experiments were performed.
Molecular docking analyses. Protein
preparation
The three-dimensional (3D) structure of BCL-w (PDB
ID: 2Y6W) (44) was selected and
downloaded from Protein Data Bank (PDB) (45). Biovia Discovery Studio (DS)
(Biovia) (46) and AutoDock Tools
1.5.6 (ADT) (47) were used to
prepare the protein for docking. The protein structure was imported
to AutoDock to remove the water molecules and add the polar
hydrogen atoms and Gasteiger charges. Finally, the protein
structures were saved in pdbqt format.
Ligand preparation. ChemSketch Software
version 2019 (Advanced Chemistry Development, Inc.) was used to
draw the 2D structures of the selected flavones, flavonols and
their glycosidic derivatives. Biovia DS was used to convert the 2D
structures of the compound into their respective 3D structures, and
the geometry was optimised using a DREIDING-like forcefield. The 3D
structures of the ligands were imported into AutoDock, where ADT
was used to prepare the ligands for docking by adding charges,
setting the rotatable bonds, and allowing all the torsions to
rotate for the ligands. All the ligands were then saved in pdbqt
format.
Identification of the binding site. The
binding site of protein structure was identified based on the
residues previously reported in the literature (48). ADT was used to determine the Grid
Box that covers the entire identified binding site of the protein.
The coordinates and sizes of the Grid Box were saved in the input
parameter file.
Molecular docking. After preparing the
protein structure, ligands and the input parameter file, molecular
docking was performed using AutoDock Vina 1.1.2. (49,50).
The prepared ligands were docked into the active sites of the
prepared target protein. All the docking parameters have remained
as default settings. The binding affinities of the ligands were
recorded and compared. The binding poses of the ligands and the
mode of interactions of the protein-ligand complex were studied
using Biovia DS.
Results
In vitro evaluation of NPC cell
viability inhibition
A preliminary screening involving four pure
compounds, apigenin, kaempferol, luteolin and quercetin, and their
respective glucosides was performed using MTS assay. Effective
concentrations required to inhibit 50% MTS signal were attained
from concentration-response curves. As a solvent control group,
DMSO did not influence the percentage of MTS signal. Generally,
apigenin, kaempferol, luteolin and quercetin exerted time- and
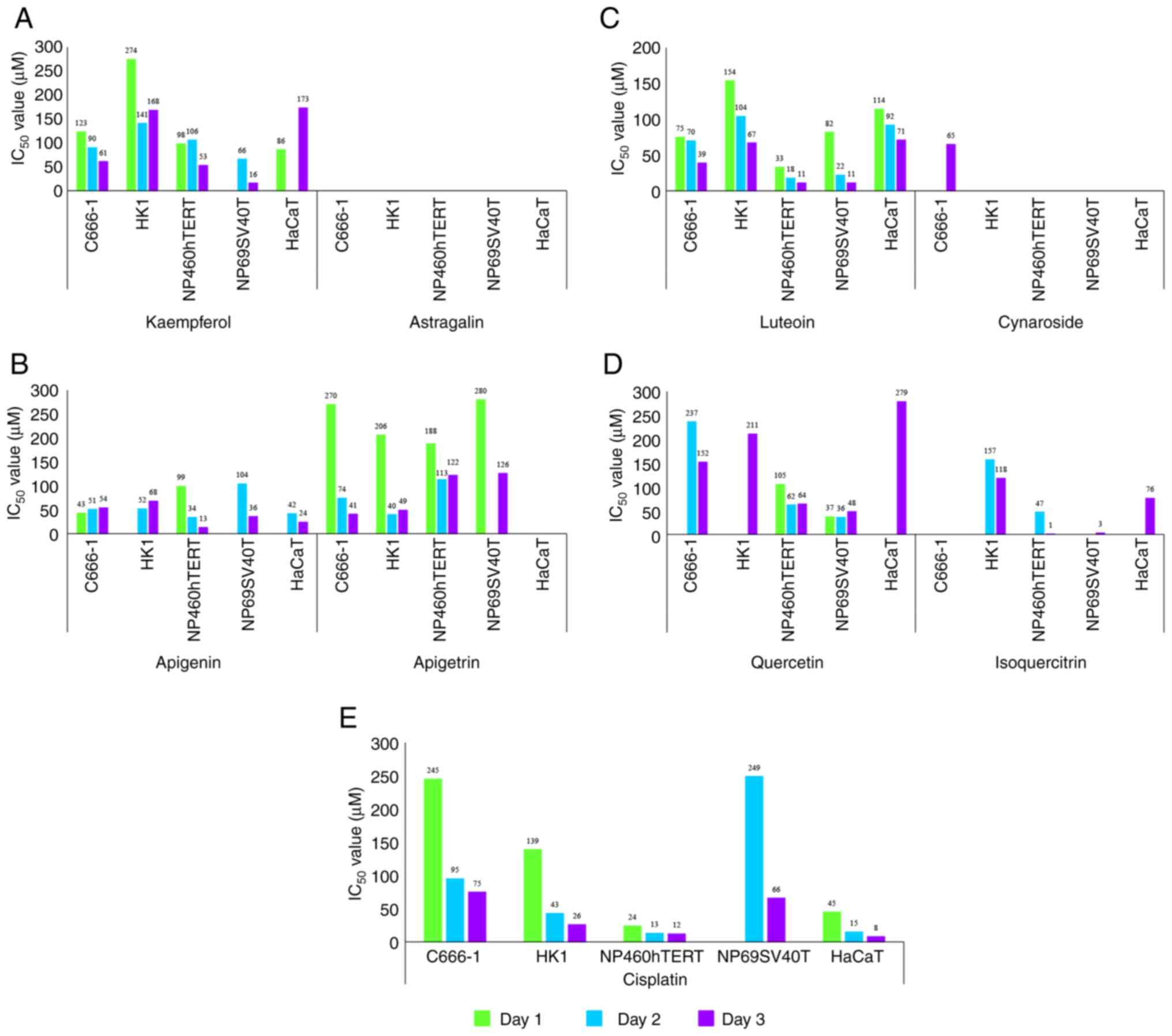
concentration-dependent inhibitory effects (Fig. 3A-D and Table SI). Apigenin,
kaempferol, luteolin, and quercetin were more effective against the
cell lines tested; in most cases, IC50 values of
apigenin, kaempferol, luteolin and quercetin were lower than their
glucoside equivalents. On the whole, IC50 values of the
glucoside equivalents were unreachable (Fig. 3A-D and Table SI). However, even at
the highest concentration tested, vitexin, isovitexin, cynaroside,
orientin, isoorientin and astragalin did not markedly inhibit the
cell lines tested (Table SI). Cisplatin, a standard
chemotherapeutic drug used for the treatment of NPC, killed
immortalised nasopharyngeal epithelial cells and immortalised human
skin keratinocytes, in addition to NPC cells (Fig. 3E and Table SI). Due to the
toxicities frequently associated with cisplatin, alternative, less
toxic treatments are essential. The SI is a calculation used to
evaluate the toxicity of compounds against normal cells (51). A higher SI value means that cancer
cells could be killed more than normal cells; thus, SI can predict
the therapeutic potential (51).
An SI>2 is favourable for a compound to be an effective
anticancer agent (43). Thus,
herein, where the IC50 could be attained, the SI values
of cisplatin, apigenin, kaempferol, luteolin, quercetin and their
glucoside equivalents on NPC cells against normal cells were
determined (Table I). Apigetrin
was the most selective flavonoid tested between normal and NPC
cells, with SI values ranging between 2.490 and 3.073 (Table I).
 | Table ISelectivity index of immortalised
nasopharyngeal epithelial cells and immortalised human skin
keratinocyte against nasopharyngeal carcinoma cells at 1-3 days
post-treatment. |
Table I
Selectivity index of immortalised
nasopharyngeal epithelial cells and immortalised human skin
keratinocyte against nasopharyngeal carcinoma cells at 1-3 days
post-treatment.
| | Epithelial,
undifferentiated carcinoma C666-1 | Epithelial,
squamous cell carcinoma HK1 |
|---|
| | Selectivity index,
IC50 NP460hTERT/IC50 C666-1 | Selectivity index,
IC50 NP69SV40T/IC50 C666-1 | Selectivity index,
IC50 HaCaT/IC50 C666-1 | Selectivity index,
IC50 NP460hTERT/IC50 HK1 | Selectivity index,
IC50 NP69SV40T/IC50 HK1 | Selectivity index,
IC50 HaCaT/IC50 HK1 |
|---|
| Standard
chemotherapeutic drug or flavonoid | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 |
|---|
| Cisplatin
Flavonoid | 0.098 | 0.137 | 0.160 | NA | 2.621 | 0.880 | 0.184 | 0.158 | 0.107 | 0.172 | 0.302 | 0.462 | NA | 5.791 | 2.538 | 0.324 | 0.349 | 0.308 |
| Apigenin | 2.302 | 0.667 | 0.240 | NA | 2.039 | 0.667 | NA | 0.823 | 0.444 | - | 0.654 | 0.191 | NA | 2 | 0.529 | NA | 0.808 | 0.353 |
| Apigetrin
(Apigenin-7-O-glucoside) | 0.696 | 1.527 | 2.976 | 1.037 | NA | 3.073 | NA | NA | NA | 0.913 | 2.825 | 2.490 | 1.359 | NA | 2.571 | NA | NA | NA |
| Kaempferol | 0.797 | 1.178 | 0.869 | NA | 0.733 | 0.262 | 0.699 | NA | 2.836 | 0.358 | 0.752 | 0.315 | NA | 0.468 | 0.095 | 0.314 | NA | 1.030 |
| Astragalin
(Kaempferol-3-O-glucoside) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Luteolin | 0.440 | 0.257 | 0.282 | 1.093 | 0.314 | 0.282 | 1.520 | 1.314 | 1.821 | 0.214 | 0.173 | 0.164 | 0.532 | 0.212 | 0.164 | 0.740 | 0.885 | 1.060 |
| Cynaroside
(Luteolin-7-O-glucoside) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Quercetin | - | 0.262 | 0.421 | - | 0.152 | 0.316 | NA | NA | 1.836 | - | - | 0.303 | - | - | 0.227 | NA | NA | 1.322 |
| Isoquercitrin
(Quercetin-3-O-glucoside) | NA | - | - | NA | NA | - | NA | NA | - | NA | 0.299 | 0.008 | NA | NA | 0.025 | NA | NA | 0.644 |
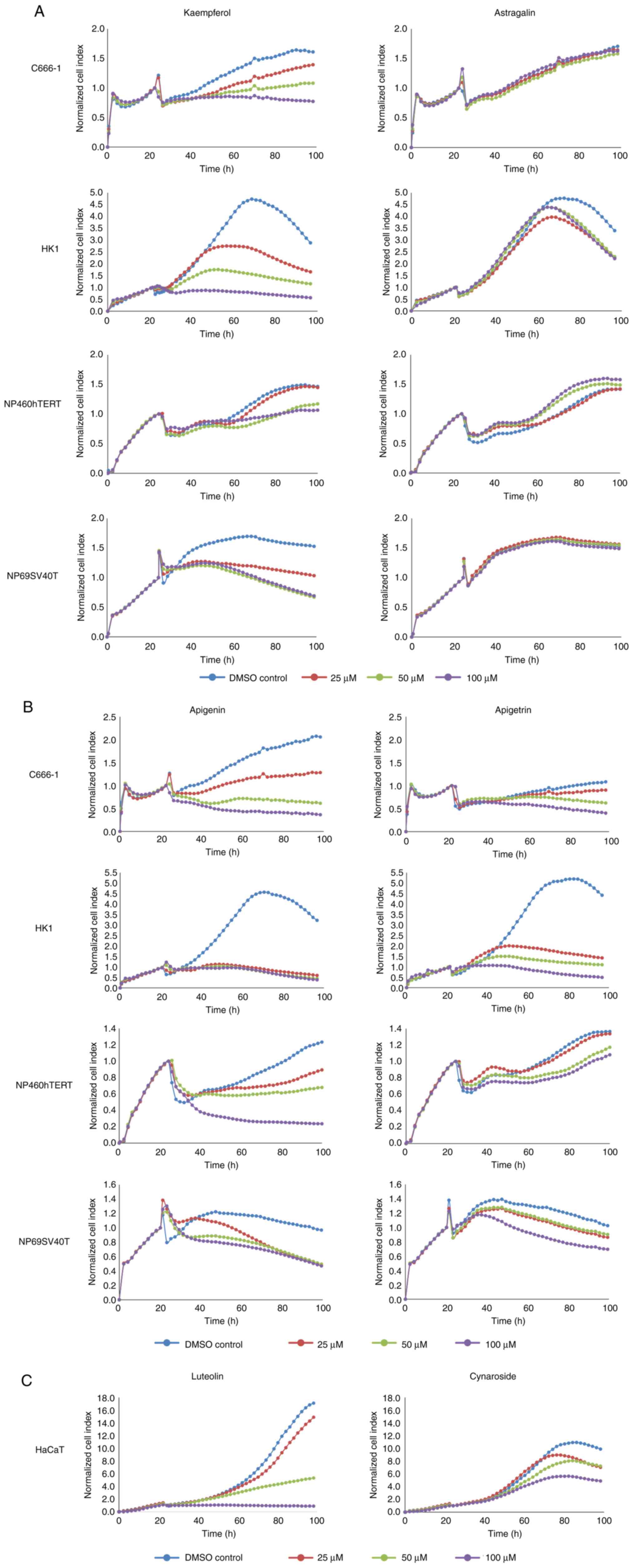
In order to verify the results of MTS assay, growth
kinetics were monitored dynamically through the xCELLigence system,
a real-time, impedance-based cell analyser used to quantify
adherent cell proliferation, cell viability, and cytotoxicity
non-invasively. The cell index, a dimensionless parameter,
increased in correspondence to impedance amplification within the
electrical circuit caused by more cells deposited on the bottom of
the well. The cell index represented growth or proliferation over
time. The normalised cell index generated by NPC cells and
nasopharyngeal epithelial cells in kaempferol was markedly lower
than that of the control cells; 100 µM kaempferol led to a
near-static normalised cell index (Fig. 4A). Apigenin essentially yielded
similar results in NPC cells and nasopharyngeal epithelial cells,
evident from the rapid decline of the normalised cell index
compared to the control cells (Fig.
4B). Luteolin produced a similar effect on human skin
keratinocytes (Fig. 4C). Cells
exposed to astragalin (Figs. 3A
and 4A) or cynaroside (Figs. 3C and 4C) were not inhibited. The normalised
cell index in astragalin or cynaroside was comparable to the
control cells (Fig. 4A and
C). Finally, dynamic monitoring
demonstrated that apigetrin inhibited NPC cells and nasopharyngeal
epithelial cells to a certain extent (Figs. 3B and 4B). Thus, the outcomes of the xCELLigence
system were in agreement with those of the MTS assay.
Docking analyses
C666-1 and HK1, two cell lines often used as cell
models for NPC, express all the anti-apoptotic genes, including
BCL-w, apart from for BFL-1 in C666-1(52). In the present study, in order to
better understand the mechanisms of action of the selected
flavones, flavonols and their glycosidic derivatives, molecular
docking analyses were carried out against BCL-w as the target site.
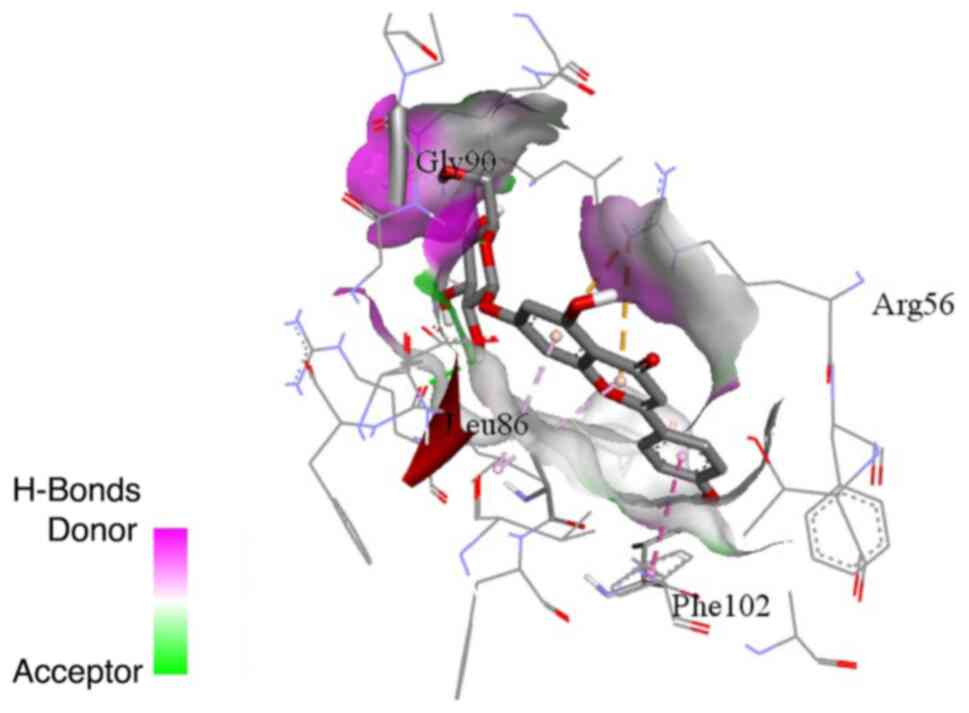
Cynaroside possessed the highest binding affinity among all
flavones, flavonols and their glycoside derivatives (Table II). Cynaroside was followed by
apigetrin, followed by both isoorientin and isovitexin. Apigenin
and kaempferol had the lowest affinities for BCL-w. In general, the
O-glycosides demonstrated the highest binding affinity,
followed by the C-glycosides, and the aglycones exhibited
the lowest affinities for BCL-w. The evaluation of the binding
modes revealed that the binding modes of cynaroside and apigetrin
were relatively similar, and they are bound to the ligand binding
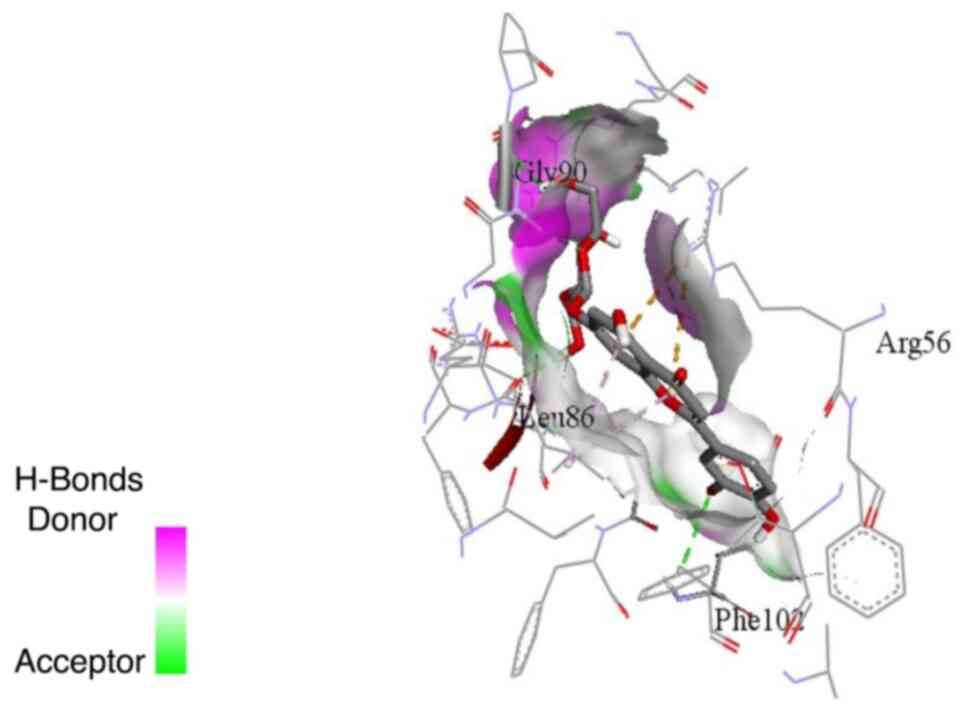
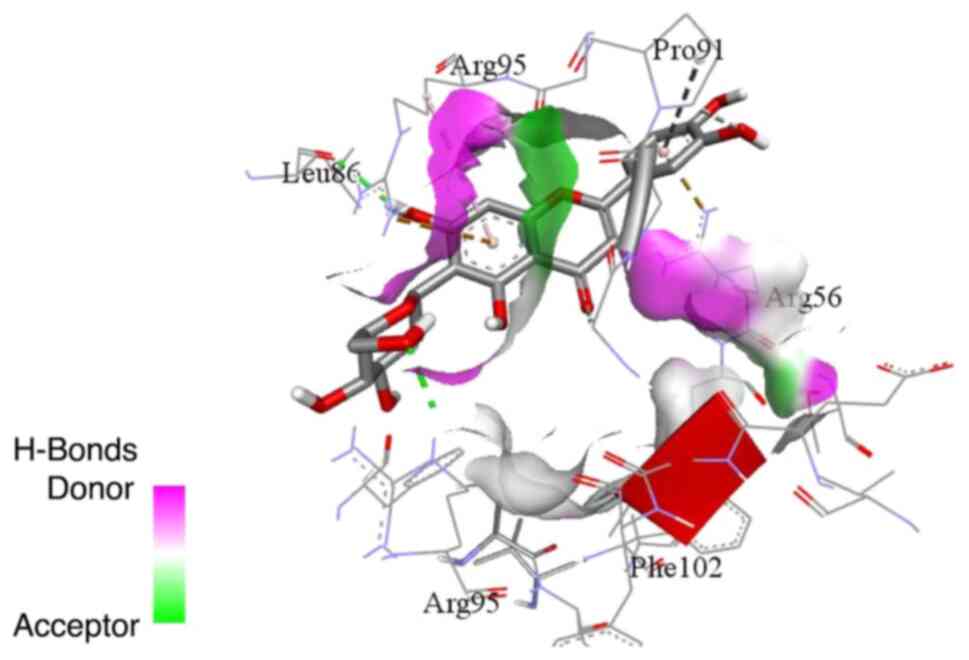
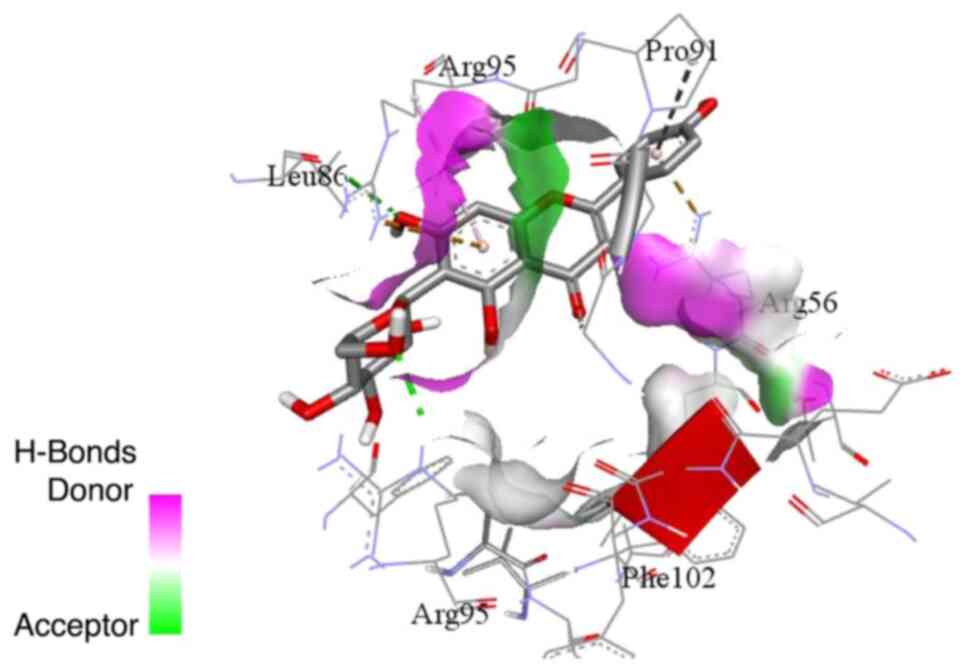
pocket (Figs. 5 and 6). On the other hand, isoorientin and
isovitexin (Figs. 7 and 8) exhibited relatively different binding
modes from cynaroside and apigetrin. Isoorientin and isovitexin
bound to a pocket adjacent to the pocket where cynaroside and
apigetrin were bound. The binding interactions demonstrated by
isoorientin and isovitexin also differed from those of cynaroside
and apigetrin. Cynaroside formed hydrogen bonds with glycine 90 and
phenylalanine 102, while it formed hydrophobic interactions via its
chromene ring with arginine 56 and leucine 86. Apigetrin formed a
hydrogen bond with glycine 90 and hydrophobic interactions via its
chromene ring with arginine 56 and leucine 86 and the phenyl ring
with phenylalanine 102. The binding interactions of isoorientin and
isovitexin with the BCL-w binding site included hydrogen bonds with
leucine 86 and arginine 95, and hydrophobic interactions via its
chromene.
 | Table IIDocking scores of the selected
flavones, flavonols and their glycosidic derivatives. |
Table II
Docking scores of the selected
flavones, flavonols and their glycosidic derivatives.
| Ligand | Binding affinity
(Kcal/mol) |
|---|
| Apigenin | -6.9 |
| Apigetrin | -8.8 |
| Astragalin | -7.9 |
| Cynaroside | -9.1 |
| Isoorientin | -8.2 |
| Isoquercitrin | -7.9 |
| Isovitexin | -8.2 |
| Kempferol | -6.9 |
| Luteolin | -7.7 |
| Orientin | -7.9 |
| Quercetin | -7.1 |
| Vitexin | -7.6 |
Discussion
Previous studies have demonstrated the inhibitory
effects of flavonoids on various human cancer cell lines. Flavones
and flavonols have been reported to be the most bioactive
flavonoids against gastric and pancreatic cancer (53,54).
Among other chemicals, kaempferol, apigenin, luteolin and quercetin
have been shown to inhibit OVCAR-3 ovarian cancer cell
proliferation (55). In another
study, in human leukaemia Jurkat T-cells, the order of potency at
24 h post-treatment was apigenin > quercetin > kaempferol
> myricetin (56). Moreover,
among various flavonoids, apigenin, genistein and luteolin were
shown to significantly reduce human colorectal cancer HCT-116 cell
growth (57). In addition, amongst
other flavones and isoflavones, apigenin was the most effective
compound for arresting cell growth (57). It was previously demonstrated that
apigenin 7-glucoside inhibited human promyelocytic leukaemia HL-60
cell proliferation (58). Luteolin
was shown to inhibit NPC CNE2(59)
and NPC HK1, C17, C666-1 and NPC 43 cell growth (42). Treatment with 100 µM quercetin for
3 days was also shown to inhibit NPC C666-1 and HK1 cells (19). Similarly, was found to quercetin
abrogate the proliferation and induce the death of NPC 5-8F and
C666-1 cells (22).
Flavones and flavonols are predominant classes of
flavonoids. The structure of flavones has a double bond between
positions 2 and 3 and a ketone in position 4 of the C ring
(Fig. 2). Flavonols, also known as
3-hydroxyflavone, are structurally similar to flavones, apart from
for the addition of a hydroxyl group in position 3 of ring C
(Fig. 2). Flavones and flavonols
occur in nature in a free state (aglycones) and as O- or
C-glycosides, where the sugar moiety is linked to the
hydroxy group of the aglycone (O-glycoside) or directly to
the carbon atom of the aglycone (C-glycoside). Apigenin,
luteolin, kaempferol and quercetin were effective against the cell
lines tested in the present study. Notably, the inhibitory activity
of apigenin and luteolin was higher than that of kaempferol and
quercetin. The number of hydroxy groups on ring B of flavone and
flavonol aglycones was observed to influence the inhibitory effect,
as apigenin exhibited better activity than luteolin, whilst
kaempferol exhibited better activity than quercetin. Apigenin and
kaempferol have one hydroxy group on ring B, while luteolin and
quercetin have two hydroxyl groups on ring B (Fig. 2). Thus, increasing the solubility
of molecules by introducing hydroxy functions may not be suitable
for the in vitro assays described herein. This was further
confirmed by comparing aglycones with their respective glycosides.
Glycosides are bioactive aglycone and glucosyl group conjugations.
Aglycones (apigenin, kaempferol, luteolin and quercetin) were more
effective against the cell lines tested as compared to the
respective O-glycosides and C-glycosides (Fig. 3 and Table SI). In vitro, the
inhibitory effect of O-glycosides was relatively higher than
their corresponding C-glycosides. Even at the highest
concentration tested, C-glycosides (vitexin, isovitexin,
orientin, and isoorientin) had no marked inhibitory effect on the
cell lines assessed (Table SI). Such a report on NPC cells has not
been made available to date, at least to the best of our knowledge.
However, one limitation of the present study is the absence of
statistical analysis.
The efficacy of flavonoids and their glycoside
derivatives in inhibiting NPC cells has been well-documented
(29). It has been shown that
these compounds exhibit significant anticancer effects, such as the
induction of apoptosis and induction of numerous signalling
pathways, thus inhibiting NPC cell growth and proliferation
(60). Flavonoids are known to
neutralize free radicals, reduce oxidative stress and modulate
inflammatory responses, which are essential in preventing cancer
progression and causing cell cycle arrest (61). It has been shown that the use of
100 µM quercetin arrested HK1 NPC cells at the G2/M phase (19). Moreover, quercetin was found to
decrease the expression of fatty acid synthase (FASN) and
Ki67(19). The expression of FASN
is elevated in highly proliferating cancer cells to accommodate
intensified fatty acid synthesis (62), while Ki67 antigen is a cell
proliferation marker. Luteolin is particularly effective in
triggering ferroptosis and inhibiting the proliferation of NPC
cells. It interferes with pathways involved in cancer cell survival
and growth (63). Additionally,
quercetin or luteolin, combined with cisplatin, produced
synergistic effects against NPC cells (19,42).
Morusin, a flavonoid obtained from the Morus plant, targets
various molecular targets and signalling pathways, hindering the
growth of NPC (64). Glycoside
derivatives of flavonoids have an advantage over aglycones due to
their enhanced bioavailability and targeted actions, which improve
their anticancer effects. Glycoside derivatives improve the
bioavailability of flavonoids, leading to increased apoptotic
protein expression and ultimately causing cell death in cancer
cells (65). Moreover, glycoside
derivatives have demonstrated targeted effects on various cancer
cells, triggering necroptosis, apoptosis, and cell cycle arrest
(65).
BCL-w is an anti-apoptotic member of the B-cell
lymphoma-2 (BCL-2) family of proteins that integrate signals that
prompt cell survival or apoptosis (66). Several signalling pathways control
the BCL-w level. BCL-w levels have been found to be elevated in
various types of cancer. Increased levels of BCL-w may be ascribed
to the abnormal activation of signalling cascades involved in
regulating BCL-w expression (67).
Increased levels of BCL-w, together with oncogene activation,
contribute to cancer development and progression. The inhibition of
BCL-w may benefit patients with cancer. To date, to the best of our
knowledge, no known agents selectively target BCL-w (67). BCL-w exhibits a high conformational
flexibility (67). Therefore, it
would be advantageous to elucidate how this conformational
flexibility could be used to develop inhibitors selective of BCL-w
(67). Drugs and natural compounds
that affect BCL-w levels, including the downregulation of BCL-w
protein expression levels by cisplatin in the JHU-029 head and neck
squamous cell carcinoma cell line, have been reviewed
comprehensively (67). Quercetin
has been shown to decrease BCL-w mRNA expression in N2a
mouse neuroblastoma cell line (68). The docking scores of matrine, used
to treat diverse cancers, including NPC, and matrine-derivatives,
have been reported (48). However,
to the best of our knowledge, no study available to date has
described the potential of flavonoids to be developed as inhibitors
selective for BCL-w in NPC.
Flavonoids induce the apoptosis of cancer cells by
binding to BCL-w and disrupting its anti-apoptotic function
(69). A comparison of the binding
sites of different flavonoids with BCL-w reveals both similarities
and differences in their molecular interactions and structural
features (70). Variations in
binding affinities to BCL-w among different flavonoids can affect
their potency and efficacy in inducing apoptosis. The specific
amino acids involved in binding interactions may differ among
flavonoids, leading to variations in their biological activity
(70). Structural features of
flavonoids, such as the presence of hydroxyl groups and
glycosylation, can influence their binding to BCL-w and overall
biological activity. The hydroxyl groups of flavonoids form
hydrogen bonds with specific amino acids in the binding site of
BCL-w, enabling interaction and potential inhibition of BCL-w
activity (71). However,
differences in the orientation and positioning of these groups
among different flavonoids can result in varying degrees of binding
affinity and biological activity. Flavonoids with higher binding
affinity and optimal structural features are more effective at
inducing apoptosis in cancer cells by disrupting BCL-w function
(67). For example, a flavonoid
with additional hydroxyl groups may exhibit stronger interactions
with key amino acids in the binding site, leading to increased
biological activity by enhancing the inhibition of BCL-w.
Conversely, another flavonoid with a different orientation of
hydroxyl groups may have reduced binding affinity and lower
biological activity. Therefore, understanding these structural
differences in binding sites and their impact on biological
activity is essential for the rational design of flavonoid-based
compounds with enhanced BCL-w inhibitory effects (70).
In the present study, cynaroside exhibited the
highest binding affinity for BCL-w, followed by apigetrin, while
isoorientin and isovitexin exhibited moderate affinities. Apigenin
and kaempferol recorded the lowest affinities for BCL-w. The
structural variances between the compounds, such as the presence
and position of the sugar groups, markedly influence their binding
affinities and, consequently, their biological activities (72). Overall, the O-glycosides
demonstrated the highest binding affinities, followed by the
C-glycoside glycosides, with aglycones having the least
affinity for BCL-w. The presence of glycoside moieties enhances the
binding affinity compared to the aglycones, which lack the sugar
groups. Additionally, the glycoside moiety enhances solubility and
bioavailability, which is crucial for the interaction with BCL-w
(73). The higher binding activity
of the O-glycosides compared to the C-glycosides may
be attributed to their flexibility. The O-glycoside bond
provides more flexibility compared to the C-glycoside bond,
facilitating better fitting and binding to the protein's active
site (65). O-glycosides
are relatively stable in physiological conditions, leading to
sustained interactions with target proteins (74). The higher the binding activity to
BCL-w, the more effective the compound will likely be in disrupting
its anti-apoptotic function and inducing apoptosis in cancer
cells.
In conclusion, in the present study, 12 flavonoids
were screened in vitro for cell inhibition. The present
study demonstrated that apigenin, kaempferol, luteolin and
quercetin consistently inhibited NPC cells, nasopharyngeal
epithelial cells, or skin keratinocytes. Inhibition by apigenin,
kaempferol, luteolin and quercetin was more notable than their
glucoside equivalents. O-glycosides inhibited cells to a
lesser extent, whilst C-glycosides, namely vitexin,
isovitexin, orientin and isoorientin, did not exert any inhibitory
effect. The molecular docking analyses highlighted the relative
binding affinities of the selected flavonoids to BCL-w, indicating
that glycosides, particularly O-glycosides, such as
cynaroside, have superior binding capabilities compared to
aglycones like apigenin and kaempferol. This suggests that
structural modifications, such as glycosylation, enhance the
anticancer efficacy of flavonoids. The molecular docking analyses
also revealed the importance of the chromene rings of flavones,
flavonols and their glycosidic derivatives in forming critical
hydrophobic interactions with the binding site residues of BCL-w.
These findings can be further used in designing new derivatives
with better binding affinity with BCL-w protein.
Acknowledgements
The authors would like to thank the Director General
of Health Malaysia for permission to publish this article. HK1,
NP69SV40T and NP460hTERT cells were from the late Professor G.S.W.
Tsao (The University of Hong Kong), whilst C666-1 cells were from
Professor K.W. Lo (The Chinese University of Hong Kong).
Funding
Funding: The present study was financially supported by the
Ministry of Health, Malaysia (NMRR-14-815-22074).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MD acquired and analysed the in vitro data
and was a major contributor to the writing of the manuscript. AG
performed the molecular docking analyses and was involved in
drafting the manuscript. GAA contributed to the conception and
design of the study, as well as the interpretation of the chemistry
data and revision of the manuscript. MD and AG confirm the
authenticity of all raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The Medical Research and Ethics Committee (MREC),
Ministry of Health Malaysia, has determined that the presaent study
does not require MREC approval as the research does not involve
human subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Ervik M, Lam F, Laversanne M,
Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I and Bray F:
Global Cancer Observatory: Cancer Today (version 1.1).
International Agency for Research on Cancer, Lyon, 2024. https://gco.iarc.fr/today. Accessde November 25,
2024.
|
|
2
|
Ferlay J, Ervik M, Lam F, Laversanne M,
Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I and Bray F:
Global Cancer Observatory: Cancer Tomorrow (version 1.1).
International Agency for Research on Cancer, Lyon, 2024. https://gco.iarc.fr/tomorrow. Accessed November 25,
2024.
|
|
3
|
Malaysia National Cancer Registry Report
2012-2016. National Cancer Registry Department, National Cancer
Institute, Ministry of Health, Malaysia, 2019.
|
|
4
|
Malaysian Study on Cancer Survival
(MySCan). National Cancer Registry, National Cancer Institute,
Ministry of Health Malaysia, Malaysia, 2018.
|
|
5
|
Incidence of Childhood Cancer in Malaysia
2007-2016. National Cancer Registry Department, Institut Kanser
Negara, Ministry of Health, Malaysia, 2021.
|
|
6
|
Chan JJC, Pilch BZ, Kuo TT, Wenig BM and
Lee AWM: Tumours of the nasopharynx. In: World Health Organization
Classification of Tumours: Pathology and Genetics of Head and Neck
Tumours. Barnes L, Eveson JW, Reichart P and Sidransky D (eds.).
IARC Press, Lyon, 2005.
|
|
7
|
Poh SS, Chua MLK and Wee JTS:
Carcinogenesis of nasopharyngeal carcinoma: Aan alternate
hypothetical mechanism. Chin J Cancer. 35(9)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chua MLK, Wee JTS, Hui EP and Chan ATC:
Nasopharyngeal carcinoma. Lancet. 387:1012–1024. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tsao SW, Yip YL, Tsang CM, Pang PS, Lau
VM, Zhang G and Lo KW: Etiological factors of nasopharyngeal
carcinoma. Oral Oncol. 50:330–338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Epidemiology of Cancer in Sarawak
2007-2011. Sarawak Cancer Registry, Sarawak Health Department,
Malaysia, 2017.
|
|
11
|
Linton RE, Daker M, Khoo ASB, Choo DCY,
Viljoen M and Neilsen PM: Nasopharyngeal carcinoma among the
Bidayuh of Sarawak, Malaysia: History and risk factors. Oncol Lett.
22(514)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shen N, Wang T, Gan Q, Liu S, Wang L and
Jin B: Plant flavonoids: Classification, distribution,
biosynthesis, and antioxidant activity. Food Chem.
383(132531)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li XY, Luo DH, Guo L, Mo HY, Sun R, Guo
SS, Liu LT, Yang ZC, Yang JH, Qiu F, et al: Deintensified
chemoradiotherapy for pretreatment Epstein-barr virus DNA-selected
low-risk locoregionally advanced nasopharyngeal carcinoma: A phase
II randomized noninferiority trial. J Clin Oncol. 40:1163–1173.
2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tang QN, Liu LT, Qi B, Guo SS, Luo DH, Sun
R, Sun XS, Chen DP, Guo L, Mo HY, et al: Effect of concurrent
chemoradiotherapy with nedaplatin vs cisplatin on the long-term
outcomes of survival and toxic effects among patients with stage II
to IVB nasopharyngeal carcinoma: A 5-year follow-up secondary
analysis of a randomized clinical trial. JAMA Netw Open.
4(e2138470)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kuhlmann MK, Horsch E, Burkhardt G, Wagner
M and Köhler H: Reduction of cisplatin toxicity in cultured renal
tubular cells by the bioflavonoid quercetin. Arch Toxicol.
72:536–540. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sanchez-Gonzalez PD, Lopez-Hernandez FJ,
Perez-Barriocanal F, Morales AI and Lopez-Novoa JM: Quercetin
reduces cisplatin nephrotoxicity in rats without compromising its
anti-tumour activity. Nephrol Dial Transplant. 26:3484–3495.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nho JH, Jung HK, Lee MJ, Jang JH, Sim MO,
Jeong DE, Cho HW and Kim JC: Beneficial effects of cynaroside on
cisplatin-induced kidney injury in vitro and in vivo. Toxicol Res.
34:133–141. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Amado NG, Cerqueira DM, Menezes FS, Mendes
da Silva JF, Neto VM and Abreu JG: Isoquercitrin isolated from
Hyptis fasciculata reduces glioblastoma cell proliferation and
changes beta-catenin cellular localization. Anticancer Drugs.
20:543–552. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Daker M, Ahmad M and Khoo ASB:
Quercetin-induced inhibition and synergistic activity with
cisplatin-a chemotherapeutic strategy for nasopharyngeal carcinoma
cells. Cancer Cell Int. 12(34)2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jeong JC, Kim MS, Kim TH and Kim YK:
Kaempferol induces cell death through ERK and Akt-dependent
down-regulation of XIAP and survivin in human glioma cells.
Neurochem Res. 34:991–1001. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lefort ÉC and Blay J: Apigenin and its
impact on gastrointestinal cancers. Mol Nutr Food Res. 57:126–144.
2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li T and Li Y: Quercetin acts as a novel
anti-cancer drug to suppress cancer aggressiveness and
cisplatin-resistance in nasopharyngeal carcinoma (NPC) through
regulating the yes-associated protein/Hippo signaling pathway.
Immunobiology. 228(152324)2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lim DY, Jeong Y, Tyner AL and Park JHY:
Induction of cell cycle arrest and apoptosis in HT-29 human colon
cancer cells by the dietary compound luteolin. Am J Physiol
Gastrointest Liver Physiol. 292:G66–G75. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pacifico S, Scognamiglio M, D'Abrosca B,
Piccolella S, Tsafantakis N, Gallicchio M, Ricci A and Fiorentino
A: Spectroscopic characterization and antiproliferative activity on
HepG2 human hepatoblastoma cells of flavonoid C-glycosides from
petrorhagia velutina. J Nat Prod. 73:1973–1978. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
González-Molina E, Domínguez-Perles R,
Moreno DA and García-Viguera C: Natural bioactive compounds of
Citrus limon for food and health. J Pharm Biomed Anal. 51:327–345.
2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Li N, Liu JH, Zhang J and Yu BY:
Comparative evaluation of cytotoxicity and antioxidative activity
of 20 flavonoids. J Agric Food Chem. 56:3876–3883. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5(e47)2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mahmud AR, Ema TI, Siddiquee MFR, Shahriar
A, Ahmed H, Mosfeq-Ul-Hasan M, Rahman N, Islam R, Uddin MR and
Mizan MFR: Natural flavonols: Actions, mechanisms, and potential
therapeutic utility for various diseases. Beni Suef Univ J Basic
Appl Sci. 12(47)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Al-Khayri JM, Sahana GR, Nagella P, Joseph
BV, Alessa FM and Al-Mssallem MQ: Flavonoids as potential
anti-inflammatory molecules: A review. Molecules.
27(2901)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hu Y, Yu C, Cheng L, Zhong C, An J, Zou M,
Liu B and Gao X: Flavokawain C inhibits glucose metabolism and
tumor angiogenesis in nasopharyngeal carcinoma by targeting the
HSP90B1/STAT3/HK2 signaling axis. Cancer Cell Int.
24(158)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu CC, Fang CY, Cheng YJ, Hsu HY, Chou SP,
Huang SY, Tsai CH and Chen JY: Inhibition of epstein-barr virus
reactivation by the flavonoid apigenin. J Biomed Sci.
24(2)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu CC, Fang CY, Hsu HY, Chen YJ, Chou SP,
Huang SY, Cheng YJ, Lin SF, Chang Y, Tsai CH and Chen JY: Luteolin
inhibits Epstein-Barr virus lytic reactivation by repressing the
promoter activities of immediate-early genes. Antiviral Res.
132:99–110. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu CC, Fang CY, Hsu HY, Chuang HY, Cheng
YJ, Chen YJ, Chou SP, Huang SY, Lin SF, Chang Y, et al: EBV
reactivation as a target of luteolin to repress NPC tumorigenesis.
Oncotarget. 7:18999–19017. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wu CC, Lee TY, Cheng YJ, Cho DY and Chen
JY: The dietary flavonol kaempferol inhibits Epstein-Barr virus
reactivation in nasopharyngeal carcinoma cells. Molecules.
27(8158)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Baloche V, Ferrand F-R, Makowska A, Even
C, Kontny U and Busson P: Emerging therapeutic targets for
nasopharyngeal carcinoma: Opportunities and challenges. Expert Opin
Ther Targets. 24:545–558. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huang DP, Ho JHC, Poon YF, Chew EC, Saw D,
Lui M, Li CL, Mak LS, Lai SH and Lau WH: Establishment of a cell
line (NPC/HK1) from a differentiated squamous carcinoma of the
nasopharynx. Int J Cancer. 26:127–132. 1980.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cheung ST, Huang DP, Hui ABY, Lo KW, Ko
CW, Tsang YS, Wong N, Whitney BM and Lee JC: Nasopharyngeal
carcinoma cell line (C666-1) consistently harbouring Epstein-Barr
virus. Int J Cancer. 83:121–126. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Boukamp P, Petrussevska RT, Breitkreutz D,
Hornung J, Markham A and Fusenig NE: Normal keratinization in a
spontaneously immortalized aneuploid human keratinocyte cell line.
J Cell Biol. 106:761–771. 1988.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li HM, Man C, Jin Y, Deng W, Yip YL, Feng
HC, Cheung YC, Lo KW, Meltzer PS, Wu ZG, et al: Molecular and
cytogenetic changes involved in the immortalization of
nasopharyngeal epithelial cells by telomerase. Int J Cancer.
119:1567–1576. 2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tsao SW, Wang X, Liu Y, Cheung YC, Feng H,
Zheng Z, Wong N, Yuen PW, Lo AK, Wong YC and Huang DP:
Establishment of two immortalized nasopharyngeal epithelial cell
lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim
Biophys Acta. 1590:150–158. 2002.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Daker M, Jayaweera U, Marzuki M,
Gunasegaran G, Chew YL, Ahmad M and Akowuah GA: Content of luteolin
and luteolin-7-О-glucoside from the leaves of Vernonia amygdalina
Del., and synergistic inhibitory effect with cisplatin on
nasopharyngeal carcinoma cells. Chem Data Collections.
45(101039)2023.
|
|
43
|
Radha Abbas Hasoon M and Jawad Kadhim N:
Improvement of the selectivity index (SI) and cytotoxicity activity
of doxorubicin drug by panax ginseng plant extract. Arch Razi Inst.
76:659–666. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lee Erinna F, Dewson G, Smith Brian J,
Evangelista M, Pettikiriarachchi A, Dogovski C, Perugini MA, Colman
PM and Fairlie WD: Crystal structure of a BCL-W Domain-swapped
dimer: Implications for the function of BCL-2 family proteins.
Structure. 19:1467–1476. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Berman HM, Westbrook J, Feng Z, Gilliland
G, Bhat TN, Weissig H, Shindyalov IN and Bourne P: The protein data
bank. Nucleic Acids Res. 28:235–242. 2000.PubMed/NCBI View Article : Google Scholar
|
|
46
|
BIOVIA Dassault Systèmes, Discovery Studio
Visualizer, Version 4.1. San Diego: Dassault Systèmes, 2022.
|
|
47
|
Morris GM, Huey R, Lindstrom W, Sanner MF,
Belew RK, Goodsell DS and Olson AJ: AutoDock4 and AutoDockTools4:
Automated docking with selective receptor flexibility. J Comput
Chem. 30:2785–2791. 2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wei J, Liang Y and Wu L: Design,
synthesis, molecular docking, and tumor resistance reversal
activity evaluation of matrine derivative with thiophene structure.
Molecules. 26:417–429. 2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Eberhardt J, Santos-Martins D, Tillack AF
and Forli S: AutoDock Vina 1.2.0: New docking methods, expanded
force field, and python bindings. J Chem Inf Model. 61:3891–3898.
2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Trott O and Olson AJ: AutoDock Vina:
Improving the speed and accuracy of docking with a new scoring
function, efficient optimization, and multithreading. J Comput
Chem. 31:455–461. 2010.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Aliyatul Fikroh R: Synthesis of halogen
substituted chalcone againts cervical cancer (HeLa) cell lines
using green method. J Tropical Chemistry Res Education. 5:36–43.
2023.
|
|
52
|
Abdul Rahman SF, Azlan A, Lo KW, Azzam G
and Mohana-Kumaran N: Dual inhibition of anti-apoptotic proteins
BCL-XL and MCL-1 enhances cytotoxicity of nasopharyngeal carcinoma
cells. Discover Oncol. 13(9)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Patil JR, Chidambara Murthy KN,
Jayaprakasha GK, Chetti MB and Patil BS: Bioactive compounds from
mexican lime (Citrus aurantifolia) Juice induce apoptosis in human
pancreatic cells. J Agric Food Chem. 57:10933–10942.
2009.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wu B, Zhang Q, Shen W and Zhu J:
Anti-proliferative and chemosensitizing effects of luteolin on
human gastric cancer AGS cell line. Mol Cellular Biochem.
313:125–132. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Luo H, Jiang BH, King SM and Chen YC:
Inhibition of cell growth and VEGF expression in ovarian cancer
cells by flavonoids. Nutr Cancer. 60:800–809. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen D, Daniel KG, Chen MS, Kuhn DJ,
Landis-Piwowar KR and Dou QP: Dietary flavonoids as proteasome
inhibitors and apoptosis inducers in human leukemia cells. Biochem
Pharmacol. 69:1421–1432. 2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhong Y, Krisanapun C, Lee SH, Nualsanit
T, Sams C, Peungvicha P and Baek SJ: Molecular targets of apigenin
in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur
J Cancer. 46:3365–3374. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nakazaki E, Tsolmon S, Han J and Isoda H:
Proteomic study of granulocytic differentiation induced by apigenin
7-glucoside in human promyelocytic leukemia HL-60 cells. Eur J
Nutr. 52:25–35. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xiong Y, Zhong W, Liu J, Cheng B, Fan J,
Zhou F, He L, Tian D and He Y: Luteolin isolated from polygonum
cuspidatum is a potential compound against nasopharyngeal
carcinoma. Biomed Res Int. 2022(9740066)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Asnaashari S, Amjad E and Sokouti B:
Synergistic effects of flavonoids and paclitaxel in cancer
treatment: A systematic review. Cancer Cell Int.
23(211)2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Pyo Y, Kwon KH and Jung YJ: Anticancer
potential of flavonoids: Their role in cancer prevention and health
benefits. Foods. 13(2253)2024.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wu Z and Qu Q: Mechanism of luteolin
induces ferroptosis in nasopharyngeal carcinoma cells. J Toxicol
Sci. 49:399–408. 2024.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Liu W, Ji Y, Wang F, Li C, Shi S, Liu R,
Li Q, Guo L, Liu Y and Cui H: Morusin shows potent antitumor
activity for melanoma through apoptosis induction and proliferation
inhibition. BMC Cancer. 23(602)2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Abusaliya A, Ha SE, Bhosale PB, Kim HH,
Park MY, Vetrivel P and Kim GS: Glycosidic flavonoids and their
potential applications in cancer research: A review. Mol Cell
Toxicol. 18:9–16. 2022.
|
|
66
|
Kale J, Osterlund EJ and Andrews DW: BCL-2
family proteins: Changing partners in the dance towards death. Cell
Death Differ. 25:65–80. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Hartman ML and Czyz M: BCL-w: Apoptotic
and non-apoptotic role in health and disease. Cell Death Dis.
11(260)2020.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Sugantha Priya E, Selvakumar K, Bavithra
S, Elumalai P, Arunkumar R, Raja Singh P, Brindha Mercy A and
Arunakaran J: Anti-cancer activity of quercetin in neuroblastoma:
An in vitro approach. Neurol Sci. 35:163–170. 2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Taghizadeh MS, Niazi A, Moghadam A and
Afsharifar A: Experimental, molecular docking and molecular dynamic
studies of natural products targeting overexpressed receptors in
breast cancer. PLoS One. 17(e0267961)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sun J and Mei H: Binding site analysis,
3D-QSAR studies, and molecular design of flavonoids derivatives as
potent neuraminidase inhibitors. Med Chemistry Res. 22:606–614.
2013.
|
|
71
|
Ghanbari-Movahed M, Shafiee S, Burcher JT,
Lagoa R, Farzaei MH and Bishayee A: Anticancer potential of
apigenin and isovitexin with focus on oncogenic metabolism in
cancer stem cells. Metabolites. 13(404)2023.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Bhagavatula D, Hasan TN, Vohra H, Khorami
S and Hussain A: Delineating the antiapoptotic property of apigenin
as an antitumor agent: A computational and in vitro study on HeLa
cells. ACS Omega. 9:24751–24760. 2024.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Ji J, Wang Z, Sun W, Li Z, Cai H, Zhao E
and Cui H: Effects of Cynaroside on cell proliferation, apoptosis,
migration and invasion though the MET/AKT/mTOR axis in gastric
cancer. Int J Mol Sci. 22(12125)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Carrillo-Martinez EJ, Flores-Hernández FY,
Salazar-Montes AM, Nario-Chaidez HF and Hernández-Ortega LD:
Quercetin, a flavonoid with great pharmacological capacity.
Molecules. 29(1000)2024.PubMed/NCBI View Article : Google Scholar
|