Introduction
Cancer is a disease in which some of the cells in
the body grow uncontrollably; it is influenced by genetic and
environmental conditions (1).
Prostate cancer (PCa) is considered a hypoxic and lipogenic tumor
(2) and it is the second most
prevalent type of cancer among males and also one of the leading
causes of cancer-related mortality worldwide (3,4).
Lipids are involved in signal transmission, maintaining the
structural integrity of cellular membranes, and regulating energy
metabolism (5). The role of lipid
and glucose metabolism in PCa differs from other types of cancer.
While the majority of cancers primarily use glycolysis, PCa uses
lipid metabolism as opposed to glycolysis for energy metabolism.
Cancer cells may increase lipid uptake and lipid synthesis due to
increased energy demands (6). The
increased amount of lipids leads to the accumulation of lipid
droplets (LDs) in the cells. A LD is a type of functional organelle
in the cell, consisting of a neutral lipid nucleus, a single-layer
phospholipid membrane and LD-associated proteins. LDs are involved
in diverse biological phenomena, including cell proliferation,
apoptosis, lipid metabolism, stress, immunity and signal
transduction (7). LDs are known to
be involved in fundamental processes of cell homeostasis and have
been shown to be associated with various disorders, including
metabolic diseases, inflammatory reactions in leukocytes and cancer
development (8). Studies have
shown that an increase in LDs is associated with cancer cell
proliferation and chemotherapy resistance (7). Other studies investigating the role
of lipids in cancer progression have revealed that aggressive
cancers can meet their lipid requirements independently from
circulation by synthesizing high rates of de novo fatty
acids (FAs) (8,9). At the same time, cancer cells enter
into a symbiotic association with tumor-associated adipocytes,
where the lipolysis of LD in adipocytes provides FA to cancer cells
for energy production (7-9).
In PCa, there are some proteins that play a role in lipid
metabolism and the formation of LDs, such as the phospholipase A2
(PLA2) group VII (PLA2G7), uncoupling protein (UCP)2 and NEDD4 like
E3 ubiquitin protein ligase (NEDD4L) proteins. PLA2G7 is a 45-kDa
monomeric protein and is a PLA2 involved in phospholipid catabolism
(10,11). Phospholipases are a family of
lipid-modifying enzymes that break down phospholipids and regulate
bioactive lipid levels (12).
PLA2s function as: i) Suppliers of free FAs from membrane
phospholipids for the synthesis of neutral lipids; ii) producers of
metabolites that can control LD metabolism; and iii) direct
modulators of organelle formation (8). Additionally, it has been shown that
the anti-proliferative effect of PLA2G7 gene silencing is enhanced
by lipid-reducing statins in PCa cells (13). UCPs belong to the family of
mitochondrial inner membrane transporters (14). These proteins are involved in the
function of the mitochondrial membrane and cellular energy
regulation. The UCP group has five members: UCP1, UCP2, UCP3, UCP4
and UCP5, which are distributed in different tissues in the body
(15). UCP2 plays a crucial role
in cancer metabolism and supports tumor growth and survival
(16). Additionally, it has been
suggested that the switch from glucose oxidation to FA oxidation
may be supported by UCP2(17).
NEDD4L is an E3 ubiquitin ligase described to be involved in a
variety of cellular activities by regulating substrate
ubiquitination (Ub) and protein degradation (18). NEDD4L is involved in the regulation
of tumor cell functions, such as proliferation, apoptosis,
migration, invasion, epithelial-mesenchymal transition and drug
resistance by controlling protein degradation through Ub (19).
Hemp (Cannabis sativa L.) is a plant that has
been used in the treatment of various diseases since ancient times.
Cannabinoids are active compounds produced by Cannabis
sativa L. and its species. These are classified in three main
groups: Phytocannabinoids, endocannabinoids and synthetic
cannabinoids (20). The function
of cannabinoids is mediated through the endocannabinoid system,
including cannabinoid receptor 1 (CB1) and cannabinoid receptor 2
(CB2). Some synthetic CB1 and CB2 agonists have been demonstrated
to inhibit tumor progression and metastasis (21). The substance known as the most
potent and main component in hemp is Δ9-tetrahydrocannabinol (THC)
(20). Benzohydrazide
N'-[(3Z)-1-(1-heksil)-2-okso-1,2-dihidro 3H-indol 3-iliden]
benzohidrazid (MDA19), an analogue of THC, is known to be a
selective agonist at CB2 (4,21).
To date, to the best of our knowledge, there is no
study available in the literature examining the effects of MDA19 on
PLA2G7, UCP2 and NEDD4L proteins, which play a role in LD
metabolism in PCa. The present study thus aimed to determine the
therapeutic efficacy of MDA19 in the treatment of PCa, and to
contribute to the literature by demonstrating its effects on LD
metabolism, an alternative pathway of cancer metabolism.
Materials and methods
Cell lines and cell culture
The human metastatic prostate carcinoma cell lines,
DU145 (ATCC HTB-81) and PC3 (EACC 95012614), were obtained from the
Department of Biotechnology, Ankara University (Ankara, Turkey).
The cells were cultured at 37˚C and 5% CO2 using
high-glucose DMEM (SLD-524-500, Serox GmbH) containing 10% FBS
(FBS-11B, Capricorn Scientific), 1% penicillin-streptomycin
(15140122; Gibco; Thermo Fisher Scientific, Inc.), and 1%
non-essential amino acid (L-glutamine) (SRL-810-100; Serox
GmbH).
Cell lysate preparation
The cell lysate was prepared by applying the
freeze-thaw method. In the first step, the medium in the 96-well
well plate was removed and washed with PBS (PBS-1A, Capricorn
Scientific). Cells treated with trypsin (Sartorius 223601012) were
neutralized by adding a medium (DMEM, Serox GmbH). The cells were
centrifuged at 1,400 x g for 3 min at 4˚C. The supernatant was
removed and washed three times with cold PBS. Following each wash,
the supernatant was discarded by centrifugation at 3,900 x g at 4˚C
for 2 min. Subsequently, 200 µl PBS were added and maintained at
-80˚C for 5 min and incubated at 37˚C for 5 min. This procedure was
replicated three times. Cell lysates were stored at -20˚C.
Preparation of BZO-HEKZOKZID
(MDA19)
The molecular weight of MDA19 (HY-15451,
MedChemExpress), whose molecular formula is
C21H23N3O2, is 349.43
g/mol. The stock concentration of MDA19 was calculated to be 10 mM
and was prepared for use by dissolving it in dimethyl sulfoxide
(DMSO) (1.167.431.000; MilliporeSigma).
Evaluation of cell viability and
proliferation
MTT assay, one of the colorimetric methods for the
detection of cytotoxicity, was used to determine the effects of
MDA19 on the viability and proliferation of DU145 and PC3 cells. To
determine the concentration at which MDA19 killed 50% of the cells,
various concentrations (0, 25, 50, 100, 150 and 200 µM) of MDA19
were applied to the cells cultured in 96 well-plates for 24, 48 and
72 h. Subsequently, 5 mg formazan, the reagent of MTT, was
dissolved in 1 ml PBS. Subsequently, 10 µl MTT reactive (20395.02;
Serva Electropheresis GmbH) was applied to each well and after 2 h,
the solvent was added for overnight incubation at 37˚C, and the
absorbance was measured at a wavelength of 570-690 nm (Epoch 2,
BioTek Instruments, Inc.). The reagent of MTT, formazan, was
dissolved in PBS. IC50 values of MDA19 were calculated
using the GraphPad Prism 8.4.2 program (Dotmatics).
Determination of the expression levels
of PLA2G7, UCP2, and NEDD4L proteins using enzyme-linked
immunosorbent assay (ELISA)
Measurements were made using ELISA to determine the
protein expression levels of PLA2G7, UCP2 and NEDD4L. For ELISA,
the PLA2G7 ELISA kit (E3993Hu, Bioassay Technology Laboratory),
UCP2 ELISA kit (E5683Hu, Bioassay Technology Laboratory) and NEDD4L
ELISA kit (E7630Hu, Bioassay Technology Laboratory) were used.
While setting up the ELISA, the standards were prepared by diluting
them at a ratio of 1:2. Subsequently, 50 µl standard solution, 40
µl cell lysate, 10 µl protein-specific biotinylated antibody, and
50 µl streptavidin-HRP were added to the wells, respectively and
incubated for 60 min at 37˚C. Following incubation, washing was
performed five times with washing solution; 50 µl substrate
solution A and 50 µl substrate solution B were then added followed
by incubation for 10 min at 37˚C. Finally, 50 µl stop solution was
applied and the absorbance was measured at 450 nm (Epoch 2, BioTek
Instruments, Inc.).
Statistical analysis
One-way ANOVA was used for the comparison of the
ELISA results. When any differences were found, post-hoc pairwise
comparisons were performed with the Dunnett's test to compare the
control group with the experimental groups. Adjusted P-values were
used for controlling the type I error rate in statistical
decisions. Statistical analysis was performed for using GraphPad
Prism 8.4.2 software (Dotmatics). A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
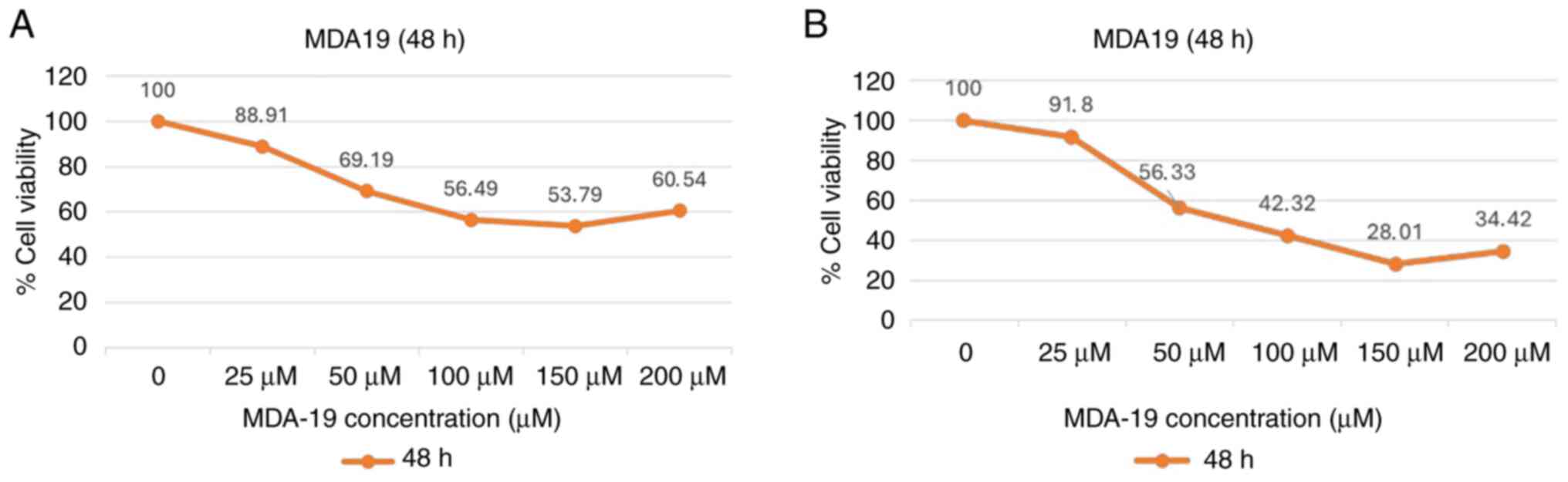
Anti-proliferative effects of MDA19
treatment
MDA19 was applied to the DU145 and PC3 PCa cell
lines at the concentrations of 0, 25, 50, 100, 150 and 200 µM for
24, 48 and 72 h. The results of MTT assay revealed that the
IC50 value at 48 h of MDA19 treatment was statistically
significant. Thus, only the images for 48 h of MDA19 treatment are
presented in Fig. 1.
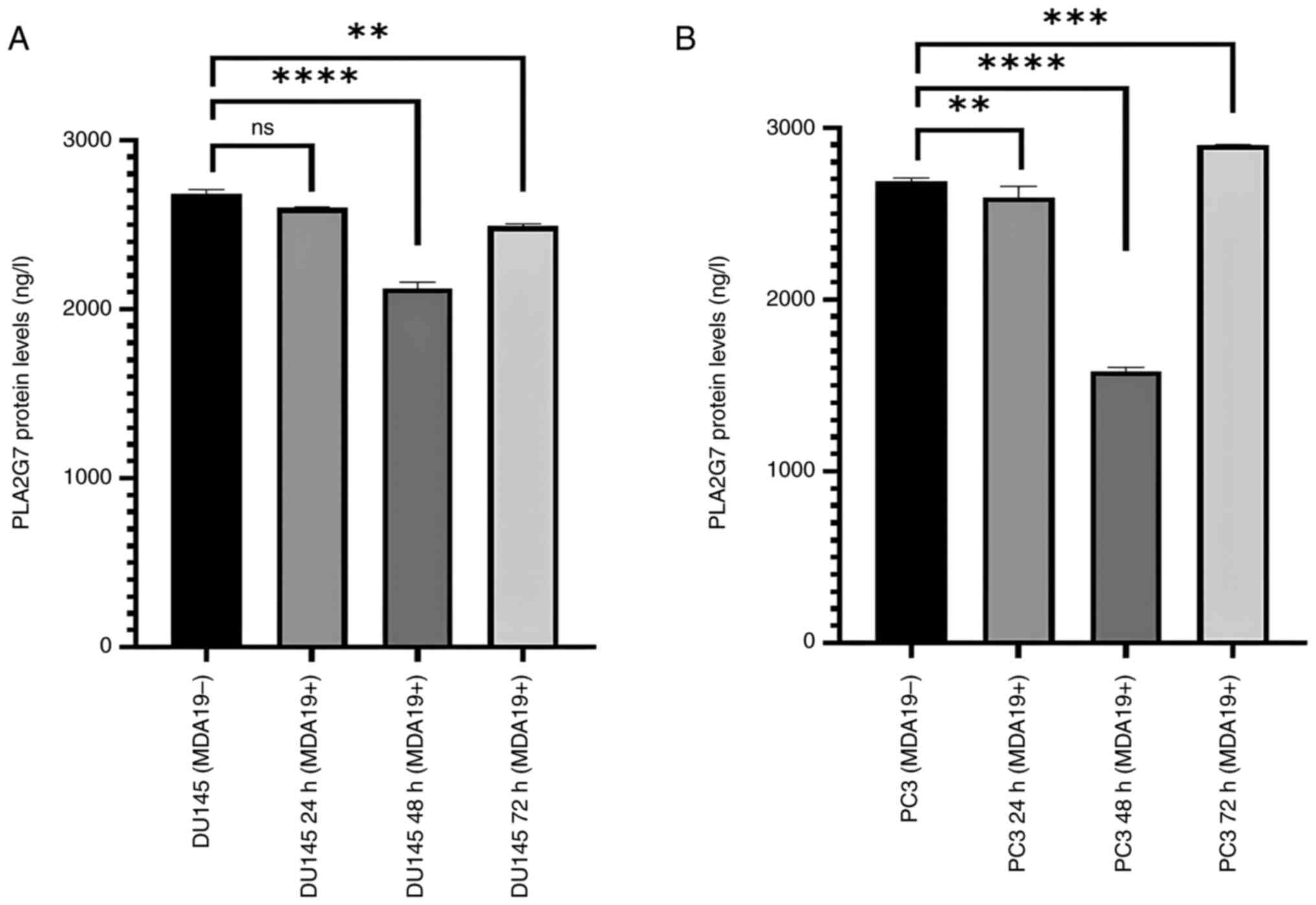
Reduction of the protein expression
level of PLA2G7
MDA19 was applied to the DU145 and PC3 PCa cell
lines at the concentration of 90 µM for 24, 48 and 72 h and ELISA
was then performed to determine the protein expression level of
PLA2G7 in the DU145 and PC3 PCa cell lines (Fig. 2). Significant differences were
observed in the protein expression level of PLA2G7 in the DU145
cell line. In the DU145 cells, in the absence of MDA19 treatment
(MDA19-), the protein expression level of PLA2G7 was determined at
2,680 ng/l, and at 24 h of treatment with MDA19 (MDA19+ 24 h), it
was determined at 2,599.5 ng/l. There was no significant
differences detected in the protein expression level of PLA2G7
between these groups (P=0.0569). The protein expression level of
PLA2G7 at 48 h treatment of MDA19 (MDA19+ 48 h) was measured at
2,124 ng/l. It was determined that there was a decrease in the
protein expression level PLA2G7 compared to the DU145 (MDA19-)
group (P<0.0001). The protein expression level of PLA2G7 was
determined at 2,490 ng/l at 72 h of treatment with MDA19 (MDA19+ 72
h). A decrease in the protein expression level of PLA2G7 was
detected compared to the MDA19- group (P=0.0028) (Fig. 2A).
In the PC3 cell line, in the MDA19- group, the
protein expression level of PLA2G7 was determined at 2,686.5 ng/l,
and at 24 h of treatment with MDA19 (MDA19+ 24 h) it was determined
at 2,595 ng/l. A considerable difference was detected between these
groups (P=0.0089). The protein expression level of PLA2G7 at 48 h
of treatment with MDA19 (MDA19+ 48 h) was determined at 1,584 ng/l
and a significant decrease in the protein expression level of
PLA2G7 was detected compared to the MDA19- group (P<0.0001). At
72 h of treatment with MDA19 (MDA19+ 72 h) the protein expression
level of PLA2G7 was determined at 2,901.06 ng/l. An increase in the
protein expression level of PLA2G7 was detected compared to the
MDA19- group (P=0.0003) (Fig.
2B).
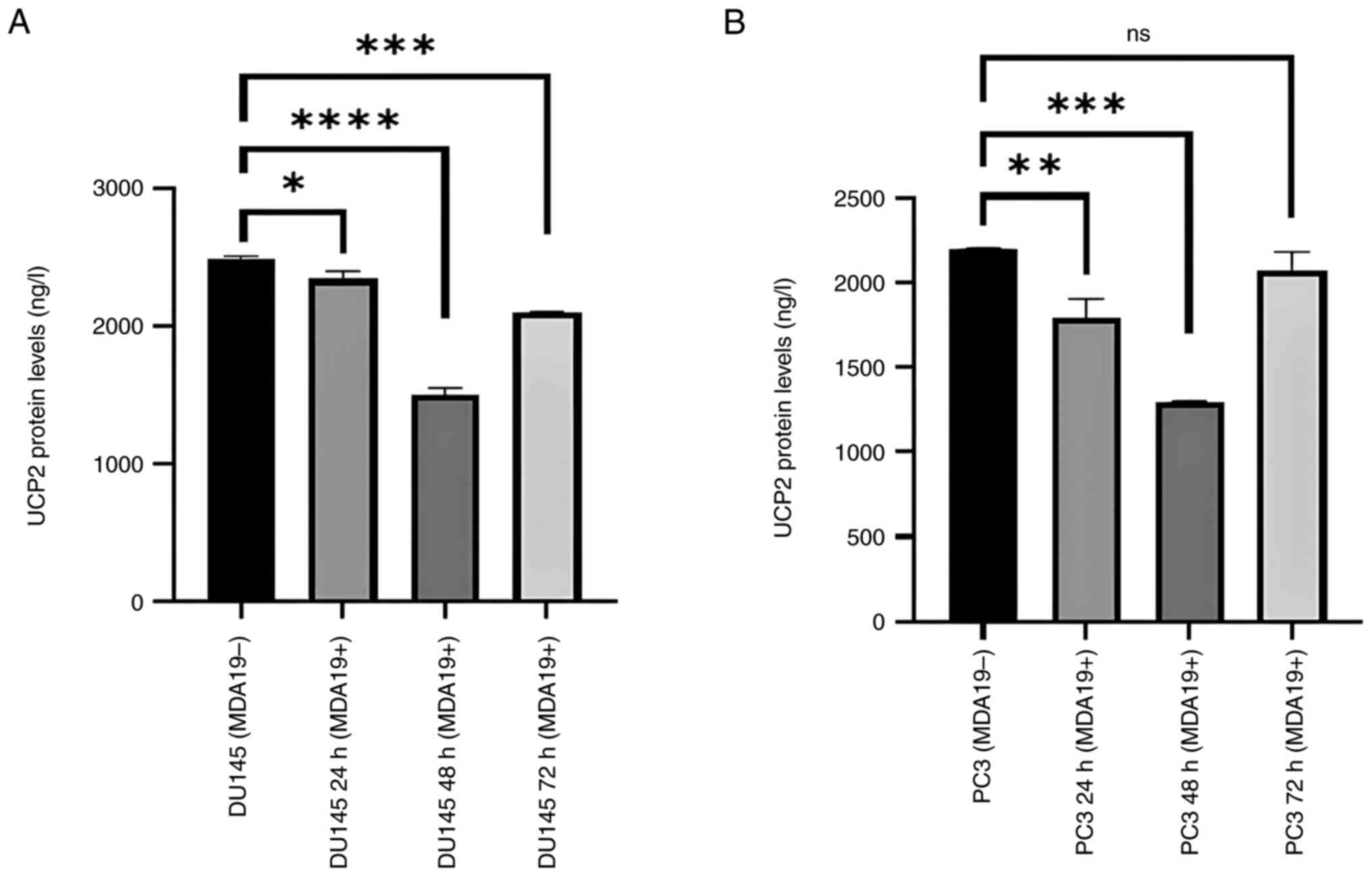
Reduction of the protein expression
level of UCP2
MDA19 was applied to the DU145 and PC3 PCa cell
lines at the concentration of 90 µM for 24, 48 and 72 h and the
results of ELISA revealed that the protein expression level of UCP2
exhibited significant differences in the DU145 and PC3 cell lines
(Fig. 3). As shown in Fig. 3A, in the DU145 cells, the protein
expression level of UCP2 was found to differ between the following
groups: MDA19- and MDA19+ at 24, 48 and 72 h of treatment with
MDA19. The protein level was determined at 2,486.13, 2,339.5, 1,496
and 2,094.88 ng/l at 0, 24, 48 and 72 h, respectively. According to
the results of the analysis, a significant difference was detected
in the protein expression level of UCP2 between the MDA19- group
and at 24 h of treatment with MDA19 (MDA19+ 24 h) (P=0.0184). When
the MDA19- group and the 48-h treatment group (MDA19+ 48 h) were
compared, a significant decrease was detected in the protein
expression level of UCP2 (P<0.0001). It was also determined that
there was a decrease in the protein expression level of UCP2
between the MDA19- group and the 72-h treatment group MDA19 (MDA19+
72 h) (P=0.0005; Fig. 3A).
In the PC3 cell line, the protein expression level
of UCP2 exhibited similar changes following treatment with MDA19 at
24 and 48 h, and the protein expression level of UCP2 was increased
at 72-h time point. The protein expression level of UCP2 was
determined at 2,494.5 ng/l for the MDA19- group and 1,791.17 ng/l
for the 24-h treatment group (MDA19+ 24 h), and the difference was
significant (P=0.0037). For the 48-h treatment group (MDA19+ 48 h),
the protein expression level of UCP2 was measured at 1,299.5 ng/l,
and a significant difference in the protein expression level of
UCP2 was observed between the MDA19- group and the MDA19+ 48 h
group (P=0.0002). In the 72-h treatment group (MDA19+ 72 h), the
protein expression level of UCP2 was determined at 2,025.5 ng/l and
no significant difference was detected in the protein expression
level of UCP2 compared to the MDA19- group (P=0.1844) (Fig. 3B).
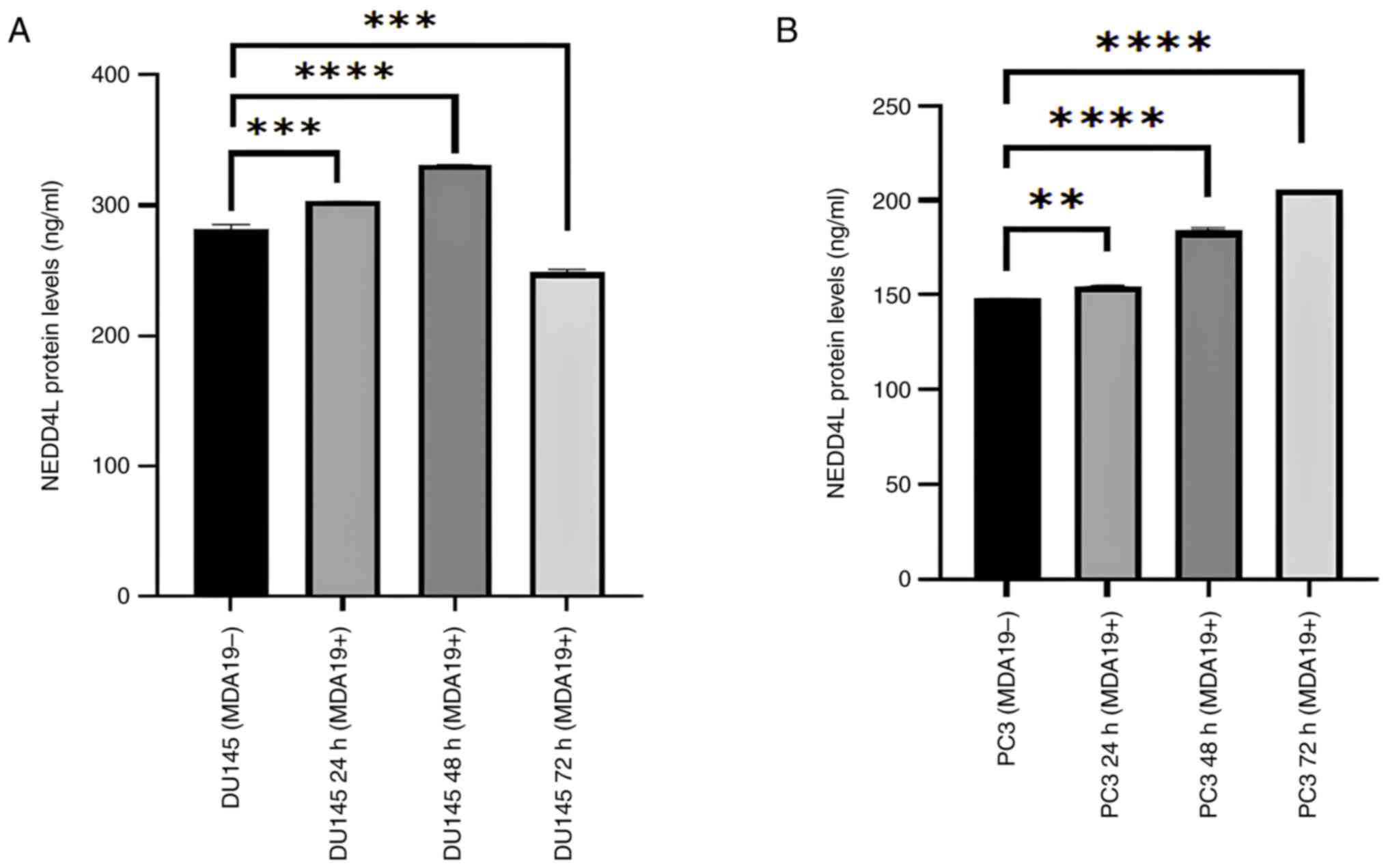
Increase in the protein expression
level of NEDD4L
MDA19 was applied to the DU145 and PC3 PCa cell
lines at the concentration of 90 µM for 24, 48 and 72 h and the
results of ELISA revealed that the protein expression level of
NEDD4L exhibited significant differences in the DU145 and PC3 cell
lines (Fig. 4). In the DU145 cell
line, the protein expression level of NEDD4L was measured in the
absence of MDA19 treatment and at the 24, 48 and 72-h treatment
time points. NEDD4L expression was determined at 282.23, 302.98,
330.73 and 248.61 ng/ml at 0, 24, 48 and 72 h, respectively. A
significant difference was detected in terms of the protein
expression level of NEDD4L between the MDA19- group and the 24-h
treatment group (MDA19+ 24 h) (P=0.0009). There was a significant
increase in the protein expression level of NEDD4L between the
MDA19- group and the 48-h treatment group (MDA19+ 48 h)
(P<0.0001). It was also determined that there was a decrease in
the protein expression level of NEDD4L between the MDA19- group and
the 72-h treatment group (MDA19+ 72 h) (P=0.0002; Fig. 4A).
The protein expression level of NEDD4L in the PC3
cell line is presented in Fig. 4B.
For the MDA19- group and the 24-h treatment group (MDA19+ 24 h),
the protein expression level of NEDD4L were determined at 148.11
and 154.73 ng/ml, respectively, and a significant difference was
detected (P=0.0031). For the 48-h treatment group (MDA19+ 48 h),
the protein expression level of NEDD4L was determined at 183.86
ng/ml, and an increase in the protein expression level of NEDD4L
was detected compared with the MDA19- group (P<0.0001). For the
72-h treatment group (MDA19+ 72 h), the protein expression level of
NEDD4L was determined at 205.98 ng/ml, and an increase in was
detected compared with the MDA19- group (P<0.0001) (Fig. 4B).
Discussion
The present study examined the effects of the
cannabinoid analogue, MDA19, on the protein expression levels of
PLA2G7, UCP2 and NEDD4L, which play a role in LD metabolism in
metastatic PCa cell lines (DU145 and PC3) using ELISA. The results
revealed that while treatment with MDA19 decreased the protein
expression levels of PLA2G7 and UCP2, it increased those of
NEDD4L.
PCa is considered a hypoxic and lipogenic tumor
(2) and PCa cells change their
metabolism to support their survival and metastasis (22). Apart from the classical metabolic
changes highlighted by the Warburg effect, it has been shown that
lipid metabolism is critical for tumorigenesis (23). These metabolic changes include
various mechanisms, including LD storage, enhanced lipid uptake,
de novo lipid synthesis and adjustments in lipolysis
(24). Lipid deposition in LDs is
a defining feature of PCa cells (25). The synthesis and utilization of
lipids in PCa cells are controlled by androgens. Androgen receptor
signaling in PCa has been reported to upregulate the levels of
lipid biosynthetic enzymes, such as fatty acid synthase (FASN) and
acetyl-CoA carboxylase alpha (4).
Additionally, Roman et al (25) demonstrated that lipid accumulation
occurred in PC3 PCa cells in their study using the Raman mapping
technique. The role of LD metabolism in the development of novel
therapies of PCa has been recently studied (26). Thus PLA2G7, UCP2 and NEDD4L
proteins play a critical role in LD metabolism in PCa.
Cannabinoids obtained from Cannabis sativa L.
and its derivatives, used in the treatment of various diseases for
palliative purposes in patients with cancer (27). Cannabinoids have been demonstrated
to modulate metabolic reprogramming and inhibit cancer cell
progress (28). Recent studies
have shown that these compounds have effects on promoting
apoptosis, arrest of the cell cycle, inhibition of cell migration
and angiogenesis (27). In
addition, cannabinoids affect AKT, EGFR and mTOR signaling pathways
and play a role in cell growth, differentiation and metabolism
(29). It has been stated that the
stimulation of the PI3K/Akt signaling pathway regulates cholesterol
uptake, glucose metabolism and lipogenesis through sterol
regulatory element binding protein (SREBP), which supplies energy
for rapid tumor growth. SREBPs play key roles in regulating
cholesterol and lipid metabolism. The study by Sun et al
(28) demonstrated that
cannabinoids inhibit cancer cell progression by inhibiting the
PI3K/Akt/mTOR pathway (27).
Due to the significance of lipid metabolism in PCa,
studies on LD have become a popular in cancer treatment. As
previously reported, PLA2G7 is a novel biomarker in 50% of primary
PCa and 70% of metastatic PCa and is associated with the
aggressiveness of cancer cells (30). PLA2s have appeared as key
modulators of LD homeostasis and have been shown to regulate their
formation at different levels (8).
In their study, Atakol et al (31) observed that when the protein levels
of PLA2G7 were compared in the DU145 and PC3 cells, the PLA2G7
protein level was higher in the DU145 cell line. However, no
significant differences were detected (31). Jayaraman et al (32) provided a promoting force for LD
formation as hydrolytic products were produced by PLA2G7,
particularly free FAs (32). In
the present study, when the time-dependent effect of MDA19 was
examined in the DU145 and PC3 cell lines, it was demonstrated that
the 90 µM concentration of MDA19 inhibited the two cell lines in a
time-dependent manner. The highest inhibitory effect was observed
at 48 h of treatment with MDA19 for the level of PLA2G7 in both
cell lines compared to the MDA19- group. No significant change was
observed in the level of PLA2G7 protein in the DU145 cell line at
the 24-h time point; therefore, further studies on the effects of
MDA19 on the PLA2G7 protein level are required.
The expression of UCP2, a regulator of cellular
metabolism, has been found to be enhanced in a number of types of
cancer, including leukemia, skin cancer, pancreatic cancer, colon
cancer and hepatocellular carcinoma. A recent study demonstrated
that targeting UCP2 inhibition in cancer treatments induces the
apoptosis of tumor cells (33).
UCP2 is involved in LD formation through FASN (7). In the study carried by Ke et
al (34), UCP2 was shown to
act as a critical regulator of lipid accumulation in vivo
and in vitro, and the accumulation and synthesis of LDs were
suppressed in UCP2-inactivated mice. Burch et al (35) investigated the UCP2 expression
level using RT-PCR and western Blot analysis in non-malignant
RC77N/E and malignant RC77T/E cells from prostate adenocarcinoma
cells. As a conclusion of their study, it was found that the UCP2
protein level was significantly increased in malignant cells
compared to non-malignant cells (36). Atakol et al (31) examined the protein level of UCP2 in
the DU145 and PC3 cell lines. It was determined that the protein
level of UCP2 in PC3 cells was higher than in DU145 cells (31). In the present study, when the
effect of MDA19 on DU145 and PC3 cell lines was examined, it was
observed that MDA19 had a significant time-dependent inhibitory
effect on the UCP2 protein level. Compared with the MDA19- group,
the highest inhibition of MDA19 on UCP2 expression was observed at
48 h in both cell lines.
Various E3 ubiquitin ligases, including NEDD4L, have
been described as regulators of lipid machinery (36). The protein expression level of
NEDD4L is lower in PCa samples compared to benign prostatic
hyperplasia (18). In addition,
Alberts and Rotin (37). noted
that spartin activated NEDD4 family ligases for the degradation of
LD proteins. Atakol et al (31) compared the protein expression level
of NEDD4L in the DU145 and PC3 cell lines. The protein level of
NEDD4L was found to be higher in DU145 than in PC3 cells in a
time-dependent manner (31). In
the present study, when the effect of MDA19 on DU145 and PC3 cell
lines was examined, a significant time-dependent promoting effect
of MDA19 on the protein level of NEDD4L was observed. The highest
promoting effect was observed at 48 h of treatment with MDA19 in
the DU145 cells (compared to the MDA19- group), while the optimal
effect was observed at 48 and 72 h of treatment with MDA19 in the
PC3 cells. It was hypothesized that MDA19 may regulate cancer cell
proliferation by increasing the NEDD4L level in PCa. In the present
preliminary study, the potential effects of MDA19 on the protein
expression levels PLA2G7, UCP2 and NEDD4L, proteins involved in LD
metabolism in metastatic prostate cell lines), were
demonstrated.
To date, to the best of our knowledge, there is no
study available in the literature demonstrating the effects of the
cannabinoid analogue, MDA19, on LD metabolism in PCa. Thus, the
present study examined the effects of MDA19 on the protein
expression levels of PLA2G7, UCP2 and NEDD4L implicated in LD
metabolism. In conclusion, it was determined that MDA19 exerts an
inhibitory effect on the protein expression levels of PLA2G7 and
UCP2, and a promoting effect on the protein expression level of
NEDD4L. The findings of the present study suggest that targeting
proteins, such as PLA2G7, UCP2 and NEDD4L, involved in LD
metabolism, by treatment with MDA19 in PCa may reduce the
proliferation of tumor cells and may provide novel treatment
options in cancer. MDA19 may prove to be a novel alternative
treatment option for PCa.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Ankara Yildirim
Beyazit University Project Coordination Application and Research
Center (grant no. 2432).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ES, SS, OOG and MEE were involved in the
conceptualization of the study. ES, SS, AS, DA, HAK and EDK were
involved in the study methodology (cytotoxicity test, MTT assay and
ELISA). ES and SS were involved in literature review. ES, SS, OOG
and MEE were involved in the formal analysis. ES and SS were
involved in the writing and preparation of the original draft of
the manuscript. ES, SS, OOG, AS, MEE, DA and HAK were involved in
the writing, reviewing and editing of the manuscript. ES was
involved in funding acquisition. ES and SS confirm the authenticity
of all the raw data. All authors have read and agreed to the
published version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baykara O: Kanser tedavisinde güncel
yaklaşimlar. BAUN Sağ Bil Derg. 5:154–165. 2016.
|
|
2
|
Ercın ME and Şimşek E: Programming of
energy metabolism in prostate carcinoma: In silico analysis.
Gümüşhane Üniv Sağlık Bilim Derg. 9:350–356. 2021.
|
|
3
|
Rawla P: Epidemiology of prostate cancer.
World J Oncol. 10:63–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Liu B, Xu L, Dai EN, Tian JX and Li JM:
Anti-tumoral potential of MDA19 in human osteosarcoma via
suppressing PI3K/Akt/mTOR signaling pathway. Biosci Rep.
38(BSR20181501)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yoon H, Shaw JL, Haigis MC and Greka A:
Lipid metabolism in sickness and in health: Emerging regulators of
lipotoxicity. Mol Cell. 81:3708–3730. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Stoykova GE and Schlaepfer IR: Lipid
metabolism and endocrine resistance in prostate cancer, and new
opportunities for therapy. Int J Mol Sci. 20(2626)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jin C and Yuan P: Implications of lipid
droplets in lung cancer: Associations with drug resistance. Oncol
Lett. 20:2091–2104. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Guijas C, Rodríguez JP, Rubio JM, Balboa
MA and Balsinde J: Phospholipase A2 regulation of lipid droplet
formation. Biochim Biophys Acta. 1841:1661–1671. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Petan T: Lipid droplets in cancer. Rev
Physiol Biochem Pharmacol. 185:53–86. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Huang F, Wang K and Shen J:
Lipoprotein-associated phospholipase A2: The story continues. Med
Res Rev. 40:79–134. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Candels LS, Becker S and Trautwein C:
PLA2G7: A new player in shaping energy metabolism and lifespan.
Signal Transduct Target Ther. 7(195)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schilke RM, Blackburn CMR, Bamgbose TT and
Woolard MD: Interface of phospholipase activity, immune cell
function, and atherosclerosis. Biomolecules.
10(1449)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vainio P, Lehtinen L, Mirtti T, Hilvo M,
Seppänen-Laakso T, Virtanen J, Sankila A, Nordling S, Lundin J,
Rannikko A, et al: Phospholipase PLA2G7, associated with aggressive
prostate cancer, promotes prostate cancer cell migration and
invasion and is inhibited by statins. Oncotarget. 2:1176–1190.
2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Luby A and Alves-Guerra MC: UCP2 as a
cancer target through energy metabolism and oxidative stress
control. Int J Mol Sci. 23(15077)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Erden Y, Tekin S, Kirbag S and Sandal S:
Mitochondrial uncoupling proteins in the brain: Their structure,
function and physiological roles. Med Sci̇. 4:2289–2307. 2014.
|
|
16
|
Sreedhar A and Zhao Y: Uncoupling protein
2 and metabolic diseases. Mitochondrion. 34:135–140.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li W, Nichols K, Nathan CA and Zhao Y:
Mitochondrial uncoupling protein 2 is up-regulated in human head
and neck, skin, pancreatic, and prostate tumors. Cancer Biomark.
13:377–383. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Xie S, Xia L, Song Y, Liu H, Wang ZW and
Zhu X: Insights into the biological role of NEDD4L E3 ubiquitin
ligase in human cancers. Front Oncol. 11(774648)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang M, Zhang Z, Tian X, Zhang E, Wang Y,
Tang J and Zhao J: NEDD4L in human tumors: Regulatory mechanisms
and dual effects on anti-tumor and pro-tumor. Front Pharmacol.
14(1291773)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Balpınar O and Aytaç S: Medical cannabis
and health: A pharmacological review. Ankara Univ Ecz Fak Derg.
45:631–635. 2021.
|
|
21
|
Dang N, Meng X, Ma S, Zhang Q, Sun X, Wei
J and Huang S: MDA-19 suppresses progression of melanoma via
inhibiting the PI3K/Akt pathway. Open Med (Wars). 13:416–424.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kubik J, Humeniuk E, Adamczuk G,
Madej-Czerwonka B and Korga-Plewko A: Targeting energy metabolism
in cancer treatment. Int J Mol Sci. 23(5572)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kostecka LG, Mendez S, Li M, Khare P,
Zhang C, Le A, Amend SR and Pienta KJ: Cancer cells employ lipid
droplets to survive toxic stress. Prostate. 84:644–655.
2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cruz ALS, Barreto EA, Fazolini NPB, Viola
JPB and Bozza PT: Lipid droplets: Platforms with multiple functions
in cancer hallmarks. Cell Death Dis. 11(105)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Roman M, Wrobel TP, Panek A, Paluszkiewicz
C and Kwiatek WM: Lipid droplets in prostate cancer cells and
effect of irradiation studied by Raman microspectroscopy. Biochim
Biophys Acta Mol Cell Biol Lipids. 1865(158753)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tousignant KD, Rockstroh A, Taherian Fard
A, Lehman ML, Wang C, McPherson SJ, Philp LK, Bartonicek N, Dinger
ME, Nelson CC and Sadowski MC: Lipid uptake is an androgen-enhanced
lipid supply pathway associated with prostate cancer disease
progression and bone metastasis. Mol Cancer Res. 17:1166–1179.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Martínez-Martínez E, Martín-Ruiz A, Martín
P, Calvo V, Provencio M and García JM: CB2 cannabinoid receptor
activation promotes colon cancer progression via AKT/GSK3β
signaling pathway. Oncotarget. 7:68781–68791. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sun D, Li X, Nie S, Liu J and Wang S:
Disorders of cancer metabolism: The therapeutic potential of
cannabinoids. Biomed Pharmacother. 157(113993)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Das S, Kaul K, Mishra S, Charan M and
Ganju RK: Cannabinoid signaling in cancer. Adv Exp Med Biol.
1162:51–61. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lehtinen L, Vainio P, Wikman H, Huhtala H,
Mueller V, Kallioniemi A, Pantel K, Kronqvist P, Kallioniemi O,
Carpèn O and Iljin K: PLA2G7 associates with hormone receptor
negativity in clinical breast cancer samples and regulates
epithelial-mesenchymal transition in cultured breast cancer cells.
J Pathol Clin Res. 3:123–138. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Atakol D, Özensoy Güler Ö, Terzi E, Yılmaz
H, Ercin ME and Şimşek E: Investigation of protein expressions of
PLA2G7, UCP2 and NEDD4L genes associated with fat droplet formation
in prostate cancer. OTJHS. 8:497–502. 2023.
|
|
32
|
Jayaraman S, Gantz DL and Gursky O:
Effects of phospholipase A(2) and its products on structural
stability of human LDL: Relevance to formation of LDL-derived lipid
droplets. J Lipid Res. 52:549–557. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li J, Jiang R, Cong X and Zhao Y: UCP2
gene polymorphisms in obesity and diabetes, and the role of UCP2 in
cancer. FEBS Lett. 593:2525–2534. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ke Q, Yuan Q, Qin N, Shi C, Luo J, Fang Y,
Xu L, Sun Q, Zen K, Jiang L, et al: UCP2-induced hypoxia promotes
lipid accumulation and tubulointerstitial fibrosis during ischemic
kidney injury. Cell Death Dis. 11(26)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Burch TC, Rhim JS and Nyalwidhe JO:
Mitochondria biogenesis and bioenergetics gene profiles in isogenic
prostate cells with different malignant phenotypes. Biomed Res Int.
2016(1785201)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Song F, Li JZ, Wu Y, Wu WY, Wang Y and Li
G: Ubiquitinated ligation protein NEDD4L participates in MiR-30a-5p
attenuated atherosclerosis by regulating macrophage polarization
and lipid metabolism. Mol Ther Nucleic Acids. 26:1303–1317.
2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Alberts P and Rotin D: Regulation of lipid
droplet turnover by ubiquitin ligases. BMC Biol.
8(94)2010.PubMed/NCBI View Article : Google Scholar
|