Introduction
Docetaxel acts as a novel type of chemotherapy drug
belonging to the taxane family. As a microtubule antagonist,
docetaxel is active to a variety of malignant tumors, including
breast cancer (1), non-small cell
lung cancer (2), head and neck
squamous carcinoma (3) and gastric
cancer (4). Docetaxel is
administered by intravenous infusion only. With the use of a
central line increasing, the incidence of extravasation has been on
the decrease in recent years. As for patients with superior vena
cava obstruction, dissection of the bilateral axilla lymph nodes or
failure to place a central catheter, peripheral intravenous line
should be considered. The incidence of chemotherapy drug
extravasation has been reported as 11 and 22% in children and
adults, respectively (5). The
vesicant potential of anthrancyclines (6), vinorelbine (7)and paclitaxel (8) extravasation have been confirmed. In
clinical practice, whether a vesicant reaction would be induced by
docetaxel extravasation has been debated previously (9), and the management of docetaxel
extravasation requires clarification. In the present study,
docetaxel extravasation was studied in a rat model. This study was
approved by the Ethics Committee of hospital and was performed
according to the Declaration of Helsinki. Written informed consent
was obtained from each patient’s family.
Materials and methods
Animals and drugs
Female Sprague-Dawley white rats (weight, 300–350 g)
were provided by Jinhua Food and Drug Administration (Jinhua,
Zhejiang, China). Docetaxel (Taxotere) and vinorelbine (Novelbine)
were produced by Sanofi Aventis (Paris, France) and Laboratories
Pierre Fabre (Castres, France), respectively.
Injection model
The experimental rats were non per os for 24 h,
watered freely, weighed and subsequently received intraperitoneal
anesthesia of pentobarbital sodium (40 mg/kg). The hair in the
bilateral lower extremities was shaved with an electric shaver. An
area of skin, 4 cm2 in diameter, was prepared. A needle
(1 ml) was used for the intradermal injection of the docetaxel
solution. The standard of successful intradermal injection was
defined as a 1-cm diameter formation of a skin rash. Docetaxel, 20
mg in 0.5 ml polysorbate 80, was diluted with 1.5 ml 13% ethanol
and stored at a 10 mg/ml concentration. Subsequently, normal saline
(NS) was used for the dilution of docetaxel into numerous
concentrations. Six levels of injection volumes were selected: 0.1,
0.2, 0.3, 0.4, 0.5 and 0.6 ml; and five levels of concentration
injection were selected: 1, 2, 4, 6 and 8 mg/ml. A total of eight
rat models were assigned to each level.
Control groups
The intradermal injection of 0.4 ml NS (1-cm
diameter skin rash formation) was considered to be the negative
control group, and 0.4 ml vinorelbine (2 mg/ml concentration) was
considered to be the positive control group. A total of eight rat
models were assigned to each control group.
Observation of the injection site
The definition of a skin ulcer was epidermal
excoriation and the loss of skin integrity. The definition of
recovery was the disappearance of the ulcer, swelling and edema.
The perpendicular widths of the skin ulcer were measured and
multiplied to yield a lesion area. The ulcer area was measured each
day from the day following extravasation and all areas were
integrated to yield the area under the curve (AUC). The AUC, the
peak area of the skin ulcer and the healing time were analyzed.
Pathological changes
The lesions induced by docetaxel and vinorelbine
were biopsied on days 4, 7, 14, 21 and 28 after the initial
injection. The entire samples, including the skin, subcutaneous
tissue and muscle, in the lesion and surrounding healthy tissue
were obtained and placed in 10% formaldehyde for fixation prior to
dehydration, paraffin-embedding and hematoxylin and eosin staining.
The pathological changes were evaluated by a pathologist who was
not present during the experimental procedure.
Statistical analysis
The differences between the AUC, peak area and
healing time among all levels of docetaxel concentrations were
analyzed by one-way analysis of variance. If the variation was
equal, the least significant difference test was used, and if it
was not equal, Tamhane’s T2 test was used. The differences of the
AUC, peak area and healing time between 6 mg/ml of docetaxel and
novelbine were analyzed by the t-test. P<0.05 was considered to
indicate a statistically significant difference. SPSS 16.0
statistical software (SPSS, Inc., Chicago, IL, USA) was used for
data analysis.
Results
Optimal volume of injection
From 0.1 to 0.4 ml, the area of the skin rash was
enlarged in correlation to the increasing injection volume. The 0.4
ml volume elaborated a 1-cm rash. Injection volumes >0.4 ml
resulted in leakage of the solution out of the hair follicles.
Therefore, the optimum volume of injection was confirmed as 0.4 ml.
The skin rash and skin ulcer were not induced by subcutaneous
injection.
Optimal concentration of injection
There was no change or subtle erythema in the
injection site following a 1 mg/ml injection, and there was no skin
ulcer formation observed. The incidence of docetaxel-induced ulcer
formation was 25.0, 50.0, 100 and 100% for the injection of 2, 4, 6
and 8 mg/ml respectively. As for the AUC, there were statistical
differences between the injection of 4, 6 and 8 mg/ml. Statistical
differences among all levels of injection were observed for the
peak area. There were statistical differences for the healing times
between the levels of 4, 6 and 8 mg/ml (Table I).
 | Table IAreas and healing time of the ulcer in
the different groups. |
Table I
Areas and healing time of the ulcer in
the different groups.
| Groups | Ulcer formation,
% | Areas under curve,
mm2 | Peak area,
mm2 | Healing time,
days |
|---|
| Docetaxel, 8
mg/ml | 100 | 1127.6±144.1a,b,c | 85.0±6.5a,b,c | 27.0±5.7a,b |
| Docetaxel, 6
mg/ml | 100 | 806.8±97.8a,b | 64.4±6.2a,b | 23.0±2.0a,b |
| Docetaxel, 4
mg/ml | 50 | 164.0±46.4 | 37.1±5.3a | 13.5±2.4.. |
| Docetaxel, 2
mg/ml | 25 | 47.9±14.3 | 14.1±1.2. | 9.0±1.4 |
| Vinorelbine | 100 | 1912.3±115.8d | 150.6±10.8d | 28.9±2.5d. |
Natural course of the injury
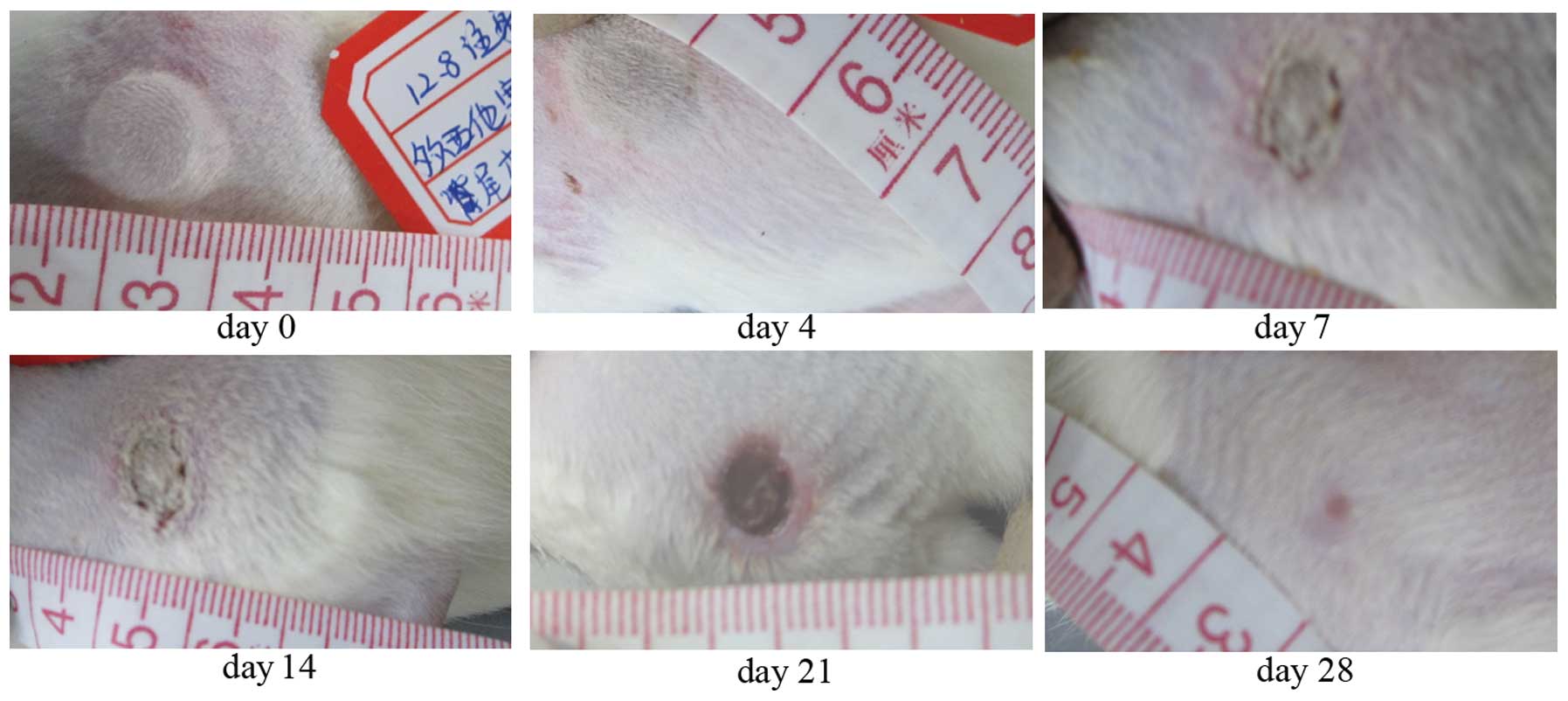
Regarding the docetaxel extravasation, mild injury
in the injection site presented as a subtle erythema, moderate
injury was whitened skin surrounded by congestion and edema, and
severe injury was necrosis. The peak area occurred on the day
following the injection. On days 7–9, the epidermal tissue
excoriated and the ulcer formed with black or white necrotic tissue
covering the ulcer surface. The ulcer was strictly limited in the
extension as observed on the day following the injection and did
not expand as time increased. On day 21, the necrotic tissue in the
bottom of the ulcer was absorbed and the granuloma began to grow
rapidly. Ulcers were recovered on days 28–42 (Fig. 1). After 12 weeks, the sequelae
presented with scar or hyperpigmentation in the injury skin.
As for the NS injection, there was no change
observed in the injection site. The extension of necrosis expanded
gradually in the positive control group with the vinorelbine
injection, and the peak area of necrosis occurred on days 3–5 after
injection. The AUC (1912.3±115.8 vs. 806.8±97.8 mm2,
P<0.005) and peak area (150.6±10.8 vs. 64.4±6.2 mm2,
P<0.005) were increased and the healing time (28.9±2.5 vs.
23.0±2.0 days, P<0.005) was longer than that of the 6 mg/ml
docetaxel concentration (Table
I).
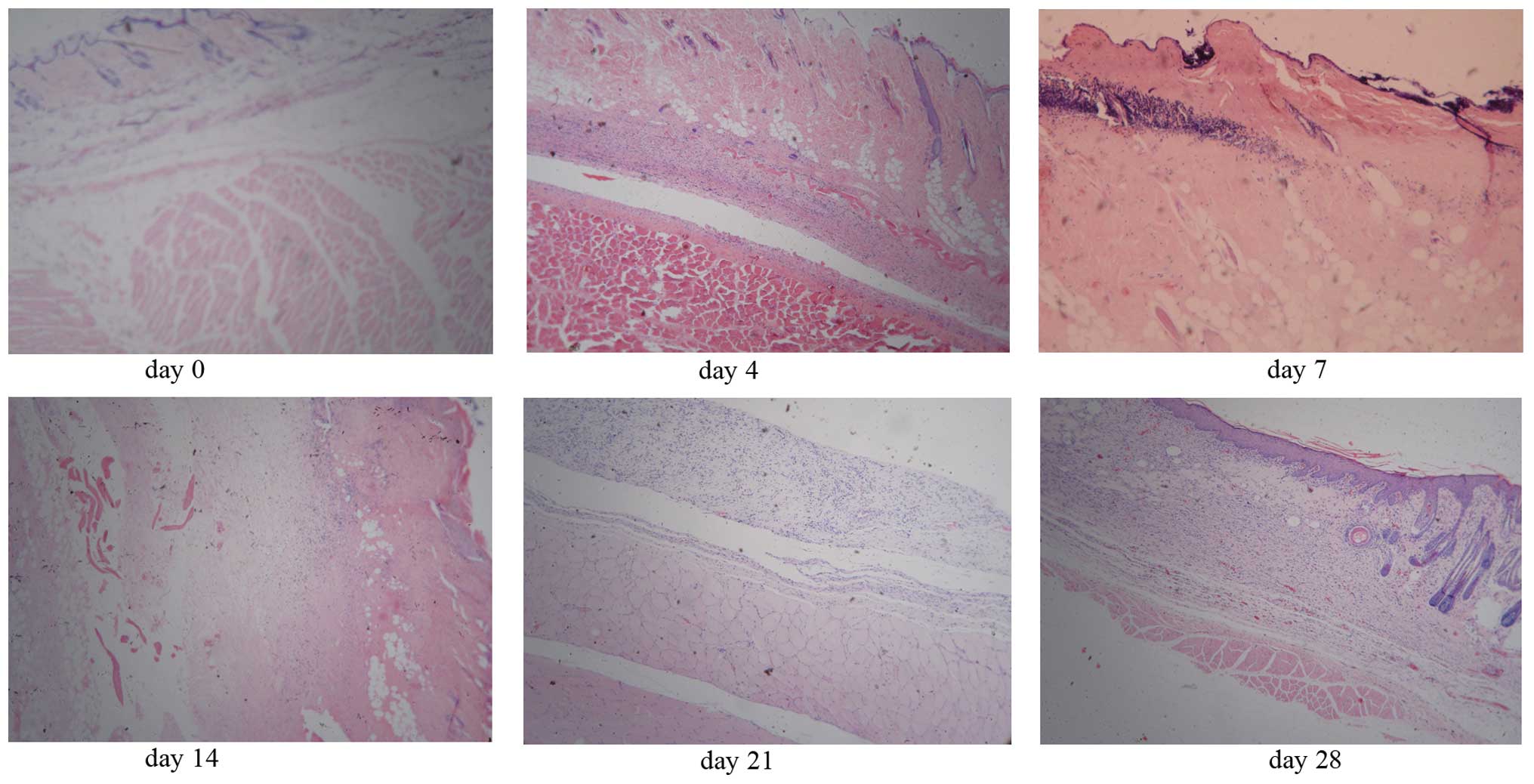
Pathological changes
In the first two weeks following injection,
epidermal and dermal degeneration were observed. The fatty necrosis
and dissolution, and diffuse nuclear debris were observed in the
subcutaneous tissue. There were nuclear debris and inflammatory
cells identified in the surficial muscles. The hair follicle, sweat
gland and sebaceous gland were damaged. In the 3rd week, granuloma
tissue formed and necrotic tissue was absorbed. In the 4th week,
the epidermis and appendix of the skin regenerated (Fig. 2). In the 12th week, the scar was
formed. As for extravasation of the injected vinorelbine, a
reverse-breaker-like ulcer formed, which deepened into the muscles
in the first two weeks.
Discussion
At a low concentration (1 mg/ml), the extravasation
of docetaxel failed to induce a skin ulcer in a rat model.
Concentrations of 2 and 4 mg/ml, formed an irregular ulcer. The
severity of the skin damage was associated with a higher
concentration. The equal concentration of docetaxel injected into
the dermis rather than the subcutaneous layer induced a skin ulcer.
However, whether the ulcer formation was associated with the
injected concentration and local anatomy component remains to be
elucidated. The peak area occurred on the day following the
injection, which indicates that docetaxel would not cause a delayed
damage and expand into the surrounding tissue in the rat model.
In clinical practice, there have been numerous
debates with regards to the vesicant potential of docetaxel
extravasation. A study by Gallo et al (10) reported that three patients who
encountered the docetaxel extravasation presented with a severe
irritant reaction. Kramer et al (11) and Ley et al (12) also reported that docetaxel
extravasation induced the skin recall phenomenon. In these studies,
the initial symptom of docetaxel extravasation was irritant
reaction. By contrast, other studies (13–19)
have indicated that docetaxel was a surficial vesicant drug. In the
study by Cifuentes et al (13), the ultrasonic image indicated change
in cell lysis in the subcutaneous layer. Berghammer et al
(17) and Chu et al
(20) described that docetaxel
extravasation induced the tissue necrosis and nerve injury. The
sequelea of the feeling of skin paralysis was reported (18,20).
In clinical practice, when 250 ml NS is used to
dilute docetaxel, the concentration is ~0.2 mg/ml in the weekly
regime (25 mg/m2) and 0.5 mg/ml in 3-week regime (75
mg/m2). However, these concentrations, which are much
lower than 1 mg/ml, failed to induce an ulcer in the rat model of
the present study. The ulcer formation is not only associated with
the concentration and volume of extravasation, but also with the
speed and site of extravasation. In addition, once the docetaxel is
adversely extravasated, treatment is provided immediately, which
also impacts the ulcer formation. All these factors may be
explanations for the observed contradictions between the rat model
and clinical practice. A study by Raley et al (18) indicated the delayed vesicant-type
reaction of docetaxel extravasation. The study by El Saghir and
Otrock (15) identified that
docetaxel extravasated into the normal breast when administrated by
infusion with a central line. The extent of the skin injury
expanded gradually. The phenomenon of the delayed reaction and
expanded damage was contradicted with the rat model of the present
study.
In the rat model, pathological changes of docetaxel
extravasation are potentially described in three phases: The
necrosis/lysis, granuloma repair and cure phases. Fatty necrosis
and dissolution, and granuloma formation were first described in
the study. Docetaxel did not induce a reverse-breaker-like ulcer,
which was characteristic of vinorelbine extravasation. The
extension of the ulcer induced by docetaxel extravasation was
smaller compared to vinorelbine induction. The depth of necrosis
induced by docetaxel extravasation was more surficial compared to
vinorelbine, and the muscle impact was weaker. The pathological
changes were further confirmed to have a surficial vesicant
property of docetaxel. Previous studies have indicated that the
pathological changes included dyskeratotic keratinocytes, the
bubble of the basal cell (10,12,20).
These changes were not observed in the rat model. The sequelea of
the docetaxel extravasation was scar formation or
hyperpigmentation, which is similar to reported clinical studies
(14,15).
In conclusion, the extravasation of a high
concentration docetaxel can induce tissue necrosis, and the
severity is weaker compared to induction by vinorelbine. Docetaxel
is a surficial vesicant agent, and it is essential that docetaxel
extravasation is prevented in the clinical practice.
Acknowledgements
The present study was supported by grants from the
Jinhua Municipal Science Technology Department (grant no.
2011-3-032).
References
|
1
|
Mackey JR, Martin M, Pienkowski T, et al;
TRIO/BCIRG 001 investigators. Adjuvant docetaxel, doxorubicin, and
cyclophosphamide in node-positive breast cancer: 10-year follow-up
of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 14:72–80.
2013.PubMed/NCBI
|
|
2
|
Fossella F, Pereira JR, von Pawel J, et
al: Randomized, multinational, phase III study of docetaxel plus
platinum combinations versus vinorelbine plus cisplatin for
advanced non-small-cell lung cancer: the TAX 326 study group. J
Clin Oncol. 21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vermorken JB, Remenar E, van Herpen C, et
al; EORTC 24971/TAX 323 Study Group. Cisplatin, fluorouracil, and
docetaxel in unresectable head and neck cancer. N Engl J Med.
357:1695–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al; V325 Study Group. Phase III study of docetaxel and cisplatin
plus fluorouracil compared with cisplatin and fluorouracil as
first-line therapy for advanced gastric cancer: a report of the
V325 Study Group. J Clin Oncol. 24:4991–4997. 2006.PubMed/NCBI
|
|
5
|
Yilmaz M, Demirdover C and Mola F:
Treatment options in extravasation injury: an experimental study in
rats. Plast Reconstr Surg. 109:2418–2423. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bowers DG Jr and Lynch JB: Adriamycin
extravasation. Plast Reconstr Surg. 61:86–92. 1978. View Article : Google Scholar
|
|
7
|
Dorr RT and Bool KL: Antidote studies of
vinorelbine-induced skin ulceration in the mouse. Cancer Chemother
Pharmacol. 36:290–292. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dorr RT, Snead K and Liddil JD: Skin
ulceration potential of paclitaxel in a mouse skin model in vivo.
Cancer. 78:152–156. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Payne AS, James WD and Weiss RB:
Dermatologic toxicity of chemotherapeutic agents. Semin Oncol.
33:86–97. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallo E, Llamas-Velasco M, Navarro R,
Fraga J and García-Diez A: Eccrine squamous syringometaplasia
secondary to cutaneous extravasation of docetaxel: report of three
cases. J Cutan Pathol. 40:326–329. 2013.PubMed/NCBI
|
|
11
|
Kramer F, Schippert C, Rinnau F,
Hillemanns P and Park-Simon TW: The first description of
docetaxel-induced recall inflammatory skin reaction after previous
drug extravasation (February). Ann Pharmacother. Jan 25–2011.(Epub
ahead of print).
|
|
12
|
Ley BD, Millán GG, Perez JS, Fraga J and
Díez AG: Docetaxel recall phenomenon at the site of previous drug
extravasation. Arch Dermatol. 146:1190–1191. 2010.PubMed/NCBI
|
|
13
|
Cifuentes L, Ring J and Brockow K:
Extravasation of docetaxel. J Dtsch Dermatol Ges. 10:662–663.
2012.(In English, German).
|
|
14
|
Barceló R, Viteri A, Muñoz A, Carrera S,
Rubio I and López-Vivanco G: Extravasation of docetaxel: a red hand
syndrome. Arch Dermatol. 141:1326–1327. 2005.PubMed/NCBI
|
|
15
|
El Saghir NS and Otrock ZK: Docetaxel
extravasation into the normal breast during breast cancer
treatment. Anticancer Drugs. 15:401–404. 2004.PubMed/NCBI
|
|
16
|
Ho CH, Yang CH and Chu CY: Vesicant-type
reaction due to docetaxel extravasation. Acta Derm Venereol.
83:467–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berghammer P, Pöhnl R, Baur M and Dittrich
C: Docetaxel extravasation. Support Care Cancer. 9:131–134. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raley J, Geisler JP, Buekers TE and
Sorosky JI: Docetaxel extravasation causing significant delayed
tissue injury. Gynecol Oncol. 78:259–260. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ascherman JA, Knowles SL and Attkiss K:
Docetaxel (taxotere) extravasation: a report of five cases with
treatment recommendations. Ann Plast Surg. 45:438–441. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu CY, Yang CH, Yang CY, Hsiao GH and
Chiu HC: Fixed erythrodysaesthesia plaque due to intravenous
injection of docetaxel. Br J Dermatol. 142:808–811. 2000.
View Article : Google Scholar : PubMed/NCBI
|