Introduction
Leptomeningeal infiltration is frequently
encountered (1), and dural
infiltration is not rare with secondary central nervous system
(CNS) lymphoma (1,2). However, unless systemic lymphoma has
already been identified, a finding of subdural and subarachnoid
abnormality would not be directly linked to lymphoma (3). Furthermore, computed tomography (CT) and
magnetic resonance imaging (MRI) abnormalities surrounding
hemorrhagic and postoperative changes make it difficult to
distinguish between onset of disease and postoperative
complications, including metal artifacts. However, malignant cells
that enter the CNS appear first in the dura and subarachnoid space
in rats with a blood brain barrier (BBB) disrupted by focal injury
following exposure to a cold temperature (4). Previous history of subarachnoid
hemorrhage (SAH) and aneurysm clipping may be associated with CNS
infiltration in systemic lymphoma. The current study describes a
particularly rare case of systemic lymphoma involving the CNS
overlapping with the area of injury to the dura and leptomeninges
due to an aneurysmal SAH. To the best of our knowledge, no similar
cases have previously been reported.
Case report
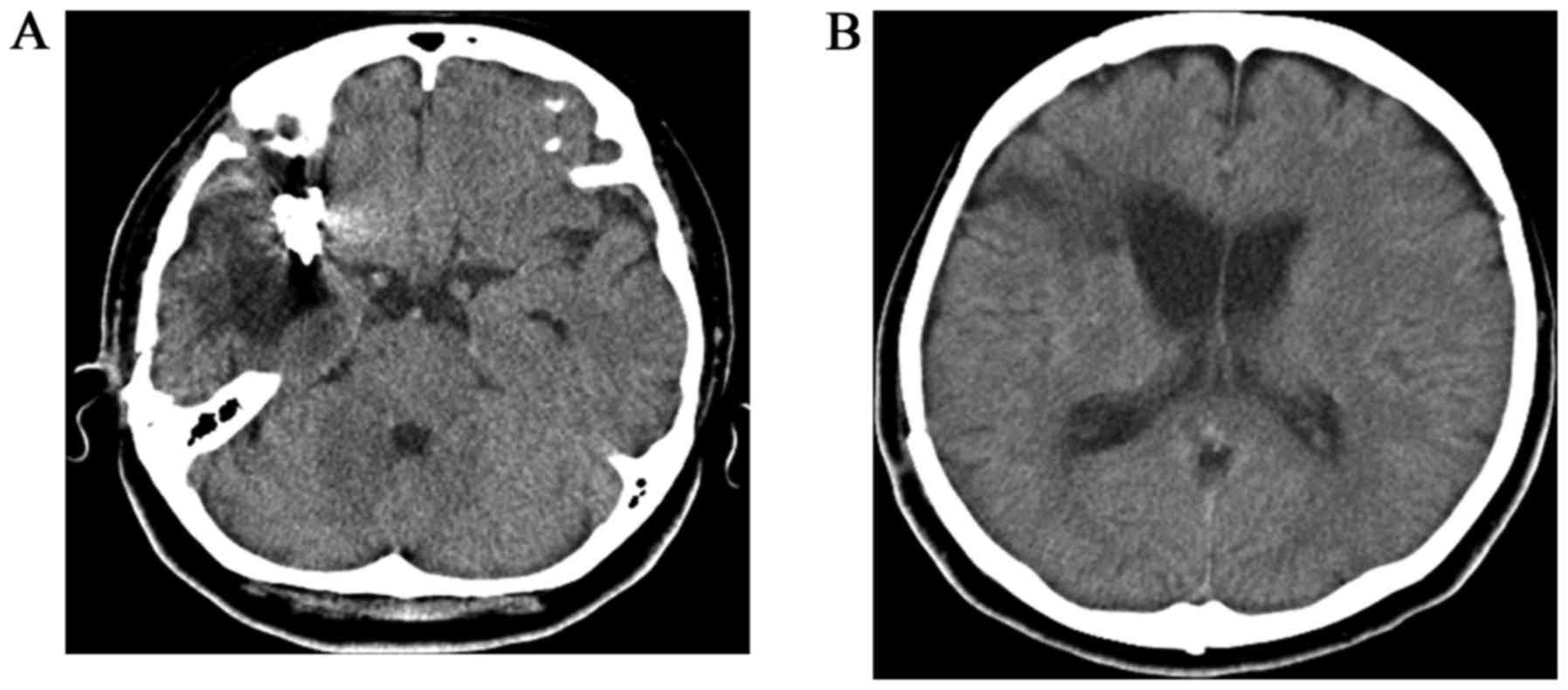
A 56-year-old woman with a history of aneurysm
clipping following acute SAH due to rupture of the right middle
cerebral arterial aneurysm 6 years earlier (Fig. 1) was hospitalized at Kochi Health
Sciences Center for sudden numbness of the left arm. The patient
had not experienced malignant or benign diseases within the 6 years
since discharge from hospital. The patient had been asymptomatic
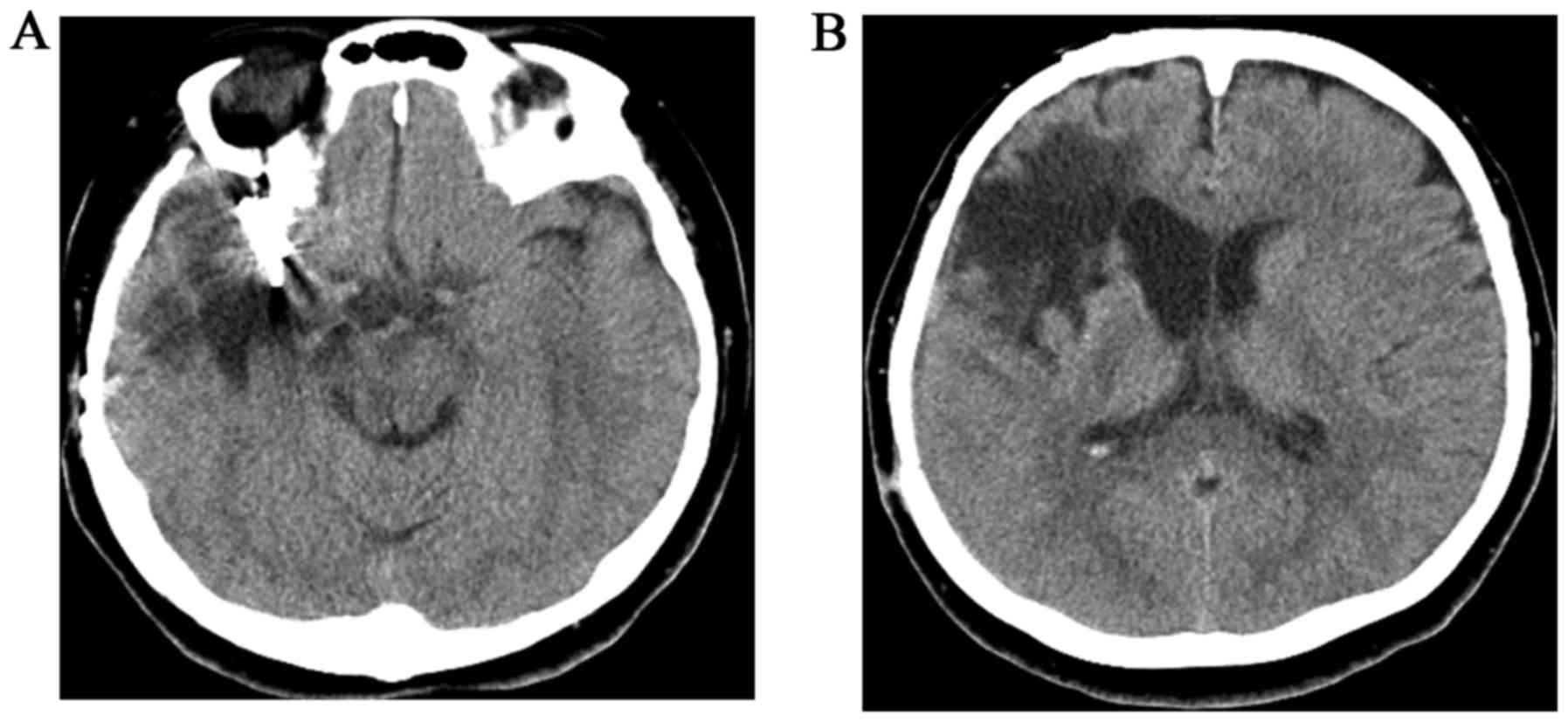
except for partial paralysis due to SAH. Unenhanced CT was
performed to exclude the recurrence of SAH, and it revealed slurred
fissures of the right parietal region and enlargement of the
low-density area surrounding the preceding hemorrhagic scar was
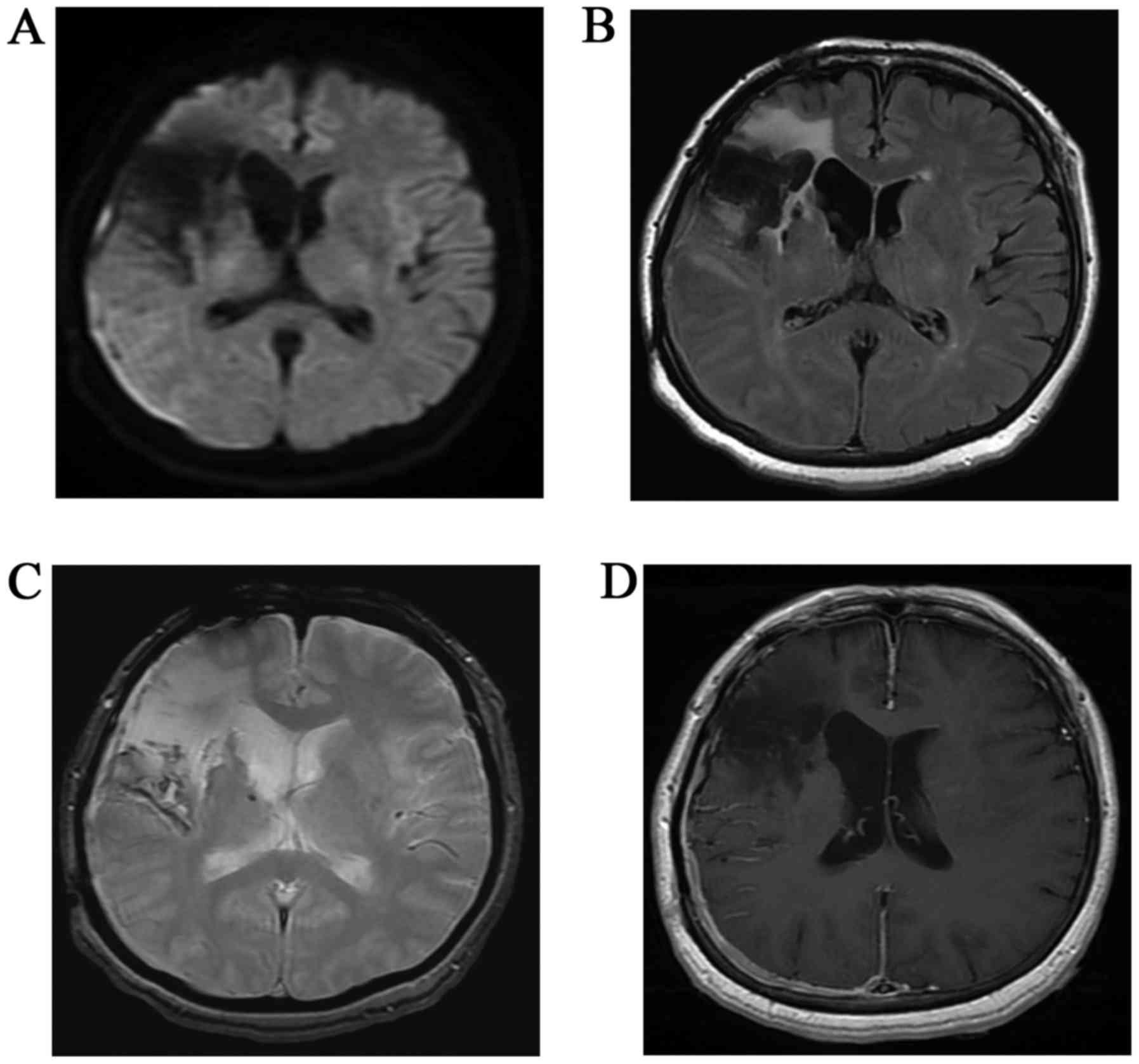
suspected (Fig. 2). In addition, MRI
was performed (Fig. 3).
Diffusion-weighted imaging (DWI) demonstrated a thin crescent of
hyperintensity in the right temporo-occipital region, although the
parenchyma near the right Sylvian fissure exhibited loss of signal
with distortion due to a magnetic susceptibility artifact.
Fluid-attenuated inversion recovery (FLAIR) demonstrated slurred
fissures of the right temporo-occipital region, and hyperintensity
near the Sylvian fissure. T2-weighted gradient echo imaging (T2WI)
demonstrated linear hypointensities along the surface of the right
cerebral hemisphere as superficial siderosis from hemosiderin
deposits caused by SAH. Enhanced axial T1-weighted imaging
demonstrated intense homogeneous enhancement along the dura, and
subarachnoid linear enhancement in the right temporo-occipital
region. A subdural mass showing enhancement was strongly suspected.
However, subarachnoid enhancement or FLAIR hyperintensity near the
right Sylvian fissure resulted in confusion between the presence of
novel lesions or postoperative complications. Prior to surgery,
meningioma with postoperative change was suspected on the basis of
subdural hyperintensity on DWI. Intraoperatively, the dura mater
and subarachnoid space exhibited a grayish-yellow mass, and
malignant lymphoma was diagnosed pathologically. Subsequent
systemic examination by gallium scintigraphy and CT revealed
multiple mediastinal and paraaortic lymph node swellings. Following
CT, neck lymph nodes began to demonstrate rapid increases in size.
Serum interleukin-2 receptor and β2-microglobulin levels were high,
at 2,170 mg/l (normal, <519 mg/ml) and 5.0 U/ml (normal, <1.9
U/ml), respectively. Bone marrow findings were consistent with CNS
lymphoma (non-Hodgkin's B-cell type; stage IV). Based upon positive
results for cyclin D1 postoperatively, mantle cell lymphoma was
proposed, although the subtype was not confirmed. At the time of
writing, the patient is undergoing systemic chemotherapy with a
combination of rituximab, methotrexate and cytalabine.
Discussion
Subdural and subarachnoid enhancement on MRI may be
indicative of a variety of differential diagnoses from benign to
malignant, such as sarcoidosis, meningitis and metastatic tumors
with leptomeningeal dissemination other than secondary lymphoma
(5). MRI findings were consistent with
CNS invasion by lymphoma (2); however,
lymphoma were not considered amongst the more likely options. CT or
MRI abnormalities overlapping a previously injured region
indicating a complication from surgery could not be excluded. In
the present case, subdural crescent enhancement identified on MRI
were exhibited as slurred fissures by CT. In addition, DWI was
distorted by magnetic susceptibility artifacts; in retrospect, the
subarachnoid findings of FLAIR and the enhanced MRI were consistent
with the surgical features of leptomeningeal infiltration (6). Prominent subarachnoid enhancement on
contrast-enhanced T1WI may depict evidence of malignancy; however,
her past history had to be taken into account.
Should systemic lymphoma be revealed in advance,
invasion of the CNS may be included in the differential diagnosis,
regardless of how distorted the CT or MRI findings are. Although
primary leptomeningeal or dural lymphoma is rare (7,8), secondary
CNS invasion is not. CNS involvement in non-Hodgkin's lymphoma
tends to occur early, at a median of 5–6 months subsequent to the
primary diagnosis of systemic lymphoma (3). Lymphoma in the current patient would
represent an aggressive subtype according to the rapid increase in
the size of neck lymph nodes.
Exactly why secondary lymphoma tends to present the
dural or leptomeningeal spread remains unknown. However, lymphoma
cells hypothetically spread from retroperitoneal lymph nodes or
bone marrow to the leptomeninges via the intervertebral nervous
plexus (9). Aho et al (4) reported that malignant cells appeared to
enter the CNS through a deficiency in the BBB around the
subarachnoid vessels. Composite meningioma and lymphoma has been
reported as a form of tumor-to-tumor metastasis (10). Previous disruption of the BBB by
meningioma may be considered as the grounds for invasion of CNS
lymphoma. In the present case, the BBB of the broad area
surrounding the right Sylvian fissure had been disrupted by acute
SAH and subsequent surgery 6 years earlier. CNS invasion by
lymphoma may be associated with disruption of the BBB. Although
confirmatory evidence is lacking, the possibility remains that
subdural and leptomeningeal invasion of lymphoma occurred through
disruption of the BBB by SAH and subsequent clipping.
In conclusion, the current study presents a case of
CNS invasion by systemic lymphoma that was difficult to
radiologically diagnose due to overlap with an area previously
affected by SAH and a subsequent surgical scar. It is proposed that
radiologists assess the possibility of invasion by CNS lymphoma in
the presence of meningeal abnormality overlapping an injured
region.
References
|
1
|
Haldorsen IS, Espeland A and Larsson EM:
Central nervous system lymphoma: Characteristic findings on
traditional and advanced imaging. AJNR Am J Neuroradiol.
32:984–992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koeller KK, Smirniotopoulos JG and Jones
RV: Primary central nervous system lymphoma: Radiologic-pathologic
correlation. Radiographics. 17:1497–1526. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hill QA and Owen RG: CNS prophylaxis in
lymphoma: Who to target and what therapy to use. Blood Rev.
20:319–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aho R, Vaittinen S, Jahnukainen K and
Kalimo H: Spread of malignant lymphoid cells into rat central
nervous system with intact and disrupted blood-brain barrier.
Neuropathol Appl Neurobiol. 20:551–561. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guermazi A, Lafitte F, Miaux Y, Adem C,
Bonneville JF and Chiras J: The dural tail sign-beyond meningioma.
Clin Radiol. 60:171–188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stuckey SL, Goh TD, Heffernan T and Rowan
D: Hyperintensity in the subarachnoid space on FLAIR MRI. AJR Am J
Roentgenol. 189:913–921. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taylor JW, Flanagan EP, O'Neill BP, Siegal
T, Omuro A, Deangelis L, Baehring J, Nishikawa R, Pinto F,
Chamberlain M, et al: Primary leptomeningeal lymphoma:
International Primary CNS Lymphoma Collaborative Group report.
Neurology. 81:1690–1696. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson BA, Fram EK, Johnson PC and
Jacobowitz R: The variable MR appearance of primary lymphoma of the
central nervous system: Comparison with histopathologic features.
AJNR Am J Neuroradiol. 18:563–572. 1997.PubMed/NCBI
|
|
9
|
Levitt LJ, Dawson DM, Rosenthal DS and
Moloney WC: CNS involvement in the non-Hodgkin's lymphomas. Cancer.
45:545–552. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin SE, Khalidi HS and Hattab EM:
Marginal zone B-cell lymphoma involving a longstanding fibrous
meningioma: An initial manifestation of systemic disease. Hum
Pathol. 44:2609–2613. 2013. View Article : Google Scholar : PubMed/NCBI
|