Periodontal diseases, which include gingivitis
(where the inflammation is confined to the gingiva and is
reversible with dental care) and periodontitis (where the
inflammation spreads, and results in tissue destruction and
alveolar bone resorption), are the most common types of disease in
humans worldwide (1). Tissue
destruction, such as the breakdown of the collagen fibres of the
periodontal ligament (PDL) and the loss of gingival tissue and
alveolar bone, are characteristic of periodontitis, resulting in
gradual weakening of the tooth-supporting tissues, which eventually
leads to tooth loss. The condition is widespread. Severe
periodontitis that threatens tooth retention affects 10–15% of
adults in the majority of populations investigated and ranged from
1%, among 20- to 29-year-old, to 39%, among individuals
>65-year-old while moderate periodontitis affects 40–60% of
adults in all populations (2).
Therefore, periodontitis is a highly prevalent, chronic
inflammatory disease, which has negative and profound impacts on
many aspects of daily life (3).
Periodontitis has been a controversial point of
interest due to the identification of correlations between it and
metabolic syndrome (MS). MS is a condition, which constitutes a
group of risk factors that occur together and increase the risk for
coronary artery disease, stroke and type 2 diabetes mellitus (DM)
(4).
Important investigations into the underlying
mechanisms linking periodontitis to systemic disorders have ensued,
resulting in the development of a novel branch of periodontics
termed ‘periodontal medicine’ (5).
Since the first report of the association between periodontitis and
systematic diseases, there have been constant novel discoveries.
Along with five other complications (diabetic
cardio-cerebrovascular disease, diabetic nephropathy, diabetic eye
disease, diabetic neuropathy and diabetic sexual dysfunction)
periodontal disease has been labeled as the sixth complication of
diabetes (6). Thus, periodontitis and
diabetes appear to interact. Clinical evidence provides strong
support to the statement that periodontal infection contributes to
the worsening of glycaemic control in individuals with diabetes
(7). Respiratory disease and chronic
obstructive pulmonary disease are other systemic disorders ranking
the third and sixth most common causes of mortality worldwide in
1990, causing 4.3 and 2.2 million mortalities, respectively
(8) and a cross-sectional study
suggested that chronic obstructive pulmonary disease may be
associated with severe periodontitis (2). The teeth and periodontium may serve as a
reservoir for respiratory infection (9), and dental plaque maybe the important
local source of the anaerobic bacteria, which cause pneumonia or
other types of respiratory infection. Several mechanisms have been
proposed to explain the mechanism by which oral bacteria
participate in the pathogenesis of respiratory infection, including
orally inhaled periodontal bacteria, periodontal disease-associated
enzymes and cytokines (10).
Cardiovascular disease (CVD) accounts for 29% of mortalities
worldwide, ranking the second leading cause of mortality following
infectious and parasitic disease (11). Until now, numerous studies that were
based upon the inflammation hypothesis of CVD, considered
periodontal diseases to be infection-triggered inflammatory
diseases (12). Furthermore, various
epidemiology experiments that have been conducted over the years
have suggested a positive association between periodontal diseases
and CVD (12). Characterized by an
imbalance between bone formation and bone resorption, osteoporosis
is the most common type of metabolic bone disease; periodontitis
and osteoporosis share similar traits with regard to bone
resorption (13). Although
substantiated studies attempting to demonstrate the positive
correlation between periodontal disease and osteoporosis have been
performed, the majority of the studies are uncontrolled and
cross-sectional in design (14);
therefore, in order to elucidate the associations between these two
diseases, further prospective studies are required.
Approximately one-quarter of the entire adult
population in the world shows excessive hepatic fat accumulation,
and NAFLD is the most common form of chronic liver disease
encountered in developed countries (15). The prevalence of NAFLD throughout the
world is speculated to be 20–30% (16), among obese patients the figure rises to
57–74% (17). NAFLD represents a wide
spectrum of conditions, ranging from NAFL to non-alcoholic
steatohepatitis (NASH) (15,18). The diagnosis of NAFLD is often
established following identification of elevated serum alanine
aminotransferase (ALT) and γ-glutamyl transferase (GGT), which is
most commonly used for screening of liver diseases in obese and
asymptomatic patients (19,20).
Thus far, a small number of studies have been
conducted to identify the association between NAFLD and
periodontitis, the majority of which have been performed in Japan.
In a study conducted in a Japanese college, male students with a
high level of serum ALT were identified to be significantly more
likely to have periodontitis than those with a low level of serum
ALT (21). As for females, the
association between a higher ALT level and an increased risk of
periodontitis was not found to be significant, which was in
contrast to a previous study that indicated that the incidence rate
of periodontitis in females aged 20–59 years was significantly
increased with elevated serum levels of ALT (22). The author of the above-mentioned
studies attributed this discrepancy to the differences in the age
of the subjects and the sample size. Notably, it seems that the
association between periodontitis and the serum levels of ALT is
mutual. In a cross-sectional study with a large sample size that
was conducted in Japan, researchers found that ALT and GGT levels
were higher in patients with periodontal pockets(depth, ≥4 mm) when
compared to healthy controls. Multiple logistic regression analysis
with GGT or ALT as the dependent variable revealed that there was a
significant association between periodontal pockets and GGT, even
after adjusting for age, gender, cigarette smoking, alcohol
drinking habits, and symptoms of MS (23). In a previous study, it was found that
periodontal treatments improved certain liver function parameters,
such as serum aspartate transaminase and ALT in NAFLD patients
(24). Such periodontal treatments
include oral hygiene procedures, including scaling, root planing
procedures and application of minocycline hydrochloride.
LC is a major, life-threatening health problem
worldwide. LC is often a result of liver injuries of numerous
different etiologies, leading to hepatocyte damage, hepatic
inflammation and fibrogenesis (25).
Furthermore, LC can lead to the development of hepatocellular
carcinoma (HCC) (26). LC is
histologically characterized by increased deposition in, and
altered composition of, the extracellular matrix and the appearance
of regenerative nodules. The destruction of the normal architecture
of the liver and the loss of its functional hepatocytes prevent the
liver from performing its normal detoxification, synthesis, and
metabolic functions, eventually leading to portal hypertension and
liver failure. From a clinical standpoint, LC is regarded as an
end-stage disease, which results in mortality, unless a liver
transplantation (LT) is performed (27).
The association between LC and periodontal disease
has been evaluated in previous studies. In one study, researchers
identified that patients with non-alcoholic cirrhosis exhibited a
tendency to have a larger clinical attachment loss (CAL) when
compared with healthy volunteers, although no significant
difference was found within any of the age groups. Notably,
significant differences between healthy controls and patients with
alcoholic cirrhosis were found in each age group (28). It has also been shown that patients
with cirrhosis exhibited a worse periodontal status compared with
healthy control individuals (29).
Furthermore, studies found that patients who had suffered with
cirrhosis for >3 years exhibited greater CAL, dental plaque and
calculus when compared to patients with <3 years cirrhosis
(30). The effects on periodontal
health, which are brought about by LC may be due to decreased blood
flow of the mucogingival junction (31) and increased levels of serum alkaline
phosphatase (32). Thus far, the
effect of periodontitis on LC has not been fully investigated.
Therefore, more studies are required to better understand the link
between these two diseases.
HCC is the sixth most common type of cancer and
accounts for ~9.2% of all cancer-associated mortalities (33). HCC is more common in males than in
females and predominantly occurs in developing countries. The
increasing trend is primarily due to a cohort effect associated
with the hepatitis B and C viruses (HBV and HCV), the incidence of
which peaked between the 1950s and 1980s (33). By contrast, in North America, Europe
and Japan, the HCV infection is the main risk factor, together with
alcohol abuse (33). Time trends of
incidence of HCC in developed countries correspond tot he timing of
HCV spread. In Japan and Europe, where the HCV infection spread
earlier than in the USA, the incidence of HCC has almost reached a
plateau and is declining in certain areas; however, in the USA, the
incidence continues to increase and the infection may have a
synergistic effect with other risk factors, such as NAFLD. In the
majority cases, HCC is a multistage disease, the occurrence of
which is linked to environmental, dietary and lifestyle factors
(34). Unlike other types of cancer,
HCC usually arises on a previously damaged organ, primarily in the
setting of chronic hepatopathy, cirrhosis (35,36), or in
association with hereditary diseases, such as hemochromatosis,
Wilson's disease and a-1-antitrypsin deficiency (34). However, in ~15–20% of cases HCC may
occur in the non-fibrotic liver or in livers with minimal portal
fibrosis without any septal fibrosis (37).
A number of studies have demonstrated the
association between periodontitis and cancer. An epidemiological
study reported a positive association between periodontitis and
lung cancer mortality, in addition to other established risk
factors for lung cancer (38). A
cross-sectional study demonstrated that the loss of alveolar bone
is considered an increased risk factor of tongue cancer (39). Furthermore, a hospital-based
case-control study demonstrated that patients presenting with
periodontitis were more likely to have poorly differentiated
squamous cell carcinoma in the oral cavity compared with those
individuals without periodontitis (40). Based on this evidence, novel
discoveries were made regarding the link between periodontitis and
HCC.
In a Japanese study, it was found that the stage of
HCC is associated with periodontitis. A method called the Japan
Integrated Staging (JIS) system (41)
was applied to assess the severity of HCC. The JIS system, which is
accepted by many institutions in Japan because of its simplicity
and validity (41,42), is based on a combination of the
Child-Pugh score (43) and the tumor
node metastasis (TNM) classification. The study demonstrated that
HCC patients with chronic periodontitis had greater JIS scores and
higher serum levels of total bilirubin when compared with the
patients who had good periodontal and gingival health. Furthermore,
a backward stepwise logistic regression model confirmed that
progression of the JIS score was significantly associated with
probing pocket depth. Increased serum levels of reactive oxygen
metabolites (ROM) were also seen in HCC patients with chronic
periodontitis when compared to those exhibiting good periodontal
and gingival health (44).
LT is a treatment for HCC, which was first performed
in the late 80s and early 90s (45).
Early studies indicated a poor outcome with regards to survival
rates and high tumor recurrence (46,47). This
was predominantly due to the fact that there were no standard
criteria for selecting patients for LT (48). In 1996, a landmark study resulted in
the development of enrolling criteria, commonly known as the Milan
Criteria (49), which is designed to
selectively enroll patients for LT and reduce the risk of
mortality. Currently, LT is considered to be the best treatment
option for HCC and cirrhosis for patients who fulfill the
eligibility criteria.
Various traits of periodontitis are associated with
LT. Periodontitis-induced immunosuppression may encourage
infections following LT. Therefore, an oral examination has long
been a requirement prior to LT with the aim of eliminating oral
foci of infection and, hence, preventing sepsis of oral origin
(50–55).
A pilot study, which recruited 16 patients with
end-stage liver disease and 16 control subjects with no liver
diseases, showed that the end-stage liver disease patients
exhibited a higher incidence of oral manifestations when compared
with the control group, and had at least one oral disease or
abnormality, which required dental treatment prior to LT (56). A study conducted in Finland, where 116
adult patients with a primary diagnosis of LC and who were due to
undergo LT were examined. The study found that the number of tooth
extractions, a surrogate marker of dental infections, was
associated with a significantly reduced time required from the
diagnosis of LD to LT. This association remained significant
following adjustment for age, gender, LD etiology and the model for
end-stage liver disease (MELD) score; additionally, alcoholic
cirrhosis was the only other significant factor in the multivariate
analysis (57). Another similar study,
also conducted in Finland, confirmed that a lower MELD score was
associated with fewer tooth extractions (58). Furthermore, the LT candidates with an
end-stage liver disease and the highest MELD score usually
exhibited various medical complications, such as severe ascites,
malnutrition, variceal bleeding and infections (58). Premedications, such as antibiotic
prophylaxis and coagulating agents, are often used to reduce the
complications associated with dental treatments of patients with a
severe LD, thus compromising the patients and rendering them at
very high risk for dental treatment complications (59–62). Another
study conducted in Brazil also showed that treating oral lesions,
such as periodontitis before and after LT, seemed to result in
reduced mortality (63).
Unfortunately, despite the important role played by periodontal
health in LT, those patients who are on the transplantation waiting
list tend to neglect their oral health resulting in poorer oral
statuses than the general population (64,65).
The microbial etiology of periodontal disease has
been the focus of research for a long time. Approximately 400
species have been detected in the gingival sulcus, among them are
Porphyromonas gingivalis (P. gingivalis) and
Tannerella forsythia, which are widely regarded as major
pathogens in periodontitis (66).
Subgingival microbiota were classified into several complexes
indicated by various colors; the colors (varying from red to
yellow) have different connotations, with red being the most
pathogenic and yellow being less invasive. Periodontal microbiota
are more heterogeneous than earlier believed. In dentistry,
gram-negative organisms were considered to be the predominant
bacteria in periodontitis; however, gram-positive organisms found
in deep, diseased sites are proposed to be the most important
pathogens in periodontitis (67).
Bacteria also have a negative effect on the liver. It is well known
that patients with cirrhosis are at greater risk of bacterial
infection (68,69) and infections rate is 4- to 5-fold
higher than the general population (70,71). It is
also reported that spontaneous bacterial peritonitis (SBP) is one
of the most encountered infectious complications by patients with
cirrhosis on the LT list (72). Thus,
the present review evaluated a selection of the most studied
bacteria that are associated with LD.
Numerous studies have demonstrated that gut-derived
bacteria may contribute to the progression of LD (83–86).
Although these studies focused on gut bacteria, it is hypothesized
that these gastrointestinal bacteria may pass through the oral
cavity and that certain bacteria may have arisen from the oral
cavity. An animal study demonstrated that gut microbiota promoted
absorption of monosaccharides in mice, which resulted in
lipogenesis, and the bacteria responsible for this effect in mice
were within the Firmicutes phylum. Additionally,
Selenomonasnoxia, a gram-negative bacteria, which is found
in the mouth and the gastro-intestinal tract, and participates in
rapidly progressive periodontitis, was the only Firmicute that was
significantly elevated in saliva (87). It has been estimated that ~1 gram of
bacteria (1011 cells) is swallowed with 500–1,500 ml
saliva each day (88). If the levels
of S. noxia are >1.05%, this represents ~109 cells
swallowed per day. It is therefore plausible that salivary
microbiology would affect the formation of bacteria in the
gastro-intestinal tract (89). The
swallowed saliva of patients with periodontitis is reported to
contain ~109 bacteria/ml, in 1.0–1.5 litres per day,
resulting in a total of >1012 bacteria per day
(90–92). As the bacterial flora of the oral
cavity is distinct from that of the gut (93), it is possible that swallowed bacteria
may affect the composition of the gut microflora. Notably, it has
been reported that oral probiotic intervention alters gut bacterial
composition (94). Thus,
orally-originating bacteria may be significant in gut-liver axis
malfunction and the link between the two requires further
investigation.
Inflammatory mediators, such as IL-12/23, TNF-α, and
IL-1 may lead to the recruitment of activated neutrophils, which
causes hepatocyte and vascular endothelial cell injuries by
releasing oxidants and proteases (95,96). Human
trials (97–105) and animal experiments (106–108) have
confirmed the production of pro-inflammatory molecules in vivo and
ex vivo in patients and animals with cirrhosis. The liver acts as
the body's first defence against bacteria and microbial components.
These pathogens, such as inflammatory mediators, which are present
in the portal blood, generate the initial immunological and
hormonal burden to the liver (109).
Increasing evidence indicates that periodontitis-induced
pro-inflammatory mediators may contribute to this burden.
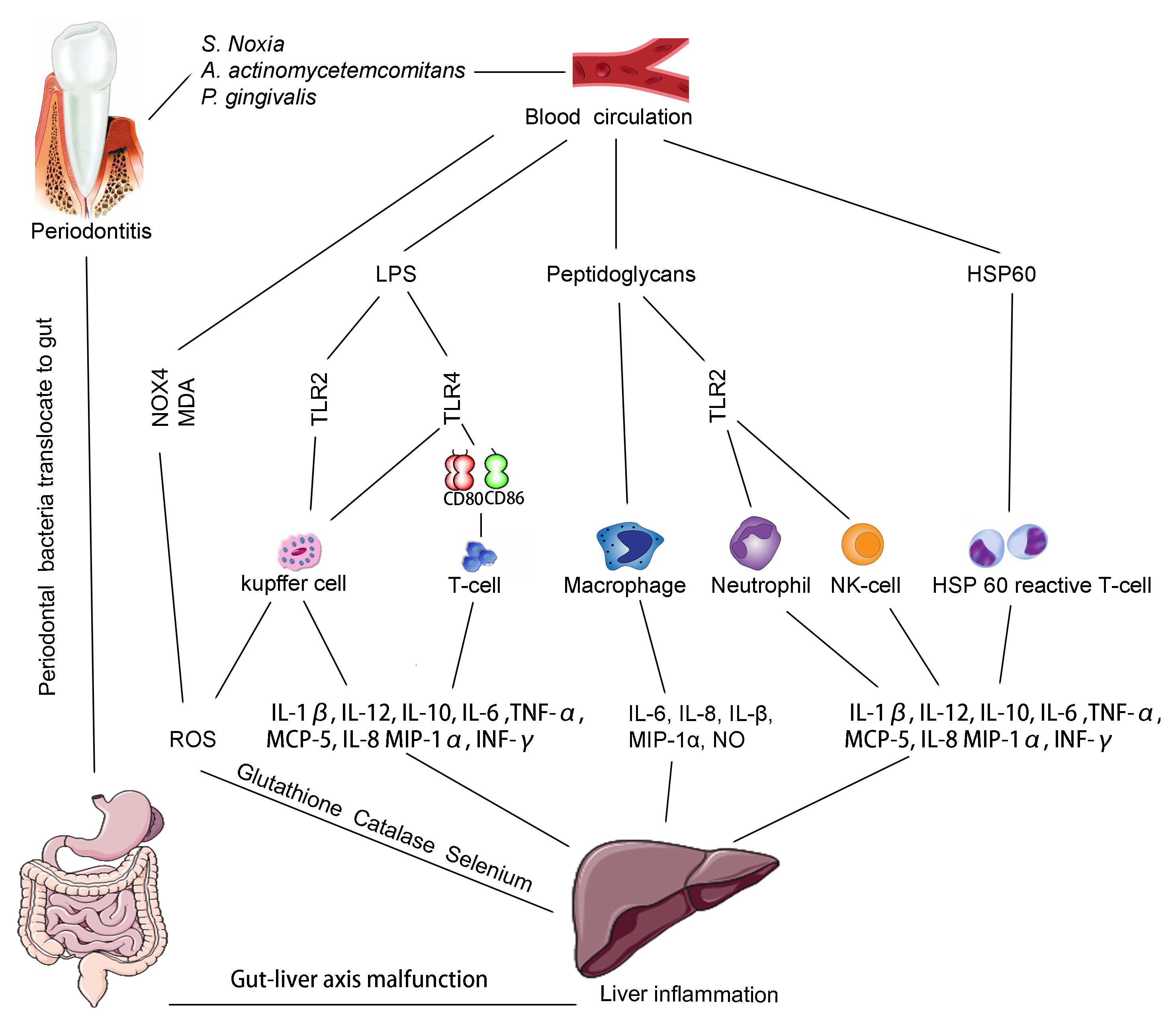
The dental plaque, partly composed of gram-negative
bacteria cell walls, which are formed of peptidoglycans,
polysaccharides, proteins, lipids, lipopolysaccharides (LPSs) and
lipoproteins (110), commonly exist
in the oral cavity of humans, particularly in those who suffer from
periodontitis. Stimulated by these components, the periodontal
tissue produces inflammatory cytokines (such as IL-1β, IL-12,
IL-10, IL-6, TNF-α and INF-γ) and chemokines [such as monocyte
chemotactic protein 5 (MCP-5), IL-8 and macrophage inflammatory
protein-1α (MIP-1α), prostaglandin E2 and nitric oxide (NO)]
(111,112). These pro-inflammatory cytokines are
involved in the progression of LD, such as cirrhosis (69,113–115).
Oral bacteria are also important in the cytokine network. LPSs,
released by periodontal bacteria, such as A.
actinomycetemcomitans and P. gingivalis, affect the
immune system by binding to Toll-like receptor (TLR)-4 or −2, oral
bacteria also stimulate the expression of co-stimulatory molecules,
cluster of differentiation (CD) 80/CD86 by binding to TLR4; and may
participate in the activation of T-cells and exacerbate liver
inflammation (116,117). Kupffer cells, which express the
highest levels of TLR4 in the liver, are the primary cells in liver
inflammation that respond to LPSs in order to produce inflammatory
cytokines, chemokines and reactive oxygen species (ROS) (117–119). In
a previous animal study, it was confirmed that the administration
of LPS generates changes in Kupffer cell function and increases
liver parenchymal sensitivity to TNF-α in genetically obese mice
(120). Additional animal experiments
demonstrated that LPSs-induced TNF-α and its subsequent interaction
with TLR2 signaling promoted NASH in mice (120,121).
HSPs are believed to be the most immunogenic
antigens produced by bacteria. The extensive homology that exists
between human and bacterial HSPs indicates that HSPs may have a
role in the progression of hepatitis. A previous study showed that
patients with periodontitis exhibited decreased proliferative
responses of peripheral blood cells to HSP when compared to control
patients (130). Additionally,
antibodies against human HSP60 and P. gingivalis GroEL were
observed in the sera and inflamed gingival tissues of patients with
periodontitis (131). Animal
experiments demonstrated that bacterial infection may lead to
elevated production of antibodies that target HSP60 (which is
expressed in the endothelium of blood vessels at their bifurcation
stressed area). The mechanism of antibodies binding to the surface
of the endothelium may be a triggering process associated with
inflammatory autoimmune disease (132). Analysis of the nucleotide sequences
of the T-cell receptor in periodontitis lesions showed that human
HSP60-reactive T-cell clones and T-cells share the same receptors.
The cytokine profile analysis showed that HSP60-reactive peripheral
blood mononuclear cells produced a significant quantity of IFN-γ in
patients with periodontitis, and it is plausible that patients with
periodontitis possess human HSP60-reactive T-cells with a type 1
cytokine profile (133).
Oxidative stress is an imbalance between the
production and elimination of ROS, reactive nitrogen species (RNS)
and free radicals, which causes DNA fragmentation, lipid
peroxidation, and protein oxidation (134) leading to the loss of membrane
integrity, structural and functional changes in proteins, and gene
mutations (135). ROS and RNS are
considered to be the most important liver toxicity mechanisms
resulting from cell damage. The sub-gingival dental plaque is the
main etiological agent for the initiation of inflammatory changes
in the periodontal tissue and the cell component of bacteria, which
exists in the dental plaque and recruits and activates hyper
responsive polymorphonucleocytes, thus accelerating the process of
ROS production.
Various studies have demonstrated the effect of
periodontitis on circulating ROS and oxidative stress. For example,
thiobarbituric acid reactive substances, such as malondialdehyde
(MDA), a biomarker commonly employed for lipid peroxidation, the
levels of which are elevated, systemically, in plasma, in
erythrocytes and locally in tissue homogenates, in patients with
periodontitis (136,137). MDA was also found to be raised in the
gingival crevicular fluid (GCF) and saliva of patients with
periodontitis, as compared to healthy controls. Furthermore, the
GCF concentrations of MDA/4-hydroxyalkanal were 200- to 400-fold
higher than the saliva concentrations, which reflected a
substantially higher quantity of ROS activity in the GCF than the
saliva (138). In a study where P.
gingivalis-generated LPS (LPS-PG) stimulated PDL fibroblasts
were established, the results demonstrated that nicotinamide
adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4) levels
significantly increased following hypoxic or inflammatory
stimulation in PDL cells, and was accompanied by a significant
upregulation of ROS and catalase (139). In a case-control study, lower levels
of glutathione, catalase, and selenium were observed in the serum
of patients with periodontitis when compared to those of the
healthy control group and the difference was significant (140). Furthermore, it has been shown that
periodontal treatment improves the circulating
pro-oxidant/antioxidant balance in chronic periodontitis patients
(141). In addition, studies have
shown that non-surgical and surgical periodontal therapy was
effective in reducing plasma ROMs (142–145).
Based on these findings, it is proposed that periodontitis-induced
ROS may be involved in liver injuries.
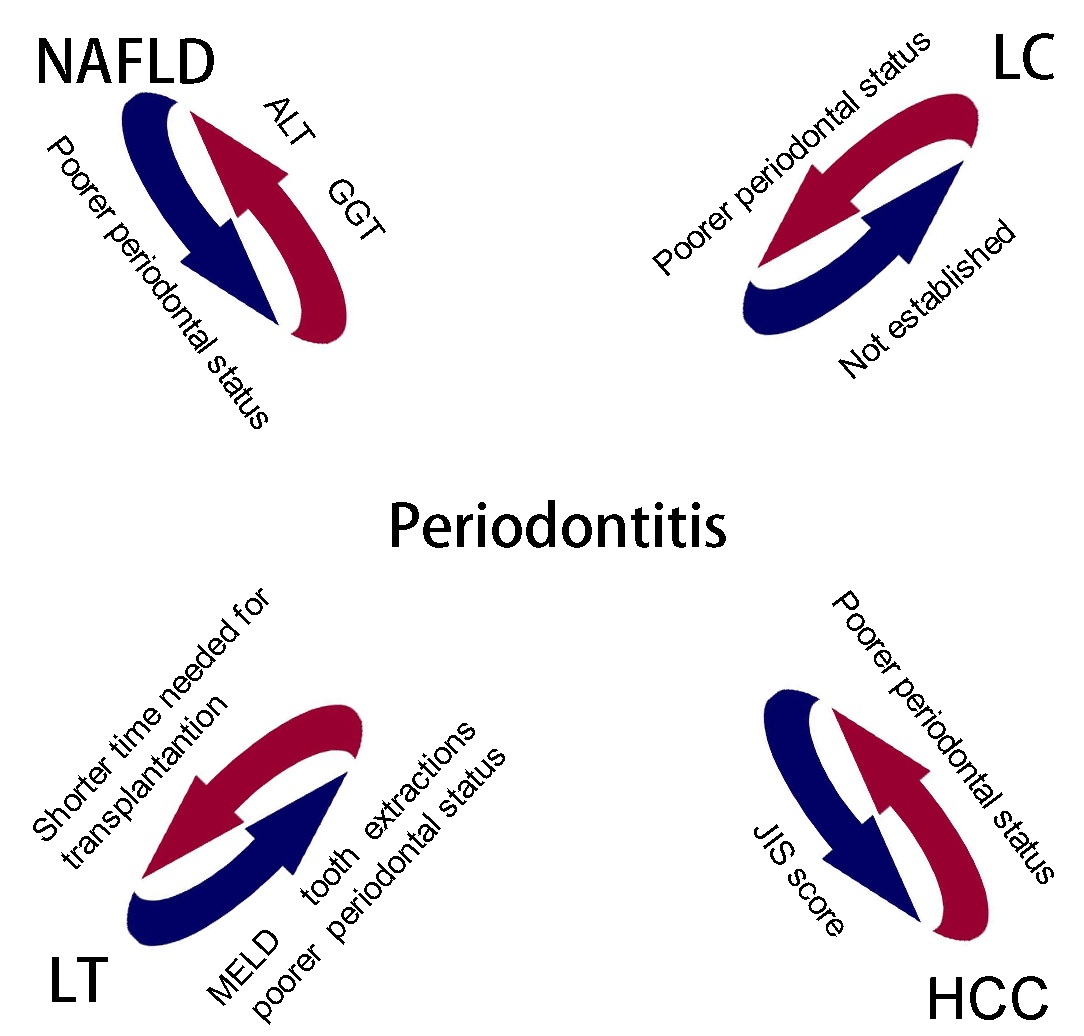
Periodontitis and LD significantly impact health.
The associations between periodontitis and NAFLD, LC, HCC, and LT
have previously been investigated (Fig.
1). Certain pathological features are shared by periodontitis
and systemic diseases, such as DM and CVDs, may exert similar
effects on the liver. Among the three mechanisms (including
bacteria, pro-inflammatory mediators and oxidative stress), various
bacteria exist in the dental plaque, some of which are more
dominant in patients with severe periodontitis, may significantly
contribute to the linking of other pathological mechanisms
(Fig. 2). Although periodontitis is a
common disease, in the majority of cases, it can be prevented and
cured. However, due to the fact that compared with other
life-threatening diseases, such as LC and CVDs, periodontitis seems
relatively harmless, it is common for patients with severe LD to
neglect to their oral hygiene, even when they present with
periodontitis. This phenomenon is not limited to patients; doctors
also neglect the potential damage caused by periodontitis to the
liver. The aim of the current review was to highlight the
association between periodontitis and LD, in the hope that
individuals who suffer from LD will attend to their periodontal
health and, by employing simple dental health strategies, improve
their liver condition.
In addition, all of the studies conducted thus far
have ruled out viral hepatitis despite its high prevalence; with
350–400 million cases of chronic HBV infection (146,147) and
170 million cases of HCV infection (148) worldwide. Furthermore, dentists are
associated with a greater (3- to 6-times) chance of HBV infection
than the general population, which is the highest rate of HBV
infection among all healthcare workers (149). The risk of HCV transmission and cross
contamination within dental practices has previously been reported
(150). It is hypothesized that by
administering dental care to patients with viral hepatitis in a
more central manner, for example, by establishing a specific dental
care facility exclusively for those patients may be beneficial to
dentists and patients. Therefore, further investigations are
required to investigate the potential effect of periodontitis on
viral hepatitis, and increased collaborations between physicians
and dentists are strongly advised.
|
1
|
Vos T, Flaxman AD, Naghavi M, Lozano R,
Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V,
et al: Years lived with disability (YLDs) for 1160 sequelae of 289
diseases and injuries 1990–2010: a systematic analysis for the
Global Burden of Disease Study 2010. Lancet. 380:2163–2196. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung JH, Hwang HJ, Kim SH and Kim TH:
Associations Between Periodontitis and Chronic Obstructive
Pulmonary Disease; the 2010–2012 Korean National Health and
Nutrition Examination Survey (KNHANES). J Periodontol. Feb
25–2016.(Epub ahead of print). View Article : Google Scholar
|
|
3
|
O'Dowd LK, Durham J, McCracken GI and
Preshaw PM: Patients' experiences of the impact of periodontal
disease. J Clin Periodontol. 37:334–339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gurav AN: The association of periodontitis
and metabolic syndrome. Dent Res J (Isfahan). 11:1–10.
2014.PubMed/NCBI
|
|
5
|
Offenbacher S: Periodontal diseases:
Pathogenesis. Ann Periodontol. 1:821–878. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mealey BL and Rethman MP: Periodontal
disease and diabetes mellitus. Bidirectional relationship. Dent
Today. 22:107–113. 2003.PubMed/NCBI
|
|
7
|
Collin HL, Uusitupa M, Niskanen L,
Kontturi-Närhi V, Markkanen H, Koivisto AM and Meurman JH:
Periodontal findings in elderly patients with non-insulin dependent
diabetes mellitus. J Periodontol. 69:962–966. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murray CJ and Lopez AD: Mortality by cause
for eight regions of the world: Global Burden of Disease Study.
Lancet. 349:1269–1276. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuo LC, Polson AM and Kang T: Associations
between periodontal diseases and systemic diseases: A review of the
inter-relationships and interactions with diabetes, respiratory
diseases, cardiovascular diseases and osteoporosis. Public Health.
122:417–433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scannapieco FA: Role of oral bacteria in
respiratory infection. J Periodontol. 70:793–802. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
No authors listed: The World Health Report
1997 - conquering suffering, enriching humanity. World Health
Forum. 18:248–260. 1997.PubMed/NCBI
|
|
12
|
Dave S, Batista EL Jr and Van Dyke TE:
Cardiovascular disease and periodontal diseases: Commonality and
causation. Compend Contin Educ Dent. 25(Suppl 1): 26–37.
2004.PubMed/NCBI
|
|
13
|
Reddy MS: Osteoporosis and periodontitis:
Discussion, conclusions, and recommendations. Ann Periodontol.
6:214–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aspalli SS, Shetty VS, Parab PG, Nagappa
G, Devnoorkar A and Devarathnamma MV: Osteoporosis and
periodontitis: Is there a possible link? Indian J Dent Res.
25:316–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Angulo P: Nonalcoholic fatty liver
disease. N Engl J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Milić S and Stimac D: Nonalcoholic fatty
liver disease/steatohepatitis: Epidemiology, pathogenesis, clinical
presentation and treatment. Dig Dis. 30:158–162. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Angulo P and Lindor KD: Non-alcoholic
fatty liver disease. J Gastroenterol Hepatol. 17:S186–S190. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liou I and Kowdley KV: Natural history of
nonalcoholic steatohepatitis. J Clin Gastroenterol. 40(Suppl 1):
S11–S16. 2006.PubMed/NCBI
|
|
19
|
Oh HJ, Kim TH, Sohn YW, Kim YS, Oh YR, Cho
EY, Shim SY, Shin SR, Han AL, Yoon SJ, et al: Association of serum
alanine aminotransferase and γ-glutamyltransferase levels within
the reference range with metabolic syndrome and nonalcoholic fatty
liver disease. Korean J Hepatol. 17:27–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adams LA, Knuiman MW, Divitini ML and
Olynyk JK: Body mass index is a stronger predictor of alanine
aminotransaminase levels than alcohol consumption. J Gastroenterol
Hepatol. 23:1089–1093. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Furuta M, Ekuni D, Yamamoto T, Irie K,
Koyama R, Sanbe T, Yamanaka R, Morita M, Kuroki K and Tobe K:
Relationship between periodontitis and hepatic abnormalities in
young adults. Acta Odontol Scand. 68:27–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saito T, Shimazaki Y, Koga T, Tsuzuki M
and Ohshima A: Relationship between periodontitis and hepatic
condition in Japanese women. J Int Acad Periodontol. 8:89–95.
2006.PubMed/NCBI
|
|
23
|
Morita T, Yamazaki Y, Fujiharu C, Ishii T,
Seto M, Nishinoue N, Sasaki Y, Kawato T, Motohashi M and Maeno M:
Serum γ-glutamyltransferase level is associated with periodontal
disease independent of drinking habits in Japanese adults. Med Sci
Monit. 20:2109–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoneda M, Naka S, Nakano K, Wada K, Endo
H, Mawatari H, Imajo K, Nomura R, Hokamura K, Ono M, et al:
Involvement of a periodontal pathogen, Porphyromonas
gingivalis on the pathogenesis of non-alcoholic fatty liver
disease. BMC Gastroenterol. 12:162012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedman SL: Mechanisms of hepatic
fibrogenesis. Gastroenterology. 134:1655–1669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang DY and Friedman SL:
Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology.
56:769–775. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Murray KF and Carithers RL Jr: AASLD:
AASLD practice guidelines: Evaluation of the patient for liver
transplantation. Hepatology. 41:1407–1432. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Novacek G, Plachetzky U, Pötzi R, Lentner
S, Slavicek R, Gangl A and Ferenci P: Dental and periodontal
disease in patients with cirrhosis - role of etiology of liver
disease. J Hepatol. 22:576–582. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sponholz H, Naethbohm K, Brügmann E and
Maass M: Correlations between chronic liver diseases and
periodontal diseases with special reference to animal experiments.
Stomatol DDR. 26:409–413. 1976.(In German). PubMed/NCBI
|
|
30
|
Movin S: Relationship between periodontal
disease and cirrhosis of the liver in humans. J Clin Periodontol.
8:450–458. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Funatsu K, Yamada M, Kawishima Y, Nishida
J, Ueno M, Ebihara Y, Mizuno Y, Oda M and Tsuchiya M:
Microcirculatory disturbances of oral mucosa and periodontal
disease in patients with liver cirrhosis. J Gastroenterol Hepatol.
4(Suppl 1): 99–102. 1989.PubMed/NCBI
|
|
32
|
Jaiswal G, Deo V, Bhongade M and Jaiswal
S: Serum alkaline phosphatase: A potential marker in the
progression of periodontal disease in cirrhosis patients.
Quintessence Int. 42:345–348. 2011.PubMed/NCBI
|
|
33
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127(Suppl 1): S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anzola M: Hepatocellular carcinoma: Role
of hepatitis B and hepatitis C viruses proteins in
hepatocarcinogenesis. J Viral Hepat. 11:383–393. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fattovich G, Stroffolini T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127(Suppl 1): S35–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bralet MP, Régimbeau JM, Pineau P, Dubois
S, Loas G, Degos F, Valla D, Belghiti J, Degott C and Terris B:
Hepatocellular carcinoma occurring in nonfibrotic liver:
Epidemiologic and histopathologic analysis of 80 French cases.
Hepatology. 32:200–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hujoel PP, Drangsholt M, Spiekerman C and
Weiss NS: An exploration of the periodontitis-cancer association.
Ann Epidemiol. 13:312–316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tezal M, Sullivan MA, Reid ME, Marshall
JR, Hyland A, Loree T, Lillis C, Hauck L, Wactawski-Wende J and
Scannapieco FA: Chronic periodontitis and the risk of tongue
cancer. Arch Otolaryngol Head Neck Surg. 133:450–454. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tezal M, Sullivan MA, Hyland A, Marshall
JR, Stoler D, Reid ME, Loree TR, Rigual NR, Merzianu M, Hauck L, et
al: Chronic periodontitis and the incidence of head and neck
squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev.
18:2406–2412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kudo M, Chung H, Haji S, Osaki Y, Oka H,
Seki T, Kasugai H, Sasaki Y and Matsunaga T: Validation of a new
prognostic staging system for hepatocellular carcinoma: The JIS
score compared with the CLIP score. Hepatology. 40:1396–1405. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo KZ, Itamoto T, Amano H, Oshita A,
Ushitora Y, Tanimoto Y, Ohdan H, Tashiro H and Asahara T:
Comparative study of the Japan Integrated Stage (JIS) and modified
JIS score as a predictor of survival after hepatectomy for
hepatocellular carcinoma. J Gastroenterol. 43:369–377. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pugh RN, Murray-Lyon IM, Dawson JL,
Pietroni MC and Williams R: Transection of the oesophagus for
bleeding oesophageal varices. Br J Surg. 60:646–649. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tamaki N, Takaki A, Tomofuji T, Endo Y,
Kasuyama K, Ekuni D, Yasunaka T, Yamamoto K and Morita M: Stage of
hepatocellular carcinoma is associated with periodontitis. J Clin
Periodontol. 38:1015–1020. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maggs JR, Suddle AR, Aluvihare V and
Heneghan MA: Systematic review: The role of liver transplantation
in the management of hepatocellular carcinoma. Aliment Pharmacol
Ther. 35:1113–1134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Penn I: Hepatic transplantation for
primary and metastatic cancers of the liver. Surgery. 110:726–734;
discussion 734–735. 1991.PubMed/NCBI
|
|
47
|
Pichlmayr R, Weimann A and Ringe B:
Indications for liver transplantation in hepatobiliary malignancy.
Hepatology. 20(1 Pt 2): 33S–40S. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yao FY, Ferrell L, Bass NM, Watson JJ,
Bacchetti P, Venook A, Ascher NL and Roberts JP: Liver
transplantation for hepatocellular carcinoma: Expansion of the
tumor size limits does not adversely impact survival. Hepatology.
33:1394–1403. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guggenheimer J, Eghtesad B and Stock DJ:
Dental management of the (solid) organ transplant patient. Oral
Surg Oral Med Oral Pathol Oral Radiol Endod. 95:383–389. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Guggenheimer J, Mayher D and Eghtesad B: A
survey of dental care protocols among US organ transplant centers.
Clin Transplant. 19:15–18. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guggenheimer J, Eghtesad B, Close JM, Shay
C and Fung JJ: Dental health status of liver transplant candidates.
Liver Transpl. 13:280–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Douglas LR, Douglass JB, Sieck JO and
Smith PJ: Oral management of the patient with end-stage liver
disease and the liver transplant patient. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 86:55–64. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Little JW and Rhodus NL: Dental treatment
of the liver transplant patient. Oral Surg Oral Med Oral Pathol.
73:419–426. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rustemeyer J and Bremerich A: Necessity of
surgical dental foci treatment prior to organ transplantation and
heart valve replacement. Clin Oral Investig. 11:171–174. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Silva Santos PS, Fernandes KS and
Gallottini MH: Assessment and management of oral health in liver
transplant candidates. J Appl Oral Sci. 20:241–245. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Aberg F, Helenius-Hietala J, Meurman J and
Isoniemi H: Association between dental infections and the clinical
course of chronic liver disease. Hepatol Res. 44:349–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Helenius-Hietala J, Meurman JH,
Höckerstedt K, Lindqvist C and Isoniemi H: Effect of the aetiology
and severity of liver disease on oral health and dental treatment
prior to transplantation. Transpl Int. 25:158–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bader G, Mesner M and Lejeune S: Oral
surgery for liver transplant patients. Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 84:5931997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Golla K, Epstein JB and Cabay RJ: Liver
disease: Current perspectives on medical and dental management.
Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 98:516–521. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Valerin MA, Napeñas JJ, Brennan MT, Fox PC
and Lockhart PB: Modified Child-Pugh score as a marker for
postoperative bleeding from invasive dental procedures. Oral Surg
Oral Med Oral Pathol Oral Radiol Endod. 104:56–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ward BB and Weideman EM: Long-term
postoperative bleeding after dentoalveolar surgery in the
pretransplant liver failure patient. J Oral Maxillofac Surg.
64:1469–1474. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lins L, Bittencourt PL, Evangelista MA,
Lins R, Codes L, Cavalcanti AR, Paraná R and Bastos J: Oral health
profile of cirrhotic patients awaiting liver transplantation in the
Brazilian Northeast. Transplant Proc. 43:1319–1321. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sheehy EC, Roberts GJ, Beighton D and
O'Brien G: Oral health in children undergoing liver
transplantation. Int J Paediatr Dent. 10:109–119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Barbero P, Garzino Demo MG, Milanesio M
and Ottobrelli A: The dental assessment of the patient waiting for
a liver transplant. Minerva Stomatol. 45:431–439. 1996.(In
Italian). PubMed/NCBI
|
|
66
|
Kumar PS, Griffen AL, Barton JA, Paster
BJ, Moeschberger ML and Leys EJ: New bacterial species associated
with chronic periodontitis. J Dent Res. 82:338–344. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Kumar PS, Griffen AL, Moeschberger ML and
Leys EJ: Identification of candidate periodontal pathogens and
beneficial species by quantitative 16S clonal analysis. J Clin
Microbiol. 43:3944–3955. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Fernández J and Gustot T: Management of
bacterial infections in cirrhosis. J Hepatol. 56(Suppl 1): S1–S12.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gustot T, Durand F, Lebrec D, Vincent JL
and Moreau R: Severe sepsis in cirrhosis. Hepatology. 50:2022–2033.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fernández J, Navasa M, Gómez J, Colmenero
J, Vila J, Arroyo V and Rodés J: Bacterial infections in cirrhosis:
Epidemiological changes with invasive procedures and norfloxacin
prophylaxis. Hepatology. 35:140–148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fernández J, Acevedo J, Castro M, Garcia
O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, et al:
Prevalence and risk factors of infections by multiresistant
bacteria in cirrhosis: A prospective study. Hepatology.
55:1551–1561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fagiuoli S, Colli A, Bruno R, Craxì A,
Gaeta GB, Grossi P, Mondelli MU, Puoti M, Sagnelli E, Stefani S, et
al: 2011 AISF Single Topic Group: Management of infections pre- and
post-liver transplantation: Report of an AISF consensus conference.
J Hepatol. 60:1075–1089. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yilmaz O: The chronicles of
Porphyromonas gingivalis: The microbium, the human oral
epithelium and their interplay. Microbiology. 154:2897–2903. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Scannapieco FA, Bush RB and Paju S:
Associations between periodontal disease and risk for
atherosclerosis, cardiovascular disease, and stroke. A systematic
review. Ann Periodontol. 8:38–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Beck J, Garcia R, Heiss G, Vokonas PS and
Offenbacher S: Periodontal disease and cardiovascular disease. J
Periodontol. 67(Suppl 10): 1123–1137. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Boggess KA, Madianos PN, Preisser JS,
Moise KJ Jr and Offenbacher S: Chronic maternal and fetal
Porphyromonas gingivalis exposure during pregnancy in
rabbits. Am J Obstet Gynecol. 192:554–557. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Furusho H, Miyauchi M, Hyogo H, Inubushi
T, Ao M, Ouhara K, Hisatune J, Kurihara H, Sugai M, Hayes CN, et
al: Dental infection of Porphyromonas gingivalis exacerbates
high fat diet-induced steatohepatitis in mice. J Gastroenterol.
48:1259–1270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Raja M, Ummer F and Dhivakar CP:
Aggregatibacter actinomycetemcomitans - a tooth killer? J
Clin Diagn Res. 8:ZE13–ZE16. 2014.PubMed/NCBI
|
|
79
|
Narayanan SK, Nagaraja TG, Chengappa MM
and Stewart GC: Leukotoxins of gram-negative bacteria. Vet
Microbiol. 84:337–356. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chen PB, Davern LB, Neiders ME, Reynolds
HS and Zambon JJ: Analysis of in vitro lymphoproliferative
responses and antibody formation following subcutaneous injection
of Actinobacillus actinomycetemcomitans and Wolinella recta
in a murine model. Oral Microbiol Immunol. 6:12–16. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Tuomainen AM, Jauhiainen M, Kovanen PT,
Metso J, Paju S and Pussinen PJ: Aggregatibacter
actinomycetemcomitans induces MMP-9 expression and
proatherogenic lipoprotein profile in apoE-deficient mice. Microb
Pathog. 44:111–117. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hyvärinen K, Tuomainen AM, Laitinen S,
Bykov IL, Törmäkangas L, Lindros K, Käkelä R, Alfthan G, Salminen
I, Jauhiainen M, et al: Chlamydial and periodontal pathogens induce
hepatic inflammation and fatty acid imbalance in apolipoprotein
E-deficient mice. Infect Immun. 77:3442–3449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Qin N, Yang F, Li A, Prifti E, Chen Y,
Shao L, Guo J, Le Chatelier E, Yao J, Wu L, et al: Alterations of
the human gut microbiome in liver cirrhosis. Nature. 513:59–64.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Goel A, Gupta M and Aggarwal R: Gut
microbiota and liver disease. J Gastroenterol Hepatol.
29:1139–1148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yoshimoto S, Loo TM, Atarashi K, Kanda H,
Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et
al: Obesity-induced gut microbial metabolite promotes liver cancer
through senescence secretome. Nature. 499:97–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wiest R, Lawson M and Geuking M:
Pathological bacterial translocation in liver cirrhosis. J Hepatol.
60:197–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
DiBaise JK, Zhang H, Crowell MD,
Krajmalnik-Brown R, Decker GA and Rittmann BE: Gut microbiota and
its possible relationship with obesity. Mayo Clin Proc. 83:460–469.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Socransky SS and Haffajee AD: Periodontal
microbial ecology. Periodontol 2000. 38:135–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Goodson JM, Groppo D, Halem S and Carpino
E: Is obesity an oral bacterial disease? J Dent Res. 88:519–523.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Boutaga K, Savelkoul PH, Winkel EG and van
Winkelhoff AJ: Comparison of subgingival bacterial sampling with
oral lavage for detection and quantification of periodontal
pathogens by real-time polymerase chain reaction. J Periodontol.
78:79–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Saygun I, Nizam N, Keskiner I, Bal V,
Kubar A, Açıkel C, Serdar M and Slots J: Salivary infectious agents
and periodontal disease status. J Periodontal Res. 46:235–239.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
von Troil-Lindén B, Torkko H, Alaluusua S,
Jousimies-Somer H and Asikainen S: Salivary levels of suspected
periodontal pathogens in relation to periodontal status and
treatment. J Dent Res. 74:1789–1795. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Koren O, Spor A, Felin J, Fåk F, Stombaugh
J, Tremaroli V, Behre CJ, Knight R, Fagerberg B, Ley RE, et al:
Human oral, gut, and plaque microbiota in patients with
atherosclerosis. Proc Natl Acad Sci USA. 108(Suppl 1): 4592–4598.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
O'Toole PW and Cooney JC: Probiotic
bacteria influence the composition and function of the intestinal
microbiota. Interdiscip Perspect Infect Dis.
2008:1752852008.PubMed/NCBI
|
|
95
|
Lentsch AB, Yoshidome H, Kato A, Warner
RL, Cheadle WG, Ward PA and Edwards MJ: Requirement for
interleukin-12 in the pathogenesis of warm hepatic
ischemia/reperfusion injury in mice. Hepatology. 30:1448–1453.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Lentsch AB, Yoshidome H, Cheadle WG,
Miller FN and Edwards MJ: Chemokine involvement in hepatic
ischemia/reperfusion injury in mice: Roles for macrophage
inflammatory protein-2 and KC. Hepatology. 27:1172–1177. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Gandoura S, Weiss E, Rautou PE, Fasseu M,
Gustot T, Lemoine F, Hurtado-Nedelec M, Hego C, Vadrot N, Elkrief
L, et al: Gene- and exon-expression profiling reveals an extensive
LPS-induced response in immune cells in patients with cirrhosis. J
Hepatol. 58:936–948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Coant N, Simon-Rudler M, Gustot T, Fasseu
M, Gandoura S, Ragot K, Abdel-Razek W, Thabut D, Lettéron P,
Ogier-Denis E, et al: Glycogen synthase kinase 3 involvement in the
excessive proinflammatory response to LPS in patients with
decompensated cirrhosis. J Hepatol. 55:784–793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Tazi KA, Quioc JJ, Saada V, Bezeaud A,
Lebrec D and Moreau R: Upregulation of TNF-alpha production
signaling pathways in monocytes from patients with advanced
cirrhosis: Possible role of Akt and IRAK-M. J Hepatol. 45:280–289.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Galbois A, Thabut D, Tazi KA, Rudler M,
Mohammadi MS, Bonnefont-Rousselot D, Bennani H, Bezeaud A, Tellier
Z, Guichard C, et al: Ex vivo effects of high-density lipoprotein
exposure on the lipopolysaccharide-induced inflammatory response in
patients with severe cirrhosis. Hepatology. 49:175–184. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Thabut D, Massard J, Gangloff A, Carbonell
N, Francoz C, Nguyen-Khac E, Duhamel C, Lebrec D, Poynard T and
Moreau R: Model for end-stage liver disease score and systemic
inflammatory response are major prognostic factors in patients with
cirrhosis and acute functional renal failure. Hepatology.
46:1872–1882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Byl B, Roucloux I, Crusiaux A, Dupont E
and Devière J: Tumor necrosis factor alpha and interleukin 6 plasma
levels in infected cirrhotic patients. Gastroenterology.
104:1492–1497. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Navasa M, Follo A, Filella X, Jiménez W,
Francitorra A, Planas R, Rimola A, Arroyo V and Rodés J: Tumor
necrosis factor and interleukin-6 in spontaneous bacterial
peritonitis in cirrhosis: Relationship with the development of
renal impairment and mortality. Hepatology. 27:1227–1232. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Devière J, Content J, Denys C,
Vandenbussche P, Schandene L, Wybran J and Dupont E: Excessive in
vitro bacterial lipopolysaccharide-induced production of monokines
in cirrhosis. Hepatology. 11:628–634. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Le Moine O, Marchant A, De Groote D, Azar
C, Goldman M and Devière J: Role of defective monocyte
interleukin-10 release in tumor necrosis factor-alpha
overproduction in alcoholics cirrhosis. Hepatology. 22:1436–1439.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Moreau R, Barrière E, Tazi KA, Lardeux B,
Dargère D, Urbanowicz W, Poirel O, Chauvelot-Moachon L, Guimont MC,
Bernuau D, et al: Terlipressin inhibits in vivo aortic iNOS
expression induced by lipopolysaccharide in rats with biliary
cirrhosis. Hepatology. 36:1070–1078. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tazi KA, Bièche I, Paradis V, Guichard C,
Laurendeau I, Dargère D, Legrand A, Fay M, Pedruzzi E, Robin MA, et
al: In vivo altered unfolded protein response and apoptosis in
livers from lipopolysaccharide-challenged cirrhotic rats. J
Hepatol. 46:1075–1088. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Thabut D, Tazi KA, Bonnefont-Rousselot D,
Aller M, Farges O, Guimont MC, Tellier Z, Guichard C, Ogier-Denis
E, Poynard T, et al: High-density lipoprotein administration
attenuates liver proinflammatory response, restores liver
endothelial nitric oxide synthase activity, and lowers portal
pressure in cirrhotic rats. Hepatology. 46:1893–1906. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Abu-Shanab A and Quigley EM: The role of
the gut microbiota in nonalcoholic fatty liver disease. Nat Rev
Gastroenterol Hepatol. 7:691–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Madianos PN, Bobetsis YA and Kinane DF:
Generation of inflammatory stimuli: How bacteria set up
inflammatory responses in the gingiva. J Clin Periodontol. 32(Suppl
6): 57–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Shapira L, Champagne C, Van Dyke TE and
Amar S: Strain-dependent activation of monocytes and inflammatory
macrophages by lipopolysaccharide of Porphyromonas
gingivalis. Infect Immun. 66:2736–2742. 1998.PubMed/NCBI
|
|
112
|
Hirschfeld M, Weis JJ, Toshchakov V,
Salkowski CA, Cody MJ, Ward DC, Qureshi N, Michalek SM and Vogel
SN: Signaling by toll-like receptor 2 and 4 agonists results in
differential gene expression in murine macrophages. Infect Immun.
69:1477–1482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Malik R, Mookerjee RP and Jalan R:
Infection and inflammation in liver failure: Two sides of the same
coin. J Hepatol. 51:426–429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Tritto G, Bechlis Z, Stadlbauer V, Davies
N, Francés R, Shah N, Mookerjee RP, Such J and Jalan R: Evidence of
neutrophil functional defect despite inflammation in stable
cirrhosis. J Hepatol. 55:574–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tilg H and Moschen AR: IL-1 cytokine
family members and NAFLD: Neglected in metabolic liver
inflammation. J Hepatol. 55:960–962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Muñoz NM, Katz LH, Shina JH, Gi YJ, Menon
VK, Gagea M, Rashid A, Chen J and Mishra L: Generation of a mouse
model of T-cell lymphoma based on chronic LPS challenge and TGF-β
signaling disruption. Genes Cancer. 5:348–352. 2014.PubMed/NCBI
|
|
117
|
Su GL, Klein RD, Aminlari A, Zhang HY,
Steinstraesser L, Alarcon WH, Remick DG and Wang SC: Kupffer cell
activation by lipopolysaccharide in rats: Role for
lipopolysaccharide binding protein and toll-like receptor 4.
Hepatology. 31:932–936. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Baffy G: Kupffer cells in non-alcoholic
fatty liver disease: The emerging view. J Hepatol. 51:212–223.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Honda Y, Yamagiwa S, Matsuda Y, Takamura
M, Ichida T and Aoyagi Y: Altered expression of TLR homolog RP105
on monocytes hypersensitive to LPS in patients with primary biliary
cirrhosis. J Hepatol. 47:404–411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Yang SQ, Lin HZ, Lane MD, Clemens M and
Diehl AM: Obesity increases sensitivity to endotoxin liver injury:
Implications for the pathogenesis of steatohepatitis. Proc Natl
Acad Sci USA. 94:2557–2562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Miura K, Yang L, van Rooijen N, Brenner
DA, Ohnishi H and Seki E: Toll-like receptor 2 and palmitic acid
cooperatively contribute to the development of nonalcoholic
steatohepatitis through inflammasome activation in mice.
Hepatology. 57:577–589. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mattsson E, Verhage L, Rollof J, Fleer A,
Verhoef J and van Dijk H: Peptidoglycan and teichoic acid from
Staphylococcus epidermidis stimulate human monocytes to
release tumour necrosis factor-alpha, interleukin-1 beta and
interleukin-6. FEMS Immunol Med Microbiol. 7:281–287. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Wang ZM, Liu C and Dziarski R: Chemokines
are the main proinflammatory mediators in human monocytes activated
by Staphylococcus aureus, peptidoglycan, and endotoxin. J
Biol Chem. 275:20260–20267. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kengatharan KM, De Kimpe S, Robson C,
Foster SJ and Thiemermann C: Mechanism of gram-positive shock:
Identification of peptidoglycan and lipoteichoic acid moieties
essential in the induction of nitric oxide synthase, shock, and
multiple organ failure. J Exp Med. 188:305–315. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Forner L, Nielsen CH, Bendtzen K, Larsen T
and Holmstrup P: Increased plasma levels of IL-6 in bacteremic
periodontis patients after scaling. J Clin Periodontol. 33:724–729.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Loos BG, Craandijk J, Hoek FJ,
Wertheim-van Dillen PM and van der Velden U: Elevation of systemic
markers related to cardiovascular diseases in the peripheral blood
of periodontitis patients. J Periodontol. 71:1528–1534. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Morré SA, Ouburg S, Klinkenberg-Knol EC,
Mulder CJ and Peña AS: The true ligand of the NOD2 receptor is
peptidoglycan instead of lipopolysaccharide: A schematic
representation of ligand-receptor interactions and NF-kappa B
activation. Gastroenterology. 126:371–373. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lichtman SN, Wang J, Schwab JH and
Lemasters JJ: Comparison of peptidoglycan-polysaccharide and
lipopolysaccharide stimulation of Kupffer cells to produce tumor
necrosis factor and interleukin-1. Hepatology. 19:1013–1022. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Petit MD, Wassenaar A, van der Velden U,
van Eden W and Loos BG: Depressed responsiveness of peripheral
blood mononuclear cells to heat-shock proteins in periodontitis
patients. J Dent Res. 78:1393–1400. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Tabeta K, Yamazaki K, Hotokezaka H, Yoshie
H and Hara K: Elevated humoral immune response to heat shock
protein 60 (hsp60) family in periodontitis patients. Clin Exp
Immunol. 120:285–293. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wick G: Atherosclerosis - an autoimmune
disease due to an immune reaction against heat-shock protein 60.
Herz. 25:87–90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Yamazaki K, Ohsawa Y, Tabeta K, Ito H,
Ueki K, Oda T, Yoshie H and Seymour GJ: Accumulation of human heat
shock protein 60-reactive T cells in the gingival tissues of
periodontitis patients. Infect Immun. 70:2492–2501. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Nencini C, Giorgi G and Micheli L:
Protective effect of silymarin on oxidative stress in rat brain.
Phytomedicine. 14:129–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Reddy BV, Sundari JS, Balamurugan E and
Menon VP: Prevention of nicotine and streptozotocin treatment
induced circulatory oxidative stress by
bis-1,7-(2-hydroxyphenyl)-hepta-1,6-diene-3,5-dione in diabetic
rats. Mol Cell Biochem. 331:127–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tüter G, Kurtiş B and Serdar M:
Interleukin-1beta and thiobarbituric acid reactive substance
(TBARS) levels after phase I periodontal therapy in patients with
chronic periodontitis. J Periodontol. 72:883–888. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY,
Wu YM and Hung CC: Lipid peroxidation: A possible role in the
induction and progression of chronic periodontitis. J Periodontal
Res. 40:378–384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Akalin FA, Baltacioğlu E, Alver A and
Karabulut E: Lipid peroxidation levels and total oxidant status in
serum, saliva and gingival crevicular fluid in patients with
chronic periodontitis. J Clin Periodontol. 34:558–565. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Golz L, Memmert S, Rath-Deschner B, Jager
A, Appel T, Baumgarten G, Götz W and Frede S: LPS from P.
gingivalis and hypoxia increases oxidative stress in
periodontal ligament fibroblasts and contributes to periodontitis.
Mediators Inflamm. 2014:9862642014.PubMed/NCBI
|
|
140
|
Thomas B, Ramesh A, Suresh S and Prasad
BR: A comparative evaluation of antioxidant enzymes and selenium in
the serum of periodontitis patients with diabetes mellitus type 2.
Contemp Clin Dent. 4:176–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tamaki N, Tomofuji T, Ekuni D, Yamanaka R
and Morita M: Periodontal treatment decreases plasma oxidized LDL
level and oxidative stress. Clin Oral Investig. 15:953–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
D'Aiuto F, Nibali L, Parkar M, Patel K,
Suvan J and Donos N: Oxidative stress, systemic inflammation, and
severe periodontitis. J Dent Res. 89:1241–1246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Tamaki N, Tomofuji T, Ekuni D, Yamanaka R,
Yamamoto T and Morita M: Short-term effects of non-surgical
periodontal treatment on plasma level of reactive oxygen
metabolites in patients with chronic periodontitis. J Periodontol.
80:901–906. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chaudhary S, Gowda TM, Mehta DS and Kumar
TAB: Comparative evaluation of plasma ROM levels in chronic
periodontitis patients before and after non-surgical and surgical
periodontal therapy: A clinical trial. J Indian Soc Periodontol.
18:140–144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wei D, Zhang XL, Wang YZ, Yang CX and Chen
G: Lipid peroxidation levels, total oxidant status and superoxide
dismutase in serum, saliva and gingival crevicular fluid in chronic
periodontitis patients before and after periodontal therapy. Aust
Dent J. 55:70–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Zacharakis G, Koskinas J, Kotsiou S, Tzara
F, Vafeiadis N, Papoutselis M, Maltezos E, Sivridis E and
Papoutselis K: The role of serial measurement of serum HBV DNA
levels in patients with chronic HBeAg(−) hepatitis B infection:
Association with liver disease progression. A prospective cohort
study. J Hepatol. 49:884–891. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
van Zonneveld M, Honkoop P, Hansen BE,
Niesters HG, Darwish Murad S, de Man RA, Schalm SW and Janssen HL:
Long-term follow-up of alpha-interferon treatment of patients with
chronic hepatitis B. Hepatology. 39:804–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Alavian SM, Izadi M, Zare AA, Lankarani
MM, Assari S and Vardi MM: Survey of the level of anti-HBs antibody
titer in vaccinated Iranian general dentists. Spec Care Dentist.
28:265–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Mahboobi N, Porter SR, Karayiannis P and
Alavian SM: Dental treatment as a risk factor for hepatitis B and C
viral infection. A review of the recent literature. J
Gastrointestin Liver Dis. 22:79–86. 2013.PubMed/NCBI
|