Introduction
Bone marrow mesenchymal stem cells (bmMSCs) are a
type of multipotent stem cells that are derived from the adult bone
marrow and are able to differentiate into a variety of cell types,
such as osteoblasts, chondrocytes, adipocytes and cardiomyocytes
(1,2). Angiotensin II (Ang II) is a peptide
hormone that plays a critical role in a series of physiological and
pathophysiological processes, including cell proliferation,
differentiation, apoptosis and inflammation (3). Recently, Ang II was used to induce the
differentiation of bmMSCs to other functional cell lineages, such
as cardiomyocytes, adipocytes and smooth muscle-like cells
(4–6). It was demonstrated that Ang II induces
inflammatory responses in vitro in several types of cells,
including endothelial cells, smooth muscle cells, fibroblasts and
kidney tubule epithelial cells (7–9).
Aspirin is a drug commonly used as analgesic,
antipyretic and occasionally anti-inflammatory medication (8). Recent studies demonstrated that
aspirin may suppress inflammatory responses in cultured endothelial
cells, fibroblasts and other cell lines, via the inhibition of
reactive oxygen species (ROS) generation (8,10,11).
Ang II, as a strong inducer of ROS generation, may induce
inflammatory responses in bmMSCs and aspirin may attenuate these
inflammatory responses. The purpose of the present study was to
investigate the effects of aspirin on Ang II-induced inflammation
in bmMSCs and the possible underlying mechanisms.
Materials and methods
Materials and reagents
Aspirin, Ang II and 2X PCR Reaction mix were
purchased from Sigma-Aldrich (St. Louis, MO, USA). The mouse tumor
necrosis factor α (TNF-α) Quantikine ELISA kit and the mouse
interleukin (IL)-6 Quantikine ELISA kit were purchased from R&D
Systems Inc. (Minneapolis, MN, USA). DNase I, RNeasy Mini kit and
SuperScript II First-Strand cDNA Synthesis kit were purchased from
Invitrogen Life Technologies (Carlsbad, CA, USA). Rabbit anti-mouse
phospho-extracellular signal-regulated protein 1/2 (ERK1/2),
ERK1/2, phospho-nuclear factor κ-light-chain-enhancer of activated
B cells (NF-κB)-p65 and NF-κB-p65 antibodies were obtained from
Cell Signaling Technology, Inc. (Danvers, MA, USA). β-actin
antibody and horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibody were purchased from Abcam
(Cambridge, MA, USA). ECL Western Blotting Substrate was purchased
from Thermo Scientific (Rockford, IL, USA). The polyvinylidene
fluoride (PVDF) membranes were obtained from GE Healthcare
(Pittsburgh, PA, USA).
Cell culture and study protocol
BmMSCs were obtained as previously described
(12,13). In brief, bone marrow was harvested
from the mouse tibia and femur, washed and cultured in Dulbecco’s
modified Eagle’s medium supplemented with 15% fetal bovine serum
for 3 h. Subsequently, the non-adherent cells were removed and the
medium was replaced. A purified population of bmMSCs was obtained
after 3 weeks of culture. The cells were plated in 6- and 12-well
plates and treated with 0, 10 nM, 100 nM, 1 μM and 10 μM Ang II for
12 h. In other experiments, the cells were pretreated with 0.1 mM
aspirin for 30 min and then exposed to 1 μM Ang II for an
additional 12 h.
Enzyme-linked immunosorbent assay
(ELISA)
Following treatment with Ang II and aspirin, the
supernatants of the growth medium were collected by centrifugation
and frozen at −80°C until use. The levels of TNF-α and IL-6 were
measured using the mouse TNF-α Quantikine ELISA kit and the mouse
IL-6 Quantikine ELISA kit, according to the manufacturer’s
instructions. Absorbance at 450 nm was read by a microplate
reader.
Western blot assay
Proteins were extracted from the treated bmMSCs and
separated by 12% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis. Following electrophoresis, the proteins were
transferred to the PVDF membranes. The membranes were blocked with
5% bovine serum albumin in Tris-buffered saline with Tween-20
(TBS-T) and then incubated with phospho-ERK1/2, ERK1/2,
phospho-NF-κB-p65, NF-κB-p65 and β-actin antibodies at 4°C
overnight. Subsequently, the blots were washed with TBS-T and
incubated with HRP-conjugated secondary antibody for 1 h at room
temperature. The immunoreactive bands were visualized by enhanced
chemiluminescence.
Reverse transcription-polymerase chain
reaction (RT-PCR) assay
Total RNA was extracted from the treated bmMSCs with
a RNeasy Mini kit and complementary DNA (cDNA) was synthesized with
a SuperScript II First-Strand cDNA Synthesis kit. To eliminate
contamination of the genomic DNA, RNA was pretreated with DNase I
prior to the synthesis of cDNA. RT-PCR was performed using 2X PCR
reaction solution with 100 ng cDNA and 0.3 μM primers. The primer
sequences and reaction cycles are listed in Table I.
 | Table IPrimers for reverse
transcription-polymerase chain reaction. |
Table I
Primers for reverse
transcription-polymerase chain reaction.
| Primer | Sequence | Product size
(bp) | Reaction cycles |
|---|
| TNF-α | Sense:
5′-CCGATGGGTTGTACCTTGTC-3′
Antisense: 5′-GGGCTGGGTAGAGAATGGAT-3′ | 352 | 32 |
| MCP-1 | Sense
5′-GGAGCATCCACGTGTTGGC-‘3
Antisense: 5′-GTAGGAGTGACCAGTGTGACAGT-3′ | 391 | 33 |
| IL-6 | Sense:
5′-GATGCTACCAAACTGGATATAATC-3′
Antisense: 5′-GGTCCTTAGCCACTCCTTCTGTG-3′ | 269 | 37 |
| IL-1β | Sense:
5′-GAAATGCCACCTTTTGACAGTG-3′
Antisense: 5′-GAAGGTCCACGGGAAAGACAC-3′ | 225 | 38 |
| β-actin | Sense:
5′-TTCTTTGCAGCTCCTTCGTTGCCG-3′
Antisense: 5′-TGGATGGCTACGTACATGGCTGGG-3′ | 458 | 32 |
Statistical analysis
Statistical analysis was performed with SPSS
software, version 11.5 (SPSS Inc., Chicago, IL, USA). Data are
presented as means ± standard deviation (SD) from 4 independent
experiments. Univariate comparisons of the means were evaluated
using the Student’s t-test and/or one-way analysis of variance with
Tukey’s post hoc adjustment for multiple comparisons when
appropriate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Ang II induces inflammation in
bmMSCs
In this study, the levels of TNF-α and IL-6 were
evaluated by ELISA. As shown in Fig. 1A
and B, TNF-α and IL-6 were increased in a dose-dependent manner
in the growth medium with the cultured bmMSCs as the cells were
exposed to 10 nM-10 μM Ang II. These data were further confirmed by
an RT-PCR assay, which demonstrated a dose-dependent increase in
TNF-α and IL-6 mRNA in the bmMSCs following exposure to 10 nM-10 μM
Ang II (Fig. 1C,D and F). In
addition, a significant increase in IL-1β and monocyte chemotactic
protein-1 (MCP-1) mRNA was also observed in the bmMSCs following
exposure to different concentrations of Ang II (Fig. 1C,E and G).
Ang II activates ERK1/2 and NF-κB signals
in bmMSCs
Western blotting demonstrated that treatment with
Ang II (10 nM-10 μM) increased the expression of phospho-ERK1/2 and
phospho-NF-κB-p65 in bmMSCs in a dose-dependent manner; however,
Ang II did not significantly affect the total expression of ERK1/2
and NF-κB-p65 (Fig. 1H–L). These
data demonstrated that ERK1/2 and NF-κB are activated in bmMSCs
following exposure to different concentrations of Ang II.
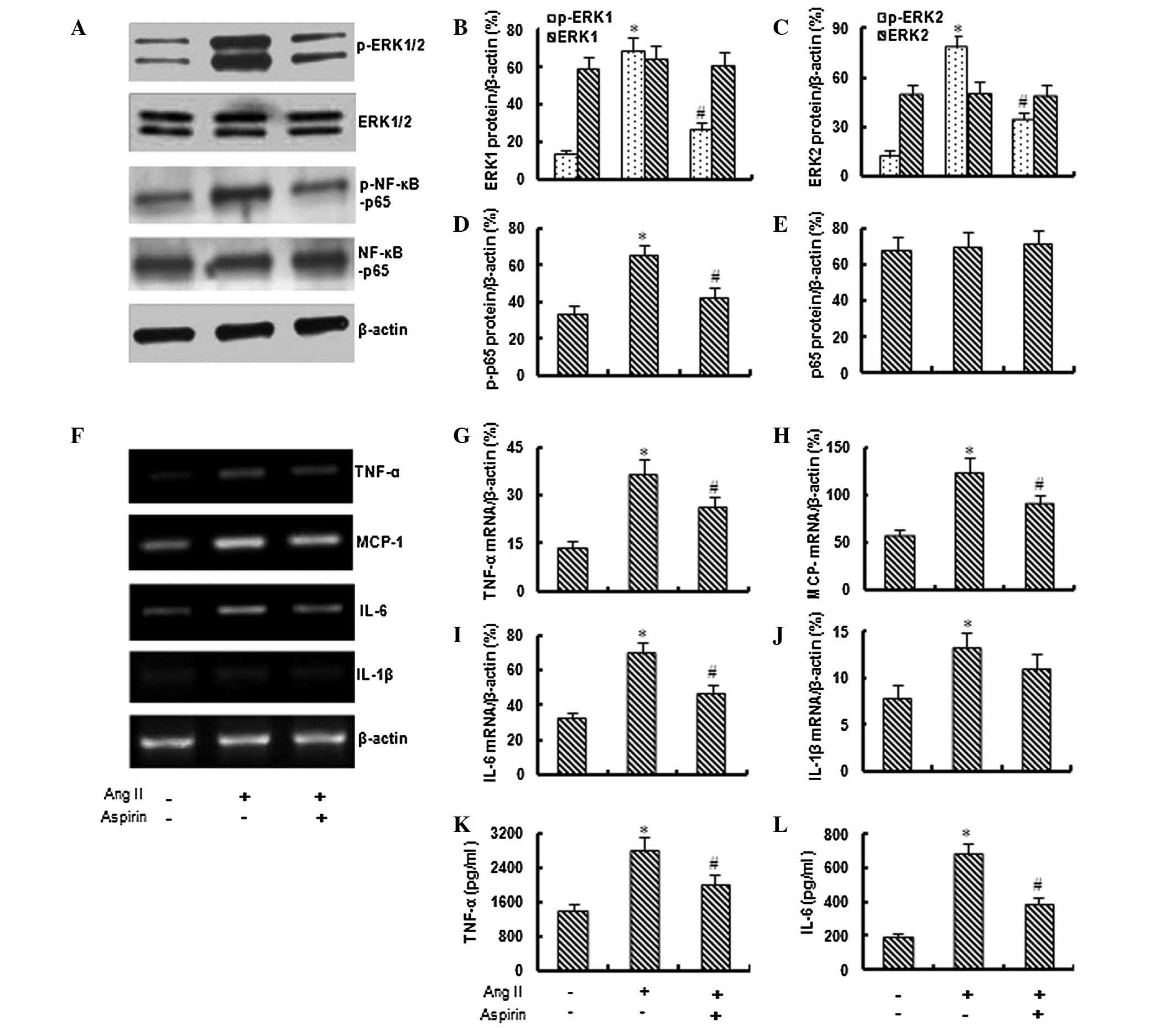
Aspirin inhibits the activation of ERK1/2
and NF-κB
Based on preliminary data, 1 μM Ang II was used in
the following experiments to investigate the effect of aspirin on
Ang II-induced inflammation. As shown in Fig. 2A–E, the application of aspirin (0.1
mM) significantly inhibited the Ang II-induced expression of
phospho-ERK1/2 and phospho-NF-κB-p65, although it did not
significantly affect the total expression of ERK1/2 and
NF-κB-p65.
Aspirin suppresses Ang II-induced
inflammation
The RT-PCR assay demonstrated that the application
of aspirin significantly inhibited the Ang II-induced expression of
TNF-α, MCP-1, IL-6 and IL-1β genes (Fig. 2F–J). In addition, the ELISA revealed
that aspirin (0.1 mM) significantly inhibited the Ang II-induced
secretion of TNF-α and IL-6 from bmMSCs (Fig. 2K and L).
Discussion
The present study demonstrated that Ang II induces
inflammatory responses and the activation of ERK1/2 and NF-κB in
bmMSCs and that the application of aspirin inhibits the Ang
II-induced activation of ERK1/2 and NF-κB and inflammatory
responses, indicating that aspirin may inhibit Ang II-induced
inflammation in bmMSCs via the inhibition of ERK1/2 and NF-κB
activation.
BmMSCs are the most promising source of stem cells
for cell transplantation therapy and have been widely used in
tissue regenerative medicine. Generally, bmMSCs must be induced
in vitro by chemical or biological reagents for directional
differentiation prior to transplantation. Ang II is one of the most
commonly used inducers of MSC differentiation. It was demonstrated
that Ang II is able to induce the differentiation of bmMSCs to
cardiomyocytes, adipocytes and smooth muscle cells (4–6). The
present study demonstrated that treatment with Ang II may also
induce inflammatory responses in bmMSCs.
It is known that the inflammatory responses in
bmMSCs limit their clinical use. Treatment with anti-inflammatory
and anti-immune rejection drugs may promote the survival of bmMSCs
in recipient organs following transplantation (14). Previous studies demonstrated that
aspirin inhibits the inflammatory responses in several cell lines,
including endothelial cells and fibroblasts, via the inhibition of
NADPH oxidase activity and ROS generation (8,10). In
the present study, we also observed that aspirin inhibited Ang
II-induced inflammatory responses in bmMSCs.
ERK1/2 are members of the mitogen-activated protein
kinase superfamily that is implicated in the development of acute
and chronic inflammatory disorders (15,16).
It was reported that aspirin inhibits the activation of ERK1/2 and
inflammatory responses in a series of pathophysiological conditions
(17,18). In the present study, we observed
that aspirin (0.1 mM) significantly inhibited the AngII-induced
activation of ERK1/2 in bmMSCs. In addition, we also observed that
aspirin markedly inhibited the Ang II-induced activation of NF-κB.
NF-κB is another important factor involved in inflammation
(19). Previous studies
demonstrated that aspirin inhibits the activation of NF-κB pathway
in certain chronic inflammatory conditions, protecting organs and
tissues from inflammation and damage (20–22).
In this study, the inhibitory effects of aspirin on the Ang
II-induced inflammation in bmMSCs may be attributed to its role in
the regulation of the ERK1/2 and NF-κB activity.
In summary, we demonstrated that Ang II induces
inflammatory responses and activation of ERK1/2 and NF-κB in bmMSCs
in a dose-dependent manner and that the application of aspirin
inhibits the Ang II-induced activation of ERK1/2, NF-κB and
inflammatory responses. These findings suggest that aspirin may be
effective in attenuating Ang II-induced inflammation in cultured
bmMSCs and may serve as an alternative drug to suppress
inflammatory responses when the cells are exposed to Ang II for
directional differentiation.
References
|
1
|
Rostovskaya M and Anastassiadis K:
Differential expression of surface markers in mouse bone marrow
mesenchymal stromal cell subpopulations with distinct lineage
commitment. PLoS One. 7:e512212012. View Article : Google Scholar
|
|
2
|
Jackson L, Jones DR, Scotting P, et al:
Adult mesenchymal stem cells: differentiation potential and
therapeutic applications. J Postgrad Med. 53:121–127. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehta PK and Griendling KK: Angiotensin II
cell signaling: physiological and pathological effects in the
cardiovascular system. Am J Physiol Cell Physiol. 292:C82–C97.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xing Y, Lv A, Wang L, et al: The
combination of angiotensin II and 5-azacytidine promotes
cardiomyocyte differentiation of rat bone marrow mesenchymal stem
cells. Mol Cell Biochem. 360:279–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsushita K, Wu Y, Okamoto Y, et al:
Local renin angiotensin expression regulates human mesenchymal stem
cell differentiation to adipocytes. Hypertension. 48:1095–1102.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim YM, Jeon ES, Kim MR, et al:
Angiotensin II-induced differentiation of adipose tissue-derived
mesenchymal stem cells to smooth muscle-like cells. Int J Biochem
Cell Biol. 40:2482–2491. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhan Y, Brown C, Maynard E, et al: Ets-1
is a critical regulator of Ang II-mediated vascular inflammation
and remodeling. J Clin Invest. 115:2508–2516. 2005. View Article : Google Scholar
|
|
8
|
Wang X, Lu J, Khaidakov M, et al: Aspirin
suppresses cardiac fibroblast proliferation and collagen formation
through downregulation of angiotensin type 1 receptor
transcription. Toxicol Appl Pharmacol. 259:346–354. 2012.
View Article : Google Scholar
|
|
9
|
Wolak T, Kim H, Ren Y, et al: Osteopontin
modulates angiotensin II-induced inflammation, oxidative stress,
and fibrosis of the kidney. Kidney Int. 76:32–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen JW, Zhou SB and Tan ZM: Aspirin and
pravastatin reduce lectin-like oxidized low density lipoprotein
receptor-1 expression, adhesion molecules and oxidative stress in
human coronary artery endothelial cells. Chin Med J (Engl).
123:1553–1556. 2010.
|
|
11
|
Wu Y, Zhai H, Wang Y, et al:
Aspirin-triggered lipoxin A4attenuates
lipopolysaccharide-induced intracellular ROS in BV2 microglia cells
by inhibiting the function of NADPH oxidase. Neurochem Res.
37:1690–1696. 2012.
|
|
12
|
Zhang F, Wang C, Jing S, et al:
Lectin-like oxidized LDL receptor-1 expresses in mouse bone
marrow-derived mesenchymal stem cells and stimulates their
proliferation. Exp Cell Res. 319:1054–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang F, Jing S, Ren T, et al:
microRNA-10b promotes the migration of mouse bone marrow-derived
mesenchymal stem cells and downregulates the expression of
E-cadherin. Mol Med Rep. Aug 6–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
14
|
van Velthoven CT, Kavelaars A and Heijnen
CJ: Mesenchymal stem cells as a treatment for neonatal ischemic
brain damage. Pediatr Res. 71:474–481. 2012.PubMed/NCBI
|
|
15
|
Pastore S, Mascia F, Mariotti F, et al:
ERK1/2 regulates epidermal chemokine expression and skin
inflammation. J Immunol. 174:5047–5056. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee WK, Chung KW, Kim GH, et al:
Gallotannin causes differentiation and inflammation via ERK-1/-2
and p38 kinase pathways in rabbit articular chondrocytes. Mol Med
Rep. 7:701–707. 2013.PubMed/NCBI
|
|
17
|
Vartiainen N, Goldsteins G,
Keksa-Goldsteine V, et al: Aspirin inhibits p44/42
mitogen-activated protein kinase and is protective against
hypoxia/reoxygenation neuronal damage. Stroke. 34:752–757. 2003.
View Article : Google Scholar
|
|
18
|
Wang YP, Wu Y, Li LY, et al:
Aspirin-triggered lipoxin A4 attenuates LPS-induced
pro-inflammatory responses by inhibiting activation of NF-κB and
MAPKs in BV-2 microglial cells. J Neuroinflammation.
8:952011.PubMed/NCBI
|
|
19
|
Ke X, Chen J, Zhang X, et al: Qing Hua
Chang Yin attenuates lipopolysaccharide-induced inflammatory
response in human intestinal cells by inhibiting NF-κB activation.
Exp Ther Med. 6:189–193. 2013.PubMed/NCBI
|
|
20
|
Yamamoto Y and Gaynor RB: Therapeutic
potential of inhibition of the NF-kappaB pathway in the treatment
of inflammation and cancer. J Clin Invest. 107:135–142. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muller DN, Heissmeyer V, Dechend R, et al:
Aspirin inhibits NF-kappaB and protects from angiotensin II-induced
organ damage. FASEB J. 15:1822–1824. 2001.PubMed/NCBI
|
|
22
|
Akyazi I, Eraslan E, Gulcubuk A, et al:
Long-term aspirin pretreatment in the prevention of
cerulein-induced acute pancreatitis in rats. World J Gastroenterol.
19:2894–2903. 2013.PubMed/NCBI
|