Introduction
Malaria is an infectious disease endemic throughout
tropical countries. Malaria is also prevalent in subtropical areas,
where the disease is contagious affecting both indigenous
population and travelers (1).
Malaria is caused by Plasmodium parasites that are
transmitted through the bite of Anopheles mosquitoes and
have a life cycle in mosquito and human hosts (1). Of all parasite types, Plasmodium
falciparum (P. falciparum) is the most dangerous
Plasmodium, causing human malaria with a mortality of 1–2
million people annually. According to surveys conducted between
1900 and 2008 in 2,366 locations in Indonesia, four species of
Plasmodium may infect humans, P. falciparum, P.
vivax, P. malariae and P. ovale. P. falciparum is
the most common parasite that is contagious in Indonesia, with
prevalence rates of 33% in Papua, 29% in Lesser Sundas and 21% in
Sumatra (2). Findings of studies
performed in other parts of Indonesia, including the Thousand
Island district (3), Nias Island
(4), Sumba Island (5) and Aceh (6), have shown that P. falciparum
was the most frequent parasite that caused malaria.
Eradication of malaria remains challenging due to
drug resistance of Plasmodium. Hyde (1) reported that the first synthetic
antimalarial drug, found in the 1930s, was chloroquine, which was
highly effective, safe and cost-effective. Since 1957, however,
resistance to administration of chloroquine was observed in
Thailand and by 1988 this resistance had spread to sub-Saharan
Africa and other areas of the world. Several factors affect
antimalarial resistance, including the overuse of drugs for
prophylaxis, incomplete therapeutic treatments of active
infections, genetic and metabolic adaptive abilities of the
parasites and a massive parasite proliferation (1). The incidence of malaria infections,
which is ~250 million cases and 80,000 mortalities annually, has
revealed the emerging requirement for identifying new classes of
medicine (7).
Medicinal plants have been used as traditional
medicines for hundreds of years. The first antimalarial agents,
including quinine as well as the next generation of antimalarial
agents, such as lapachol and artemisinin, have been isolated from
plants (7,8). Traditional medicines have a high
potential as novel drug candidates, can provide valuable clues to
find novel drugs and may shift the drug discovery paradigm from
‘finding new-entity drugs’ to ‘combining existing agents’ (9,10).
Certain approaches in finding novel drugs use plants that are
consumed by particular groups (11). Primates have a close similarity with
human physiology and also have similar characteristics of disease
as humans. Humans use drugs to cure these diseases, whereas
primates can only rely on the foods they eat to protect themselves
against these diseases.
Due to the high degree of physiological similarity
between primates and humans, primate disease often exhibits
similarities to human disease, including cancer and malaria.
Notably, certain human diseases are known to have originated from
primates (11). As the survival
rates of primates is mainly dependent on daily food intake, the
food consumed is considered to be a promising source of products
applicable for the management of human disease. In a previous
study, 19 primate-consumed plants were collected and their
anti-tumor promoting activity was confirmed in vitro
(11), with two species, Kadsura
scandens (Blume) Blume (family Schisandraceae) and
Schima wallichii (S. wallichii) (family
Theaceae), showing antimutagenic activities in further
investigations (12). The potential
of S. wallichii to inhibit MCF-7 breast cancer cell
proliferation has also been reported (13). Findings of an ethnobotanical study
in Mizoram showed that S. wallichii is used for snake and
insect bites (14). S.
wallichii is a tree with a height of 5–30 m and is usually
found in tropical countries, including Indonesia, the Philippines,
Nepal, Sikkim, Assam, Myanmar, South China and the Malay Peninsula
(15). In the present study, the
antiplasmodial properties of the S. wallichii leaves were
investigated and its active compound was identified.
Materials and methods
Plant collection
S. wallichii leaves were collected from the
Pangandaran Beach conservation area in the West Java province of
Indonesia. The leaf of S. wallichii was identified in the
School of Biological Science and Technology, Bandung Institute of
Technology, Bandung, Indonesia.
Extraction, fractionation and
isolation
The S. wallichii leaves were dried and
extracted with 70% ethanol at room temperature three times for 24 h
each. A concentrated extract was obtained in vacuo at 50°C.
The ethanol extract (86.94 g) was partitioned into n-hexane
(3.03 g), ethyl acetate (3.27 g) and aqueous phases (7.19 g),
respectively. Column chromatography on a Wakogel C-200 (Wako Pure
Chemical Industries, Ltd., Chuo-ku, Osaka, Japan) column was
performed on the most active ethyl acetate fraction, using a
mixture of n-hexane, ethyl acetate and methanol with
increasing polarity. The major compound observed was purified using
silica G-60 with sulfuric acid-ethanol (1:9) and was found to be
the most active fraction of S. wallichii, which was
characterized and analyzed as described previously (13). The isolate was, however, identified
by spectroscopic methods [ultraviolet, infrared, nuclear magnetic
resonance (NMR)] and liquid chromatography mass spectrometry
(16).
P. falciparum parasite culture
The chloroquine-resistant P. falciparum
strain, K-1, was cultured asynchronously as described in a previous
study (17). The culture was grown
in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) containing
10% type B or O human serum (serum type showed no significant
effect on parasite growth), 25 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Wako
Pure Chemical Industries, Ltd.), 25 μg/ml gentamycin
(Sigma-Aldrich), 25 mM sodium bicarbonate (Wako Pure Chemical
Industries, Ltd.) and human type O red blood cells (RBCs) to
generate the final 5% hematocrit mixture. The parasite was cultured
in a humidified incubator in 5% CO2 and 5% O2
at 37°C.
Growth inhibitory assay
The growth inhibitory effect of the extract,
fractions or isolate of S. wallichii against P.
falciparum was determined by culturing the parasite with an
initial parasitemia of 0.1% in 5% hematocrit. The culture medium
containing the extract, fractions or isolate of S. wallichii
was changed every 24 h and the number of RBCs containing the
parasite (pRBCs) was counted. Thin-smeared slides of Giemsa-stained
RBCs were prepared, pRBCs were counted, and the number of pRBCs in
3,000 RBCs was determined under a light microscope at a
magnification of ×1,000. The experiment was terminated at 72 h.
Each concentration was created in triplicate and the experiment was
performed in triplicate. The parasite morphology was observed prior
to counting the pRBCs in the same culture plate using the same
method.
Determination of the IC50
concentration against P. falciparum
The IC50 determination of
kaempferol-3-O-rhamnoside against P. falciparum was
performed using a similar method to that of the ‘growth inhibitory
assay’, as described in a previous report (18). The culture medium containing
kaempferol-3-O-rhamnoside with concentrations of 115, 230
and 460 μM was incubated for 24 h and the number of pRBCs was
counted. Thin-smeared slides of Giemsa-stained RBCs were prepared,
the pRBCs were counted, and the number of pRBCs in 3,000 RBCs was
determined under a light microscope at a magnification of ×1,000.
The IC50 values that represent the concentrations
required to inhibit 50% of plasmodium growth were calculated from a
calibration curve by linear regression.
Statistical analysis
Statistically significant differences were
determined by the Student’s t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Growth of P. falciparum is inhibited by
the ethyl acetate fraction of S. wallichii
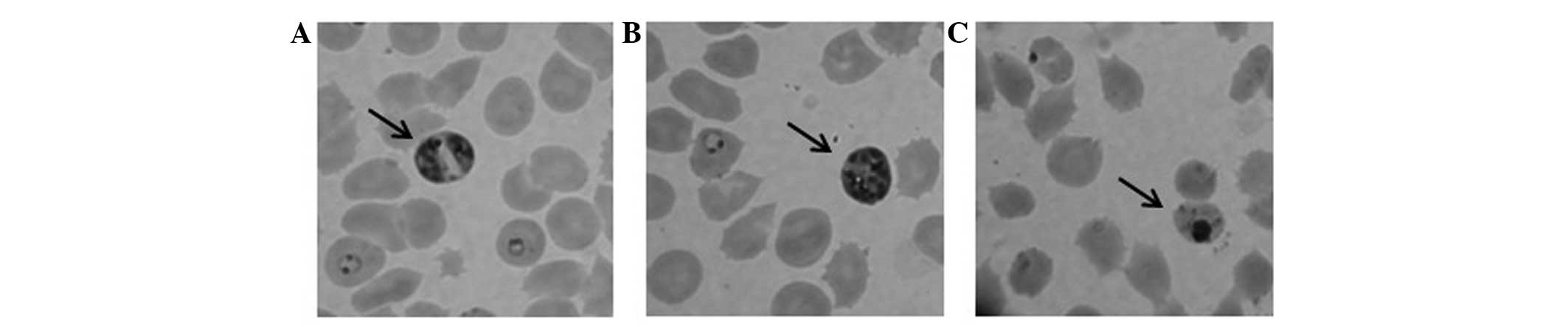
The S. wallichii extract (100 μg/ml) was
shown to inhibit P. falciparum growth after a 24-h
treatment. P. falciparum morphological changes were detected
following treatment with the extract (Fig. 1). The extract was partitioned into
n-hexane, ethyl acetate and water. The extract and each
fraction at a concentration of 100 μg/ml were then used to treat
the parasites cultured in RBCs for 72 h. The extract,
n-hexane and ethyl acetate fractions significantly inhibited
parasite growth in the RBC culture, which was observed by the
decreasing parasitemia level of 3,000 RBCs compared with that in
the untreated parasite culture (Fig.
2). These results indicated that the ethyl acetate fraction was
the most active fraction that inhibited parasite growth.
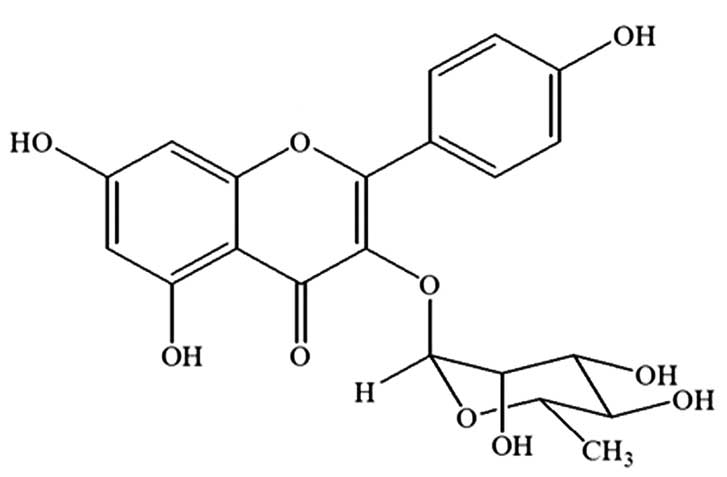
Kaempferol-3-O-rhamnoside is the major
compound of the ethyl acetate fraction of the S. wallichii
extract
The strongest parasite growth inhibition effect was
observed following the treatment with the ethyl acetate fraction of
S. wallichii. Based on a previous study (13), it was found that the major compound
of the ethyl acetate fraction of the S. wallichii extract
was kaempferol-3-O-rhamnoside. The compound was purified,
isolated and identified as kaempferol-3-O-rhamnoside
(C21H20O10) with a molecular
weight of 432 (Fig. 3).
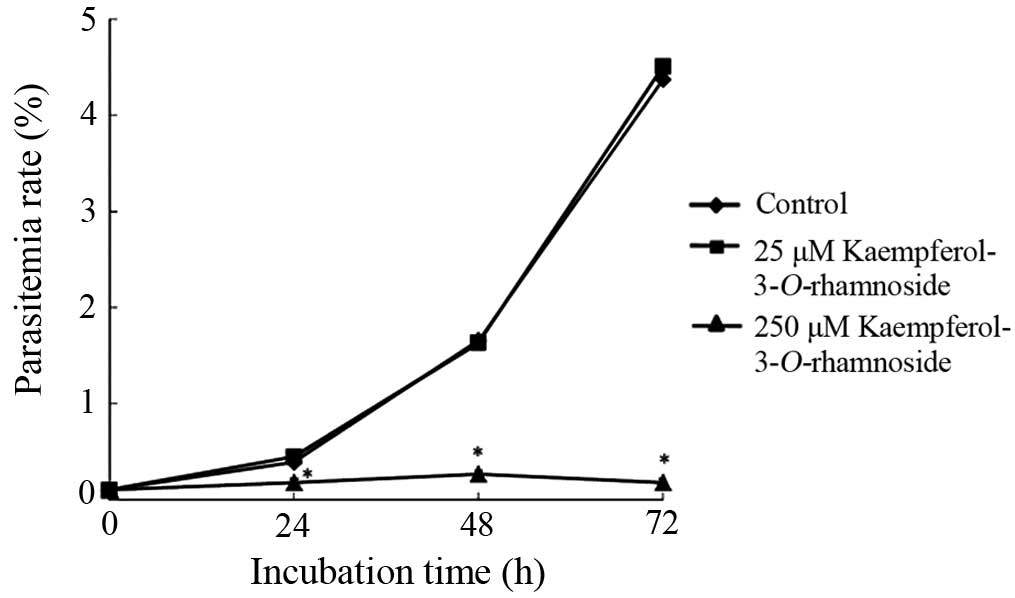
Kaempferol-3-O-rhamnoside inhibits P.
falciparum culture growth
The antiplasmodial effect of
kaempferol-3-O-rhamnoside was observed 24–72 h following the
treatment with kaempferol-3-O-rhamnoside in P.
falciparum culture (Fig. 4).
Parasite growth was significantly inhibited by 250 μM
kaempferol-3-O-rhamnoside to 54.3% at 24 h of treatment,
83.9% at 48 h and 96% at 72 h compared with the untreated control.
The results shown in Fig. 4 also
show that the antiplasmodial properties of
kaempferol-3-O-rhamnoside were dose-dependent. Incubation
with 25 μM kaempferol-3-O-rhamnoside did not show
significant parasite growth inhibitory activity (almost similar to
the negative control).
Subsequent analysis of the antiplasmodial activities
against P. falciparum culture using various concentrations
(0, 115, 230, 345 and 460 μM) of kaempferol-3-O-rhamnoside
for 24 h showed that the IC50 value of
kaempferol-3-O-rhamnoside was 106 μM (Fig. 5). At this concentration,
kaempferol-3-O-rhamnoside actively inhibited the growth rate
of P. falciparum and selectively affected the parasite, but
not the human RBCs as the host cells.
Discussion
In the present study, treatment with 100 μg/ml S.
wallichii extract caused shrinkage and pyknotic bodies in the
parasite morphology, but not in the human RBCs as the host cells in
P. falciparum culture. Pyknosis is an irreversible condition
involving chromatin condensation in the nucleus. This condition
shows ongoing necrosis or apoptosis inside the cells, which is
followed by nucleus fragmentation (19). This finding showed that S.
wallichii Korth. extract has selective antimalarial
activity.
The results also demonstrate that the ethanol
extract of S. wallichii inhibited parasite growth. Results
of the present study also show that the ethyl acetate fraction of
S. wallichii had the strongest antiplasmodial activity in a
time-dependent manner. This result suggests that this fraction has
the potential to be explored for its active antiplasmodial
compounds.
The characterized compound in the ethyl acetate
fraction was determined to be kaempferol-3-O-rhamnoside
following purification by column chromatography and identification
by NMR. In addition, kaempferol-3-O-rhamnoside that was
isolated from S. wallichii has also been reported to have
anti-cancer activity by inducing the apoptotic mechanism through
the caspase-cascade pathway in MCF-7 breast cancer cells (13). The results of the present study show
that at a concentration of 250 μM, kaempferol-3-O-rhamnoside
inhibited parasite growth in a time-dependent manner. In subsequent
investigations, the IC50 was also confirmed to be 106 μM
in vitro against chloroquine-resistant P. falciparum
after the 24-h treatment.
Oxidative stress through the generation of reactive
oxygen species (ROS) plays an important role in the pathogenesis of
malarial infection. During rapid growth and multiplying, plasmodium
produces toxic redox active by-products that cause host haemoglobin
degradation (20–22). Additionally, ROS are also produced
by recruited and activated monocytes and neutrophils during
infections that attack infected and uninfected erythrocytes, which
increases the ROS level (22–24).
Plasmodium cause damage in the membrane cells of infected
and uninfected erythrocytes by inducing lipid peroxidation, leading
to an aging-like process, eventually resulting in anemia (24). As a polyphenol,
kaempferol-3-O-rhamnoside is able to inhibit lipid
peroxidation and cyclooxygenase (COX) enzymes (COX-1 and COX-2)
(25). Thus, it can be hypothesized
that these antioxidant properties may be responsible for the
antiplasmodial acitivity of kaempferol-3-O-rhamnoside.
The present study has shown the antiplasmodial
activity of S. wallichii, which was previously reported for
its anticancer properties (13).
This is similar to another study, which focused on Cryptolepis
sanguinolenta (Lindl.) Schltr (Periplocaceae), a traditional
antimalarial in West Africa, for its anticancer activity by its
cytotoxic effect in mammalian cells (26).
As the study is in a preliminary stage, follow-up
studies on the antiplasmodial mechanisms in various plasmodium life
stages in vitro an in vivo are currently being
conducted in our laboratory. However, these findings provide a
basis for subsequent investigations of
kaempferol-3-O-rhamnoside as a candidate compound for
potential antimalarial in drug development.
References
|
1
|
Hyde JE: Drug-resistant malaria - an
insight. FEBS J. 274:4688–4698. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Elyazar IR, Hay SI and Baird JK: Malaria
distribution, prevalence, drug resistance and control in Indonesia.
Adv Parasitol. 74:41–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maguire JD, Tuti S, Sismadi P, et al:
Endemic coastal malaria in the Thousand Islands District, near
Jakarta, Indonesia. Trop Med Int Health. 10:489–496. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Syafruddin D, Asih PB, Wahid I, et al:
Malaria prevalence in Nias District, North Sumatra Province,
Indonesia. Malar J. 6:1162007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asih PB, Rogers WO, Susanti AI, et al:
Seasonal distribution of anti-malarial drug resistance alleles on
the island of Sumba, Indonesia. Malar J. 8:2222009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asih PB, Rozi IE, Herdiana, et al: The
baseline distribution of malaria in the initial phase of
elimination in Sabang Municipality, Aceh Province, Indonesia. Malar
J. 11:2912012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wells TN: Natural products as starting
points for future anti-malarial therapies: going back to our roots?
Malar J. 10(Suppl 1): S32011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ginsburg H and Deharo E: A call for using
natural compounds in the development of new antimalarial treatments
- an introduction. Malar J. 10(Suppl 1): S12011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kong DX, Li XJ and Zhang HY: Where is the
hope for drug discovery? Let history tell the future. Drug Discov
Today. 14:115–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wagner H and Ulrich-Merzenich G: Synergy
research: approaching a new generation of phytopharmaceuticals.
Phytomedicine. 16:97–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koshimizu K, Murakami A, Hayashi H,
Ohigashi H, Subarnas A, Gurmaya KJ and Ali A: Biological activities
of edible and medicinal plants from Indonesia and Malaysia. In:
Proceedings of The Tokyo International Forum on Conservation and
Sustainable Use of Tropical Bioresources; Japan Bioindustry
Association; Tokyo: pp. 203–208. 1998
|
|
12
|
Subarnas A, Hadiansyah C, Gurmaya KJ and
Muhtadi A: Characterization of antimutagenic compound from
primates- consumed plant Schima wallichii. Biotika. 2:7–13.
2003.
|
|
13
|
Diantini A, Subarnas A, Lestari K, et al:
Kaempferol-3-O- rhamnoside isolated from the leaves of
Schima wallichii Korth. inhibits MCF-7 breast cancer cell
proliferation through activation of the caspase cascade pathway.
Oncol Lett. 3:1069–1072. 2012.
|
|
14
|
Lalfakzuala R, Lalramnghinglova H and
Kayang H: Ethnobotanical usages of plants in western Mizoram.
Indian J Tradit Knowl. 6:486–493. 2007.
|
|
15
|
Keng H: Flora malesianae precursores -
LVIII, part four. The genus Schima(Theaceae) in
Malesia. Gard Bull Singapore. 46:77–88. 1994.
|
|
16
|
Silverstein RM, Webster FX and Kiemle DJ:
Spectrometric Identification of Organic Compounds. 7th edition.
John Wiley & Sons; New Jersey, NJ: pp. 72–229. 2005
|
|
17
|
Taguchi N, Hatabu T, Yamaguchi H, Suzuki
M, Sato K and Kano S: Plasmodium falciparum:
selenium-induced cytotoxicity to P. falciparum. Exp
Parasitol. 106:50–55. 2004. View Article : Google Scholar
|
|
18
|
Suradji EW, Hatabu T, Kobayashi K, et al:
Selenium-induced apoptosis-like cell death in Plasmodium
falciparum. Parasitology. 19:1–11. 2011.
|
|
19
|
Baehrecke EH: How death shapes life during
development. Nat Rev Mol Cell Biol. 3:779–787. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ginsburg H and Atamna H: The redox status
of malaria-infected erythrocytes: an overview with an emphasis on
unresolved problems. Parasite. 1:5–13. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Becker K, Tilley L, Vennerstrom JL,
Roberts D, Rogerson S and Ginsburg H: Oxidative stress in malaria
parasite-infected erythrocytes: host-parasite interactions. Int J
Parasitol. 34:163–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ibrahim MA, Zuwahu MM, Isah MB, Jatau ID,
Aliyu AB and Umar IA: Effects of vitamin E administration on
Plasmodium berghei induced pathological changes and
oxidative stress in mice. Trop Biomed. 29:98–106. 2012.
|
|
23
|
Postma NS, Mommers EC, Eling WM and
Zuidema J: Oxidative stress in malaria: implications for prevention
and therapy. Pharm World Sci. 18:121–129. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omodeo-Salè F, Motti A, Basilico N,
Parapini S, Olliaro P and Taramelli D: Accelerated senescence of
human erythrocytes cultured with Plasmodium falciparum.
Blood. 102:705–711. 2003.PubMed/NCBI
|
|
25
|
Vareed SK, Schutzki RE and Nair MG: Lipid
peroxidation, cyclooxygenase enzyme and tumor cell proliferation
inhibitory compounds in Cornus kousa fruits. Phytomedicine.
14:706–709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ansah C and Mensah KB: A review of the
anticancer potential of the antimalarial herbal Cryptolepis
sanguinolenta and its major alkaloid cryptolepine. Ghana Med J.
47:137–147. 2013.PubMed/NCBI
|