Introduction
Schistosomiasis is considered one of the most
important neglected tropical diseases and remains a major public
health problem in endemic countries (1,2).
Although schistosomiasis may be effectively treated with
praziquantel (3), the high
reinfection rate limits its overall success and repeated
administration is often required multiple times during the first
two decades (4,5). Therefore, the development of a safe
and effective vaccine may improve the long-term treatment of
schistosomiasis and the efficacy of chemotherapeutic interventions
(6,7). Despite decades of research towards the
development of vaccines against Schistosoma japonicum (S.
japonicum), however, the current schistosoma vaccine only
induces limited protection, the reasons for which have not yet been
elucidated.
A potential factor limiting the response of the
immune system to vaccination is the presence of regulatory T cells
(Tregs) which suppress T-cell activation (8,9).
Previous studies demonstrated that Tregs dampen the immune response
against the pathogen in rats harboring S. japonicum
(10,11). Toll-like receptors (TLRs) are
mediators of innate immune responses detecting conserved
pathogen-associated molecules. Thus, selecting the optimal TLR
ligands for a given vaccine may prove to be a crucial component in
maximizing the anti-pathogen immune response. However, few TLR
ligands affecting the schistosome vaccine have been characterized
thus far (12).
In the present study, we investigated whether the
pVAX1-Sj26GST vaccine in combination with the CpG
oligodeoxynucleotide (ODN) 1826 (CpG) conferred an improved immune
response against S. japonicum and assessed the impact of
these TLR ligands on the regulatory function of Tregs in
vitro. It was observed that pVAX1-Sj26GST vaccination combined
with CpG led to the detection of higher levels of interferon
(IFN)-γ and tumor necrosis factor (TNF)-α in the supernatant of
splenocytes and improved the protection against S.
japonicum. CpG inhibited Treg immunosuppressive function and
upregulated the production of IFN-γ, TNF-α, interleukin (IL)-4,
IL-10, IL-2 and IL-6 in vitro, which may contribute to the
escape from Treg-mediated suppression during vaccination, allowing
expansion of antigen-specific T cells against pathogens.
Immunization combined with the CpG TLR ligand therefore is a
promising novel approach in the design of schistosome vaccines.
Materials and methods
Animals
Six-week-old C57BL/6 female rats were provided by
the Center of Experimental Animals (Nanjing University, Nanjing,
China) and bred in university facilities. The animal experiments
were performed in accordance with the Chinese laws for animal
protection and in adherence to experimental guidelines and
procedures approved by the Institutional Animal Care and Use
Committee (IACUC) and the Ethics Review Committee of Nanjing
Medical University for the use of laboratory animals (permit no.
NJMU 09-1107). Oncomelania hupensis (Chinese mainland snail
strain) harboring S. japonicum cercariae were purchased from
the Jiangsu Institute of Parasitic Diseases (Wuxi, China).
Reagents
The TLR9 ligand CpG, with a nuclease-resistant
phosphorothioate backbone and no detectable endotoxin, was
purchased from the Coley Pharmaceutical Group (Wellesley, MA, USA).
The sequence of CpG was 5′-TCCATGACGTTCCT GACGTT-3′. Soluble
schistosome worm antigen was prepared as previously described
(13,14). pVAX1-Sj26GST was purchased from the
Institute of Parasitic Diseases (Nanjing University, Nanjing,
China).
Immunization infection
In each experiment, C57BL/6 rats were divided into
seven groups (n= 8/group). Each rat was intramuscularly injected
with pVAX1-Sj26GST (50 μg), with or without CpG (25
μg). Immunization was repeated three times at 14-day
intervals. Two weeks after the final vaccination, all the rats from
each group were challenged percutaneously with 40±1 S.
japonicum cercariae. After six weeks, rats were sacrificed and
perfused to determine the adult worm and liver egg burdens.
Reductions in worm/liver egg burdens were expressed as the
percentage of the burden recorded in the control groups.
Cell isolation and culture
Single-cell suspensions were prepared by teasing
apart spleens, inguinal and mesenteric lymph nodes from 6
rats/group in PBS containing 1% FCS and 1% EDTA, followed by red
blood cell lysis with Tris-ammonium chloride buffer.
CD4+ T cells were purified from single-cell suspensions
with a CD4+ T Cell Negative-Isolation kit (Miltenyi
Biotec, Auburn, CA, USA) and a magnetic-activated cell sorter,
according to the manufacturer’s recommendations (>97%
CD4+ T cells by flow cytometric analysis).
CD4+CD25+ and
CD4+CD25− cell populations were separated
from purified CD4+ T cells using a mouse regulatory T
Cell Isolation kit (Miltenyi Biotec), according to the
manufacturer’s protocol. The CD25+ populations were
>95% CD4+CD25+ and the
CD4+CD25− populations were 98% pure, as
determined by flow cytometry. CD4+CD25+ cells
were cultured in 96-well U-bottom plates with 1×105
antigen-presenting cells (APCs)/well in triplicate for 72 h at 37°C
in complete RPMI-1640 medium (0.2 ml/well). Cultures were
stimulated with 1 g/ml soluble anti-CD3 (BD Pharmingen, Inc., San
Diego, CA, USA) in the presence or absence of 3 μg/ml CpG.
Proliferation was measured by incubating with 0.5 μCi/well
of 3H-thymidine and measuring incorporation during the
final 16 h of a 3-day culturing period. The supernatants were
collected and quantified for IFN-γ, TNF-α, IL-4, IL-10, IL-2 and
IL-6 production using the FlowCytomix Mouse Cytokine kit (Bender
MedSystems, Vienna, Austria) according to the manufacturer’s
instructions.
Flow cytometry
The Mouse Regulatory T Cell Staining kit
(eBioscience, San Diego, CA, USA) was used for the analysis of
CD4+CD25+Foxp3+ T cells.
Splenocytes from immunized or naïve rats, in the presence or
absence of CpG (3 μg/ml) for 48 h, were surface-stained with
PerCP anti-CD3 monoclonal antibody (eBioscience), FITC anti-CD4
mAbs and APC anti-CD25 mAbs, followed by fixation and
permeabilization with Cytofix/Cytoperm and were then stained
intracellularly with phycoerythrin mouse anti-Foxp3 immunoglobulin
control antibody, according to the manufacturer’s protocol. Data
were collected on a FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA) and analyzed with FlowJo software (Tree
Star, San Carlos, CA, USA).
Statistical analysis
Data were expressed as the means ±standard
deviation. Statistical analysis was performed using SPSS software
version 12.0 (SPSS Inc., Chicago, IL, USA). Statistical
significance was determined by the Student’s t-test and P<0.05
was considered to indicate a statistically significant
difference.
Results
CpG inhibits
CD4+CD25+ Treg function in vitro
TLR9 ligands have been shown to directly impair Treg
function in humans or rats (15,16).
To investigate the effects of CpG and R848 on Treg activity in our
series, CD4+CD25− T cells (responder cells)
were sorted and co-cultured with CD4+CD25+ T
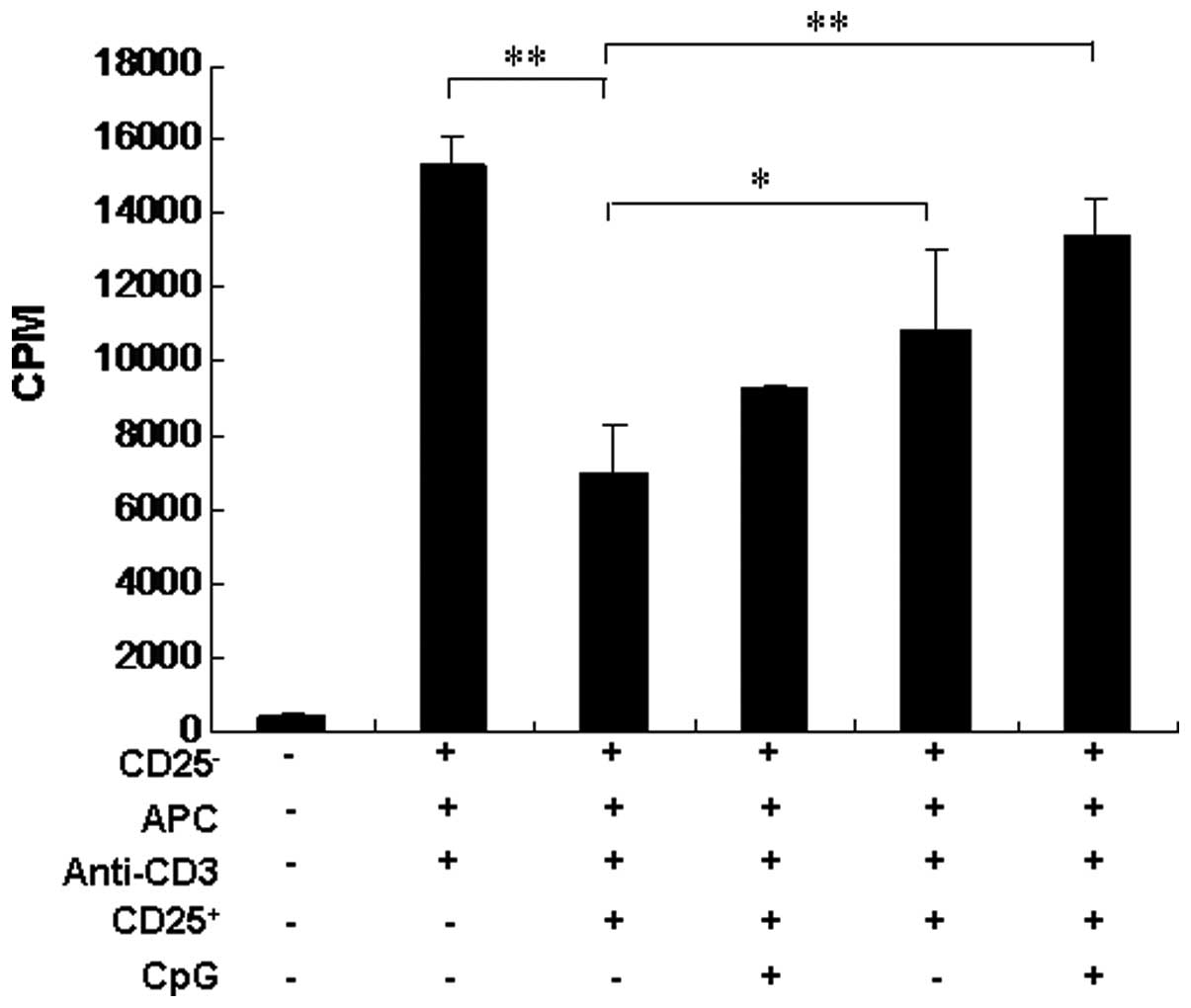
cells from naïve rats. The results shown in Fig. 1 demonstrate that, following
stimulation with anti-CD3 antibody, CD4+CD25+
T cells were highly effective in suppressing the
CD4+CD25− T-cell proliferation. Conversely,
the addition of CpG significantly inhibited Treg function (Fig. 1). These results suggest that the
combination with CpG reduces Treg frequency in vaccinated rats
in vivo and inhibits Treg function in vitro.
Combination with CpG induces higher
levels of proinflammatory cytokines in a conventional suppression
assay in vitro
CpG was shown to significantly increase the levels
of these cytokines in the above-mentioned supernatant. CpG induced
the production of higher IFN-γ, TNF-α, IL-4, IL-2 and IL-6 levels.
Compared to IL-10 levels, the secretion of IFN-γ, TNF-α, IL-4, IL-2
and IL-6 was significantly higher in a conventional in vitro
suppression assay following the addition of CpG (Fig. 2). Thus, these results suggest that
the combination with CpG induced higher levels of proinflammatory
cytokines that may help hinder the immunosuppression of
CD4+CD25+ Tregs in a conventional in
vitro suppression assay.
CpG reduces induction of Foxp3-expressing
T cells in vitro
Foxp3 expression was analyzed by flow cytometry,
which demonstrated that a significantly reduced percentage of
Foxp3-expressing cells were observed among splenocytes in the
presence of CpG. CpG alone induced a reduction in Foxp3 expression
which was not statistically significant compared to the medium
alone (Fig. 3).
Discussion
TLR ligands coordinate innate, adaptive and
regulatory immune responses and as vaccine adjuvants they represent
a promising approach to stimulating strong immune responses and
enhancing vaccine-induced protection (17). TLR9 ligands, including CpG, enhance
immune responses to co-delivered antigens in animal models and are
currently being developed for clinical use as either vaccine
adjuvants or immune therapeutics by Coley Pharmaceuticals (Pfizer,
New York, NY, USA) and Dynavax Technologies. However, the effect of
CpG on vaccines against schistosomiasis, a disease that poses a
significant public health concern in numerous tropical countries,
has not been elucidated and was the subject of this
investigation.
In the present study, we demonstrated that
immunization with pVAX1-Sj26GST including CpG as an adjuvant may
induce stronger protection compared to pVAX1-Sj26GST alone or
single ligands combined. It was reported that TLR ligands as
adjuvants may elicit more vigorous immune responses against
infection and cancer (17,18). The quantification of cytokines in
splenocyte culture supernatants indicated that pVAX1-Sj26GST
vaccination induced a significant increase in IFN-γ levels and a
reduction in IL-4 and IL-10 levels in vaccinated control
pVAX-treated rats. However, the combination with CpG enhanced the
production of IFN-γ and TNF-α, although it also increased the
secretion of IL-4 and IL-10 in rats vaccinated by pVAX1-Sj26GST
plus CpG, which, however, remained lower compared to that in
pVAX1-vaccinated rats. The elevated IFN-γ and TNF-α levels in
response to the combination with CpG may be associated with the
enhancement of protection conferred by pVAX1-Sj26GST vaccination
since the protection induced by several schistosoma vaccines was
associated with elevated production of IFN-γ and TNF-α (19,20).
Our data also suggested that triggering more than one TLR may be an
effective approach to optimize immune responses to vaccination.
This finding is consistent with those of several previous studies
(21).
There is evidence that TLR signaling may modulate
the suppressive functions of Tregs (22,23).
It has been suggested that exposure to inflammatory cytokines
released by APCs may render Tregs defective in mediating their
suppressive effects (24).
Furthermore, exposure to TNF may inhibit the function of Tregs by
TNF receptor II signaling (25).
Consistent with a previous study which reported that
CD4+CD25+ Tregs are able to suppress T-cell
proliferation and cytokine production (26), our study demonstrated that the
presence of CpG in a conventional in vitro suppression assay
induced a panel of inflammatory cytokines, including IFN-γ, TNF-α,
IL-4, IL-10, IL-2 and IL-6, and these elevated cytokines may
inhibit the Treg suppressive function, since a variety of cytokines
have been reported to inhibit Treg function in autoimmune
reactions, including TNF-γ, IL-4, IL-6 and IL-12 (27). Although IL-10, as a major
anti-inflammatory cytokine induced by TLR signaling, inhibits the
production of TLR-induced proinflammatory mediators, such as TNF
(13), this study demonstrated that
elevated levels of IL-10 in the presence of CpG in an in
vitro suppression assay were insufficient to overcome the
strong inflammatory response mediated by the other cytokines.
Furthermore, CpG reduced the expression of Foxp3 in
CD4+ T cells in vitro, which is indispensable in
Treg development and function. Although the in vitro assays
of TLR ligands on Tregs failed to completely mimic the in
vivo milieu, they may lead to the hypothesis that the
downregulation of Foxp3 expression, not only affects Treg function
in vitro, but may also impair Treg generation following
vaccination in vivo, thereby reducing the frequency of
CD4+CD25+ Tregs in rats vaccinated by
pVAX1-Sj26GST combined with CpG, since Foxp3 is critical for the
development and differentiation of Tregs and transduction of Foxp3
into naïve CD4+ T cells resulted in a suppressive
phenotype in rats (14,28). These results are consistent with a
previous study that demonstrated that activation of DCs by TLR7
ligands leads to the down-regulation of Foxp3 expression following
the initial induction and consequently reduces Treg numbers in the
DC-T-cell cocultures in vitro. Furthermore, single TLR
ligands were less effective in decreasing
CD4+Foxp3+ T cells, whereas the combination
of TLR ligands may prevent expansion of Foxp3+ Tregs,
thereby improving T-cell responses (29).
In conclusion, this study has demonstrated that CpG
may impair Treg development and function by upregulating the
secretion of pro-inflammatory cytokines. Defective Tregs provide
the effector response freedom to generate protective immunity when
in combination with the vaccine, in order to elicit the most
appropriate immune response in rats.
Acknowledgements
This study was supported by a grant
from the National Natural Science Foundation of China (NSFC nos.
30801046 and 81172778) and the grant KJ2010A087 from the Natural
Science Foundation of Anhui Province.
References
|
1.
|
Hotez PJ, Brindley PJ, Bethony JM, King
CH, Pearce EJ, et al: Helminth infections: the great neglected
tropical diseases. J Clin Invest. 118:1311–1321. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
King CH: Toward the elimination of
schistosomiasis. N Engl J Med. 360:106–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Doenhoff MJ, Cioli D and Utzinger J:
Praziquantel: mechanisms of action, resistance and new derivatives
for schistosomiasis. Curr Opin Infect Dis. 21:659–667. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Abdul-Ghani R, Loutfy N, el-Sahn A and
Hassan A: Current chemotherapy arsenal for schistosomiasis mansoni:
alternatives and challenges. Parasitol Res. 104:955–965. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Fenwick A, Webster JP, Bosque-Oliva E,
Blair L, Fleming FM, et al: The Schistosomiasis Control Initiative
(SCI): rationale, development and implementation from 2002–2008.
Parasitology. 136:1719–1730. 2009.PubMed/NCBI
|
|
6.
|
McManus DP and Loukas A: Current status of
vaccines for schistosomiasis. Clin Microbiol Rev. 21:225–242. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Bergquist NR, Leonardo LR and Mitchell GF:
Vaccine-linked chemotherapy: can schistosomiasis control benefit
from an integrated approach? Trends Parasitol. 21:112–117. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Aloysius MM, Mc Kechnie AJ, Robins RA,
Verma C, Eremin JM, et al: Generation in vivo of peptide-specific
cytotoxic T cells and presence of regulatory T cells during
vaccination with hTERT (class I and II) peptide-pulsed DCs. J
Transl Med. 7:182009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Toka FN, Suvas S and Rouse BT:
CD4+CD25+T cells regulate vaccine-generated
primary and memory CD8+T-cell responses against herpes
simplex virus type 1. J Virol. 78:13082–13089. 2004.
|
|
10.
|
Wang X, Zhou S, Chi Y, Wen X, Hoellwarth
J, et al: CD4+CD25+Treg induction by an
HSP60-derived peptide SJMHE1 from Schistosoma japonicum is
TLR2 dependent. Eur J Immunol. 39:3052–3065. 2009.
|
|
11.
|
Tang CL, Lei JH, Wang T, Lu SJ, Guan F, et
al: Effect of CD4+CD25+regulatory T cells on
the immune evasion of Schistosoma japonicum. Parasitol Res.
108:477–480. 2010.
|
|
12.
|
Ahmad G, Zhang W, Torben W, Noor Z and
Siddiqui AA: Protective effects of Sm-p80 in the presence of
resiquimod as an adjuvant against challenge infection with
Schistosoma mansoni in mice. Int J Infect Dis. 14:e781–e787.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Mosser DM and Zhang X: Interleukin-10: new
perspectives on an old cytokine. Immunol Rev. 226:205–218. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Schmetterer KG, Neunkirchner A and Pickl
WF: Naturally occurring regulatory T cells: markers, mechanisms,
and manipulation. FASEB J. 26:2253–2276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
LaRosa DF, Gelman AE, Rahman AH, Zhang J,
Turka LA and Walsh PT: CpG DNA inhibits
CD4+CD25+Treg suppression through direct
MyD88-dependent costimulation of effector CD4+T cells.
Immunol Lett. 108:183–188. 2007.PubMed/NCBI
|
|
16.
|
Peng G, Guo Z, Kiniwa Y, Voo KS, Peng W,
et al: Toll-like receptor 8-mediated reversal of
CD4+regulatory T cell function. Science. 309:1380–1384.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Duthie MS, Windish HP, Fox CB and Reed SG:
Use of defined TLR ligands as adjuvants within human vaccines.
Immunol Rev. 239:178–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Engel AL, Holt GE and Lu H: The
pharmacokinetics of Toll-like receptor agonists and the impact on
the immune system. Expert Rev Clin Pharmacol. 4:275–289. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Farias LP, Cardoso FC, Miyasato PA,
Montoya BO, Tararam CA, et al: Schistosoma mansoni Stomatin
like protein-2 is located in the tegument and induces partial
protection against challenge infection. PLoS Negl Trop Dis.
4:e5972010. View Article : Google Scholar
|
|
20.
|
Cardoso FC, Macedo GC, Gava E, Kitten GT,
Mati VL, et al: Schistosoma mansoni tegument protein Sm29 is
able to induce a Th1-type of immune response and protection against
parasite infection. PLoS Negl Trop Dis. 2:e3082008. View Article : Google Scholar
|
|
21.
|
Napolitani G, Rinaldi A, Bertoni F,
Sallusto F and Lanzavecchia A: Selected Toll-like receptor agonist
combinations synergistically trigger a T helper type 1-polarizing
program in dendritic cells. Nat Immunol. 6:769–776. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Liu G and Zhao Y: Toll-like receptors and
immune regulation: their direct and indirect modulation on
regulatory CD4+CD25+T cells. Immunology.
122:149–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Walker LS: Regulatory T cells overturned:
the effectors fight back. Immunology. 126:466–474. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Andre S, Tough DF, Lacroix-Desmazes S,
Kaveri SV and Bayry J: Surveillance of antigen-presenting cells by
CD4+CD25+regulatory T cells in autoimmunity:
immunopathogenesis and therapeutic implications. Am J Pathol.
174:1575–1587. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Valencia X, Stephens G, Goldbach-Mansky R,
Wilson M, Shevach EM, et al: TNF downmodulates the function of
human CD4+CD25hi T-regulatory cells. Blood. 108:253–261.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Sakaguchi S, Wing K, Onishi Y,
Prieto-Martin P and Yamaguchi T: Regulatory T cells: how do they
suppress immune responses? Int Immunol. 21:1105–1111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Buckner JH: Mechanisms of impaired
regulation by CD4(+) CD25(+)FOXP3(+) regulatory T cells in human
autoimmune diseases. Nat Rev Immunol. 10:849–859. 2010.
|
|
28.
|
Lu LF and Rudensky A: Molecular
orchestration of differentiation and function of regulatory T
cells. Genes Dev. 23:1270–1282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Zhu Q, Egelston C, Gagnon S, Sui Y,
Belyakov IM, et al: Using 3 TLR ligands as a combination adjuvant
induces qualitative changes in T cell responses needed for
antiviral protection in mice. J Clin Invest. 120:607–616. 2010.
View Article : Google Scholar : PubMed/NCBI
|