Introduction
Hepatitis B virus (HBV) infection is a major health
problem, with approximately 350 million individuals having chronic
infection and approximately 600,000 hepatitis B-related deaths
annually. The main purpose of treating chronic hepatitis B (CHB) is
to achieve sustained suppression of HBV replication and to improve
quality of life and survival by preventing progression to cirrhosis
and liver cancer (1–3). HBs antigen (HBsAg) is a classical
serological marker used to screen for HBV infection. In recent
years, serum HBsAg titer has been established as a useful marker
for monitoring the response to antiviral therapy and is associated
with the severity of fibrosis among HBeAg-positive CHB patients
(4,5).
Moreover, achieving HBsAg seroclearance is an ultimate aim of
antiviral therapy and is related to favorable clinical outcomes of
CHB (6).
Currently, nucleotide(s) analogs (NA) and interferon
(IFN) are commonly used to treat CHB (7). Although several kinds of NAs are
available, including lamivudine, telbivudine, entecavir,
emtricitabine, adefovir dipivoxil (ADV), and tenofovir (8), patients cannot stop treatment because
covalently closed circular (ccc)DNA is stably incorporated into the
nucleus of hepatocytes, even after treatment with an NA (6,8). Currently,
however, the efficacy of IFN in combination with an NA is not well
established and some controversial findings have been published
(9,10).
It was reported that the combination of pegylated (PEG)-IFNα with
lamivudine was associated with a greater virological response
during treatment but not after treatment (11,12). A
recent study revealed that PEG-IFN in combination with telbivudine
(LDF) increased the risk of peripheral neuropathy (13). By contrast, IFN in combination with ADV
was effective in terms of reducing intrahepatic cccDNA and reducing
the serum HBsAg titer (14–16). Based on this complicated situation,
combination therapy is not recommended in the international
guidelines proposed by the Asian-Pacific Association, the American
Association for the Study of Liver Diseases and the European
Association for the Study of the Liver (1–3). However,
recent meta-analyses revealed that NA and IFN combination therapy
was associated with improved outcomes than NA or IFN monotherapy
(10,17). In addition, it is unclear whether the
HBsAg titer is decreased by combination therapy.
In the present study, we examined the efficacy of
IFN monotherapy, and IFN and ADV combination therapy and the
factors associated with a decrease in HBsAg of >1 log IU/ml from
baseline.
Materials and methods
Thirty five patients with CHB who received IFN-based
therapy were enrolled in the study (mean ± standard deviation age,
36.7±8.5 years; 27 males and 8 females). Overall, 21 received
PEG-IFN monotherapy and 14 received IFN and ADV combination
therapy. The decision to use IFN monotherapy or IFN and ADV
combination therapy was made by the attending physician. The serum
HBsAg titer was determined using an Abbott Architect HBsAg
quantative II assay (Abbott Laboratories, Abbott Park, IL, USA).
Serum HBV DNA levels were measured using a quantitative PCR assay
(Abbott Laboratories). Serum aspartate aminotransferase (AST),
alanine aminotransferase (ALT), albumin, and total bilirubin
concentrations, and the international normalized ratio (INR) for
prothrombin time were measured using standard laboratory
procedures. We examined the factors associated with a decrease in
the HBsAg titer of >1.0 log IU/ml from baseline to the end of
treatment and at 24 weeks after treatment.
The present study was conducted between November
2011 and June 2013 by Kobe University Hospital and three affiliated
hospitals in Hyogo prefecture. The study protocol was approved by
the Ethics Committee at Kobe University Hospital, and written
informed consent was obtained from each patient before starting
treatment.
Statistical analysis
Statistical analyses were performed using SPSS v16
software (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Serological and virological
characteristics of the patients
The patient backgrounds are summarized in Table I. The mean age of the patients was
36.7±8.5 years. Overall, 29 patients had genotype C and 1 patient
was genotype A. Twenty patients (57%) were positive for HB envelope
antigen (HBeAg). Serum HBsAg, HBcrAg, and HBV-DNA were
significantly lower in patients who received IFN monotherapy than
in patients who received combination therapy.
 | Table I.Clinical characteristics of patients
before treatment. |
Table I.
Clinical characteristics of patients
before treatment.
| Variables | IFN monotherapy | IFN + ADV
combination | All patients |
|---|
| n | 21 | 14 | 35 |
| Age (years) | 38.2±8.5 | 34.4±8.0 | 36.7±8.5 |
| Sex (M:F) | 16:5 | 11:3 | 27:8 |
| Genotype (A:B:C) | 1:2:17 | 0:2:12 | 1:5:29 |
| HBeAg-positive | 8 (38%) | 12 (86%) | 20 (57%) |
| PLT count
(×104/mm3) | 21.0±3.8 | 20.6±4.1 | 20.8±4.0 |
| AST (IU/l) | 106.2±181.1 | 94.8±110.7 | 101.3±154.7 |
| ALT (IU/l) | 158.6±306.9 | 115.9±105.2 | 141.5±247.8 |
| T-Bil (mg/dl) | 0.80±0.59 | 0.72±0.24 | 0.77±0.48 |
| HBsAg titer (log
IU/ml) |
3.28±0.86a |
4.39±0.69a | 3.72±0.97 |
| HBcrAg titer (log
IU/ml) |
5.20±1.58a |
6.53±1.18a | 5.78±1.04 |
| HBV-DNA (log
copies/ml) |
6.80±1.70a |
8.71±1.39a | 7.53±1.84 |
HBsAg, HBV-DNA, and ALT levels during
and after therapy
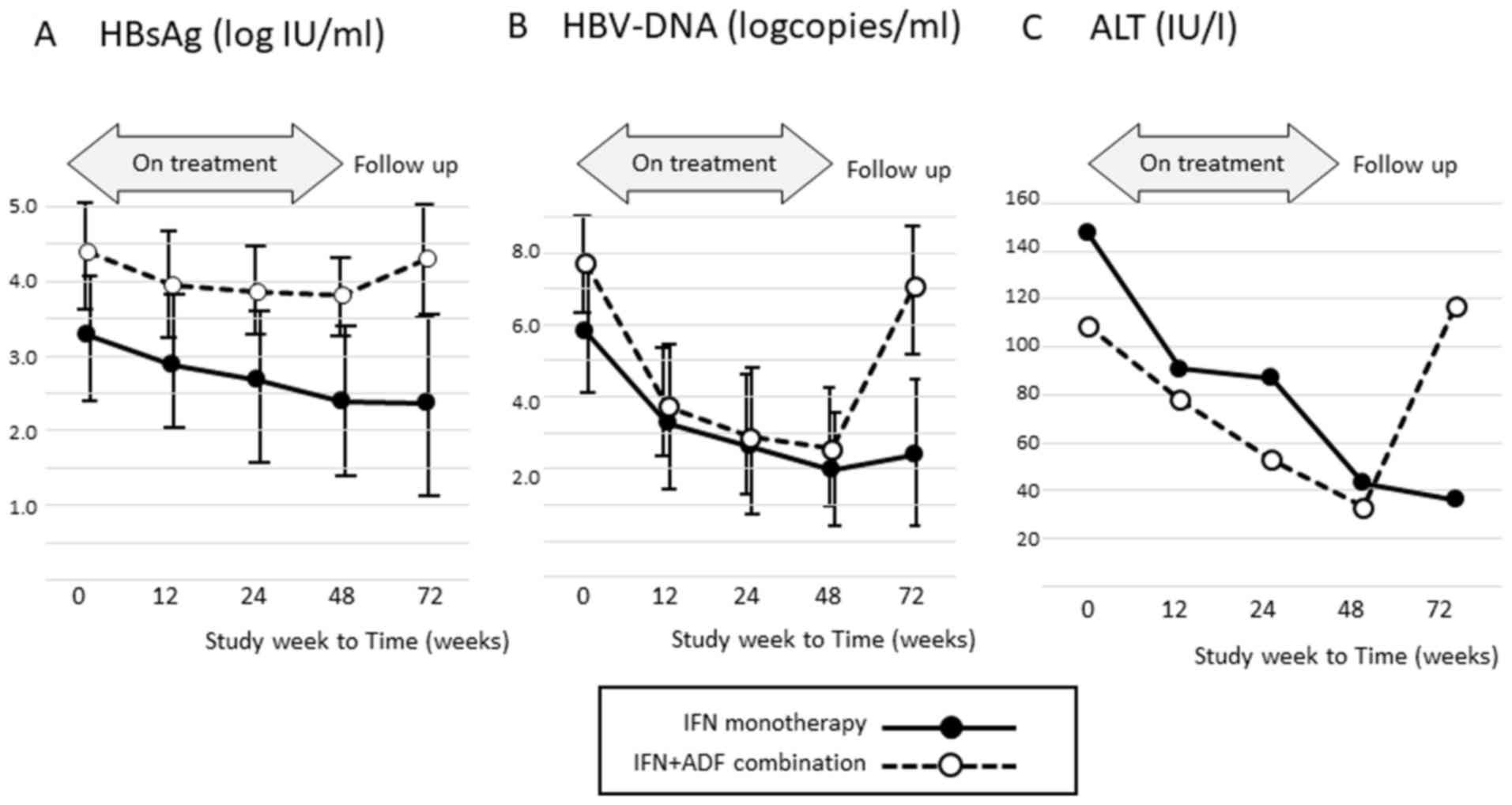
The mean HBsAg titer, HBV-DNA level, and ALT level
in patients who received IFN monotherapy or combination therapy are
shown in Fig. 1. The mean HBsAg titer
and HBV-DNA level before therapy were much greater in patients who
received combination therapy than in those who received IFN
monotherapy. The mean ALT and HBV-DNA levels in patients who
received combination therapy had re-increased at 24 weeks after
treatment.
Factors associated with decreases in
HBsAg titers during and after therapy
Although 13/35 (37%) patients achieved a decrease in
HBsAg of >1 log IU/ml from baseline to the end of treatment, 6
(17%) patients experienced a relapse after therapy. In 7 (20%)
patients, the reduction in HBsAg was maintained until 24 weeks
after treatment (Tables II and
III). HBcrAg before therapy
(6.56±0.78 vs. 5.30±1.66 log IU/ml, P<0.05) and the proportion
of patients with an increase in ALT of >2 times from baseline
(62 vs. 14%, P<0.05) were significantly greater in patients with
a decrease in HBsAg of >1 log IU/ml at the end of treatment than
in patients whose HBsAg did not decrease.
 | Table II.Factors associated with a decrease in
HBsAg of >1 log IU/ml at the end of treatment. |
Table II.
Factors associated with a decrease in
HBsAg of >1 log IU/ml at the end of treatment.
| Variables | Patients with a
decrease in HBsAg | Patients without a
decrease in HBsAg | P-value |
|---|
| n | 13 (37%) | 22 (63%) |
|
| Age (years) | 34.2±4.7 | 38.2±9.83 | n.s. |
| Sex (M:F) | 11:2 | 16:6 | n.s. |
| Genotype
(A:B:C) | 1:1:11 | 0:4:18 | n.s. |
| HBeAg-positive | 10 (77%) | 10 (45%) | n.s. |
| Combined
therapy | 4 (31%) | 10 (45%) | n.s. |
| PLT count
(×104/mm3) | 20.5±4.2 | 22.3±2.2 | n.s. |
| AST (IU/l) | 142.2±214.4 | 69.9±69.5 | n.s. |
| ALT (IU/l) | 226.6±370.0 | 91.2±99.8 | n.s. |
| T-Bil (mg/dl) | 0.82±0.52 | 0.54±0.17 | n.s. |
| HBsAg (log
IU/ml) | 4.01±0.62 | 3.56±1.09 | n.s. |
| HBcrAg (log
IU/ml) | 6.56±0.78 | 5.30±1.66 | 0.007a |
| HBV-DNA (log
copies/ml) | 8.00±1.60 | 7.24±1.92 | n.s. |
| Increase in ALT at
12 weeks after starting | 8
(62%)a | 3
(14%)a | 0.014a |
 | Table III.Factors associated with a decrease in
the HBsAg titer of >1 log IU/mL at 24 weeks after therapy. |
Table III.
Factors associated with a decrease in
the HBsAg titer of >1 log IU/mL at 24 weeks after therapy.
| Variables | Patients with a
decrease in HBsAg | Patients without a
decrease in HBsAg | P-value |
|---|
| n | 7 (20%) | 28 (80%) |
|
| Age (years) | 34.1±5.8 | 37.4±9.0 | n.s. |
| Sex (M:F) | 6:1 | 21:7 | n.s. |
| Genotype
(A:B:C) | 1:0:6 | 0:5:23 | n.s. |
| HBeAg positive | 5 (71%) | 15 (54%) | n.s. |
| Combined
therapy | 1 (14%) | 13 (46%) | n.s. |
| PLT count
(×104/mm3) | 20.8±4.0 | 20.8±4.0 | n.s. |
| AST (IU/l) | 179.3±257.9 | 77.5±92.5 | n.s. |
| ALT (IU/l) | 332.1±476.0 | 93.9±93.5 | n.s. |
| T-Bil (mg/dl) | 0.77±0.48 | 0.77±0.48 | n.s. |
| HBsAg (log
IU/ml) | 4.01±0.62 | 3.56±1.09 | n.s. |
| HBcrAg (log
IU/ml) | 6.66±0.43 | 5.42±1.69 | 0.006a |
| HBV-DNA (log
copies/ml) | 7.62±1.82 | 7.51±1.84 | n.s. |
| Increase in ALT at
12 weeks after starting | 5 (71%) | 6 (27%) | n.s. |
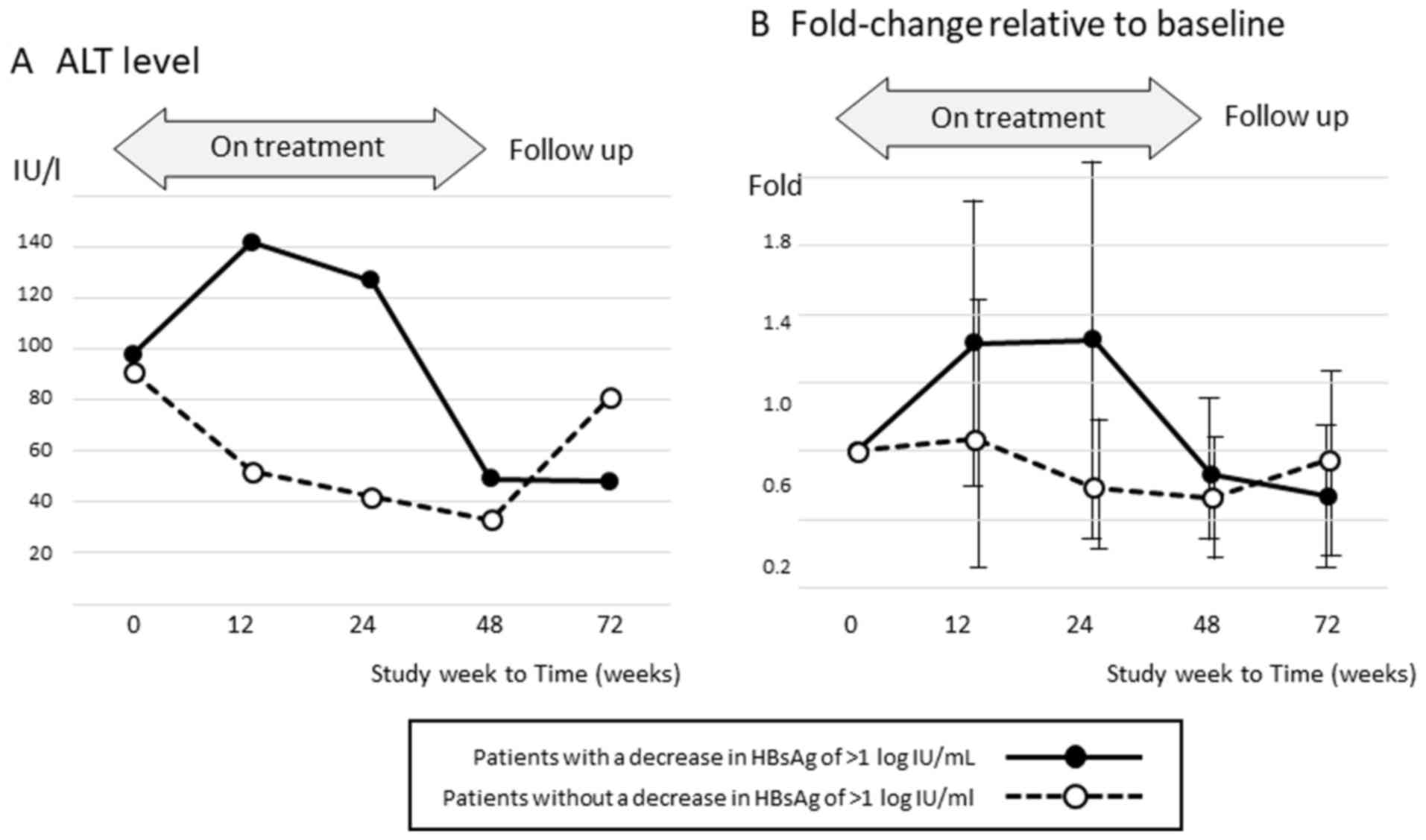
The mean ALT levels during and after therapy are
shown in Fig. 2 according to the
achievement of HBsAg reduction. Among patients without a decrease
in HBsAg of >1 log IU/ml at the end of therapy, the ALT level
increased markedly after completing treatment.
Discussion
IFN is commonly used to treat CHB, and studies have
demonstrated its efficacy in HBeAg-negative patients in particular
(18,19). In the present study, HBe seroconversion
was detected in just 2 (10%) of 20 HBeAg-positive subjects, which
indicates that IFN is less effective in HBeAg-positive patients.
The therapeutic efficacy of IFN is usually evaluated in terms of
undetectable HBV-DNA and normalization of ALT levels at the end of
treatment and at 24–96 weeks after treatment. In the present study,
16 (45.7%) and 21 (60%) patients had undetectable HBV-DNA and
normalization of ALT levels, respectively, at the end of
treatment.
It is also well known that the efficacy of IFN
differs among genotypes. In particular, the efficacy of IFN for
treating CHB with genotype A and D, which are prevalent in European
countries, is much greater than that of CHB with genotypes B and C,
which are prevalent in Asian countries. In addition, its efficacy
is less effective in genotype C, which is spreading throughout
Japan, than in genotype B, which is spreading in South China,
Taiwan, and Thailand (20,21). Although none of the patients with
genotype C achieved loss of HBsAg, one patient with genotype A had
undetectable HBV-DNA and loss of HBsAg at the end of treatment.
These findings suggest that the HBV genotype is associated with the
efficacy of IFN.
HBsAg is produced from HBV/S protein and the HBsAg
titer is correlated with the HBV viral quantity in the liver
(22). Recently, HBsAg seronegativity
has become a focus of the long-term goal of treatment, and the
decrease in the HBsAg titer is an important factor for evaluating
the outcome of antiviral therapy (23,24). Of
note, the decrease in the HBsAg titer during the early phase of IFN
therapy is strongly associated with a sustained virological
response (25). In the present study,
a decrease in HBsAg of >1 log IU/ml was detected in 13 (37%)
patients at the end of therapy and in 7 (20%) patients at 24 weeks
after treatment. Thus, the HBsAg titer before starting treatment is
an important factor associated with the outcome of antiviral
therapy.
ADV is a NA of adenine and was reported to be
effective in terms of reducing cccDNA (26,27). The
efficacy of IFN and ADV combination therapy was clearly
demonstrated in a meta-analysis (10),
which showed that the proportions of patients with undetectable
HBV-DNA, HBeAg seroconversion, and normalization of ALT levels were
significantly greater in patients who received combination therapy
than in those who received IFN monotherapy (10). The present study also revealed that the
proportion of patients with normalization of ALT was slightly
greater in those who received combination therapy than in those who
received monotherapy (71.4 vs. 66.7%, respectively). However, the
proportion of patients with undetectable HBV was lower in patients
who received combination therapy (35.7 vs. 66.7%, respectively) and
the proportion of patients with an increase in ALT levels after
therapy was higher in patients with combination therapy. These
findings may be explained by the higher viral load before therapy
and the greater proportion of HBeAg-positive patients among the
group who received combination therapy.
The present study revealed that HBcrAg before
treatment and on-treatment ALT flares were associated with the
therapeutic efficacy. It was reported that on-treatment ALT flares
were associated with the response to IFN, and are related to the
immune response derived from IFN therapy (24,28). In the
present study, the proportion of patients with an ALT flare was
similar between patients who received IFN monotherapy or
combination therapy (33.3 vs. 35.7%, respectively). HBcrAg is used
mainly as a serum marker reflecting intrahepatic cccDNA (29,30). In the
present study, the HBcrAg titer before starting treatment was
associated with the decrease in HBsAg at the end of treatment,
although HBV-DNA was not associated with the reduction in HBsAg
after treatment. These findings suggest that the HBcrAg titer is a
superior serological marker for predicting the decrease in HBsAg
elicited by IFN-based therapy.
In conclusion, the HBcrAg titer before starting
treatment and the increase in ALT after treatment (host instruction
flare) are important factors associated with the decrease in HBsAg
after IFN-based therapy. The efficacy of IFN and ADV combination
therapy was not apparent in terms of a decrease in HBsAg
titers.
References
|
1
|
Lok AS and McMahon BJ: Chronic hepatitis
B: Update 2009. Hepatology. 50:661–662. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
European Association For The Study Of The
Liver, . EASL clinical practice guidelines: Management of chronic
hepatitis B virus infection. J Hepatol. 57:167–185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liaw YF, Kao JH, Piratvisuth T, Chan HLY,
Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH, et al:
Asian-Pacific consensus statement on the management of chronic
hepatitis B: A 2012 update. Hepatol Int. 6:531–561. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marcellin P, Martinot-Peignoux M, Asselah
T, Batrla R, Messinger D, Rothe V, Lau G and Liaw YF: Serum levels
of Hepatitis B surface antigen predict severity of fibrosis in
patients with E antigen-positive chronic Hepatitis B. Clin
Gastroenterol Hepatol. 13:1532–9.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shouval D: Focus: Quantitative HBsAg
measurement as a new surrogate marker for assessment of hepatic
fibrosis in HBeAg+ chronic hepatitis B. J Hepatol. 58:1063–1064.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim GA, Lim YS, An J, Lee D, Shim JH, Kim
KM, Lee HC, Chung YH, Lee YS and Suh DJ: HBsAg seroclearance after
nucleoside analogue therapy in patients with chronic hepatitis B:
Clinical outcomes and durability. Gut. 63:1325–1332. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hynicka LM, Yunker N and Patel PH: A
review of oral antiretroviral therapy for the treatment of chronic
hepatitis B. Ann Pharmacother. 44:1271–1286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lam YF, Yuen MF, Seto WK and Lai CL:
Current antiviral therapy of chronic hepatitis B: Efficacy and
safety. Curr Hepat Rep. 10:235–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Y, Wu YH, Shu ZY, Zhang WJ, Yang J and
Chen Z: Interferon and lamivudine combination therapy versus
lamivudine monotherapy for hepatitis B e antigen-negative hepatitis
B treatment: A meta-analysis of randomized controlled trials.
Hepatobiliary Pancreat Dis Int. 9:462–472. 2010.PubMed/NCBI
|
|
10
|
Huang R, Hao Y, Zhang J and Wu C:
Interferon-alpha plus adefovir combination therapy versus
interferon-alpha monotherapy for chronic hepatitis B treatment: A
meta-analysis. Hepatol Res. 43:1040–1051. 2013.PubMed/NCBI
|
|
11
|
Lau GK, Piratvisuth T, Luo KX, Marcellin
P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, et
al: Peginterferon alfa-2a HBeAg-positive chronic Hepatitis B study
group: Peginterferon Alfa-2a, lamivudine, and the combination for
HBeAg-positive chronic hepatitis B. N Engl J Med. 352:2682–2695.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marcellin P, Lau GK, Bonino F, Farci P,
Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin
C, et al: Peginterferon alfa-2a HBeAg-negative chronic hepatitis B
study group: Peginterferon alfa-2a alone, lamivudine alone, and the
two in combination in patients with HBeAg-negative chronic
hepatitis B. N Engl J Med. 351:1206–1217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marcellin P, Wursthorn K, Wedemeyer H,
Chuang WL, Lau G, Avila C, Peng CY, Gane E, Lim SG, Fainboim H, et
al: Telbivudine plus pegylated interferon alfa-2a in a randomized
study in chronic hepatitis B is associated with an unexpected high
rate of peripheral neuropathy. J Hepatol. 62:41–47. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wursthorn K, Lutgehetmann M, Dandri M,
Volz T, Buggisch P, Zollner B, Longerich T, Schirmacher P, Metzler
F, Zankel M, et al: Peginterferon alpha-2b plus adefovir induce
strong cccDNA decline and HBsAg reduction in patients with chronic
hepatitis B. Hepatology. 44:675–684. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takkenberg B, Terpstra V, Zaaijer H,
Weegink C, Dijkgraaf M, Jansen P, Beld M and Reesink H:
Intrahepatic response markers in chronic hepatitis B patients
treated with peginterferon alpha-2a and adefovir. J Gastroenterol
Hepatol. 26:1527–1535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takkenberg RB, Jansen L, de Niet A,
Zaaijer HL, Weegink CJ, Terpstra V, Dijkgraaf MG, Molenkamp R,
Jansen PL, Koot M, et al: Baseline hepatitis B surface antigen
(HBsAg) as predictor of sustained HBsAg loss in chronic hepatitis B
patients treated with pegylated interferon-α2a and adefovir.
Antivir Ther. 18:895–904. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie QL, Zhu Y, Wu LH, Fu LL and Xiang Y:
The efficacy and safety of entecavir and interferon combination
therapy for chronic hepatitis B virus infection: A meta-analysis.
PLoS One. 10:e01322192015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buster EH, Flink HJ, Cakaloglu Y, Simon K,
Trojan J, Tabak F, So TM, Feinman SV, Mach T, Akarca US, et al:
Sustained HBeAg and HBsAg loss after long-term follow-up of
HBeAg-positive patients treated with peginterferon alpha-2b.
Gastroenterology. 135:459–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moucari R, Korevaar A, Lada O,
Martinot-Peignoux M, Boyer N, Mackiewicz V, Dauvergne A, Cardoso
AC, Asselah T, Nicolas-Chanoine MH, et al: High rates of HBsAg
seroconversion in HBeAg-positive chronic hepatitis B patients
responding to interferon: A long-term follow-up study. J Hepatol.
50:1084–1092. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Seo Y and Yano Y: Short- and long-term
outcome of interferon therapy for chronic hepatitis B infection.
World J Gastroenterol. 20:13284–13292. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Croagh CM, Desmond PV and Bell SJ:
Genotypes and viral variants in chronic hepatitis B: A review of
epidemiology and clinical relevance. World J Hepatol. 7:289–303.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Howard CR: The structure of hepatitis B
envelope and molecular variants of hepatitis B virus. J Viral
Hepat. 2:165–170. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Höner Zu, Siederdissen C and Cornberg M:
The role of HBsAg levels in the current management of chronic HBV
infection. Ann Gastroenterol. 27:105–112. 2014.PubMed/NCBI
|
|
24
|
Brunetto MR, Moriconi F, Bonino F, Lau GK,
Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S,
et al: Hepatitis B virus surface antigen levels: A guide to
sustained response to peginterferon alfa-2a in HBeAg-negative
chronic hepatitis B. Hepatology. 49:1141–1150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moucari R, Mackiewicz V, Lada O, Ripault
MP, Castelnau C, Martinot-Peignoux M, Dauvergne A, Asselah T, Boyer
N, Bedossa P, et al: Early serum HBsAg drop: A strong predictor of
sustained virological response to pegylated interferon alfa-2a in
HBeAg-negative patients. Hepatology. 49:1151–1157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dando T and Plosker G: Adefovir dipivoxil:
A review of its use in chronic hepatitis B. Drugs. 63:2215–2234.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng PN, Liu WC, Tsai HW, Wu IC, Chang TT
and Young KC: Association of intrahepatic cccDNA reduction with the
improvement of liver histology in chronic hepatitis B patients
receiving oral antiviral agents. J Med Virol. 83:602–607. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hansen BE, Rijckborst V, Ter Borg MJ and
Janssen HL: HBV DNA suppression in HBeAg-positive chronic hepatitis
B patients treated with peginterferon or placebo. J Med Virol.
83:1917–1923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong DK, Tanaka Y, Lai CL, Mizokami M,
Fung J and Yuen MF: Hepatitis B virus core-related antigens as
markers for monitoring chronic hepatitis B infection. J Clin
Microbiol. 45:3942–3947. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Suzuki F, Miyakoshi H, Kobayashi M and
Kumada H: Correlation between serum hepatitis B virus core-related
antigen and intrahepatic covalently closed circular DNA in chronic
hepatitis B patients. J Med Virol. 81:27–33. 2009. View Article : Google Scholar : PubMed/NCBI
|