1. Introduction
Non-small-cell lung cancer (NSCLC) is the leading
cause of cancer-related mortality in several industrialized
countries and its incidence is increasing worldwide. It is
estimated that 40% of patients with newly diagnosed NSCLC have
incurable stage IV disease (1). The
remaining patients receive radical treatment, such as surgery or
chemoradiotherapy. However, approximately half of the patients who
receive surgery eventually relapse and, among the patients who
receive chemoradiotherapy, only a small proportion are cured.
Consequently, the majority of NSCLC patients are eventually
classified as having stage IV disease.
Although exact data are unavailable, the incidence
of brain metastases in NSCLC patients is reportedly 24–44% and it
is considered to be increasing with the advances in diagnostic
techniques, such as magnetic resonance imaging (2). In a retrospective review of 809
patients with NSCLC and brain metastases, 181 patients (22%) had
brain metastases at initial staging, of whom 61 (34%) were
asymptomatic. Patients with non-squamous NSCLC had a significantly
higher risk of brain metastases compared to patients with squamous
cell NSCLC (3).
The available survival data on NSCLC patients with
brain metastases are limited, since such patients are generally
excluded from clinical trials. However, previous retrospective
analyses reported that the median survival time (MST) of such
patients is ~3–6 months (4–6). According to a recent retrospective
analysis (7) of 81 patients with
brain metastases who were diagnosed between January, 1996 and June,
2007, the MST was 5 months and the 1- and 2-year survival rates
were 14 and 7.6%, respectively. In addition, neurologically
symptomatic patients exhibited significantly shorter survival
compared to asymptomatic patients, with an MST of 4.0 and 7.5
months, respectively (P=0.02) (7).
At present, there are three available treatment
options for NSCLC patients with brain metastases: surgery,
radiotherapy and chemotherapy. These therapeutic approaches should
be selected appropriately, based on each patient’s clinical
condition. In this review, we aimed to summarize the currently
available treatment options and present a therapeutic strategy for
NSCLC patients with brain metastases, with particular emphasis on
bevacizumab.
2. Whole-brain radiotherapy
Whole-brain radiotherapy (WBRT) is the classical
treatment approach for brain metastases and the schedule of 10
fractions of 3-Gy over 2 weeks (total dose of 30 Gy) is most
commonly used. Thus far, eight randomized controlled trials
comparing the standard dose schedule (30 Gy divided into 10
fractions) with altered dose schedules have been conducted on
patients with brain metastases from various primary cancers,
including NSCLC, with no reported significant differences in
overall survival (OS) and symptom control rate between the two
groups (8).
Concerns have been raised that neurocognitive
function may deteriorate following WBRT. According to a previous
study, 11% of patients who received WBRT and survived for 1 year
developed severe radiation-induced dementia (9). Another study reported that brain
atrophy developed in ≤30% of patients who received ≤50 Gy of WBRT.
However, radiographic brain atrophy was not necessarily accompanied
by a Mini Mental State Examination (MMSE) score decrease and
approximately half of the cases exhibiting a decrease in MMSE
scores could be attributed to a decrease in performance status (PS)
caused by systemic disease progression (10). Evidence suggests that the
deterioration of neurocognitive function is significantly
associated with brain tumor growth and the view that the benefits
greatly outweigh the disadvantages of WBRT is currently predominant
(11–13).
3. Surgery
Surgery has occasionally been selected for patients
with a single brain metastasis and its role has been established
based on randomized controlled trials (Table I) (14–16).
In the first study conducted by Patchell et al(14), 48 patients with a single brain
metastasis, of whom 37 had NSCLC, were randomized to either surgery
followed by WBRT or WBRT alone groups. The frequency of brain
metastasis recurrence was significantly reduced in the surgery plus
WBRT compared to the WBRT alone group (52 vs. 20%, respectively,
P<0.02) and the OS was significantly better in the surgery plus
WBRT compared to the WBRT alone group (MST, 40 vs. 15 weeks,
respectively; P<0.01) (14). In
the second study, the OS was significantly better in the surgery
plus WBRT compared to the WBRT alone group (MST, 43 vs. 26 weeks,
respectively; P=0.04). However, the survival advantage was
prominent in patients with stable extracranial disease (MST, 12 vs.
7 months), whereas in patients with progressive extracranial
disease the MST was 5 months in both groups (15). Furthermore, a third study reported
no significant differences in survival time: the MST was 6.3 months
in the WBRT alone group and 5.6 months in the surgery plus WBRT
group (P=0.24) (16).
 | Table IRandomized studies of whole-brain
radiotherapy (WBRT) with or without surgery in solitary brain
metastasis. |
Table I
Randomized studies of whole-brain
radiotherapy (WBRT) with or without surgery in solitary brain
metastasis.
| Study | Treatment arm | Patient no. | Median survival
(weeks) | Refs. |
|---|
| Patchell et
al | WBRT alone | 23 | 15 | (14) |
| WBRT + surgery | 25 | 40 | |
| Vecht et
al | WBRT alone | 31 | 26 | (15) |
| WBRT + surgery | 32 | 43 | |
| Mintz et
al | WBRT alone | 43 | 6.3 | (16) |
| WBRT + surgery | 41 | 5.6 | |
Regarding the discrepant results among these
studies, it should be noted that 73% of the patients included in
the third study had either extracranial metastases or
uncontrollable primary disease, which is higher compared to the
other two studies (17).
Collectively, surgery followed by WBRT is recommended for NSCLC
patients with a single brain metastasis when extracranial disease
is controlled.
By contrast, there has been no prospective study of
surgery in patients with multiple brain metastases. According to a
retrospective study, patients with multiple brain metastases
achieved survival times similar to those of patients with a single
brain metastasis who underwent surgery, provided all the brain
metastases were surgically resected. However, patients with
multiple brain metastases who did not have all the brain metastases
completely resected exhibited significantly shorter survival times
(18). The role of surgery for
multiple brain metastases remains uncertain and surgery is not
generally recommended for patients with more than one brain
metastasis outside a clinical trial.
4. Radiosurgery
Stereotactic radiosurgery (SRS) has recently emerged
as an alternative option for the treatment of brain metastases. SRS
has an advantage over surgical resection: it is less invasive and
allows more than one lesion to be treated, including those in areas
not surgically accessible.
The largest randomized study comparing WBRT plus SRS
and WBRT alone was conducted by the Radiation Therapy Oncology
Group (RTOG), in which 333 patients with 1–3 brain metastases, of
whom 63% had lung cancer, were randomly assigned to WBRT plus SRS
and WBRT alone groups. No significant survival improvement was
observed in the entire population in that study: the MST was 5.7
months in the WBRT alone group and 6.5 months in the WBRT plus SRS
group (P=0.1356). However, a survival advantage in the WBRT plus
SRS over the WBRT alone group was demonstrated in patients with a
single brain metastasis (MST, 6.5 vs. 4.9 months, respectively;
P=0.0393). In addition, patients in the WBRT plus SRS group were
more likely to have stable or improved PS at 6-month follow-up
compared to patients in the WBRT alone group (43 vs. 27%, P=0.03)
(19).
In another study, SRS plus WBRT was compared to SRS
alone in patients with 1–4 brain metastases, of whom 67% had lung
cancer, and 132 patients were randomized to each group. The OS was
almost identical between the two groups; the MST was 7.5 months in
the SRS alone group and 8.0 months in the combination group
(P=0.42). However, the brain tumor recurrence rate at 1 year was
significantly lower in the combination group (46.8 vs. 76.4%,
P<0.001). The 1-year actual rate of developing new brain
metastases was also significantly reduced in the combination group
(41.5 vs. 63.7%, P=0.003). Consequently, salvage brain treatment
was less frequently required in the combination group (20).
Therefore, the combination of WBRT and SRS is
recommended for NSCLC patients with a single brain metastasis and
may be a viable option for patients with ≤4 brain metastases. There
has been no direct comparison between surgery and SRS in patients
with a single brain metastasis.
5. Chemotherapy
The brain has been traditionally considered to be a
‘sanctuary’ for metastases, under the hypothesis that the
blood-brain barrier (BBB), a mechanism found across species that
protects the brain from exposure to toxins, limits the delivery of
chemotherapeutic agents to the brain (21). Therefore, WBRT has been the standard
treatment for NSCLC patients with multiple brain metastases.
However, certain studies suggested that patients who have developed
brain metastases may have an inherently compromised BBB (22,23).
Robinet et al(24) conducted a randomized phase III study
to determine whether the timing of WBRT with respect to
chemotherapy affects survival in patients with NSCLC and concurrent
brain metastases. Of the patients included in the study, 64% had
multiple brain metastases and 36% had an inoperable solitary brain
metastasis. All the patients received chemotherapy with cisplatin
and vinorelbine for a maximum of 6 cycles. A total of 171 eligible
patients were randomized to receive chemotherapy alone for at least
the first 2 cycles or chemotherapy and early concurrent WBRT (30 Gy
divided in 10 fractions). Patients in the chemotherapy alone group
received the same WBRT i) at any time in the case of proven
clinical progression of brain lesions; ii) after 2 or 4 cycles of
chemotherapy in the case of stable brain lesions; and iii) after 6
cycles of chemotherapy, as for other patients. The intracranial
response rate was 27% in the chemotherapy alone group and 33% in
the chemotherapy with concurrent WBRT group (P=0.12). Survival time
was not significantly different between the two groups: the MST was
24 weeks in the chemotherapy alone group and 21 weeks in the
chemotherapy with concurrent WBRT group (P=0.83). Those results
suggested that the timing of WBRT does not affect the outcome of
NSCLC patients with brain metastases treated with chemotherapy
(24).
More recently, Lee et al(25) conducted a similar study in which 48
NSCLC patients with clinically silent and inoperable brain
metastases were randomized to upfront chemotherapy followed by WBRT
or WBRT followed by chemotherapy groups. Sixty-four percent of the
patients had ≥3 brain metastases and the remaining patients had
<3 brain metastases. The intracranial response rate and the
disease control rate were 28.0 and 72.0% for the chemotherapy first
and 39.1 and 56.5% for the WBRT first arm, respectively. There was
no significant difference in survival time: the MST was 9.1 months
for the chemotherapy first and 9.9 months for the WBRT first arm
(P=0.61). It is noteworthy that 4 patients (17.4%) did not receive
further chemotherapy due to early death or poor PS following WBRT
in the WBRT first arm (25).
The results of recent retrospective studies have
also suggested that upfront chemotherapy is as effective as upfront
WBRT in patients with asymptomatic brain metastases (26,27).
According to the current European Society of Medical Oncology
clinical practice guidelines for metastatic NSCLC, systemic therapy
is a viable option for patients with no or relatively minor
symptoms from brain metastases with early radiotherapy intervention
in the case of the development or progression of symptoms while on
treatment (II, B) (28). Overall,
upfront chemotherapy may be a reasonable option for NSCLC patients
with asymptomatic brain metastases.
6. Bevacizumab
Although an intracranial response comparable to the
extracranial response has been reported with a
pemetrexed-containing regimens, the survival time of patients with
brain metastases is still poor compared to stage IV patients
without brain metastases (29,30).
More effective treatment is required to improve the prognosis of
these patients.
Clinical efficacy of bevacizumab in
NSCLC
Bevacizumab (Avastin; F. Hoffmann-La Roche, Ltd.,
Basel, Switzerland) is a humanized monoclonal antibody that
inhibits tumor angiogenesis by neutralizing the vascular
endothelial growth factor. The first phase III study of bevacizumab
in combination with paclitaxel and carboplatin vs. paclitaxel and
carboplatin in patients with non-squamous NSCLC (E4599) was
conducted in the USA. The progression-free survival (PFS) (6.2 vs.
4.5 months, P<0.001) and OS (12.3 vs. 10.3 months, P=0.003) were
significantly better in the bevacizumab arm (31). In the second phase III study
(AVAiL), conducted in the EU, bevacizumab (7.5 and 15 mg/kg) was
investigated in combination with gemcitabine and cisplatin. PFS,
the primary endpoint, was found to be significantly better in the
bevacizumab arm (32). According to
a recent meta-analysis, bevacizumab in combination with
platinum-based chemotherapy significantly prolonged OS and PFS
(33). Major clinical guidelines
currently recommend the use of bevacizumab in NSCLC patients
without contraindications, such as squamous cell histology and
history of hemoptysis (28,34).
Safety data of bevacizumab in patients
with brain metastases
Despite the promising preclinical data (35–39),
patients with brain metastases have been excluded from clinical
trials of bevacizumab, following a single serious bleeding event in
a phase I trial in one patient with hepatocellular carcinoma and
occult brain metastasis (35).
In an attempt to elucidate whether brain metastases
are a significant risk factor for intracranial hemorrhage (ICH),
Srivastava et al(36)
conducted a retrospective analysis of patients with advanced NSCLC
using data from the MD Anderson Cancer Center Tumor Registry. This
study included 776 patients with and 1,367 patients without brain
metastases and the rates of spontaneous ICH were compared. The
actual number of patients that developed ICH and the incidence rate
(per 1,000 individuals/year) was 9 and 15.5 in patients with brain
metastases and 4 and 3.2 in patients without brain metastases,
respectively (crude incidence rate ratio, 4.79; P=0.0076). However,
the actual number of patients developing symptomatic ICH and the
incidence rate (per 1,000 individuals/year) were 4 and 6.9 in
patients with brain metastases and 4 and 3.2 in patients without
brain metastases, respectively (crude incidence rate ratio, 2.13;
P=0.31). These results demonstrated that the rate of spontaneous
ICH appeared to be higher among patients with brain metastases
compared to those without, although the rate was very low in both
groups and the rates of symptomatic ICH were not significantly
different between the two groups (36). A later study by Khasraw et
al(37) conducted a
retrospective analysis to investigate the association between
treatment with bevacizumab and ICH in various types of tumors. It
was concluded that bevacizumab does not increase the incidence of
ICH in cancer patients, despite the presence of brain metastases.
In the NSCLC population, the incidence rate of ICH was 1.00%
(29/2,914) in patients treated without bevacizumab and 1.24%
(3/242) in patients treated with bevacizumab. Moreover, the
incidence rates of ICH were 3.6% (28/789) in patients with brain
metastases treated without bevacizumab and 3.9% (3/77) in patients
with brain metastases treated with bevacizumab (37). Retrospective exploratory analyses of
randomized controlled trials reported similar results, indicating
that bevacizumab is not a significant risk factor for ICH, even in
patients with brain metastases (38,39).
In the prospective setting, the safety of
bevacizumab for brain metastases was first investigated in patients
with treated brain metastases (PASSPORT study) (40). Of the 115 enrolled NSCLC patients,
67 (58.7%) received WBRT alone, 25 (21.7%) received WBRT with SRS
or surgery, 22 (19.1%) received SRS alone and 1 (0.9%) received
surgery alone. The chemotherapy consisted of carboplatin and
paclitaxel (33%), carboplatin and other agents (28%), pemetrexed
(19%), erlotinib (10%) and others (10%). Among the 106
safety-evaluable patients, there was no reported grade 1–5 ICH (95%
CI: 0.0–3.3%) (40). Thus,
bevacizumab was safely administered to patients with treated brain
metastases. In the subsequent study, NSCLC patients with
asymptomatic untreated brain metastases were treated with
bevacizumab (BRAIN study) (41). In
the first-line arm (n=67), the combination of bevacizumab,
paclitaxel and carboplatin was administered, whereas bevacizumab
and erlotinib were administered in the second-line arm (n=24). The
median PFS was 6.7 months in the first-line and 6.3 months in the
second-line arm. The median OS was 15.1 months in the first-line
and 13.6 months in the second-line arm. The response rates for
intracranial and extracranial metastases were almost identical in
the two arms. One grade 1 ICH event as reported in the first-line
arm and none were reported in the second-line arm. In this study,
bevacizumab exhibited an acceptable safety profile in patients with
asymptomatic untreated brain metastases and the survival data were
comparable to those of patients without brain metastases treated
with bevacizumab (41). Due to the
fact that patients with brain metastases generally exhibit lower
survival times compared to patients without brain metastases, these
data encouraged clinicians to use bevacizumab in this patient
population.
There has been no prospective study of bevacizumab
in patients with active or symptomatic brain metastases. However,
certain case series recently reported that bevacizumab was safe and
effective for progressive brain metastases (42,43). A
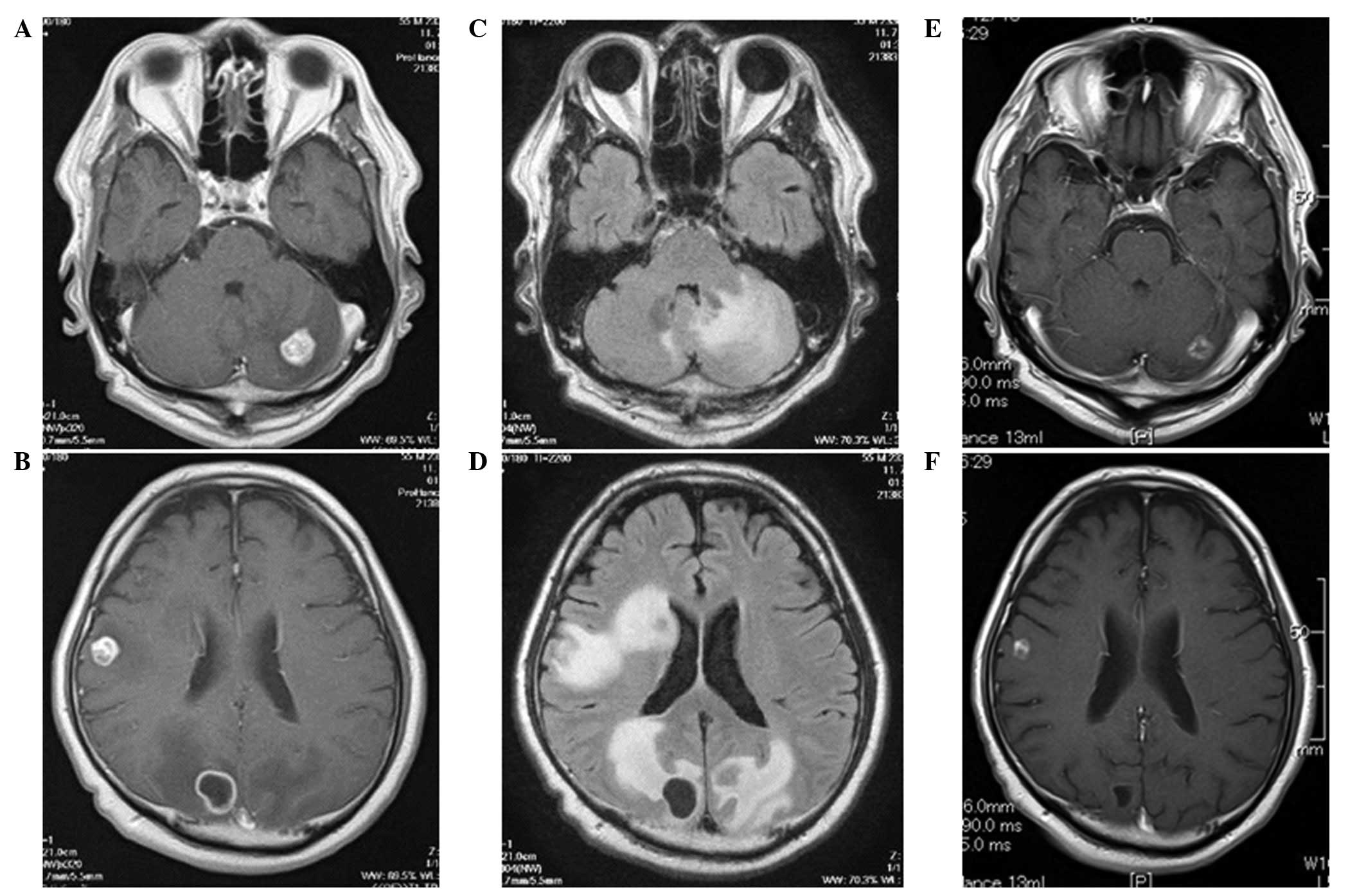
case of progressive brain metastases treated at our hospital is
shown in Fig. 1. The patient’s
brain tumors did not respond to WBRT and he developed seizures. The
patient subsequently received a combination chemotherapy with
bevacizumab, paclitaxel and carboplatin as second-line chemotherapy
and the brain metastases were significantly reduced in size,
without ICH. These results suggest that bevacizumab may merit
further investigation in patients with active or symptomatic brain
metastases as well.
7. Treatment algorithm for NSCLC patients
with brain metastases
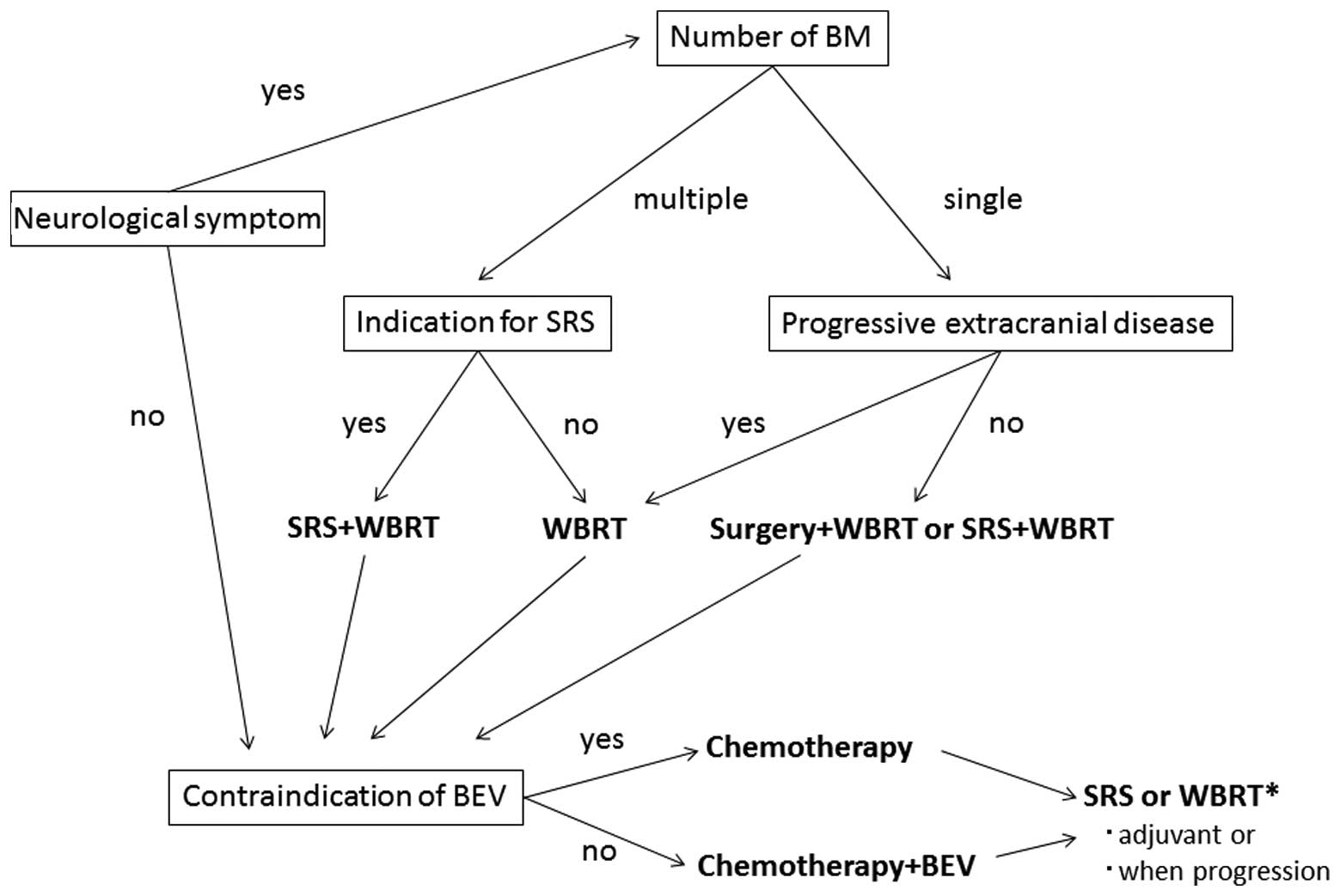
An example of the algorithm for the treatment of
NSCLC patients with brain metastases is shown in Fig. 2. Patients with symptomatic brain
metastases should be treated with local therapy prior to
administration of chemotherapy and the treatment modality should be
selected according to the number of brain metastases and the
extracranial disease status. Surgery followed by WBRT or SRS+WBRT
is recommended for patients with a single brain metastasis.
Although there are no established criteria, SRS+WBRT may be
considered for patients with ≤4 brain metastases (≤4 cm) and
controlled extracranial disease (44). Patients with asymptomatic brain
metastases and those who have received prior local therapy should
be evaluated for indications for bevacizumab and the administration
of bevacizumab is recommended for patients without specific
contraindications for this drug.
8. Conclusions
In this review we summarized the currently available
treatment options for NSCLC patients with brain metastases and
highlighted the safety and efficacy of bevacizumab. Considering
their dismal prognosis, bevacizumab is recommended particularly for
patients with brain metastases. Molecularly-targeted agents were
not included in the review to simplify the discussion. Should
molecularly-targeted agents be included, the treatment algorithm
becomes more complicated. For example, it has not yet been
established whether WBRT or tyrosine kinase inhibitor of epidermal
growth factor receptor (EGFR) is preferred as initial treatment for
patients with activating mutation of EGFR, or which treatment
option is preferred for neurologically symptomatic patients. There
remain several unanswered questions regarding the treatment of
NSCLC patients with brain metastases. A more sophisticated
treatment strategy, including molecularly-targeted agents, should
be identified to cater for personalized medicine.
References
|
1
|
Socinski MA, Crowell R, Hensing TE, et al:
Treatment of non-small cell lung cancer, stage IV: ACCP
evidence-based clinical practice guidelines (2nd edition). Chest.
132:S277–S289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nayak L, Lee EQ and Wen PY: Epidemiology
of brain metastases. Curr Oncol Rep. 14:48–54. 2012. View Article : Google Scholar
|
|
3
|
Shi AA, Digumarthy SR, Temel JS, et al:
Does initial staging or tumor histology better identify
asymptomatic brain metastases in patients with non-small cell lung
cancer? J Thorac Oncol. 1:205–210. 2006.PubMed/NCBI
|
|
4
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
Radiation Therapy Oncology Group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Penel N, Brichet A, Prevost B, et al:
Prognostic factors of synchronous brain metastases from lung
cancer. Lung Cancer. 33:143–154. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ampil F, Caldito G, Milligan S, et al: The
elderly with synchronous non-small cell lung cancer and solitary
brain metastasis: does palliative thoracic radiotherapy have a
useful role? Lung Cancer. 57:60–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanchez de Cos J, Sojo Gonzalez MA,
Montero MV, et al: Non-small cell lung cancer and silent brain
metastasis. Survival and prognostic factors. Lung Cancer.
63:140–145. 2009.PubMed/NCBI
|
|
8
|
Tsao MN, Lloyd N, Wong RK, et al: Whole
brain radiotherapy for the treatment of newly diagnosed multiple
brain metastases. Cochrane Database Syst Rev. 4:CD0038692012.
|
|
9
|
DeAngelis LM, Mandell LR, Thaler HT, et
al: The role of postoperative radiotherapy after resection of
single brain metastases. Neurosurgery. 24:798–805. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shibamoto Y, Baba F, Oda K, et al:
Incidence of brain atrophy and decline in mini-mental state
examination score after whole-brain radiotherapy in patients with
brain metastases: a prospective study. Int J Radiat Oncol Biol
Phys. 72:1168–1173. 2008. View Article : Google Scholar
|
|
11
|
Meyers CA, Smith JA, Bezjak A, et al:
Neurocognitive function and progression in patients with brain
metastases treated with whole-brain radiation and motexafin
gadolinium: results of a randomized phase III trial. J Clin Oncol.
22:157–165. 2004. View Article : Google Scholar
|
|
12
|
Li J, Bentzen SM, Renschler M and Mehta
MP: Regression after whole-brain radiation therapy for brain
metastases correlates with survival and improved neurocognitive
function. J Clin Oncol. 25:1260–1266. 2007. View Article : Google Scholar
|
|
13
|
Tallet AV, Azria D, Barlesi F, et al:
Neurocognitive function impairment after whole brain radiotherapy
for brain metastases: actual assessment. Radiat Oncol. 7:772012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patchell RA, Tibbs PA, Walsh JW, et al: A
randomized trial of surgery in the treatment of single metastases
to the brain. N Engl J Med. 322:494–500. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vecht CJ, Haaxma-Reiche H, Noordijk EM, et
al: Treatment of single brain metastasis: radiotherapy alone or
combined with neurosurgery? Ann Neurol. 33:583–590. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mintz AH, Kestle J, Rathbone MP, et al: A
randomized trial to assess the efficacy of surgery in addition to
radiotherapy in patients with a single cerebral metastasis. Cancer.
78:1470–1476. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wronski M and Lederman G: A randomized
trial to assess the efficacy of surgery in addition to radiotherapy
in patients with a single cerebral metastasis. Cancer.
80:1002–1004. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bindal RK, Sawaya R, Leavens ME and Lee
JJ: Surgical treatment of multiple brain metastases. J Neurosurg.
79:210–216. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andrews DW, Scott CB, Sperduto PW, et al:
Whole brain radiation therapy with or without stereotactic
radiosurgery boost for patients with one to three brain metastases:
phase III results of the RTOG 9508 randomised trial. Lancet.
363:1665–1672. 2004. View Article : Google Scholar
|
|
20
|
Aoyama H, Shirato H, Tago M, et al:
Stereotactic radiosurgery plus whole-brain radiation therapy vs.
stereotactic radiosurgery alone for treatment of brain metastases:
a randomized controlled trial. JAMA. 295:2483–2491. 2006.
View Article : Google Scholar
|
|
21
|
Walbert T and Gilbert MR: The role of
chemotherapy in the treatment of patients with brain metastases
from solid tumors. Int J Clin Oncol. 14:299–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deeken JF and Loscher W: The blood-brain
barrier and cancer: transporters, treatment, and Trojan horses.
Clin Cancer Res. 13:1663–1674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mehta MP, Paleologos NA, Mikkelsen T, et
al: The role of chemotherapy in the management of newly diagnosed
brain metastases: a systematic review and evidence-based clinical
practice guideline. J Neurooncol. 96:71–83. 2010. View Article : Google Scholar
|
|
24
|
Robinet G, Thomas P, Breton JL, et al:
Results of a phase III study of early versus delayed whole brain
radiotherapy with concurrent cisplatin and vinorelbine combination
in inoperable brain meta- stasis of non-small-cell lung cancer:
Groupe Français de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann
Oncol. 12:59–67. 2001.
|
|
25
|
Lee DH, Han JY, Kim HT, et al: Primary
chemotherapy for newly diagnosed nonsmall cell lung cancer patients
with synchronous brain metastases compared with whole-brain
radiotherapy administered first: result of a randomized pilot
study. Cancer. 113:143–149. 2008. View Article : Google Scholar
|
|
26
|
Moscetti L, Nelli F, Felici A, et al:
Up-front chemotherapy and radiation treatment in newly diagnosed
nonsmall cell lung cancer with brain metastases: survey by Outcome
Research Network for Evaluation of Treatment Results in Oncology.
Cancer. 109:274–281. 2007. View Article : Google Scholar
|
|
27
|
Kim KH, Lee J, Lee JI, et al: Can upfront
systemic chemotherapy replace stereotactic radiosurgery or whole
brain radiotherapy in the treatment of non-small cell lung cancer
patients with asymptomatic brain metastases? Lung Cancer.
68:258–263. 2010. View Article : Google Scholar
|
|
28
|
Peters S, Adjei AA, Gridelli C, et al:
Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 23(Suppl 7): vii56–vii64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barlesi F, Gervais R, Lena H, et al:
Pemetrexed and cisplatin as first-line chemotherapy for advanced
non-small-cell lung cancer (NSCLC) with asymptomatic inoperable
brain metastases: a multicenter phase II trial (GFPC 07-01). Ann
Oncol. 22:2466–2470. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bailon O, Chouahnia K, Augier A, et al:
Upfront association of carboplatin plus pemetrexed in patients with
brain metastases of lung adenocarcinoma. Neuro Oncol. 14:491–495.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reck M, von Pawel J, Zatloukal P, et al:
Overall survival with cisplatin-gemcitabine and bevacizumab or
placebo as first-line therapy for nonsquamous non-small-cell lung
cancer: results from a randomised phase III trial (AVAiL). Ann
Oncol. 21:1804–1809. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Soria JC, Mauguen A, Reck M, et al:
Systematic review and meta-analysis of randomised, phase II/III
trials adding bevacizumab to platinum-based chemotherapy as
first-line treatment in patients with advanced non-small-cell lung
cancer. Ann Oncol. 24:20–30. 2013. View Article : Google Scholar
|
|
34
|
Azzoli CG, Baker S Jr and Temin S;
American Society of Clinical Oncology. American Society of Clinical
Oncology Clinical Practice Guideline update on chemotherapy for
stage IV non-small-cell lung cancer. J Clin Oncol. 27:6251–6266.
2009. View Article : Google Scholar
|
|
35
|
Gordon MS, Margolin K, Talpaz M, et al:
Phase I safety and pharmacokinetic study of recombinant human
anti-vascular endothelial growth factor in patients with advanced
cancer. J Clin Oncol. 19:843–850. 2001.PubMed/NCBI
|
|
36
|
Srivastava G, Rana V, Wallace S, et al:
Risk of intracranial hemorrhage and cerebrovascular accidents in
non-small cell lung cancer brain metastasis patients. J Thorac
Oncol. 4:333–337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khasraw M, Holodny A, Goldlust SA and
DeAngelis LM: Intracranial hemorrhage in patients with cancer
treated with bevacizumab: the Memorial Sloan-Kettering experience.
Ann Oncol. 23:458–463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carden CP, Larkin JM and Rosenthal MA:
What is the risk of intracranial bleeding during anti-VEGF therapy?
Neuro Oncol. 10:624–630. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Besse B, Lasserre SF, Compton P, et al:
Bevacizumab safety in patients with central nervous system
metastases. Clin Cancer Res. 16:269–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Socinski MA, Langer CJ, Huang JE, et al:
Safety of bevacizumab in patients with non-small-cell lung cancer
and brain metastases. J Clin Oncol. 27:5255–5261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Besse B, Le Moulec S, Senellart H, et al:
Phase II study of bevacizumab in combination with first-line
chemotherapy or second-line erlotinib in non-squamous NSCLC
patients with asymptomatic untreated brain metastases (ML21823).
Ann Oncol. 23:ix4262012.
|
|
42
|
De Braganca KC, Janjigian YY, Azzoli CG,
et al: Efficacy and safety of bevacizumab in active brain
metastases from non-small cell lung cancer. J Neurooncol.
100:443–447. 2010.PubMed/NCBI
|
|
43
|
Yamamoto D, Iwase S, Tsubota Y, et al:
Bevacizumab in the treatment of five patients with breast cancer
and brain metastases: Japan Breast Cancer Research Network-07
trial. Onco Targets Ther. 5:185–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tsao MN, Lloyd NS and Wong RK; Supportive
Care Guidelines Group of Cancer Care Ontario’s Program in
Evidence-based Care. Clinical practice guideline on the optimal
radiotherapeutic management of brain metastases. BMC Cancer.
5:342005. View Article : Google Scholar : PubMed/NCBI
|