Introduction
The gold standard in pathology used to this day is
slide analysis through optical microscopy, which was invented in
the 17th century (1). Advancements in
technology have enabled constant improvements in resolution and
photography. The first electromagnetic lens was developed by Hans
Busch in the late 1920s (2), and
similar to optical microscopy, it too experienced major
developments as technology increased (3). Amongst the technological advancements of
the mid 1980s, an important one was digital microscopy. However, it
was not until the late 1990s (when personal computers finally
became powerful enough to process and store the large quantities of
information derived from glass slides) that digital microscopy
became reliable to be used in medical practice and research
worldwide (4–7).
With additional advancements, this technology became
so trustworthy that scientific methods using informatics are now
standard procedure (8). This is
particularly true for contemporary trends, such as the construction
of three-dimensional tissue models and in vivo imaging, for
which optical microscopes were replaced by digital imaging
(9,10).
Scanners nowadays are able to create a digital image of the entire
slide, which can then be viewed virtually on smartphones, tablets
and laptops (11,12). The possibility of analyzing data
on-the-go, or even on the other side of the planet, is a marked
benefit of digital imaging and virtual microscopy (13). In this context, open-source software
programs were developed featuring data availability, collaboration
and providing more transparency in research, as researchers are
able to assess the analysis of others more freely.
While the use of digital imaging has also
contributed to the re-analysis of old biopsies, confirming or
disproving diagnoses in clinical practice (9,14), digital
imaging also benefits the teaching process (15). The image of a single scanned slide may
be distributed to numerous students who may have access either in
the same room or remotely. Thus, for teaching purposes, digital and
virtual microscopy replace the box containing glass slides and the
textbook images. Rather than having multiple boxes with similar
tissue sections, virtual microscopy allows the creation of a more
extensive library and the evaluation of numerous different cases.
However, handling a microscope continues to be an important skill
and this experience should be offered to students when possible
(4,16).
One of the early important drawbacks of digital
microscopy was the elevated cost of the equipment (17), limiting this method to larger research
labs, particularly in underdeveloped countries. In recent years,
however, the costs have rapidly reduced (11). A potential disadvantage of digital and
virtual slides is the file size, which can be particularly large,
thus requiring better and more expensive computers for optimal
performance and transmission over the internet (4,5,11).
As prices have reduced in recent years and computers
deal better with large size files, our graduate program (at the
Federal Fluminense University, Niterói, Brazil) applied for and
received a grant to purchase a slide scanner in 2014. Although this
was a great achievement, the students and faculty were skeptical
about its reliability when compared with traditional microscopy.
This skepticism is also apparent in developed countries, although
digital microscopy usage has been growing steadily in clinical
practice (18). Thus, in the present
study analysis of the duodenum of mice with normal and inflamed
guts was compared when using traditional and digital
microscopy.
Materials and methods
Tissue samples
A mouse model of gut inflammation developed by
Teixeira et al (19) was used
in the present study. Briefly, adult male C57BL/6 mice bred in the
local animal facility of Federal Fluminense University (Rio de
Janeiro, Brazil) were given free access to food and water. They
were 2-months-old and weighed ~25 g at the beginning of the
experiment. The animals were immunized subcutaneously twice (with a
21 day interval) with 100 µg specific protein with (primary) or
without (booster) adjuvant, 1 mg aluminum hydroxide
[Al(OH)3]. Subsequent to immunization, the animals
received a challenge diet containing only the allergenic protein
for 30 days. They were maintained at the university's bioterium
(temperature of 22°C, ~60% humidity and 12 h light/12 h dark cycle)
and the number of animals per cage varied from 4–6. This study was
approved by the Ethical Committee of the Federal Fluminense
University (Niterói, Brazil).
Tissue preparation
The experiment was terminated after the challenge
diet period, with an overdose of anesthetics, totaling 200 µl per
animal (100 µl of xylazine + 100 µl of ketamine, concentrations at
60 mg/kg and 350 mg/kg, respectively, produced by Sespo
Industries®, Paulinia, Sao Paulo, Brazil). After examining the
peritoneal cavity, a 2 cm segment of the gastro-duodenum junction
was collected for histopathology. These were fixed with 10%
buffered formaldehyde, and stained for 5 min with hematoxylin and
for 3 min with eosin at 23°C.
Tissue analyses
All microscopic analyses were performed using
optical and digital microscopy. For optical microscopy (OM), an
Olympus BX41 with magnifications of ×480 (40X objective + 12X
ocular) and ×1,200 (100X objective + 12X ocular) was used. A
reticle with a 100 µm ruler was placed in the eyepiece such that
the image of the ruler was imposed onto the tissue sample. For the
digital microscopy (DM), the slides were scanned using the APERIO
ScanScope CS System® with a 20X objective lens. To evaluate the
histological parameters, the ImageScope® software (v11.2.0.780;
Leica Microsystems GmbH, Wetzlar, Germany) tools were used with a
7.2 digital zoom for cell counting. The evaluated parameters were
as follows: Integrity of the intestinal structure, number of villi
per field (OM) and per 4,000 µm tissue (DM); villi height, width
and area; number of intestinal epithelial cells (IECs) and number
of intraepithelial leukocytes (IELs). The software calculated the
zoom and placed the ruler automatically.
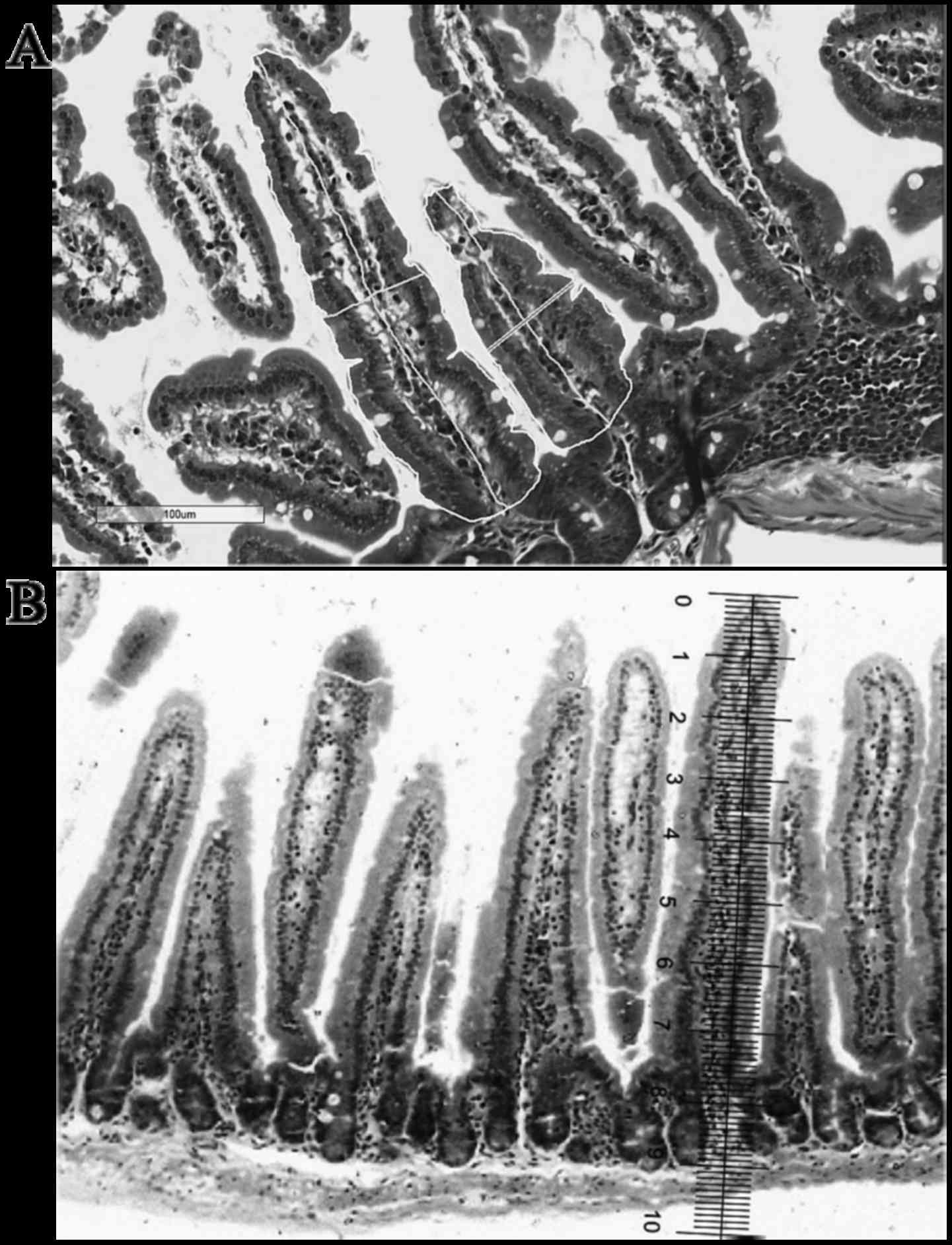
Villi height, width and area
For OM analyses the height and width of all villi in
the chosen field were measured. The width was measured
approximately in the middle of the villus height. The villi area
was obtained later by multiplying the height with the width of each
villus.
For DM analyses the height and width of all villi in
the 4,000 µm of chosen tissue were measured using a touch pen and a
trackpad (Apple Inc., Cupertino, CA, USA). To calculate the area,
the edge of each villus was traced using a touch pen and a trackpad
(Fig. 1).
IEC and IEL counts
For OM, IEC and IEL counts were performed with a
100X immersion lens (magnification, ×1,200), and immersion oil (1
ml; Newprov, Pinhais, Brazil) was placed on each slide. For DM, an
iPhone 6 (Apple Inc.) application, Touch Counter® (v1.0; Nexbrain,
Seoul, Korea) was used to count IECs and IELs (optical
magnification of the scanner, 20x plus digital magnification in the
software, 20x).
Statistical analysis
Data are expressed as means ± standard deviation. A
two-way analysis of variance with Bonferroni post hoc test was used
to determine the minimum significant difference using Graphpad
Prism 6 Software (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference. All analyses were performed with a minimum of five
animals per cage.
Results
Villi number
No significant differences were observed in villi
number between either method of analysis (data not shown).
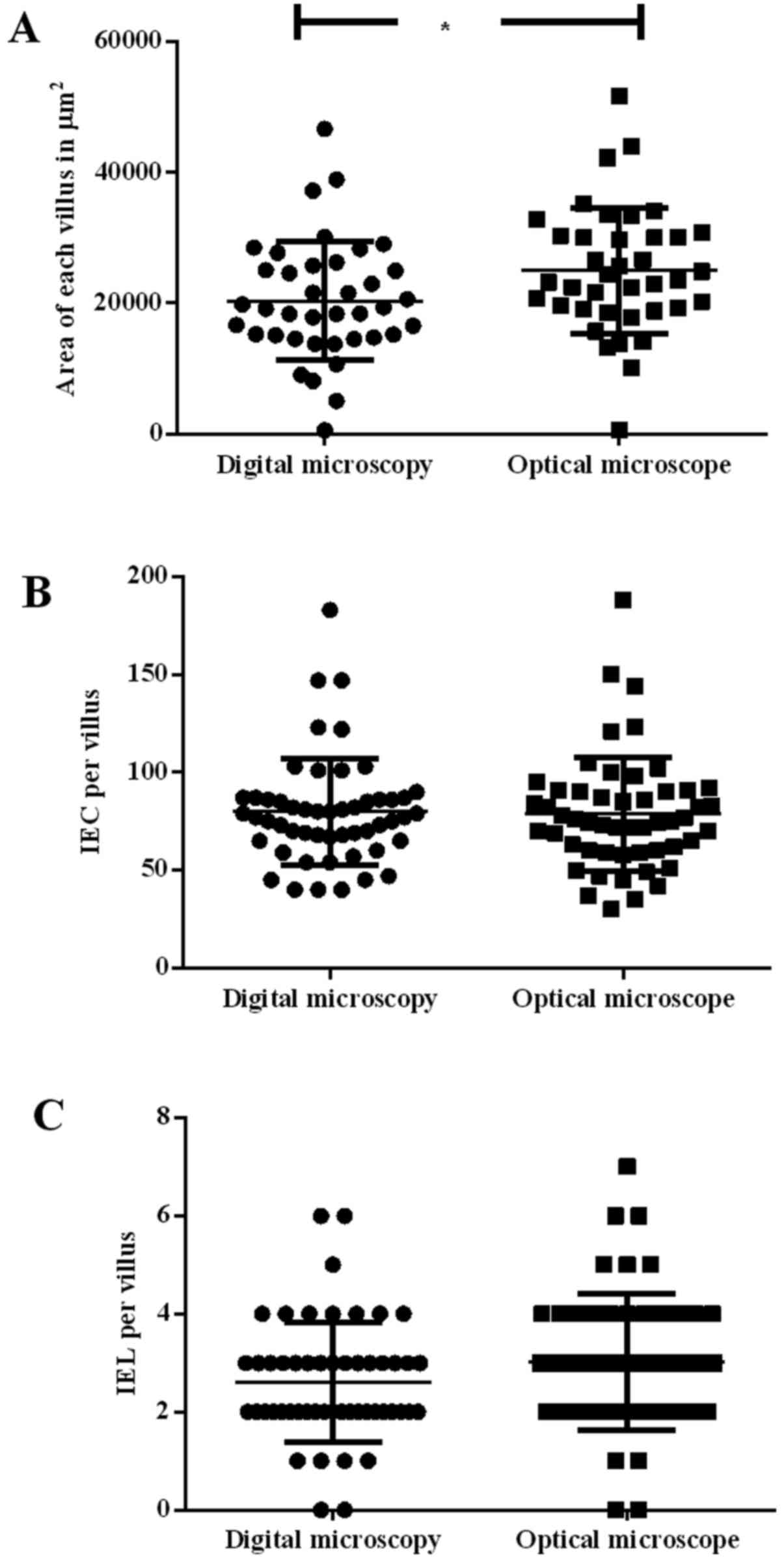
Villi area
It was observed that the measurements of villi area
using DM (20,391.87±9,064.49 µm2) were significantly
smaller (P<0.05) than those measured by OM (24,996.54±9,620.67
µm2; Figs. 1A and 2A).
IECs and IELs
As shown in Fig. 2B and
C, no significant differences were observed between the two
methods, although counting using DM demonstrated a smaller
deviation when compared with OM (DM, 79.90±27.34 vs. OM,
78.71±29.18) and (DM, 2.62±1.22 vs. OM, 3.06±1.32). Thus, no
difference was observed for the ratio of IEC to IEL.
Discussion
The primary strategies used to diagnose a food
allergy are evaluating clinical history and performing physical
examination, whereas the definitive method is to submit the patient
to an oral challenge with the suspected food and assess the
allergic reactions at an organ level (20,21). For
researchers, the state of the organs is particularly interesting to
enable investigation of the pathological mechanisms of the disease
and the development of novel treatment methods (21,22).
In the present study, we analyzed two methods of
analysis (OM and DM) of the inflammatory status of the duodenum of
mice submitted to a food allergy induction protocol was compared.
Previous studies from our group reported the inflammatory milieu of
the gut of allergic animals submitted to oral challenge, as well as
the normal millieu of the gut of allergic animals that were not
submitted to oral challenge (23,24). The
analysis performed in these papers used either OM or DM; however,
no comparison was performed. Historically, OM is considered to be
the gold standard by many of the students and professors in the
authors' department (Department of Immunobiology, Federal
Fluminense University, Rio de Janeiro, Brazil) and in other
universities in Brazil. Thus, all analyses were performed using
this method. With the acquisition of a slide scanner by the Federal
Fluminense University and the use of digital analysis software, a
comparison between the two methods was considered to be mandatory.
Thus, the present study reveals that digital imaging analysis a
reliable method, and it a novel standard was established, as it is
very accurate in determining the staging of the inflammatory state
of the gut. As soon as slides have been digitalized, analyses may
be performed wherever there is access to a good computer and/or
internet. It is important to mention, however, that both methods
(OM and DM) offer different perspectives to the user, therefore one
is not better than the others. They are all complimentary to each
other, allowing students and researchers to learn and discover
novel methods of analysis.
The results described in the present study indicate
that the two methods are largely equivalent, as the majority of the
results were not statistically different. The most important
disadvantages of OM are the lack of reproducibility due to operator
bias (25,26) and the requirement of more than one high
image quality microscope in the lab. However, Dee (5) identified numerous advantages of virtual
microscopy over traditional microscopy, including accessibility,
costs, and efficiency, amongst others.
The only result that demonstrated a statistically
significant difference was the measurement of villi area, which may
be explained by the operator bias involved in OM (14), as stated by Fisher and Parsons
(27) when he compared his study to
that of others (28). Another reason
is that villi do not have regular borders, thus the fact that the
villi (including the indents) are delimited with the pen explains
the observed difference and deviation. However, the small
differences between the methods of analysis did not impact upon the
diagnosis of gut inflammation when compared with the inflammatory
status of the gut, which was established using the inflammation
score developed by Marsh (29) and
modified later by Oberhuber et al (30,31).
In conclusion, in our mouse model the usage of
either method of intestine analysis did not differ significantly
from each other, thus showing that digital imaging is a trustworthy
method for study purposes.
Acknowledgements
The authors would like to thank Professor Maira
Platais for the English review and the grant provided by FAPERJ
(Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio
de Janeiro - ‘Carlos Chagas Filho Foundation for Supporting of
Research of the State of Rio de Janeiro’) (grant no.
E-26/110/409/2011) and the doctorate fellowship provided by CNPq
(Conselho Nacional de Desenvolvimento Científico e Tecnológico -
‘National Council for Scientific and Tecnological
Development’).
References
|
1
|
Boyce BF: Whole slide imaging: Uses and
limitations for surgical pathology and teaching. Biotech Histochem.
90:321–330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bogner A, Jouneau PH, Thollet G, Basset D
and Gauthier C: A history of scanning electron microscopy
developments: Towards ‘wet-STEM’ imaging. Micron. 38:390–401. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hawkes PW: The correction of electron lens
aberrations. Ultramicroscopy. 156:A1–A64. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Banavar SR, Chippagiri P, Pandurangappa R,
Annavajjula S and Rajashekaraiah PB: Image montaging for creating a
virtual pathology slide: An innovative and economical tool to
obtain a whole slide image. Anal Cell Pathol (Amst).
2016:90849092016.PubMed/NCBI
|
|
5
|
Dee FR: Virtual microscopy in pathology
education. Hum Pathol. 40:1112–1121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Silage DA and Gil J: Digital image tiles:
A method for the processing of large sections. J Microsc.
138:221–227. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Westerkamp D and Gahm T: Non-distorted
assemblage of the digital images of adjacent fields in histological
sections. Anal Cell Pathol. 5:235–247. 1993.PubMed/NCBI
|
|
8
|
Furness PN: The use of digital images in
pathology. J Pathol. 183:253–263. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arena ET, Rueden CT, Hiner MC, Wang S,
Yuan M and Eliceiri KW: Quantitating the cell: Turning images into
numbers with ImageJ. Wiley Interdiscip Rev Dev Biol.
2016.PubMed/NCBI
|
|
10
|
Entenberg D, Rodriguez-Tirado C, Kato Y,
Kitamura T, Pollard JW and Condeelis J: In vivo subcellular
resolution optical imaging in the lung reveals early metastatic
proliferation and motility. Intravital. 4:1–11. 2015. View Article : Google Scholar
|
|
11
|
Indu M, Rathy R and Binu MP: ‘Slide less
pathology’: Fairy tale or reality? J Oral Maxillofac Pathol.
20:284–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pantanowitz L, Valenstein PN, Evans AJ,
Kaplan KJ, Pfeifer JD, Wilbur DC, Collins LC and Colgan TJ: Review
of the current state of whole slide imaging in pathology. J Pathol
Inform. 2:362011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
García-Rojo M, Sánchez J, de la Santa E,
Durán E, Ruiz JL, Silva A, Rubio FJ, Rodríguez AM, Meléndez B,
González L, et al: Automated image analysis in the study of
lymphocyte subpopulation in eosinophilic oesophagitis. Diagn
Pathol. 9:S72014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Higgins C: Applications and challenges of
digital pathology and whole slide imaging. Biotech Histochem.
90:341–347. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rocha R, Vassallo J, Soares F, Miller K
and Gobbi H: Digital slides: Present status of a tool for
consultation, teaching, and quality control in pathology. Pathol
Res Pract. 205:735–741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamilton PW, Wang Y and McCullough SJ:
Virtual microscopy and digital pathology in training and education.
APMIS. 120:305–315. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camparo P, Egevad L, Algaba F, Berney DM,
Boccon-Gibod L, Compérat E, Evans AJ, Grobholz R, Kristiansen G,
Langner C, et al: Utility of whole slide imaging and virtual
microscopy in prostate pathology. APMIS. 120:298–304. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lundström C, Thorstenson S, Waltersson M,
Persson A and Treanor D: Summary of 2(nd) Nordic symposium on
digital pathology. J Pathol Inform. 6:52015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Teixeira G, Paschoal PO, de Oliveira VL,
Pedruzzi MM, Campos SM, Andrade L and Nóbrega A: Diet selection in
immunologically manipulated mice. Immunobiology. 213:1–12. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu W, Freeland DMH and Nadeau KC: Food
allergy: Immune mechanisms, diagnosis and immunotherapy. Nat Rev
Immunol. 16:751–765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dogan S, Astvatsatourov A, Deserno TM,
Bock F, Shah-Hosseini K, Michels A and Mösges R: Objectifying the
conjunctival provocation test: Photography-based rating and digital
analysis. Int Arch Allergy Immunol. 163:59–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bachert C, Borchard U, Wedi B, Klimek L,
Rasp G, Riechelmann H, Schultze-Werninghaus G, Wahn U and Ring J:
Allergic rhinoconjunctivitis. Guidelines of the DGAI in association
with the DDG. J Dtsch Dermatol Ges. 4:264–275. 2006.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Campos SM, de Oliveira VL, Lessa L, Vita
M, Conceição M, Andrade LA and Teixeira GA: Maternal
immunomodulation of the offspring's immunological system.
Immunobiology. 219:813–821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paschoal PO, Campos SM, Pedruzzi MM,
Garrido V, Bisso M, Antunes DM, Nobrega AF and Teixeira G: Food
allergy/hypersensitivity: Antigenicity or timing? Immunobiology.
214:269–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Casuccio GS, Janocko PB, Lee RJ, Kelly JF,
Dattner SL and Mgebroff JS: The use of computer controlled scanning
electron microscopy in environmental studies. J Air Pollut Control
Assoc. 33:937–943. 1983. View Article : Google Scholar
|
|
26
|
Apotheker H and Jako GJ: A microscope for
use in dentistry. J Microsurg. 3:7–10. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fisher RB and Parsons DS: The gradient of
mucosal surface area in the small intestine of the rat. J Anat.
84:272–282. 1950.PubMed/NCBI
|
|
28
|
Wood HO: The surface area of the
intestinal mucosa in the rat and in the cat. J Anat. 78:103–105.
1944.PubMed/NCBI
|
|
29
|
Marsh MN: Intestinal Manifestations of
food hypersensitivity. 34. Raven Press; New York, NY: 1995
|
|
30
|
Oberhuber G, Granditsch G and Vogelsang H:
The histopathology of coeliac disease: Time for a standardized
report scheme for pathologists. Eur J Gastroenterol Hepatol.
11:1185–1194. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dickson BC, Streutker CJ and Chetty R:
Coeliac disease: An update for pathologists. J Clin Pathol.
59:1008–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|