Introduction
Cognitive impairment is the result of schizophrenia
and influences the quality and function of life (1). Learning and attention are the most
affected aspects of working memory disruptions. Furthermore, an
impaired mental status renders self-care more difficult and results
in increased frequency of hospitalization with significant costs to
society (2).
One of the prevailing hypotheses is that cognitive
impairments are caused by the hypofunction of the
N-methyl-D-aspartate (NMDA) receptor (3). The antagonists of the NMDA receptor, such
as ketamine, MK-801 and phencyclidine, provoke a schizophrenia-like
syndrome in normal subjects, and positive, negative and cognitive
symptoms may be observed (3). In
addition, the NMDA receptor antagonists disrupt learning and cause
memory impairments in animals that have similar psychosis
conditions; therefore, these agents are used as a model to
demonstrate cognitive dysfunctions (4,5).
Currently, various atypical antipsychotic drugs
demonstrate improved efficacy when compared with classical
antipsychotics to treat cognitive dysfunction. Recent clinical
studies indicated that second generation atypical antipsychotic
agents (clozapine, ziprasidone, quetiapine and olanzapine) improve
cognitive impairment, whereas typical agents, such as haloperidol,
exerted no effect (6,7). The cognitive tests of the atypical
antipsychotics in preclinical studies demonstrate normal cognitive
functions, which is in contrast to the classical antipsychotics.
Previous studies reported the influences of the classical
antipsychotics on cognitive functions, with controversial results
(8–10).
The atypical antipsychotic drugs, quetiapine and
aripiprazole are widely administered worldwide; however,
iloperidone is a recently developed atypical antipsychotic drug
that is only administered in the USA. The aim of the current study
was to determine the effects of atypical antipsychotics, such as
quetiapine, aripiprazole and iloperidone, on olfactory memory in
naive and MK-801-treated mice using the social transmission of food
preference (STFP) test.
Materials and methods
Animals
The main subjects of the experiments were 144
female, inbred BALB/cByJ mice (age, 7–8 week; Uludag University,
Bursa, Turkey). They were housed with 8 mice per cage. The mice
were maintained under standardized laboratory conditions (12-h
light/dark cycle, lights on at 7:00 a.m.; temperature, 21±1°C) with
access to food and water ad libitum, for two weeks before
commencing the tests. All procedures were conducted in accordance
with the European Community Council's Directive for the ethical
treatment of animals (Directive 86/609/EEC) and with approval from
the Kocaeli University Medical Faculty (Kocaeli, Turkey; approval
no. 4/3/2015).
Experimental groups and drug
administration
Quetiapine was provided by Santa Farma Ilac
(Istanbul, Turkey). Aripiprazole was purchased from Bal Pharma Ltd.
(Bangalore, India) and iloperidone was purchased from AuSun Gaoba
Pharmaceutical Co., Ltd. (Hebei, China). Quetiapine and
aripiprazole were dissolved in saline plus 1% acetic acid.
Iloperidone was dissolved in saline supplemented with 1% dimethyl
sulfoxide. Quetiapine, aripiprazole and iloperidone were
administered at a dose of 0.01 ml/g. Three control groups (n=8 per
group) received the same volume of excipients. The animals were
grouped randomly (n=8 per group) and treated intraperitoneally with
the following: Quetiapine (5 and 10 mg/kg) for 60 min; aripiprazole
(3 and 6 mg/kg) for 30 min; iloperidone (0.5 and 1 mg/kg) for 60
min or MK-801 (0.1 mg/kg) for 30 min alone or concurrently with
quetiapine, aripiprazole and iloperidone, before the retention
session of the STFP test. The effective doses of each drug were
administered according to previous behavioral and neurochemical
studies (11).
STFP test
The STFP test evaluates the hippocampus-dependent
non-spatial olfactory memory (12).
According to the test design, the scent of food, which was smelled
on the muzzle of a demonstrator mouse 24 h before, is remembered by
the observer mice with normal olfactory memory who will eat a
greater proportion of the familiar cued food compared with a novel
food.
The experiment was designed in three phases as
follows: i) Flavored food habituation; ii) interaction between
‘demonstrator’ and ‘observer’ mice; and iii) test of the food
preference in the ‘observer’ mice (12). Mice were kept at a ratio of 3–4
observer mice to 1 demonstrator mouse. In the habituation phase, a
demonstrator mouse was selected from each cage. Demonstrators were
housed alone in a separate cage for 3 h, with access to water ad
libitum, but restricted food consumption. After 3 h of food
restriction, the demonstrators were allowed to consume the ground
chow scented with either cinnamon (1% w/w) or cocoa (2% w/w)
flavored powder (12). Half of the
demonstrators received cocoa-flavored food and the other half
received cinnamon-flavored food. The demonstrators were allowed to
eat the flavored food for 2 h. The pellets were weighed before and
after being given to the demonstrators. The inclusion criterion was
the consumption of at least 0.2 g. The demonstrators were housed
with their observer mates for flavor interaction for 30 min
(12). Subsequent to the interaction
period, the demonstrator mouse was removed from the interaction
cage and placed in its individual cage.
In the final phase of the experiment, the 24-h food
preference of the observer mice was evaluated following the
interaction with the demonstrator. Five hours prior to the test,
the observer mice were caged individually with free access to water
and a pair of food pellets. One pellet contained the flavor of food
eaten by the demonstrator (cued) and the other contained the novel
flavor (novel). Therefore, half of the observers were tested with
the cinnamon-flavored cued food eaten by their demonstrator versus
the novel cocoa-flavored food; the other half of the observers were
tested with the cocoa flavored cued food eaten by their
demonstrator vs. the novel cinnamon-flavored food (12). After 2 h, all of the food pellets were
removed and weighed to quantify the food consumption of the
observer mice at 3 h prior to the preference test. The weight ratio
of the eaten cued food and the total eaten food were determined as
a measure of food preference (12).
Statistical analysis
IBM SPSS Statistics 22.0 (IBM SPSS, Armonk, NY, USA)
was used for statistical analysis. A two-way variance analysis and
post hoc Tukey test were used to analyze the cued food/total food
eaten percentage during the STFP test. The associated data are
expressed as the mean values ± standard error of the mean and
P<0.05 was considered to indicate a statistically significant
difference.
Results
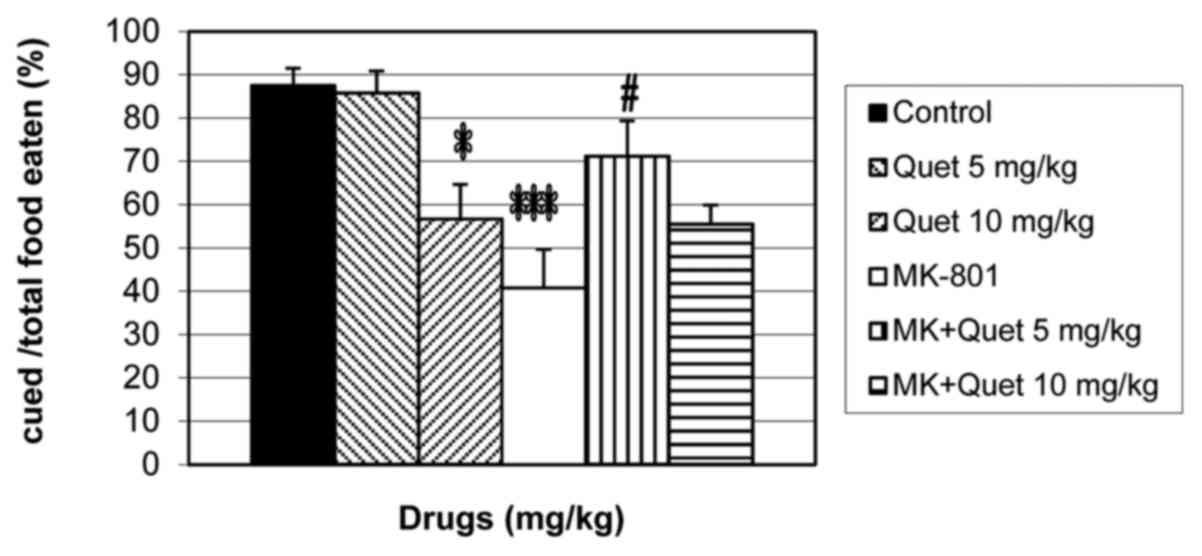
Effects of quetiapine on olfactory
memory in naive and MK-801-treated mice during the STFP test
When quetiapine (5 and 10 mg/kg) and MK-801 (0.1
mg/kg) were administered alone or concurrently before the retention
session of the STFP test, the percentage of cued food/total food
eaten was significantly different [F(5,42)=7.53; P<0.0001;
Fig. 1]. Treatment with quetiapine (10
mg/kg; P<0.05) and MK-801 (P<0.001) significantly decreased
the percentage of cued food/total food eaten when compared with the
control group (Fig. 1). Treatment with
quetiapine (5 mg/kg; P<0.05) significantly increased the
percentage of cued food/total food eaten when compared with the
MK-801 alone group; however, quetiapine (10 mg/kg) treatment
resulted in no significant effect (Fig.
1).
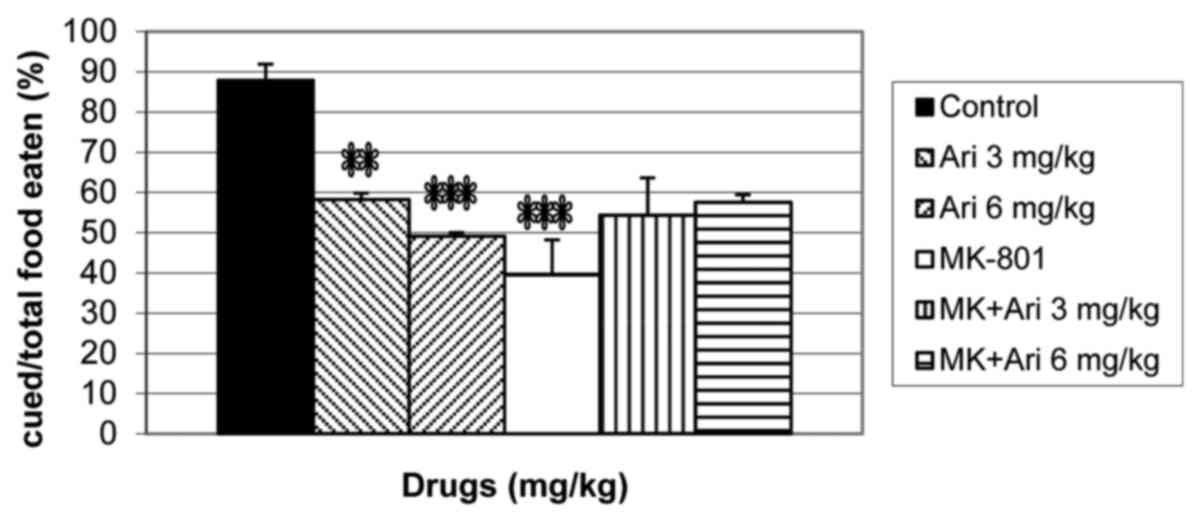
Effects of aripiprazole on olfactory
memory in naive and MK-801-treated mice during the STFP test
When aripiprazole (3 and 6 mg/kg) and MK-801 (0.1
mg/kg) were administered alone or concurrently before the retention
session of the STFP test, a significant difference was observed
among the groups for the percentage of cued food/total food eaten
[F(5,42)=8.61; P<0.0001; Fig. 2].
Aripiprazole (3 and 6 mg/kg; P<0.01 and P<0.001,
respectively) and MK-801 (P<0.001) treatment significantly
decreased the percentage of cued food/total food eaten compared
with the control group (Fig. 2).
Aripiprazole (3 and 6 mg/kg) treatment partially increased the
percentage of cued food/total food eaten compared with the MK-801
alone group, although the effect was not significant (Fig. 2).
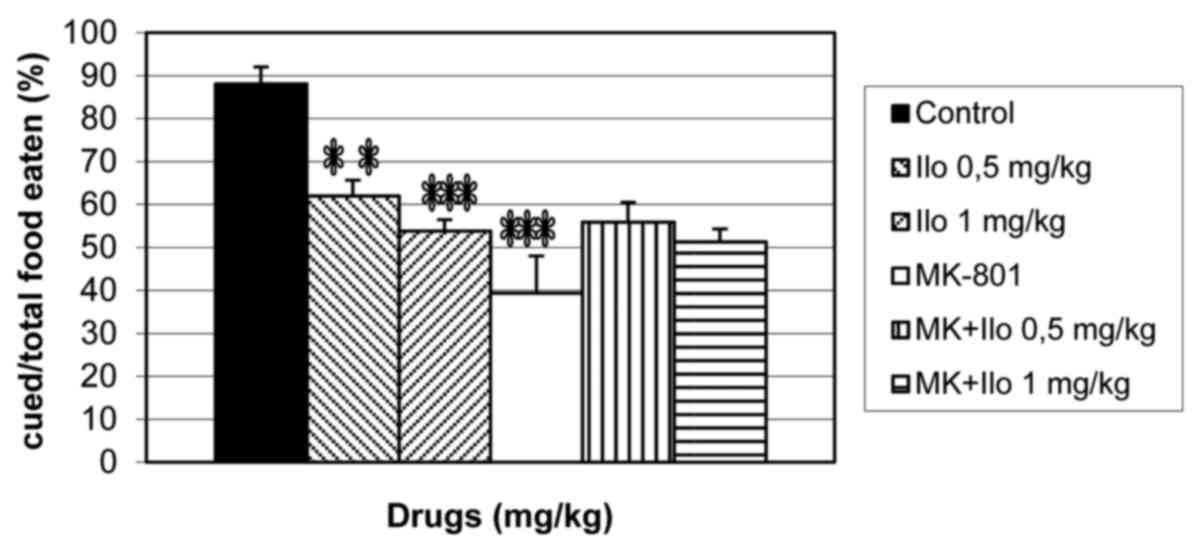
Effects of iloperidone on olfactory
memory in naive and MK-801-treated mice during the STFP test
When iloperidone (0.5 and 1 mg/kg) and MK-801 (0.1
mg/kg) were administered alone or concurrently before the retention
session of the STFP test, a significant difference among groups for
the percentage of cued food/total food eaten was demonstrated [F
(5,42)=11.31; P<0.0001; Fig. 3].
Treatment with iloperidone (0.5 and 1 mg/kg; P<0.01 and
P<0.001, respectively) and MK-801 (P<0.001) significantly
decreased the percentage of cued food/total food eaten when
compared with the control group (Fig.
3). Iloperidone (0.5 and 1 mg/kg) treatment partially increased
the percentage of cued food/total food eaten compared with the
MK-801 alone group although the effect was not significant
(Fig. 3).
Discussion
In the current study, treatment with quetiapine (10
mg/kg), aripiprazole (3 and 6 mg/kg), iloperidone (0.5 and 1 mg/kg)
and MK-801 (0.1 mg/kg) decreased the percentage of cued/total food
eaten during the STFP test. Quetiapine (5 mg/kg) significantly
increased the MK-801-induced decreases in the percentage of
cued/total food eaten; however, aripiprazole and iloperidone
treatment exerted no significant effects.
The effects of antipsychotics on learning and memory
remain controversial. Although haloperidol and risperidone are
strongly associated with cognition impairment from the effective
dosage that is required to treat psychosis, clozapine and
sertindole efficiently treat psychosis without negative effects on
cognition (6,9,10).
Skarsfeldt (10) reported the impaired
performance of rats in a water maze associated with haloperidol
usage (10) and in a delayed
non-matching to position test (8).
According to previous studies, atypical antipsychotics offered
greater efficacy for improving cognitive function when compared
with typical antipsychotics; however, certain studies reported
controversial effects from associated drugs (13–15). For
example, administration of atypical antipsychotics, such as
clozapine and olanzapine, more effectively attenuates the cognitive
deficits in schizophrenic patients when compared with haloperidol
administration (16). In the present
study, the atypical antipsychotic drug quetiapine improved
MK-801-induced olfactory memory impairment; however, aripiprazole
and iloperidone administration exerted no effects.
Quetiapine is a dopamine, serotonin and adrenergic
antagonist, and is a potent antihistamine with clinically
negligible anticholinergic properties. Aripiprazole is an atypical
antipsychotic with a novel pharmacological profile that acts as a
partial agonist of dopamine D2 and D3 and
serotonin (5-HT) 5-HT1A receptors, and as an antagonist
for 5-HT2A receptors (17,18).
Aripiprazole has moderate affinity for histamine, α-adrenergic, and
D4 receptors, as well as the serotonin transporter;
however, it has no appreciable affinity for cholinergic muscarinic
receptors (17,18). Iloperidone is an antagonist of
serotonin, D2, D3, D4,
5-HT6, and noradrenaline α1 receptors and has low
affinity for the serotonin 5-HT1A, dopamine
D1 and histamine H1 receptors. The effects of
quetiapine, aripiprazole and iloperidone that are associated with
these receptors modify the positive and negative symptoms of
schizophrenia.
Antipsychotic drugs that target the D2
receptors may treat the positive symptoms of schizophrenia while
modification on non-D2 receptors (D1,
D3 and D4), serotonin receptors
(5-HT2A, 5-HT1A, 5-HT3,
5-HT6 and 5-HT7) and α-adrenergic receptors
affect the negative symptoms of schizophrenia (19,20). The
impact on particular receptors is important for the reversal of
MK-801-induced cognitive impairment by quetiapine.
The 5-HT2A receptor regulates
mesocortical dopamine projections. The atypical antipsychotic drugs
diminish the cognitive impairments in schizophrenic patients. The
underlying mechanism is the blockage of 5-HT2A receptors
within the prefrontal cortex and enhancement of dopamine
transmission. The success on controlling the negative symptoms of
schizophrenia is correlated with the enhanced affinity on
5-HT2A/dopamine D2 receptors; therefore, the
reported interaction may be valuable for relieving cognitive
deficits (21).
Different NMDA receptor antagonist-induced
experimental models have been investigated to demonstrate the
cognitive impairments, and selective ligands for serotonin and
adrenoceptors have been examined in these models (22,23). In
addition, post-training administration of the specific 5-HT7
receptor antagonists, SB-269970 and DR-4004, improved
MK-801-induced memory impairments in rat auto-shaping tasks
(22). Quetiapine reversed
MK-801-induced deficits in the current study and this may be linked
via the interactions between serotonin receptors and
adrenoceptors.
The antihistaminic and anticholinergic effects of
drugs also result in changes in cognitive performance. Increased
histamine occupancy on the receptors may disturb cognitive
performance. The olfactory memory disturbing effects of quetiapine,
aripiprazole and iloperidone in naive mice may be associated with
their antihistaminergic and anticholinergic activities. The present
study identified the beneficial effects of quetiapine
administration at a low dose (5 mg/kg); however, this activity
disappeared at a high dose (10 mg/kg). This may be due to certain
non-specific effects of quetiapine on motor activity and total food
consumption at the high dose.
In conclusion, quetiapine, aripiprazole and
iloperidone disturbed olfactory memory in naive mice; however, only
quetiapine reversed MK-801-induced memory impairment during the
STFP test. This study demonstrates the superior effect of
quetiapine administration, compared with aripiprazole and
iloperidone, for cognitive dysfunctions of schizophrenic patients.
The superior effects of quetiapine compared with aripiprazole and
iloperidone in the present study may be associated with the
different features of olfactory memory, drug doses, administration
time, strain and gender differences of the animals used in the
current study. Further studies with different doses and learning
tasks are required to determine the underlying mechanisms of these
drugs.
References
|
1
|
Krivoy A, Fischel T and Weizman A: The
cognitive deficit in schizophrenia. Harefuah. 151:277–280, 319.
2012.(In Hebrew). PubMed/NCBI
|
|
2
|
McGurk SR, Mueser KT, Covell NH, Cicerone
KD, Drake RE, Silverstein SM, Medialia A, Myers R, Bellack AS, Bell
MD, et al: Mental health system funding of cognitive enhancement
interventions for schizophrenia: Summary and update of the New York
Office of Mental Health expert panel and stakeholder meeting.
Psychiatr Rehabil J. 36:133–145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SY, Lee H, Kim HJ, Bang E, Lee SH, Lee
DW, Woo DC, Choi CB, Hong KS, Lee C, et al: In vivo and ex vivo
evidence for ketamine-induced hyperglutamatergic activity in the
cerebral cortex of the rat: Potential relevance to schizophrenia.
NMR Biomed. 24:1235–1242. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zimnisky R, Chang G, Gyertyán I, Kiss B,
Adham N and Schmauss C: Cariprazine, a dopamine
D(3)-receptor-preferring partial agonist, blocks
phencyclidine-induced impairments of working memory, attention
set-shifting, and recognition memory in the mouse.
Psychopharmacology (Berl). 226:91–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ihalainen J, Savolainen K, Tanila H and
Forsberg MM: Comparison of phencyclidine-induced spatial learning
and memory deficits and reversal by sertindole and risperidone
between Lister Hooded and Wistar rats. Behav Brain Res.
305:140–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gopalakrishna G, Ithman MH and Lauriello
J: Update on New and Emerging Treatments for Schizophrenia.
Psychiatr Clin North Am. 39:217–238. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amr M, Lakhan SE, Sanhan S, Al-Rhaddad D,
Hassan M, Thiabh M and Shams T: Efficacy and tolerability of
quetiapine versus haloperidol in first-episode schizophrenia: A
randomized clinical trial. Int Arch Med. 6:472013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tuplin EW, Stocco MR and Holahan MR:
Attenuation of MK-801-induced behavioral perseveration by typical
and atypical antipsychotic pretreatment in rats. Behav Neurosci.
129:399–411. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mutlu O, Ulak G, Celikyurt IK, Akar FY,
Erden F and Tanyeri P: Effects of olanzapine, sertindole and
clozapine on MK-801 induced visual memory deficits in mice.
Pharmacol Biochem Behav. 99:557–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Skarsfeldt T: Differential effect of
antipsychotics on place navigation of rats in the Morris water
maze. A comparative study between novel and reference
antipsychotics. Psychopharmacology (Berl). 124:126–133. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bourin M, Chenu F, Prica C and Hascoët M:
Augmentation effect of combination therapy of aripiprazole and
antidepressants on forced swimming test in mice. Psychopharmacology
(Berl). 206:97–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bunsey M and Eichenbaum H: Selective
damage to the hippocampal region blocks long-term retention of a
natural and nonspatial stimulus-stimulus association. Hippocampus.
5:546–556. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lally J and MacCabe JH: Antipsychotic
medication in schizophrenia: A review. Br Med Bull. 114:169–179.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumari V, Ettinger U, Lee SE, Deuschl C,
Anilkumar AP, Schmechtig A, Corr PJ, Ffytche DH and Williams SC:
Common and distinct neural effects of risperidone and olanzapine
during procedural learning in schizophrenia: A randomised
longitudinal fMRI study. Psychopharmacology (Berl). 232:3135–3147.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mutlu O, Ulak G, Celikyurt IK, Akar FY and
Erden F: Effects of olanzapine, sertindole and clozapine on
learning and memory in the Morris water maze test in naive and
MK-801-treated mice. Pharmacol Biochem Behav. 98:398–404. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buoli M, Kahn RS, Serati M, Altamura AC
and Cahn W: Haloperidol versus second-generation antipsychotics in
the long-term treatment of schizophrenia. Hum Psychopharmacol.
31:325–331. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tadori Y, Forbes RA, McQuade RD and
Kikuchi T: In vitro pharmacology of aripiprazole, its metabolite
and experimental dopamine partial agonists at human dopamine D2 and
D3 receptors. Eur J Pharmacol. 668:355–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Topolov MK and Getova DP: Cognitive
Impairment in Schizophrenia, Neurotransmitters and the New Atypical
Antipsychotic Aripiprazole. Folia Med (Plovdiv). 58:12–18.
2016.PubMed/NCBI
|
|
19
|
de Bartolomeis A, Buonaguro EF and
Iasevoli F: Serotonin-glutamate and serotonin-dopamine reciprocal
interactions as putative molecular targets for novel antipsychotic
treatments: From receptor heterodimers to postsynaptic scaffolding
and effector proteins. Psychopharmacology (Berl). 225:1–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eggers AE: A serotonin hypothesis of
schizophrenia. Med Hypotheses. 80:791–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dall'Olio R, Gandolfi O and Gaggi R:
Blockade of the serotonergic system counteracts the
dizocilpine-induced changes in dopaminergic function. Behav
Pharmacol. 11:29–36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meltzer HY, Rajagopal L, Huang M, Oyamada
Y, Kwon S and Horiguchi M: Translating the N-methyl-D-aspartate
receptor antagonist model of schizophrenia to treatments for
cognitive impairment in schizophrenia. Int J Neuropsychopharmacol.
16:2181–2194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Talpos J, Aerts N, Waddell J and Steckler
T: MK-801 and amphetamine result in dissociable profiles of
cognitive impairment in a rodent paired associates learning task
with relevance for schizophrenia. Psychopharmacology (Berl).
232:3911–3920. 2015. View Article : Google Scholar : PubMed/NCBI
|