Introduction
Currently, there is significant interest in
generating mature hepatocytes from stem cells either for disease
modelling, predictive drug toxicology studies or, in the longer
term, for transplantation. The scarcity of adult human hepatocytes,
and their functional deterioration and poor replication in culture
are the main motivations behind this research (1). The extensive in vitro replication
capability of pluripotent stem cells has made them an attractive
alternative cell source. Numerous studies show that embryonic stem
cells (ESCs), induced pluripotent stem cells and adult stem cells
can be differentiated towards the hepatic lineage (2–4).
Hepatocytes are used primarily in the pharmaceutical
industry for screening new compounds. This screening helps to
eliminate potentially toxic drugs at the preliminary stage,
avoiding unanticipated effects at later stages of clinical trials
and reducing the time and expense required for the
commercialisation of new drugs. Due to their simplicity, commonly
used materials, such as microsomal fractions, hepatocyte
suspensions and short-term cultures, have their limitations for
evaluating complex toxic reactions (5). A highly polarised hepatocyte model is
necessary for evaluating hepatobiliary excretion and potential
liver injury, and studying viral infections (6,7).
Polarised hepatocytes have an asymmetric cell
membrane characterised by the segregated expression of membrane
proteins in different domains. Tight junctions are responsible for
this segregation of apical and basolateral membrane domains. Apical
poles of adjacent hepatocytes join to form a continuous channel,
which is the bile canaliculi that harbours numerous transport
proteins, such as multidrug resistance-associated protein 2 (MRP2),
to excrete bile salts from the cell. The basal membrane domain
(sinusoidal pole) is responsible for the uptake of recycled biliary
salts and nutrients, and the secretion of various hepatic proteins
into the blood circulation. Bile canaliculi formation is an
important feature of polarised hepatocytes and can be observed
microscopically as channels between adjacent hepatocytes. The
formation of hepatic polarity in cultured cells can be assessed by
the expression of the tight junction [zona occludin 1 (ZO-1)],
localised expression of transport protein MRP2 to the bile
canalicular side and by the excretion and localisation of traceable
compounds, such as fluorescein, within bile canaliculi.
In the present study, human ESCs (hESCs) and
mesenchymal stem cells (hMSCs) were differentiated to
hepatocyte-like cells. These differentiated stem cells were
examined for the expression of hepatic genes and functions. Hepatic
polarisation, particularly the expression of tight junction protein
ZO-1, the apical bile canalicular MRP2, bile canalicular protein
dipeptidyl peptidase 4 (DPP4) and the excretion of fluorescein by
these stem cell-derived hepatocytes was extensively evaluated and
compared with that of the human foetal hepatocytes and HepG2
cells.
Materials and methods
hESC culture and hepatic
differentiation (monolayer culture)
The RCM1 hESC line was cultured and propagated in
Matrigel-coated plates with mTeSR 1 medium (Stem Cell Technologies,
Vancouver, BC, Canada) under feeder-free and serum-free conditions
as previously described (8).
Differentiation was initiated when the hESCs reached ~30%
confluency by replacing MTeSR-1 medium with priming medium
[RPMI-1640 medium (Biosera, ZI du Bousquet, Boussens, France)
containing 1X B27 supplement (Invitrogen Life Technologies,
Carlsbad, CA, USA), 100 ng/ml Activin A (PeproTech, Rocky Hill, NJ,
USA) and 50 ng/ml Wnt-3A (PeproTech)] for 3 days. Cells were
subsequently maintained in differentiation medium [knockout
Dulbecco's modified eagle medium (DMEM) (Invitrogen Life
Technologies) containing 20% serum replacement, 1 mmol/l glutamine,
1% non-essential amino acids, 0.1 mmol/l β-mercaptoethanol (all
from Invitrogen Life Technologies) and 1% dimethyl sulfoxide (DMSO)
(Sigma, St. Louis, MO, USA)] for 5 days. Finally, the cells were
cultured in maturation medium [L15 medium containing 8.3% fetal
bovine serum (FBS), 8.3% tryptose phosphate broth, 10 mmol/l
hydrocortisone 21-hemisuccinate, 1 mmol/l insulin (all from Sigma),
2 mmol/l glutamine, 10 ng/ml hepatocyte growth factor (PeproTech)
and 20 ng/ml oncostatin M (PeproTech)] for 9 days. Medium was
changed every 2 days during differentiation.
Collagen sandwich culture of
differentiated hESC (sandwich culture)
Neutralised collagen solution (100 µl, 1 mg/ml)
(Roche Diagnostics, Basel, Switzerland) was overlaid on hESCs that
had differentiated for 12 days. Collagen was allowed to form a gel
by incubating for 30 min at 37°C in a CO2 incubator.
Sufficient medium was added to the wells and cells were
subsequently cultured for 5 days.
Isolation and culture of hMSCs
Foetal pancreas samples from mid-trimester
therapeutic terminations of pregnancy were obtained from the Royal
Infirmary of Edinburgh with informed consent from patients and
approval of the Local Research Ethics Committee. hMSCs were
isolated as described previously (9).
In brief, the pancreas was collected in ice-cold medium, finely
sliced and incubated for 3 days undisturbed. Thereafter,
plastic-adherent hMSCs were maintained in DMEM (Biosera) with 10%
FBS.
Differentiation of hMSCs to
hepatocytes
Cells from passages 6–15 were used for hepatic
differentiation. Hepatic differentiation was carried out as
previously described (2). Induction
was initiated when cells were 80% confluent by treating hMSCs with
differentiation medium [DMEM containing 20 ng/ml hepatocyte growth
factor, 10 ng/ml basic fibroblast growth factor (PeproTech) and
nicotinamide (0.61 g/l) (Sigma)] for 7 days. Subsequently, cells
were cultured in maturation medium [DMEM containing 20 ng/ml
oncostatin M, 1 mmol/l dexamethasone (Sigma) and 1X
Insulin-Transferrin-Selenium (Invitrogen Life Technologies)]. Cells
were cultured for ≤41 days in differentiation medium and the medium
was changed twice/week.
Isolation and culture of foetal
hepatocytes
Livers from mid-trimester therapeutic terminations
of pregnancy were obtained from the Royal Infirmary of Edinburgh
with informed consent from patients and approval of the Local
Research Ethics Committee. Foetal hepatocytes were isolated as
described previously (10). In brief,
livers were collected in ice-cold Williams' E medium (Invitrogen
Life Technologies), dissected free of fibrous material, finely
sliced and washed in Hanks' balanced salt solution (HBSS) to remove
the red blood cells. Tissue fragments were digested with 0.1%
collagenase type II (Worthington Biochemical Corporation, Lakewood,
NJ, USA) in HBSS. Digestion was stopped by the addition of DMEM
with 10% FBS. Cells were centrifuged and resuspended in Williams' E
medium supplemented with 10% FBS, 50 U/ml penicillin, 50 mg/ml
streptomycin, 2 mmol/l glutamine, 1X Insulin-Transferrin-Selenium
(all from Invitrogen Life Technologies) and 10−7 M
dexamethasone. Viability was determined with 0.2% trypan blue using
a Neubauer hemocytometer. Cells (2.7×106
cells/cm2) were cultured at 37°C in 5% CO2 on
collagen-coated plates [for coating plates, 250 µl of collagen
solution (type I collagen, 1.5 mg/ml) was added to the wells,
excess solution removed and plates incubated at 37°C for 1 h].
Estimation of secreted proteins by
enzyme-linked immunosorbent assay
Cells were cultured in 12-well plates in 500 µl of
appropriate media and the supernatant was collected for the protein
assay after 24 h. Hepatic proteins were measured using sandwich
enzyme-linked immunosorbent assays (ELISAs), as described by the
manufacturer (Dako, Glostrup, Denmark). High-binding EIA plates
were coated with a rabbit anti-human antibody (Dako) specific for
the protein of interest (Dako) overnight at 4°C. Sample
supernatants were diluted 1:10 and added to 96-well plates in
triplicate. Plates were subsequently incubated for 2 h at room
temperature. Peroxidase-conjugated rabbit anti-human antibody
directed against the appropriate protein (Dako) was added and the
plates were incubated for 1 h at room temperature. The substrate
o-phenylenediamine was added and the reaction was stopped
with 0.5 M sulphuric acid. The plates were read at 490 nm with a
reference wavelength of 630 nm using MRX II Endpoint software
(Dynex Technologies, Chantilly, VA, USA). To normalise the ELISA
results, cells were lysed and the total protein was estimated using
the DC protein assay kit (Bio-Rad, Hercules, CA, USA).
Immunostaining and flow cytometry
For immunostaining, cells were fixed in 4%
paraformaldehyde for 10 min at room temperature and washed with
phosphate-buffered saline. After blocking with 2% appropriate
serum, cells were incubated with the primary antibody at 4°C
overnight followed by incubation with the appropriate secondary
antibody for 1 h at room temperature. Nuclei were counterstained
with 4′,6-diamidino-2-phenylindole. For flow cytometry, cells were
harvested and incubated in blocking buffer containing 2% rabbit
serum for 30 min followed by incubation for 30 min with the primary
antibody, according to the manufacturer's instructions. The cells
were counterstained with rabbit anti-mouse secondary antibody for
30 min and were analysed in a Beckman Coulter EPICS flow cytometer
(Brea, CA, USA) after washing. Primary antibodies were replaced
with the corresponding immunoglobulin G antibodies for the control
conditions. Antibodies used for immunostaining and flow cytometry
are listed in Table I.
 | Table I.Antibodies and the dilutions
used. |
Table I.
Antibodies and the dilutions
used.
| Antibody | Catalogue no. | Supplier | Country | Dilution |
|---|
| Alexa Fluor 594
F(ab)2 fragment of goat anti-mouse IgG (H+L) | A11020 | Molecular
Probes/Invitrogen Life Technologies | USA | 1:100 |
| Alexa Fluor 594
goat anti-rabbit IgG | A11037 | Molecular
Probes/Invitrogen Life Technologies | USA | 1:100 |
| Anti-human albumin
FITC conjugated | CLFAG2140 | Cedarlane | Canada | 1:50 |
| Anti-mouse IgG FITC
conjugated | F2883 | Sigma | USA | 1:50 |
| Monoclonal
anti-human hepatocyte specific antigen | NCL-HSA |
Novocastra/Leica | Germany | 1:20 |
| Monoclonal mouse
anti-human CK18 | M7010 | Dako | Denmark | 1:20 |
| Mouse
anti-CD13 | MCA1270 | ABD Serotec | UK | 1:100 |
| Mouse anti-human
CD105 | MCA1557 | ABD Serotec | UK | 1:50 |
| Mouse anti-human
CD117 | MCA1841 | ABD Serotec | UK | 1:20 |
| Mouse anti-human
CD29 | MCA1949GA | ABD Serotec | UK | 1:100 |
| Mouse anti-human
CD31 (PECAM-1) | MCA1738 | ABD Serotec | UK | 1:100 |
| Mouse anti-human
CD34 | MCA547G | ABD Serotec | UK | 1:200 |
| Mouse anti-human
CD44 | MCA89 | ABD Serotec | UK | 1:20 |
| Mouse anti-human
CD45 | MCA87GA | ABD Serotec | UK | 1:50 |
| Mouse anti-human
CD49b | MCA743GA | ABD Serotec | UK | 1:100 |
| Mouse anti-human
CD54 | MCA1615GA | ABD Serotec | UK | 1:100 |
| Mouse anti-human
CD90 | MCA90 | ABD Serotec | UK | 1:50 |
| Mouse anti-human
DPP4 (CD26) | 555435 | BD Pharmingen | USA | 1:100 |
| Mouse IgG1-negative
control | MCA928 | ABD Serotec | UK | 1:100 |
| Mouse
IgG2a-negative control | MCA929 | ABD Serotec | UK | 1:100 |
| Mouse monoclonal
antibody to MRP2 | ab3373 | Abcam | UK | 1:250 |
| Rabbit polyclonal
to ZO-1 | ab59720 | Abcam | UK | 1:25 |
Excretion of fluorescein
diacetate
The functional reconstitution of bile canaliculi was
assessed by the localisation of secreted fluorescein within the
bile canaliculi. Cells were treated with fluorescein diacetate (10
µg/ml) (Sigma) for 10 min and immediately observed by fluorescence
microscopy (DM IRB; Leica, Mannheim, Germany) to visualise
fluorescein localisation.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using the TRI reagent (Sigma)
following the manufacturer's instructions. cDNA was synthesised
using 1 mg total RNA with RT in a 20 ml volume following the
manufacturer's instructions (Promega Corporation, Madison, WI,
USA). The PCR was performed using the primer sequences and PCR
conditions provided in Table II for
35 cycles. PCR products were analysed on a 2% agarose gel and the
product size was estimated using a 100-bp DNA ladder.
 | Table II.Primer sequence, product length and
annealing temperature. |
Table II.
Primer sequence, product length and
annealing temperature.
| Gene | Primer
sequence | Product length,
bp | Annealing
temperature, °C |
|---|
| ALB |
5′-TGAGAAAACGCCAGTAAGTGAC-3′ | 265 | 49 |
|
|
5′-TGCGAAATCATCCATAACAGC-3′ |
|
|
| CPS |
5′-TGAGGGATGCTGACCCCATT-3′ | 255 | 53 |
|
|
5′-CATTGTTGGCGTTGAGCCAG-3′ |
|
|
| CYP1A1 |
5′-TTCGTCCCCTTCACCATC-3′ | 302 | 49 |
|
|
5′-CTGAATCCACCCGTTGC-3′ |
|
|
| CK18 |
5′-CCCGCTACGCCCTACAGAT-3′ | 271 | 53 |
|
|
5′-ACCACTTTGCCATCCACTATCC-3′ |
|
|
| CK19 |
5′-TCCAGATGAGCAGGTCCGAGGTTA-3′ | 281 | 57 |
|
|
5′-GCTGCGGTAGGTGGCAATCTCC-3′ |
|
|
| HNF4α |
5′-GCCTACCTCAAAGCCATCAT-3′ | 275 | 53 |
|
|
5′-GACCCTCCCAGCAGCATCTC-3′ |
|
|
| MRP2 |
5′-ACAGAGGCTGGTGGCAACC-3′ | 227 | 54 |
|
|
5′-ACCATTACCTTGTCACTGTCCATGA-3′ |
|
|
| TDO |
5′-GGTTTAGAGCCACATGGATT-3′ | 425 | 62 |
|
|
5′-ACAGTTGATCGCAGGTAGTG-3′ |
|
|
| AFP |
5′-GCTTGGTGGTGGATGAAACA-3′ | 157 | 50 |
|
|
5′-TCCTCTGTTATTTGTGGCTTTTG-3′ |
|
|
| ACTB |
5′-GCACTCTTCCAGCCTTTC-3′ | 385 | 51 |
|
|
5′-AGAAAGGGTGTAACGCAACTAAG-3′ |
|
|
Quantitative PCR (qPCR) analysis
qPCR was performed according to the TaqMan® Fast
Universal PCR Master mix instructions. The reaction mixture was
prepared and PCR amplification was performed using the TaqMan® Gene
Expression Assay mix for hepatocyte nuclear factor 4α (HNF4α),
cytochrome P450 2D6 (CYP2D6), β2-macroglobulin and
β-actin. The optimal baseline and threshold for each gene was set
appropriately and the cycle threshold (CT) value was
determined. The CT value of each sample was analysed and
the sample group CT mean and standard deviation were
calculated. β2-macroglobulin was considered as the
reference gene (β2-macroglobulin showed the same level
of expression in all the samples: CT standard deviation
<1). The gene transcripts were quantified by ΔΔCT
analysis, normalised with adult human hepatocytes and the results
are expressed as fold change.
Periodic-acid Schiff (PAS)
staining
Culture dishes containing cells were fixed in 4%
formaldehyde and permeabilised with 0.1% Triton X-100 for 10 min.
Samples were oxidised in 2% periodic acid for 5 min, treated with
Schiff's reagent for 15 min and rinsed in deionised water for 5 to
10 min. Samples were counterstained with Mayer's haematoxylin for
20 sec, rinsed and assessed by light microscopy.
Indocyanin uptake
To determine the cellular uptake of indocyanin green
(ICG; Sigma), 1 mg/ml of ICG in DMEM containing 10% FBS was added
to cell cultures and incubated at 37°C for 15 min. Cells were
subsequently rinsed and cellular uptake of ICG was examined by
microscopy.
Statistical analysis
Data obtained from 3 independent experiments with 3
replicates were evaluated by Student's t-test. Data are expressed
as mean ± standard deviation and P<0.05 was considered to
indicate a statistically significant difference.
Results
Characterisation of hMSCs
MSCs were isolated from human foetal pancreas by
plastic adherence and were characterised for the expression of
essential MSC markers by flow cytometry (11,12). hMSCs
were positive for cluster of differentiation 90 (CD90) (90±1%),
CD105 (56±12%), CD49b (95±1%), CD73 (10±2%), CD29 (70±10%), CD13
(78±5%) and CD44 (96±1%) and were negative for CD34, CD117, CD31
and CD45 (Fig. 1).
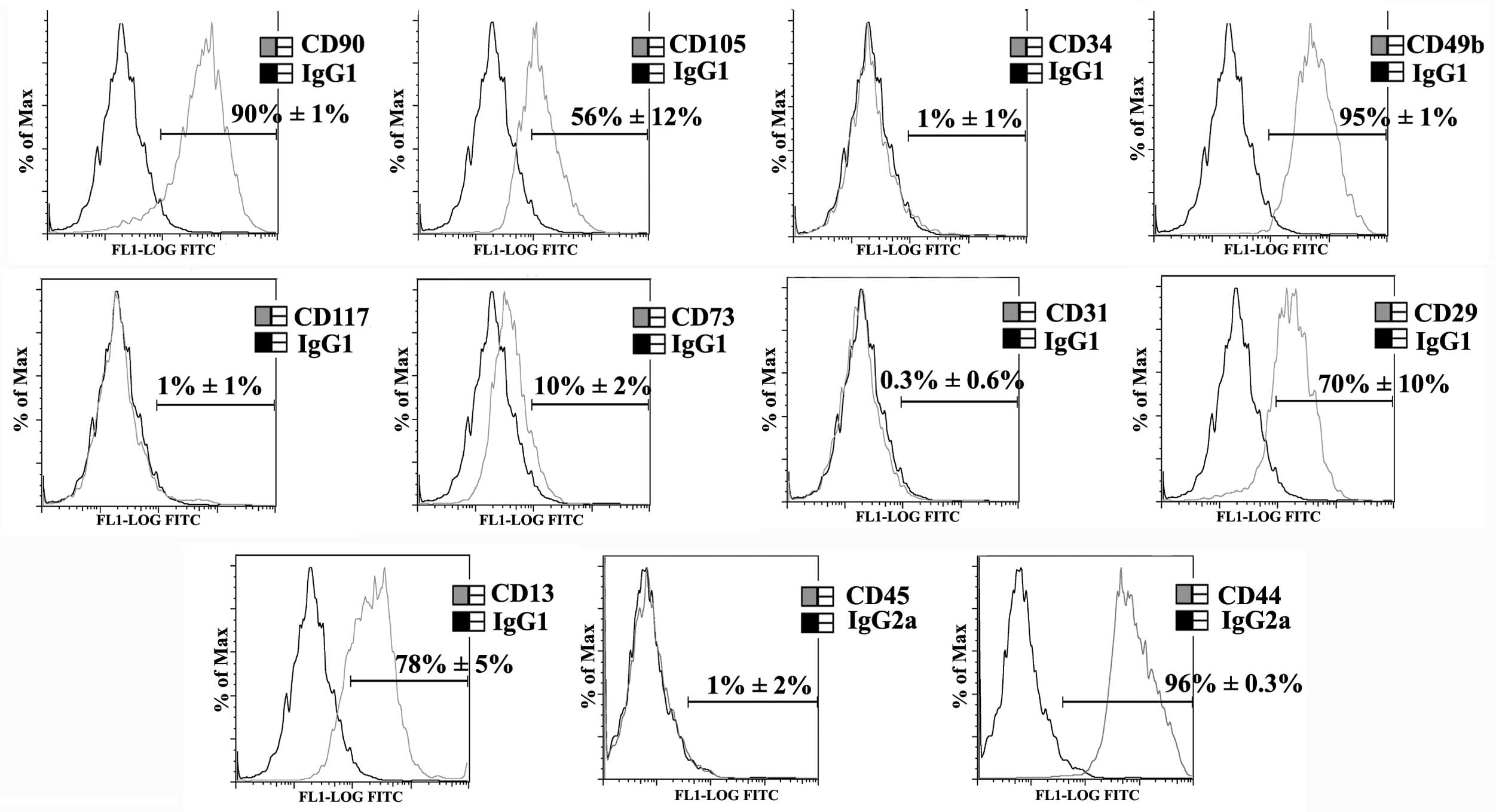 | Figure 1.Characterisation of mesenchymal stem
cells (MSCs). MSCs isolated from human foetal pancreas were
characterised by flow cytometry. The black and gray histograms
represent isotype control and test, respectively. Inset value
denotes the percentage of the positive population. MSCs were
positive for cluster of differentiation 90 (CD90) (90±1%), CD105
(56±12%), CD49b (95±1%), CD73 (10±2%), CD29 (70±10%), CD13 (78±5%)
and CD44 (96±0.3%) and were negative for CD34 (1±1%), CD117 (1±1%),
CD31 (0.3±0.6%) and CD45 (1±2%). IgG, immunoglobulin G; FITC,
fluorescein isothiocyanate. |
Differentiation of hMSCs to
hepatocytes
Although MSCs are highly proliferative, their growth
slows during differentiation. During the initial phase of
differentiation (the first week post-induction), the fibroblast
spindle morphology of hMSCs became more broad and elongated. During
the maturation phase, in the presence of oncostatin M and
dexamethasone, a retraction of elongated ends was observed and the
cells developed a cubical morphology by the second week
post-induction. Following prolonged culture, the hepatic morphology
further matured with a polygonal shape and the cells became much
more compact (Fig. 2A).
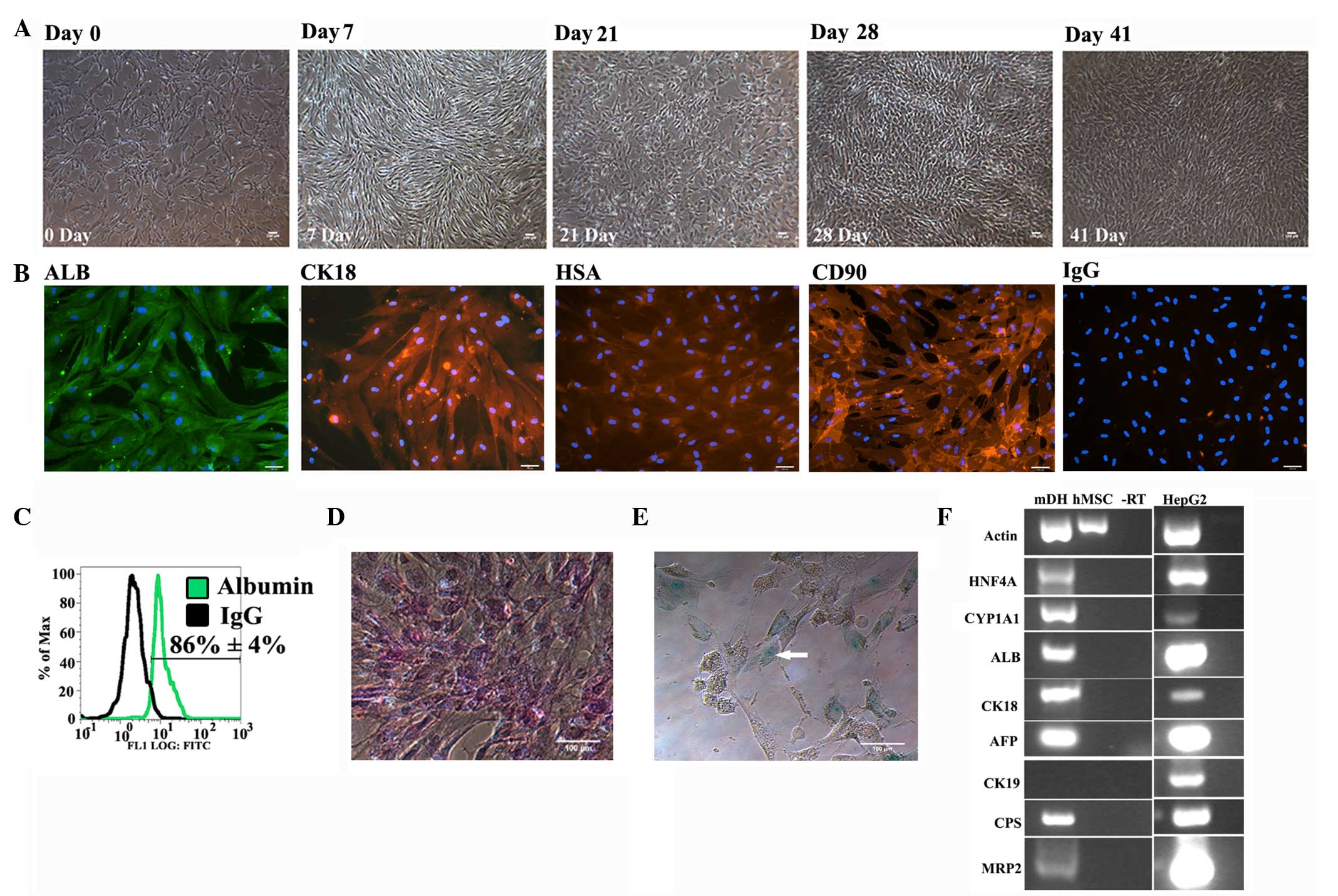 | Figure 2.Characterisation of human mesenchymal
stem cell (hMSC)-derived hepatocytes. (A) Phase contrast images
showing the change in morphology during differentiation. (B)
Immunostaining showing the expression of ALB, CK18,
hepatocyte-specific antigen (HSA) and CD90 on day 41.
Immunoglobulin G (IgG) represents the negative control. (C) Flow
cytometry showing the expression of albumin on day 41. Bright field
image showing (D) positive periodic-acid Schiff staining (pink) and
(E) indocyanin green uptake (arrowhead point cells showing
indocyanin uptake) on day 41. In all the images, the scale bar
denotes 100 µm. (F) Reverse transcription-polymerase chain reaction
showing the expression of hepatic genes on day 41. Actin, β-actin;
HNF4α, hepatocyte nuclear factor 4α; CYP1A1, cytochrome P450 1A1;
ALB, albumin; CK18, cytokeratin 18; AFP, α-fetoprotein; CK19,
cytokeratin 19; CPS, carbomyl phosphate synthetase 1; MRP2,
multidrug resistance-associated protein 2; mDH, hMSC-derived
hepatocytes; hMSC, undifferentiated hMSCs; -RT, negative reverse
transcription; HepG2, HepG2 cells; FITC, fluorescein
isothiocyanate. |
The expression of hepatic markers by hMSC-derived
hepatocytes (mDHs) was analysed. mDHs were positive for albumin,
cytokeratin 18 (CK18) and hepatocyte-specific antigen, confirming
their hepatic differentiation (Fig.
2B). Furthermore, flow cytometry showed that 86±4% of
MSC-derived hepatocytes were positive for albumin, confirming the
efficient hepatic differentiation of hMSCs (Fig. 2C).
The glycogen storage function was also examined in
mDHs using PAS staining. mDHs exhibited evident glycogen storage
(Fig. 2D), a function of the liver in
carbohydrate metabolism. As hepatocytes are the major cells
involved in xenobiotic metabolism, the uptake of xenobiotic
compounds by mDHs was tested using ICG. MSC-derived hepatocytes
exhibited evident indocyanin uptake (Fig.
2E), confirming their ability to uptake xenobiotic compounds.
RT-PCR analysis confirmed that differentiated hMSCs expressed
various hepatic genes, including α-fetoprotein (foetal form of
serum albumin), albumin (a serum protein produced by hepatocytes),
CK18 (hepatic cytoskeleton protein), carbomyl phosphate synthase
(an enzyme of urea cycle), cytochrome P450 1A1 (CYP1A1; an enzyme
involved in xenobiotic metabolism), HNF4α (hepatic transcription
factor) and MRP2 (export protein) (Fig.
2F).
Differentiation of hESCs to
hepatocytes
For differentiation, hESCs routinely cultured
without feeder cells were treated with Activin A and Wnt-3A for 3
days. The cells proliferated extensively and reached a confluency
of 80–90% during the initial 3 days (priming stage). Furthermore,
the shape of the cells became spiky and triangular during this
stage. The cells were subsequently changed to SR-DMSO
differentiation medium and cultured for 5 days. Cell death was
observed during the initial 24 h in differentiation medium,
although cell death was lower in cultures with higher confluency
and better cell-cell contacts. Cells in differentiation medium
gradually exhibited a morphological change from a triangular to a
polygonal outline (Fig. 3A). Medium
was finally changed to maturation medium and the cells were
cultured for 10 days, during which they exhibited the
characteristic hepatocyte morphology; polygonal shape with large
nuclei.
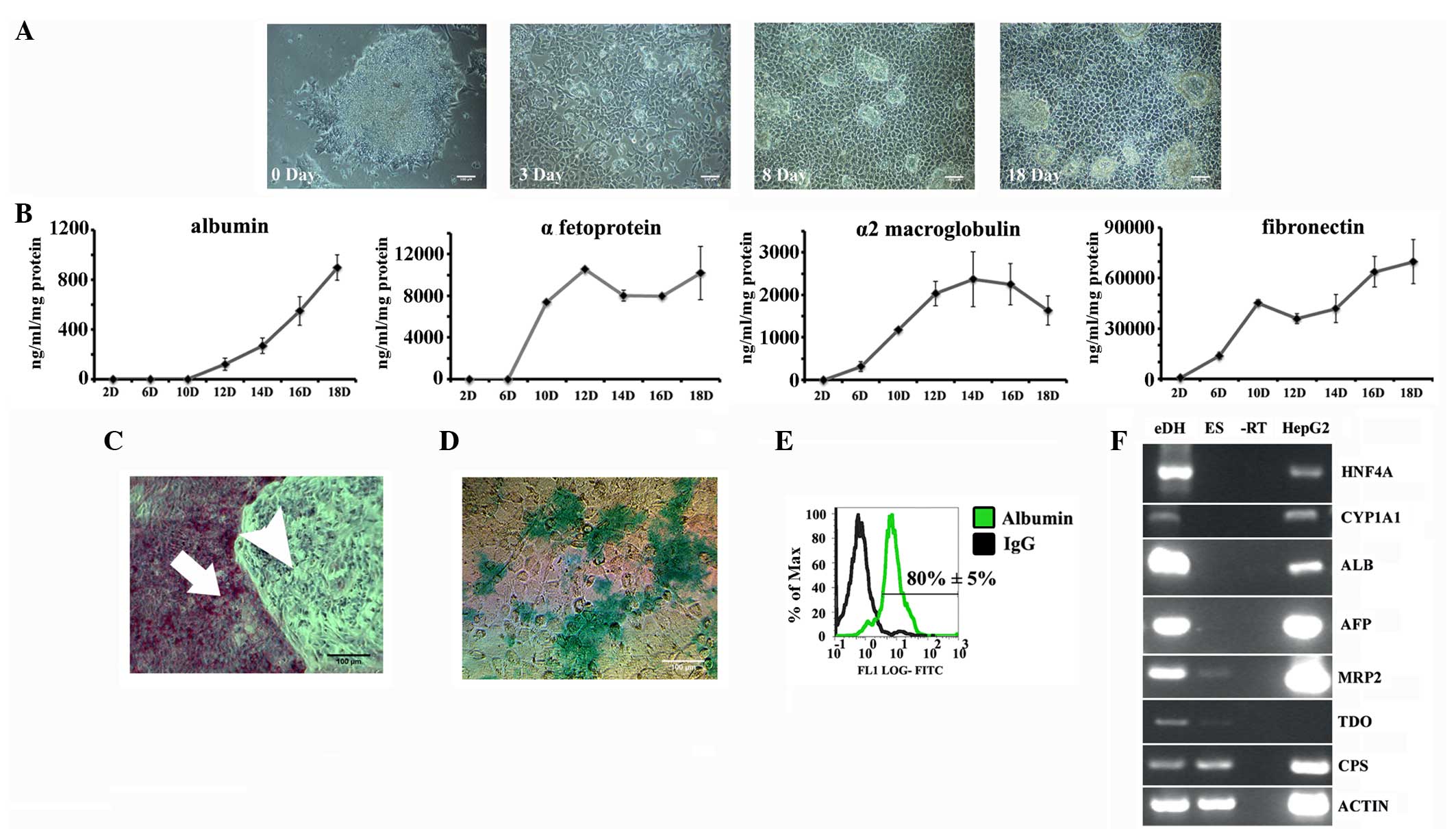 | Figure 3.Characterisation of embryonic stem
cell-derived hepatocytes. (A) Phase contrast images showing the
change in morphology during differentiation. (B) Human embryonic
stem cell (hESC)-derived hepatocytes (eDHs) showing the secretion
of hepatic proteins. The x-axis represents the day of
differentiation and the y-axis represents protein concentration
(ng/ml/mg of total protein). (C) Bright field image showing
periodic-acid Schiff stained eDHs (arrow with the tail points to
eDHs and arrow without the tail points to differentiation resistant
hESCs) on day 17. (D) Bright field image showing indocyanin green
uptake on day 17. In all the images, the scale bar denotes 100 µm.
(E) Flow cytometry showing the expression of albumin on day 17. (F)
Reverse transcription-polymerase chain reaction showing the
expression of hepatic genes on day 17. HNF4α, hepatocyte nuclear
factor 4α; CYP1A1, cytochrome P450 1A1; ALB, albumin; AFP,
α-fetoprotein; MRP2, multidrug resistance-associated protein 2;
TDO, tryptophan 2,3-dioxygenase; CPS, carbomyl phosphate synthetase
1; Actin, β-actin; eDH, hESC-derived hepatocytes; EC,
undifferentiated hESCs; -RT, negative reverse transcription; HepG2,
HepG2 cells; IgG, immunoglobulin G; FITC, fluorescein
isothiocyanate. |
One of the functions of hepatocytes in the liver is
the secretion of plasma proteins, such as albumin (essential for
maintaining blood osmolarity), α-fetoprotein (the major protein of
foetal serum), α2-macroglobulin (a protease inhibitor) and
fibronectin (an extracellular protein capable of binding to
receptor proteins). To examine the secretion of these hepatic
proteins by hESC-derived hepatocytes (eDHs), the culture medium
from these cells was analysed by ELISA. The secretion of plasma
proteins by eDHs significantly increased at different stages of
differentiation, and this secretion peaked during the later
differentiation stage (Fig. 3B).
PAS staining revealed evident glycogen storage in
eDHs (Fig. 3C). eDHs also exhibited
evident indocyanin uptake, confirming the generation of functional
hepatocytes (Fig. 3D). Flow cytometry
showed that 80±5% of eDHs were positive for albumin, confirming the
efficient hepatic differentiation of hESCs (Fig. 3E). Furthermore, PCR analysis revealed
that eDHs expressed HNF4α (a hepatic transcription factor), CYP1A1
(an enzyme involved in xenobiotic metabolism), albumin (a serum
protein produced by hepatocytes), α-fetoprotein (a major foetal
serum protein), MRP2 (also known as canalicular multispecific
organic anion transporter 1 or ATP-binding cassette subfamily C
member-ABCC2), tryptophan 2,3-dioxygenase (an enzyme involved in
amino acid metabolism) and carbomyl phosphate synthase (an enzyme
involved in the urea cycle) (Fig.
3F).
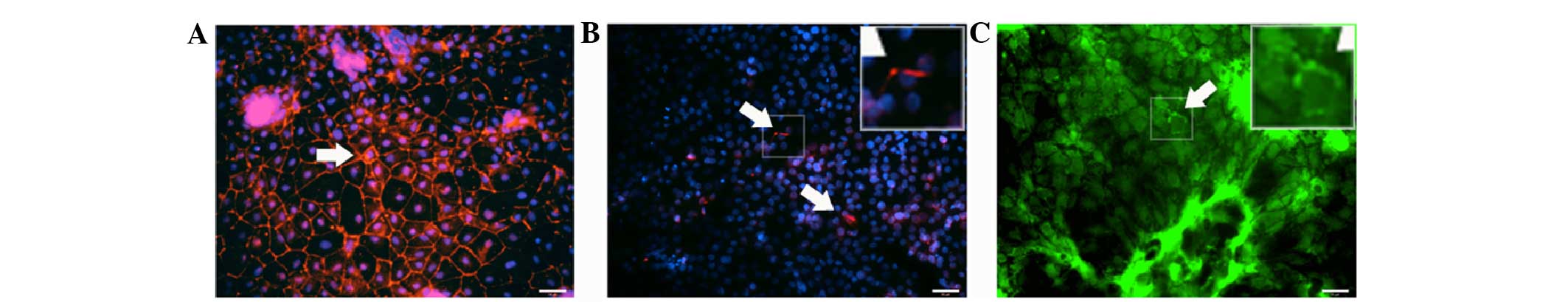
Polarisation of stem cell-derived
hepatocytes
Polarisation of ESC-derived hepatocytes was
evaluated by the expression of the tight junction protein ZO-1 and
the apical export protein MRP2 and bile canalicular protein DPP4.
The functional ability of formed bile canaliculi was further
evaluated by accumulation of fluorescein. eDHs cultured as a
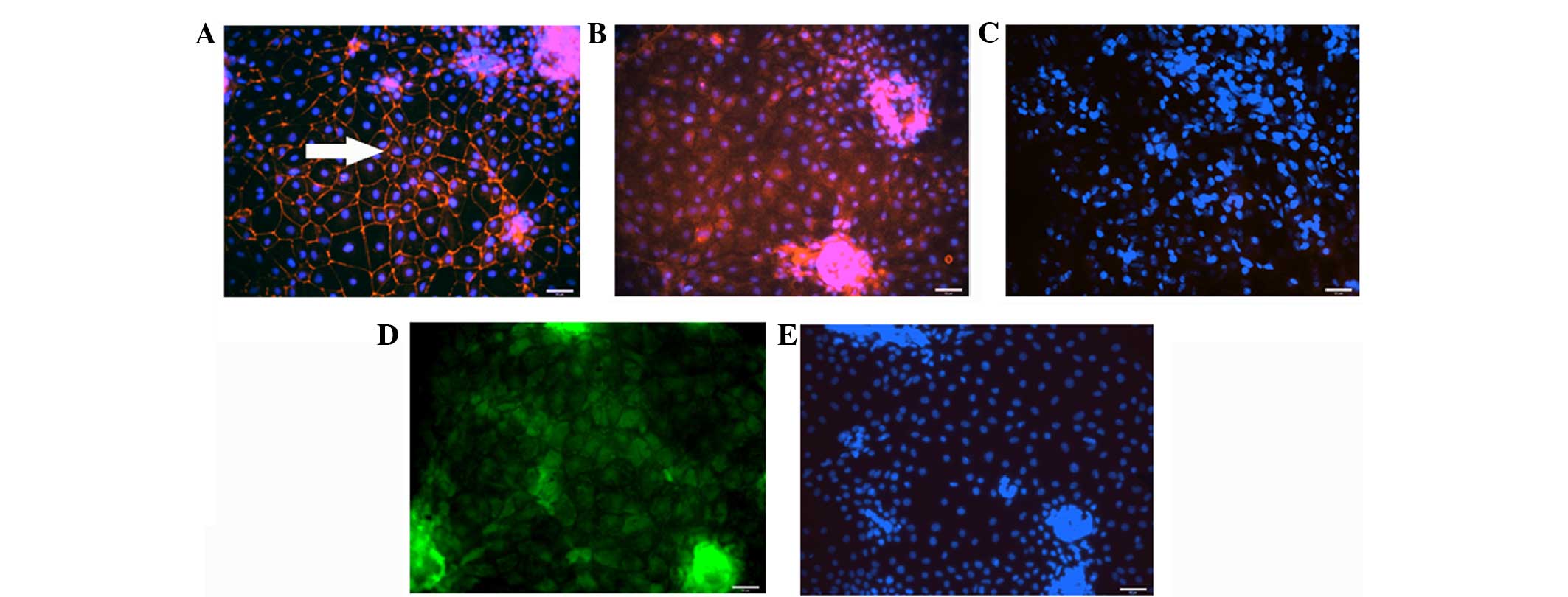
monolayer expressed the tight junction protein ZO-1 (Fig. 4A), however, they did not show localised
expression of MRP2 (Fig. 4B) and DPP4
(Fig. 4C) at their apical sides.
Additionally, fluorescein accumulation was not visible in these
cells (Fig. 4D).
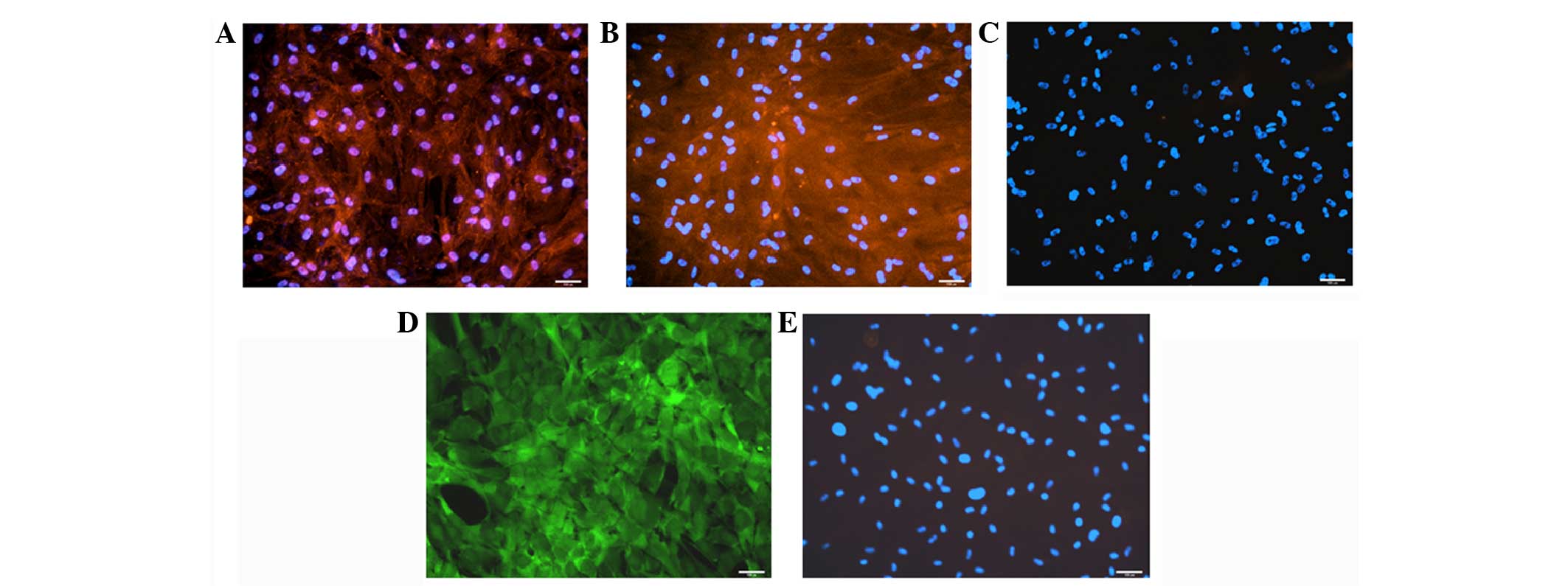
Although mDHs showed the expression of tight
junction protein ZO-1, the expression was not localised to the cell
borders (Fig. 5A). Similarly, MRP2
expression was not localised to the apical side of the cell
(Fig. 5B) and the DPP4 expression was
not observed (Fig. 5C). mDHs were also
not able to localise fluorescein (Fig.
5D).
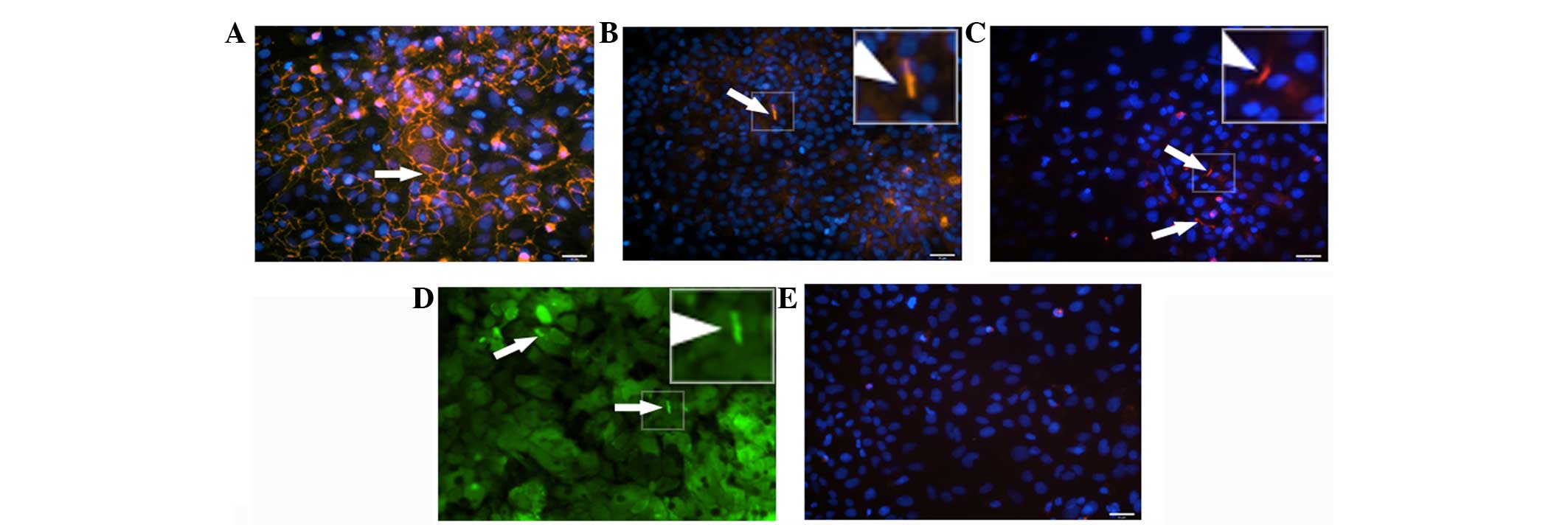
Foetal hepatocytes showed expression of tight
junctions at cell borders (Fig. 6A)
and localised expression of MRP2 (Fig.
6B) and DPP4 (Fig. 6C) at the
apical side of the cell. In addition, foetal hepatocytes showed
fluorescein excretion localised to bile canaliculi (Fig. 6D).
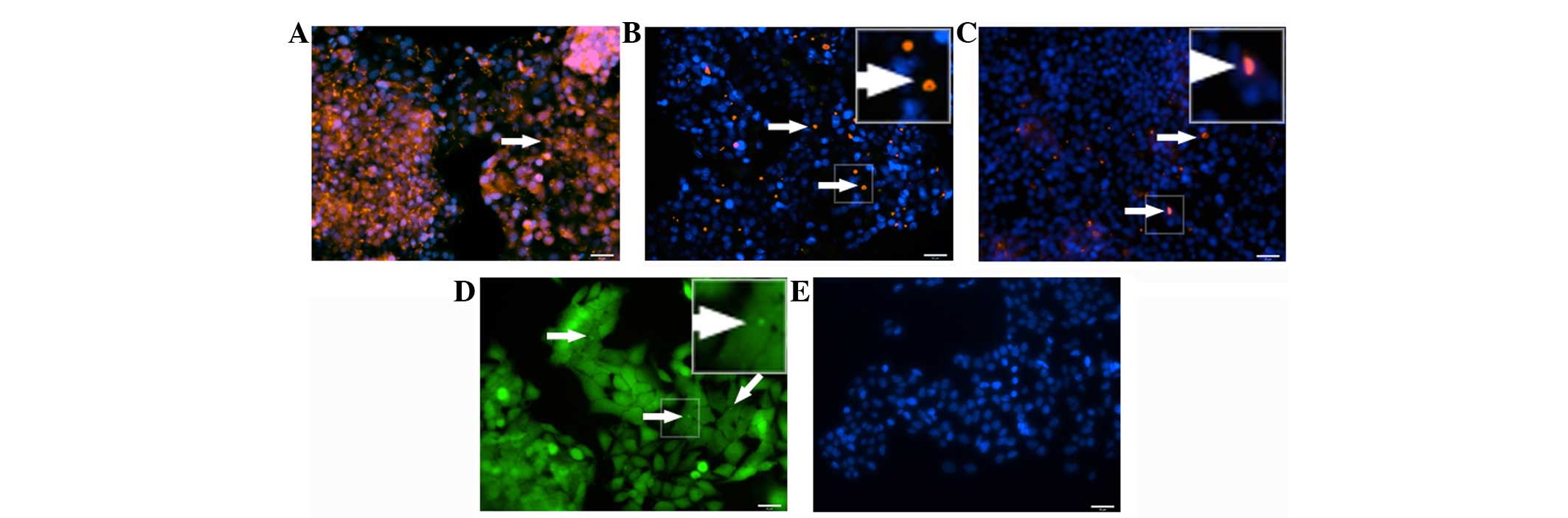
In the HepG2 hepatic cell line, expression of the
tight junction protein ZO-1 was not uniform (Fig. 7A) and MRP2 (Fig. 7B) and DPP4 (Fig. 7C) expression was confined between a few
cells.
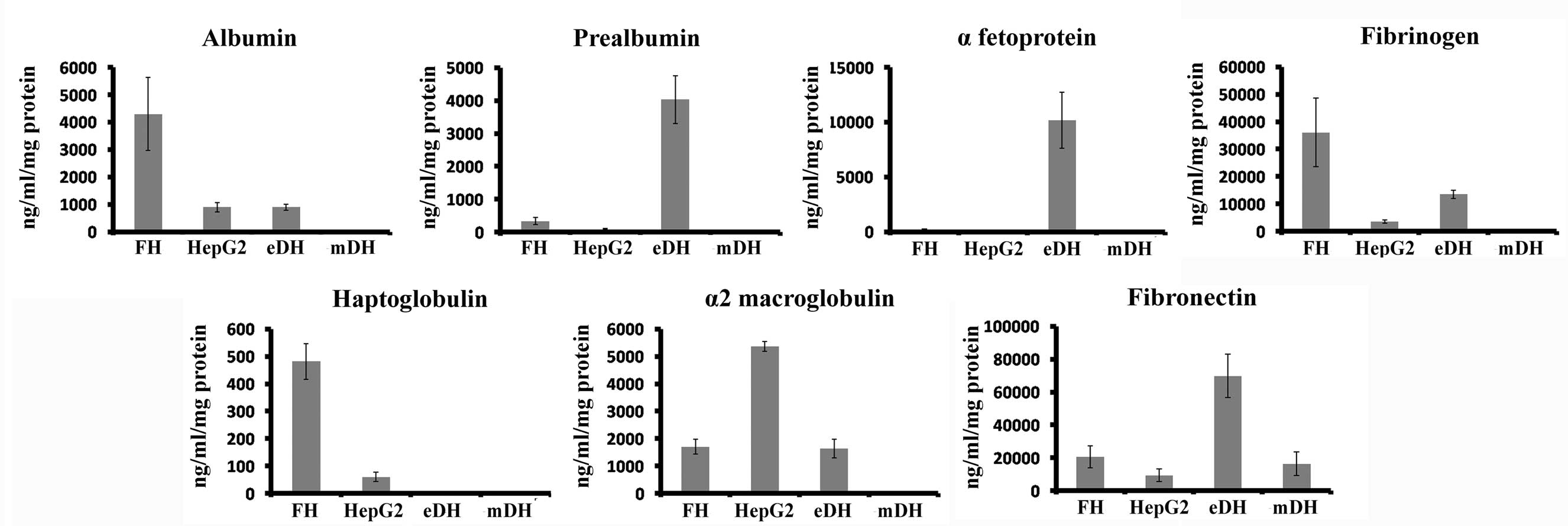
Secretion of hepatic proteins by stem
cell-derived hepatocytes
The functionality of eDHs, mDHs, human foetal
hepatocytes and HepG2 cells were compared by analysing the
secretion of hepatic proteins (albumin, prealbumin, α-fetoprotein,
fibrinogen, haptoglobulin, α2-macroglobulin and fibronectin) by
ELISA (Fig. 8). The secretion of
mature hepatic proteins albumin, fibrinogen and haptoglobulin was
higher in foetal hepatocytes, while α2-macroglobulin secretion was
higher in HepG2 cells. Higher secretion of premature hepatic
proteins, prealbumin and α-fetoprotein was observed in eDHs.
MSC-derived hepatocytes showed extremely low expression of all
hepatic proteins.
Expression of hepatic genes by stem
cell-derived hepatocytes
The expression of hepatic genes (MRP2,
HNF4α and CYP1A1) by MSC-derived hepatocytes, eDHs,
human foetal hepatocytes and HepG2 cells were examined by RT-PCR
(Fig. 9A).
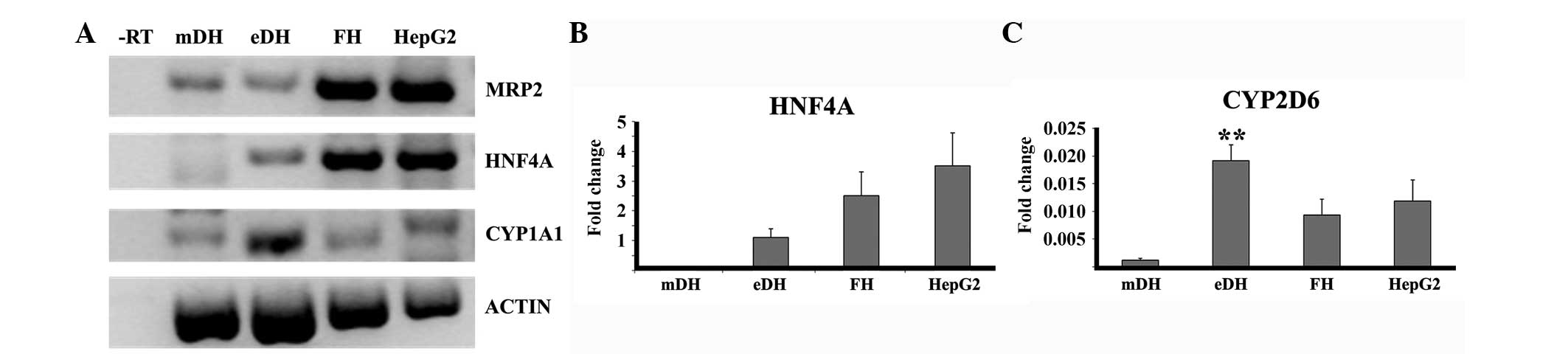 | Figure 9.Expression of hepatic genes by stem
cell-derived hepatocytes. (A) Reverse transcription-polymerase
chain reaction (RT-PCR) showing the expression of hepatic genes in
different cell types. Quantitative PCR (qPCR) analysis showing the
relative expression of (B) HNF4α and (C) CYP2D6 in
the different cell types. **P<0.05. Actin, β-actin;
CYP1A1, cytochrome P450 1A1; CYP2D6, cytochrome P450
2D6; HNF4α, hepatocyte nuclear factor 4α; MRP2,
multidrug resistance-associated protein 2; -RT, negative reverse
transcription control; mDH, MSC-derived hepatocytes; eDH,
hESC-derived hepatocytes; FH, foetal hepatocyte; HepG2, HepG2
cells. |
In order to validate the RT-PCR result for HNF4α and
CYP enzyme expression, qPCR analysis was performed. qPCR analysis
confirmed that HNF4α expression was in the order: HepG2 cells
>foetal hepatocytes >eDHs >mDHs (Fig. 9B). qPCR analysis also confirmed the
higher expression of CYP2D6 by eDHs compared to the HepG2 cells and
foetal hepatocytes (Fig. 9C).
Effect of extracellular matrix in
polarising eDHs
To evaluate the effect of extracellular matrix in
polarising eDHs, the polarity of sandwich-cultured eDHs was
assessed by the expression of ZO-1, DPP4 and fluorescein excretion
(Fig. 10). Apart from ZO-1
expression, eDHs showed modest localised expression of DPP4. eDHs
also exhibited localised fluorescein excretion at their apical
sides. The results confirm the ability of eDHs to form tight
junctions (indicated by ZO-1 expression), hepatic polarity
(indicated by localised DPP4 expression) and functional bile
canaliculi (indicated by localised fluorescein excretion).
Discussion
Similar to other epithelial cells, adult hepatocytes
are polarised with an apical and basolateral surface, separated by
tight junction proteins. In the liver, the apical poles of
hepatocytes join to form channels (bile canaliculi), through which
bile salts and other metabolites are expelled. Tight junction
proteins support the formation of hepatic polarity by sealing
neighbouring cells to prevent intermembrane diffusions, thus
segregating bile canaliculi and cellular lumen. They also promote
differential expression of membrane proteins on the apical and
basal side of the hepatocytes. Due to this membrane
compartmentalisation, export proteins that are actively involved in
excreting bile salts/xenobiotic compounds are localised to the
canalicular side of hepatocytes. The present study aimed to
evaluate the hepatic functions and polarity of stem cell-derived
hepatocytes (eDHs and mDHs) and foetal heptocytes.
The hESC line RCM1 (13) was used for the study. RCM1 cells were
differentiated using the same protocol that was developed for
differentiating hESCs and pluripotent stem cells to hepatocytes
efficiently (3,4). During differentiation, RCM1 cells showed
prominent changes in morphology, expressed hepatic genes (albumin,
α-fetoprotein, carbomyl phosphate synthetase, MRP2, CYP1A1 and
HNF4α) and secreted hepatic proteins (albumin, α-fetoprotein,
α2-macroglobulin and fibronectin). They also exhibited mature
hepatocyte characteristics, such as ICG uptake and glycogen
deposition. These results confirmed the formation of functional
hepatocytes from the RCM1 hESC line, as has been reported with
other hES cell lines (3,4). However, the study showed the presence of
differentiation-resistant colonies within the eDH population,
suggesting their unsuitability for transplantations, as the
presence of contaminating ES cell colonies can result in teratoma
formation following transplantation (14).
Differentiation of hMSCs from bone marrow (2), umbilical cord blood (2), adipose tissues (15), dental pulp (16) and foetal bone marrow (17) to hepatocyte-like cells has been
reported previously. In the present study, human foetal pancreatic
MSCs were isolated, cultured and analysed for the expression of
essential MSC markers (11,12). Cells were positive for CD90, CD105,
CD49b, CD73, CD29 and CD44, and were negative for CD34, CD117, CD31
and CD45, confirming that the cultured cells were MSCs. The
differentiation of hMSCs to a hepatic fate was carried out by
following the 3-stage protocol described previously (2), however, the cells were maintained in
maturation medium for 35 days. This extended period in the
maturation medium was designed to enhance their functional maturity
(2). During differentiation the
morphology of hMSCs changed and the cells expressed albumin, CK18
and hepatocyte-specific antigen, confirming hepatic
differentiation. Flow cytometry analysis (albumin) and hepatic gene
expression (albumin, α-fetoprotein, carbomyl phosphate synthetase,
HNF4α, CYP1A1 and MRP2) further confirmed the differentiation
(16). The derived hepatocytes in the
present study were negative for the expression of the biliary
epithelial marker cytokeratin 19 (a biliary epithelial cytoskeleton
protein) (18), confirming that the
differentiation was specific to the hepatocyte lineage. mDHs also
exhibited facets of hepatic phenotype, such as ICG uptake and
glycogen deposition (3). Such results
confirmed the formation of functional hepatocytes from hMSCs
isolated from the foetal pancreas. mDHs also expressed CD90,
indicating a putative oval stem cell phenotype (10). However, they showed less secretion of
hepatic proteins and a low level of CYP gene expression
compared to other hepatocyte sources. They also showed
heterogeneity in ICG uptake, indicating the functional
heterogeneity of mDHs. This demonstrates their functional
inefficiency for bioartificial liver (BAL) and in vitro
toxicity studies, as these applications require highly functional
hepatocytes.
In order to analyse the formation of functional
hepatocyte polarity in stem cell-derived hepatocytes, the
expression of the tight junction protein ZO-1, the bile canalicular
export protein MRP2, bile canalicular protein DPP4 and excretion of
fluorescein was examined. eDHs showed functional tight junctions,
however, bile canaliculi formation was not observed. Extracellular
matrix is a requisite for polarising epithelial cells (19,20) and
collagen is widely used for polarising primary hepatocytes
(21,22). Collagen was used to make a layer of
extracellular matrix over eDHs in the present study. eDHs cultured
with collagen showed modest localised expression of DPP4 and
fluorescein, confirming the formation of functional bile canaliculi
in sandwich culture. These results show that the extracellular
matrix has a profound influence even on the topology of stem
cell-derived hepatocytes and emphasise the requirement to provide
specific extracellular matrix components and factors required by
adult hepatocytes/epithelial cells for their functional maturation.
A recent study (23) reports similar
advantages using a 3-dimensional (3D) system of culture.
Cell junctions are frequently involved in acute
hepatotoxicity (24) and chronic
hepatic diseases (25) and their loss
is commonly evident during hepatocellular carcinoma (26). Therefore, junction proteins are used as
an indicator for studying acute hepatotoxicity and chronic
diseases, and for screening of non-genotoxic carcinogens (27). As eDHs form tight junctions, they can
be considered useful as in vitro-based tight junction
studies on acute and chronic hepatotoxicity and for screening
genotoxic carcinogens. In addition, eDHs showed higher expression
of CYP enzymes compared to the other alternate hepatocyte sources,
confirming their suitability for in vitro toxicity studies
and for BAL. As eDHs showed formation of polarity in sandwich
culture, they may be able to perform better in the 3D environment
of a BAL (28). As the 3-stage
protocol of ESC hepatic differentiation mimics the embryonic
developmental stages of the hepatocyte (3), differentiated hESC can also be used as a
tool to study the development and formation of tight junctions and
the process of hepatocyte polarisation.
Human foetal hepatocytes are also considered as a
potent alternative human hepatocyte source (29,30). A major
source of human foetal hepatocyte is the liver of a therapeutically
aborted foetus, and such abortions usually occur at the early
stages of pregnancy. In the present study, human foetal hepatocytes
isolated from the liver of terminated midtrimester foetuses showed
albumin expression, glycogen storage and uptake of ICG confirming
the efficient culture of functional human foetal hepatocytes
(31). Additionally, human foetal
hepatocytes showed hepatic polarisation with functional bile
canaliculi formation. These results demonstrate the more mature
aspects of human foetal hepatocytes compared to the pluripotent
stem cell-derived hepatocytes. However, based on protein secretion
and gene expression data, foetal hepatocytes were not as
functionally mature as adult hepatocytes.
HNF4α is an important hepatic transcription factor
with active roles in hepatogenesis (32) and maintenance of hepatic functions
(33,34). Among the stem cell-derived hepatocytes
examined in the present study, the expression of HNF4α was much
lower in less polarised mDHs compared with the more polarised HepG2
cells and isolated human foetal hepatocytes. This demonstrates the
profound role of HNF4α in creating and maintaining cell
polarisation even during the development of hepatocytes from stem
cells and supports the previous reports in mature hepatocytes
(32,35,36). In
addition, the more polarised cells, HepG2 and human foetal
hepatocytes, showed less secretion of primitive hepatic proteins,
such as α-fetoprotein. These results correlate with previous
studies on the effect of polarisation on hepatic functions, such as
albumin secretion (37), bile
secretion (38), glycogenolysis
(39) and xenobiotic metabolism
(26,40,41) in adult
hepatocytes. This correlation between functional efficiency and
polarisation may be due to the better protein trafficking and
compartmentalisation of cellular functions in polarised
hepatocytes.
In conclusion, the present study has demonstrated
that ESC-derived hepatocytes form functional tight junctions, have
higher secretion of hepatic proteins and improved expression of CYP
enzymes and hepatocyte transcription factor compared with mDHs.
Collagen overlay to form a 2D-matrix sandwich improves the
functional polarisation of ESC-derived hepatocytes and promotes the
formation of bile canaliculi, emphasising the requirement for
improved extracellular matrix environments, natural or synthetic,
to further improve the function of pluripotent stem cell-derived
hepatocytes for toxicology studies and BAL devices.
Acknowledgements
The authors would like to thank the Commonwealth
Scholarship Commission for financial support (AAP) for the project.
We thank all the members of the laboratories who have provided
assistance throughout the project.
References
|
1
|
Palakkan AA, Hay DC, Anil Kumar PR, Kumary
TV and Ross JA: Liver tissue engineering and cell sources: Issues
and challenges. Liver Int. 33:666–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee KD, Kuo TK, Whang-Peng J, Chung YF,
Lin CT, Chou SH, Chen JR, Chen YP and Lee OK: In vitro hepatic
differentiation of human mesenchymal stem cells. Hepatology.
40:1275–1284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hay DC, Zhao D, Fletcher J, Hewitt ZA,
McLean D, Urruticoechea-Uriguen A, Black JR, Elcombe C, Ross JA,
Wolf R, et al: Efficient differentiation of hepatocytes from human
embryonic stem cells exhibiting markers recapitulating liver
development in vivo. Stem Cells. 26:894–902. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sullivan GJ, Hay DC, Park IH, Fletcher J,
Hannoun Z, Payne CM, Dalgetty D, Black JR, Ross JA, Samuel K, et
al: Generation of functional human hepatic endoderm from human
induced pluripotent stem cells. Hepatology. 51:329–335. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gómez-Lechón MJ, Donato MT, Castell JV and
Jover R: Human hepatocytes in primary culture: The choice to
investigate drug metabolism in man. Curr Drug Metab. 5:443–462.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuschl G, Hrach J, Walter Y, Hewitt PG and
Mueller SO: Serum-free collagen sandwich cultures of adult rat
hepatocytes maintain liver-like properties long term: A valuable
model for in vitro toxicity and drug-drug interaction studies. Chem
Biol Interact. 181:124–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schulze A, Mills K, Weiss TS and Urban S:
Hepatocyte polarization is essential for the productive entry of
the hepatitis B virus. Hepatology. 55:373–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hannoun Z, Fletcher J, Greenhough S,
Medine C, Samuel K, Sharma R, Pryde A, Black JR, Ross JA, Wilmut I,
et al: The comparison between conditioned media and serum-free
media in human embryonic stem cell culture and differentiation.
Cell Reprogram. 12:133–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Y, Liao L, Wang Q, Ma L, Ma G, Jiang X
and Zhao RC: Isolation and identification of mesenchymal stem cells
from human fetal pancreas. J Lab Clin Med. 141:342–349. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masson NM, Currie IS, Terrace JD, Garden
OJ, Parks RW and Ross JA: Hepatic progenitor cells in human fetal
liver express the oval cell marker Thy-1. Am J Physiol Gastrointest
Liver Physiol. 291:G45–G54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Sousa PA, Gardner J, Sneddon S, Pells
S, Tye BJ, Dand P, Collins DM, Stewart K, Shaw L, Przyborski S, et
al: Clinically failed eggs as a source of normal human embryo stem
cells. Stem Cell Res (Amst). 2:188–197. 2009. View Article : Google Scholar
|
|
14
|
Tang C, Weissman IL and Drukker M: The
safety of embryonic stem cell therapy relies on teratoma removal.
Oncotarget. 3:7–8. 2012.PubMed/NCBI
|
|
15
|
Taléns-Visconti R, Bonora A, Jover R,
Mirabet V, Carbonell F, Castell JV and Gómez-Lechón MJ: Hepatogenic
differentiation of human mesenchymal stem cells from adipose tissue
in comparison with bone marrow mesenchymal stem cells. World J
Gastroenterol. 12:5834–5845. 2006.PubMed/NCBI
|
|
16
|
Ishkitiev N, Yaegaki K, Imai T, Tanaka T,
Nakahara T, Ishikawa H, Mitev V and Haapasalo M: High-purity
hepatic lineage differentiated from dental pulp stem cells in
serum-free medium. J Endod. 38:475–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei X, Wang CY, Liu QP, Li J, Li D, Zhao
FT, Lian JQ, Xie YM, Wang PZ, Bai XF, et al: In vitro hepatic
differentiation of mesenchymal stem cells from human fetal bone
marrow. J Int Med Res. 36:721–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terrace JD, Currie IS, Hay DC, Masson NM,
Anderson RA, Forbes SJ, Parks RW and Ross JA: Progenitor cell
characterization and location in the developing human liver. Stem
Cells Dev. 16:771–778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chambard M, Gabrion J and Mauchamp J:
Influence of collagen gel on the orientation of epithelial cell
polarity: Follicle formation from isolated thyroid cells and from
preformed monolayers. J Cell Biol. 91:157–166. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roskelley CD, Desprez PY and Bissell MJ:
Extracellular matrix-dependent tissue-specific gene expression in
mammary epithelial cells requires both physical and biochemical
signal transduction. Proc Natl Acad Sci USA. 91:12378–12382. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu D, Wakabayashi Y, Ido Y,
Lippincott-Schwartz J and Arias IM: Regulation of bile canalicular
network formation and maintenance by AMP-activated protein kinase
and LKB1. J Cell Sci. 123:3294–3302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Palakkan AA, Anil Kumar PR and Kumary TV:
An in vitro fluorometric assay for evaluating functional polarity
of hepatocyte. Int J Bioassays. 3:1630–1636. 2014.
|
|
23
|
Gieseck RL III, Hannan NR, Bort R, Hanley
NA, Drake RA, Cameron GW, Wynn TA and Vallier L: Maturation of
induced pluripotent stem cell derived hepatocytes by 3D-culture.
PLoS One. 9:e863722014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kojima T, Sawada N, Zhong Y, Oyamada M and
Mori M: Sequential changes in intercellular junctions between
hepatocytes during the course of acute liver injury and restoration
after thioacetamide treatment. Virchows Arch. 425:407–412. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakisaka S, Kawaguchi T, Taniguchi E,
Hanada S, Sasatomi K, Koga H, Harada M, Kimura R, Sata M, Sawada N,
et al: Alterations in tight junctions differ between primary
biliary cirrhosis and primary sclerosing cholangitis. Hepatology.
33:1460–1468. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Neveu MJ, Hully JR, Babcock KL, Hertzberg
EL, Nicholson BJ, Paul DL and Pitot HC: Multiple mechanisms are
responsible for altered expression of gap junction genes during
oncogenesis in rat liver. J Cell Sci. 107:83–95. 1994.PubMed/NCBI
|
|
27
|
Mally A and Chipman JK: Non-genotoxic
carcinogens: Early effects on gap junctions, cell proliferation and
apoptosis in the rat. Toxicology. 180:233–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palakkan AA, Raj DK, Rojan J, Raj RGS,
Anil Kumar PR, Muraleedharan CV and Kumary TV: Evaluation of
polypropylene hollow-fiber prototype bioreactor for bioartificial
liver. Tissue Eng Part A. 19:1056–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Khan AA, Habeeb A, Parveen N, Naseem B,
Babu RP, Capoor AK and Habibullah CM: Peritoneal transplantation of
human fetal hepatocytes for the treatment of acute fatty liver of
pregnancy: A case report. Trop Gastroenterol. 25:141–143.
2004.PubMed/NCBI
|
|
30
|
Chen Y, Li J, Liu X, Zhao W, Wang Y and
Wang X: Transplantation of immortalized human fetal hepatocytes
prevents acute liver failure in 90% hepatectomized mice. Transplant
Proc. 42:1907–1914. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weber A, Touboul T, Mainot S, Branger J
and Mahieu-Caputo D: Human foetal hepatocytes: Isolation,
characterization, and transplantation. Methods Mol Biol. 640:41–55.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parviz F, Matullo C, Garrison WD, Savatski
L, Adamson JW, Ning G, Kaestner KH, Rossi JM, Zaret KS and Duncan
SA: Hepatocyte nuclear factor 4alpha controls the development of a
hepatic epithelium and liver morphogenesis. Nat Genet. 34:292–296.
2003.PubMed/NCBI
|
|
33
|
Hayhurst GP, Lee YH, Lambert G, Ward JM
and Gonzalez FJ: Hepatocyte nuclear factor 4alpha (nuclear receptor
2A1) is essential for maintenance of hepatic gene expression and
lipid homeostasis. Mol Cell Biol. 21:1393–1403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jover R, Bort R, Gómez-Lechón MJ and
Castell JV: Cytochrome P450 regulation by hepatocyte nuclear factor
4 in human hepatocytes: A study using adenovirus-mediated antisense
targeting. Hepatology. 33:668–675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Battle MA, Konopka G, Parviz F, Gaggl AL,
Yang C, Sladek FM and Duncan SA: Hepatocyte nuclear factor 4alpha
orchestrates expression of cell adhesion proteins during the
epithelial transformation of the developing liver. Proc Natl Acad
Sci USA. 103:8419–8424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cattin AL, Le Beyec J, Barreau F,
Saint-Just S, Houllier A, Gonzalez FJ, Robine S, Pinçon-Raymond M,
Cardot P, Lacasa M, et al: Hepatocyte nuclear factor 4alpha, a key
factor for homeostasis, cell architecture, and barrier function of
the adult intestinal epithelium. Mol Cell Biol. 29:6294–6308. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hou DX, Arimura M, Fukuda M, Oka T and
Fujii M: Expression of cell adhesion molecule and albumin genes in
primary culture of rat hepatocytes. Cell Biol Int. 25:239–244.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nathanson MH, Rios-Velez L, Burgstahler AD
and Mennone A: Communication via gap junctions modulates bile
secretion in the isolated perfused rat liver. Gastroenterology.
116:1176–1183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stümpel F, Ott T, Willecke K and
Jungermann K: Connexin 32 gap junctions enhance stimulation of
glucose output by glucagon and noradrenaline in mouse liver.
Hepatology. 28:1616–1620. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shoda T, Mitsumori K, Onodera H, Toyoda K,
Uneyama C, Imazawa T and Hirose M: The relationship between
decrease in Cx32 and induction of P450 isozymes in the early phase
of clofibrate hepatocarcinogenesis in the rat. Arch Toxicol.
73:373–380. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hamilton GA, Jolley SL, Gilbert D, Coon
DJ, Barros S and LeCluyse EL: Regulation of cell morphology and
cytochrome P450 expression in human hepatocytes by extracellular
matrix and cell-cell interactions. Cell Tissue Res. 306:85–99.
2001. View Article : Google Scholar : PubMed/NCBI
|