Introduction
In the diagnosis of prostate cancer with ultrasound
(US) imaging, gray scale US, Doppler US, dynamic contrast enhanced
(DCE)-US and elastography have been widely used (1). The majority of prostate cancers are
harder than normal prostatic tissue, due to increased
micro-vascularity and a stromal response leading to increased
collagen deposition around the tumor (2). Elastography evaluates tissue stiffness
instead of echogenicity, which provides a novel method of detecting
pathological abnormality that may otherwise be missed by
conventional US (3). At present,
there are two types of US elastography: Strain elastography and
shear wave elastography (SWE) (4).
Strain elastography requires user-applied uniform compression at
the surface of the body to cause deformation of the tissue. The US
scanner then calculates and displays the induced deformation in the
imaging plane. Due to its reliance on the individual applying
pressure to the body, users have reported poor reproducibility and
intra-operator variability with this method (4). Thus, in strain elastography, only
relative information is obtained regarding the stiffness of the
measured tissue, and it is not a quantitative imaging mode. By
contrast, SWE provides a quantitative value of stiffness, thus
yielding useful information of a pathological abnormality (4). Shear wave is a technique that uses a
sonographic push pulse to generate a shear wave in the tissues.
Shear wave velocity (Vs) through the tissue is affected
by tissue stiffness, with stiffer tissues accommodating faster
movement (5). Tissue stiffness may be
expressed as Young's modulus or as the ratio of stress on a
material to the tissue deformation caused by the stress (6). The Vs or Young's modulus
(kPa) for each pixel is color-coded and overlaid on a B-mode image
(7). The SWE, or tissue elasticity,
is detected by tissue response to an operator-independent
compression wave pulsed into the tissue by an ultrasound probe
(7). Woo et al (8) reported that the intra-observer
reproducibility of SWE in terms of Young's modulus (kPa) was high
[intraclass correlation coefficients =0.876, 95% confidence
interval (CI) =0.864–0.887]. Barr et al (9) reported that a value of 37 kPa may be
used as a cut-off between benign and malignant prostate tissue on
the basis of the receiver operating characteristic (ROC) curve.
This achieved a sensitivity of 96.2%, a specificity of 96.2%, a
positive predictive value (PPV) of 69.4%, and a negative predictive
value (NPV) of 99.6% in 318 sextants from 53 patients (9). Furthermore, this method was more
sensitive and specific in diagnosing the prostate cancer than
strain elastography (9).
Recently, clinically significant prostate cancer,
defined by at least one biopsy core with a Gleason score of 3+4 or
6 with a maximum cancer core length greater than 4 mm, has been
considered to be associated with cancer progression (10). In the detection of significant
prostate cancer, multi-parametric magnetic resonance imaging
(mpMRI) is becoming more used due to its increased availability and
capacity to combine anatomical and functional data (11,12). To
standardize the evaluation and reporting of MRI findings of the
prostate, the European Society of Urogenital Radiology published
guidelines, termed the Prostate Imaging Reporting and Data System
(PI-RADS) (13). In this system,
suspicious areas, known as ‘regions of interest (ROI)’, are defined
and radiologists provide a likelihood score that clinically
significant cancer is present for each ROI from 1 to 5 on the
PI-RAD classification14: 1, most probably benign; 2, probably
benign; 3, intermediate; 4, probably malignant; 5, highly
suspicious of malignancy (13,14). In a
diagnostic meta-analysis of the standardized evaluation system of
PI-RADS, the sensitivity and specificity for pooled studies were
78% (95% CI: 70–84) and 79% (95% CI: 68–86), respectively (15). Although mpMRI findings may be useful
for detecting significant prostate cancer, the differential
diagnosis of certain prostate cancers from benign prostatic
hyperplasia and inflammation is challenging in mpMRI, as the signal
intensity of these lesions can be similar to that of the tumor
tissue (16). Therefore, elastography
may be more effective, as a separate technique to mpMRI, in the
detection of significant prostate cancer.
The development of three-dimensional (3D) US has
enabled 3D visualization of the prostate, allowing diagnosis and
localization of prostatic lesions. In the present study, the
applications of 3D SWE in the detection of significant prostate
cancer were evaluated. To the best of our knowledge, the current
study is the first to determine the efficacy of 3D SWE in the
detection of significant prostate cancer.
Materials and methods
Study population
The study prospectively recruited patients with
serum prostate specific antigen (PSA) levels of 4.0–20.0 ng/ml who
were suspected of having prostate cancer from mpMRI scans taken
from May 2016 to June 2016 at Tokai University Hachioji Hospital
(Tokyo, Japan). The study was approved by the Institutional Review
Board for Clinical Research of Tokai University School of Medicine
(Shimokasuya, Japan) and informed consent was obtained from all
patients prior to enrollment.
mpMRI
MRI examination was conducted as described in our
previous study (17). Briefly, the
MRI examination was performed using a 1.5-Tesla magnet (Signa
HDx®; GE Healthcare Life Sciences, Little Chalfont, UK)
with an 8-channel cardiac coil. T1-weighted fat-saturated axial
fast spin-echo images [repetition time (TR), 450 ms; echo time
(TE), 8.8 ms; slice thickness, 3 mm; resolution, 0.9×1.3 mm] were
obtained prior to injection. An intravenous bolus of 0.2 ml/kg
meglumine gadopentetate (Magnevist Syringe®; Bayer AG,
Leverkusen, Germany) was then injected. All MRI examinations were
performed using the same protocol, and included non-enhanced
T2-weighted images (TR, 5,000 ms; TE, 125 ms; slice thickness, 3
mm; resolution, 0.6×0.9 mm) acquired in the axial and sagittal
planes, diffusion weighted images and apparent diffusion
coefficient (ADC) maps (b-value =1,500 s/mm2), and DCE
imaging (resolution, 0.9×1.3 mm) using a fat-saturated T1-weighted
fast-field echo sequence in the axial plane. All mpMRI images were
reviewed by two experienced radiologists with no prior clinical
information. Suspicious areas, or ROIs, were defined and the
radiologists provided a likelihood score that clinically
significant cancer was present for each ROI from 1 to 5 on the
PI-RADS (14): 1, most probably
benign; 2, probably benign; 3, intermediate; 4, probably malignant;
5, highly suspicious of malignancy (13,14).
3D SWE
At pre-biopsy, 3D SWE was performed using the
Aixplorer® equipped with an SE12-3 146° Super Endocavity
Volumetric Array (SuperSonic Imagine, Aix-en-Provence, France) on
patients in lithotomy position under spinal anesthesia with 1.2 ml
high specific gravity type 0.5% marcaine (Aspen Japan K.K., Tokyo,
Japan). The minimum amount of pressure on the prostate was applied
while maintaining contact with the probe. For each imaging
procedure, the probe was held steady for 30 sec. Measurement of
Young's modulus was performed in all biopsy-punctured lesions with
SWE Vs above that of the baseline prostate tissue. Images were
stored digitally.
MRI-TRUS fusion image-guided prostate
biopsy
MRI-TRUS fusion image-guided prostate biopsy was
performed with a BioJet® system version 2.0 (D&K
Technologies GmbH, Barum, Germany). The biopsy was performed as
described previously (17). Targeted
biopsies for cancer-suspicious lesions were initially performed,
followed by 12-core systematic biopsies, using the transperineal
method. Immediately after each biopsy, the spatial punctured needle
orbits were recorded in the 3D model reconstructed from MRI.
Pathological analysis
All biopsy samples were examined by two senior
pathologists in cohesion. Clinically significant cancer was defined
as follows: A minimum of one core with a Gleason score (18) of 3+4 or 6 with a maximum cancer core
length >4 mm (10). The
pathological biopsy results were compared with the images from
mpMRI and 3D SWE.
Statistical analysis
All statistical analyses were performed using IBM
SPSS® software version 19.0 (IBM Corp., Armonk, NY,
USA). For the detected and un-detected lesions by biopsy, the
difference in median tissue elasticity values was analyzed using
the Mann-Whitney U-test. The association between Young's modulus,
mpMRI data and pathological findings for the cancer-detected
lesions was assessed by Pearson correlation. P<0.05 was
considered to indicate statistically significant differences.
Young's modulus was evaluated in the detection of prostate cancer
using ROC analyses.
Results
Patient characteristics and cancer
detection by mpMRI
A total of 12 patients were included in the present
study. The patient characteristics are presented in Table I. The median age of the patients was
65 years (range, 49–78). The median pre-biopsy PSA value was 5.65
ng/ml (range, 4.14–10.91). The median prostate volume was 28 ml
(range, 22–32). The median number of biopsies for each patient was
13 cores (range, 13–15). The median number of targeted biopsies for
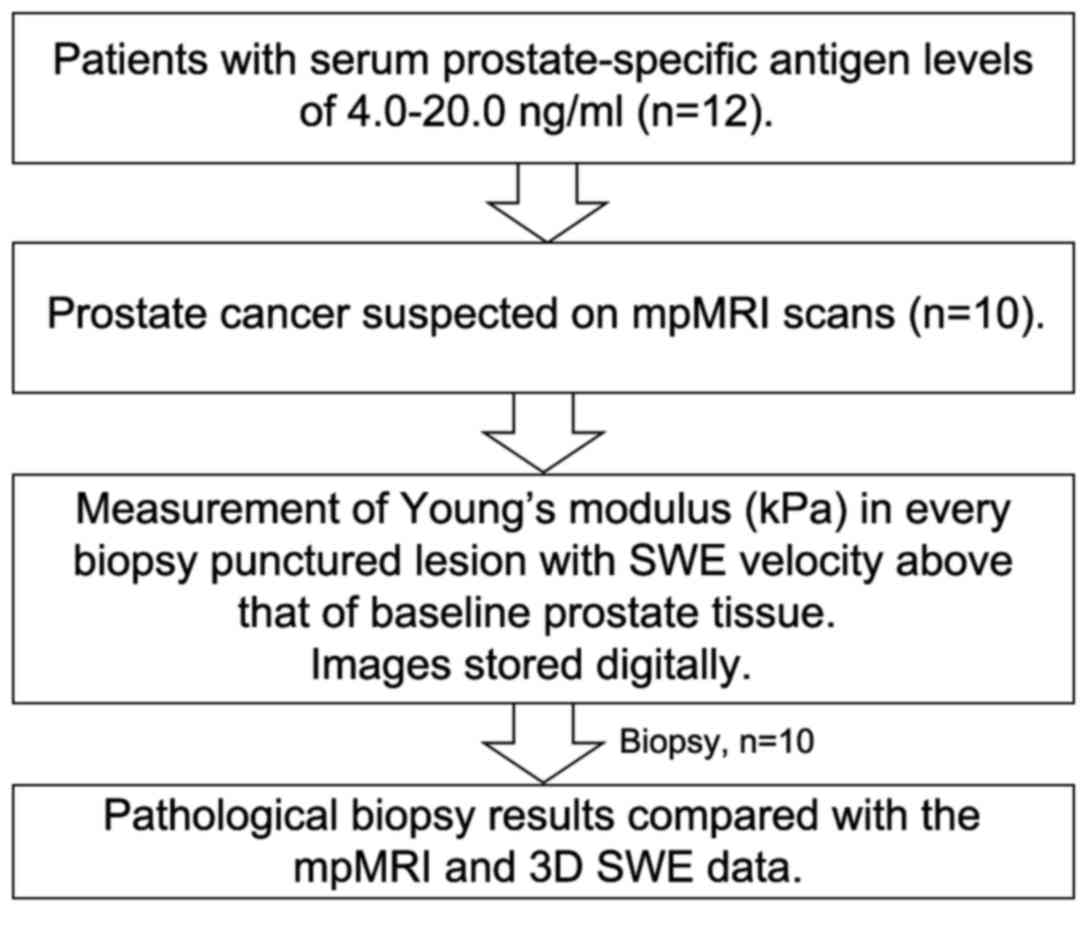
each patient was 2 cores (range, 1–3). The diagnostic procedure is
depicted in Fig. 1. Of the 12
patients, 10 patients were diagnosed with prostate cancer by mpMRI.
The median lengths of the maximum and minimum diameters of the
cancer-detected lesions on T2-weighted image MRI were 10.2 mm
(range, 4.8–24.0) and 5.8 mm (range, 3.0–12.0), respectively. Using
mpMRI, 28 suspected cancer lesions were diagnosed, comprising of 13
lesions of PI-RADS score 3, 10 lesions of PI-RADS score 4and 5
lesions of PI-RADS score 5. Cancer diagnosis was confirmed for 21
of the 28 lesions (75%), including 62% (8/13) of the lesions with
PI-RADS score 3, 80% (8/10) of the lesions with PI-RADS score 4 and
100% (5/5) of the lesions with PI-RADS score 5. The sensitivity,
specificity, PPV and NPV of cancer detection based on PI-RADS score
≥3 were 70, 94, 75 and 92%, respectively.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Value |
|---|
| Median age, years
(range) | 65 (49–78) |
| Median
prostate-specific antigen, ng/ml (range) | 5.65
(4.14–10.91) |
| Median prostate
volume, ml (range) | 28 (22–32) |
| Median number of
biopsies for each patient, n (range) | 13 (13–15) |
| Median number of
targeted biopsies for each patient, n (range) | 2 (1–3) |
| Median length of
maximum diameter of cancer-detected lesions on T2WI MRI, mm
(range) | 10.2 (4.8–24.0) |
| Median length of
minimum diameter of cancer-detected lesions on T2WI MRI, mm
(range) | 5.8 (3.0–12.0) |
Cancer detection by 3D SWE and
mpMRI
Using 3D SWE, all of the suspected cancer lesions
assessed by mpMRI were detected and measured for tissue elasticity
value. The median tissue elasticity value for the cancer-detected
areas was significantly higher compared with that of the undetected
areas [63.6 kPa (range, 18.8–85.7) vs. 24.1 kPa (range 5.8–58.9),
P<0.0001]. Similarly, in the targeted biopsy lesions, tissue
elasticity was significantly higher in the cancer-detected areas
(median 64.1, range 17.2–91.5, n=20) compared with the undetected
areas (median 30.8, range 11.2–38.8, n=8), respectively
(P<0.0001; data not shown). On ROC analysis, the cut-off value
of the Young's modulus was determined to be 41.0 kPa for the
detection of clinically significant cancer, with which the
sensitivity, specificity, PPV and NPV of cancer detection were 58,
97, 86 and 87%, respectively. When this value for Young's modulus
was used in conjunction with PI-RADS score, the cancer detection
rates in suspected cancer lesions (PI-RADS score ≥3) with Young's
modulus >41.0 kPa and <41.0 kPa were 91% (21/23 lesions) and
0% (0/5 lesions), respectively. Thus, by combining the cut-off
value of Young's modulus with PI-RADS score, cancer was diagnosed
in 21 of 23 lesions (91%), including 89% (8/9) of the lesions with
PI-RADS score 3, 89% (8/9) of the lesions with PI-RADS score 4 and
100% (5/5) of the lesions with PI-RADS score 5. Furthermore, when
combining the cut-off value of Young's modulus with PI-RADS score,
the sensitivity, specificity, PPV and NPV of cancer detection in
confirmed cancer lesions were 70, 98, 91 and 92%, respectively
(data not shown). In these cancer-detected lesions, a significant
association was identified between the tissue elasticity value of
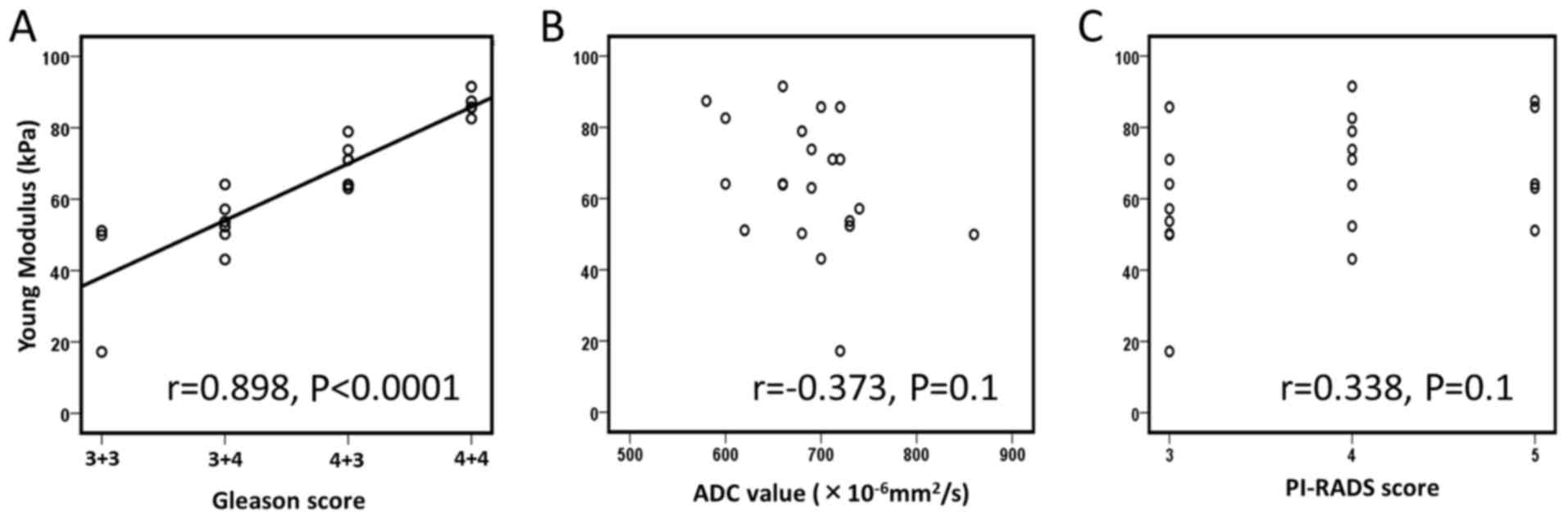
the lesions and Gleason score (r=0.898, P<0.0001); however,
there was no association between the tissue elasticity value of the
lesions with ADC value (r=−0.373, P=0.1) or PI-RADS score (r=0.338,
P=0.1), respectively (Fig. 2).
Fig. 3 depicts representative images
of the mpMRI, TRUS and 3D SWE scans performed on patients.
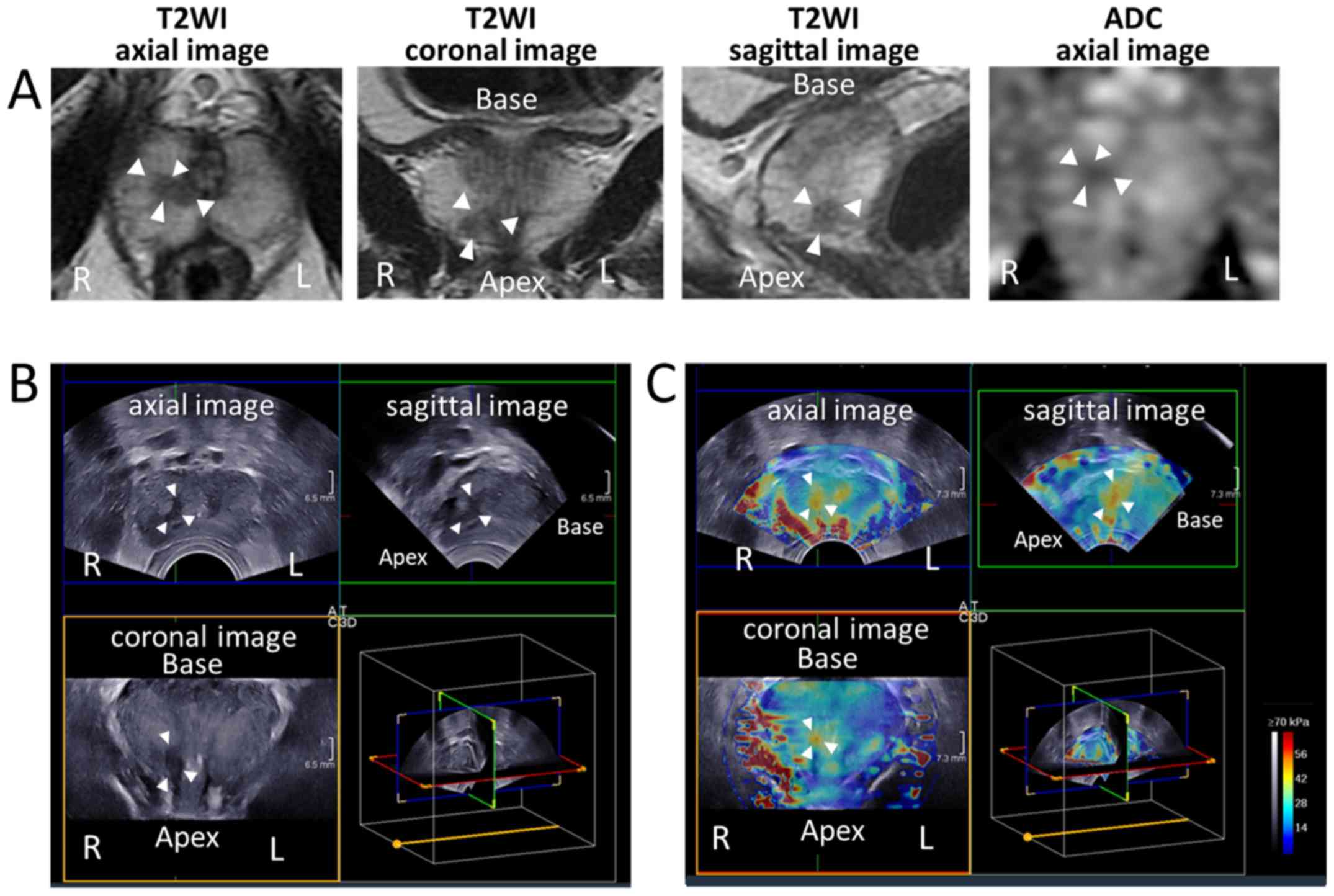 | Figure 3.Representative images of mpMRI, 3D
gray scale TRUS and 3D SWE scans of patients. (A) MRI, (B) 3D gray
scale TRUS and (C) 3D SWE images of the prostate in a patient
determined to have clinically significant cancer on mpMRI. White
arrowheads indicate the cancer-suspected lesion. The tissue
elasticity value of the lesion was 43.1 kPa, and pathological
examination of biopsy specimens from the lesion indicated
adenocarcinoma of Gleason score 3+3=6. mpMRI, multi-parametric
magnetic resonance imaging; TRUS, transrectal ultrasound; SWE,
shear wave elastography; 3D, three-dimensional; T2WI, T2-weighted
image; ADC, apparent diffusion coefficient; R, right; L, left. |
Discussion
SWE evaluates tissue elasticity, or Vs, by using a
sonographic push pulse to generate a shear wave in the tissues; Vs
through the tissue is affected by tissue stiffness, with stiffer
tissues accommodating faster movement (5). Previous reports have indicated the
clinical applications of SWE (19,20). Ahmad
et al (19) reported that data
analyzed per core regarding SWE findings indicated that for
patients with serum PSA <20 ng/ml, the sensitivity and
specificity of SWE for prostate cancer detection were 90 and 88%,
respectively, while in patients with PSA >20 ng/ml, the
sensitivity and specificity were 93 and 93%, respectively. Correas
et al (20) reported that the
sensitivity, specificity, PPV, NPV and AUC for SWE with a cut-off
of 35 kPa for differentiating benign from malignant lesions in
1,040 peripheral zone sextants from 184 patients were 96% (95% CI:
95–97), 85% (95% CI: 83–87), 48% (95% CI: 46–50), 99% (95% CI:
98–100) and 95% (95% CI: 93–97), respectively. In the present
study, when combining the cut-off value of 41.0 kPa for tissue
elasticity to PI-RADS score, the sensitivity, specificity, PPV and
NPV of cancer detection were 70, 98, 91 and 92%, respectively.
Based on these results, 3D SWE may have potential for improving the
detection of significant prostate cancer.
Although the majority of prostate cancers are harder
than normal prostatic tissue (2),
elastography is not included in mpMRI. The applications of
elastography in the detection of significant prostate cancer was
evaluated using 3D SWE in the present study. In a previous report
on SWE, it was observed that prostate cores with a Gleason score of
7 had a higher mean Young's modulus (163±63 kPa) compared with
cores with a Gleason score of 6 (95±28.5 kPa; P=0.007) (19). Similarly, Woo et al (21) reported that Young's modulus was
significantly correlated with Gleason score (r=0.343, P=0.002). In
the present study, a significant association was identified between
the tissue elasticity value of prostatic lesions and Gleason score
(r=0.898, P<0.0001), while there were no associations between
the tissue elasticity value and ADC value (P=0.1) or PI-RADS score
(P=0.1). Additionally, there was no significant association between
ADC value and Gleason score in the cancer-detected lesions in the
present series (P=0.1). These results indicate the potential of
Young's modulus to predict Gleason scores more accurately compared
with ADC value, possibly due to the different approach of measuring
the targeted lesion.
The current study had several limitations. First, it
was a single-institutional study and patients were not randomized
to facilitate comparison of biopsy techniques. Furthermore,
although previous reports have demonstrated high reproducibility of
SWE (8), a single operator performed
the 3D SWE in the present study. Therefore, a multi-institutional
randomized study is now required to confirm the efficacy of the
biopsy methods. Second, the study lacked a comparison of biopsy
results and pathological findings of whole-gland specimens.
Therefore, although the locations and pathological grades of
clinically significant cancers corresponded to the results of the
targeted biopsies, it is possible that a clinically significant
cancer was omitted in the absence of large-scale pathological
analysis of whole-gland specimens.
In conclusion, the tissue elasticity values of
cancer-detected areas were significantly higher compared with those
of undetected areas (P<0.0001), and PI-RADS combined with
measurement of Young's modulus by 3D SWE may have potential for
improving the diagnosis of clinically significant prostate cancer.
However, additional multi-institutional studies are now required to
validate the usefulness of 3D SWE in detecting clinically
significant prostate cancer.
Glossary
Abbreviations
Abbreviations:
|
PCa
|
prostate cancer
|
|
SWE
|
shear wave elastography
|
|
2D
|
two-dimensional
|
|
PSA
|
prostate-specific antigen
|
|
mpMRI
|
multi-parametric magnetic resonance
imaging
|
|
US
|
ultrasound
|
|
3D
|
three-dimensional
|
|
TRUS
|
transrectal ultrasound
|
|
DCE
|
dynamic contrast enhanced
|
|
PI-RADS
|
Prostate Imaging Reporting and Data
System
|
|
ROC
|
receiver operating characteristic
|
|
Vs
|
shear wave velocity
|
|
APC
|
apparent diffusion coefficient
|
|
PPV
|
positive predictive value
|
|
NPV
|
negative predictive value
|
|
ROI
|
regions of interest
|
|
CI
|
confidence interval
|
References
|
1
|
Postema A, Mischi M, de la Rosette J and
Wijkstra H: Multiparametric ultrasound in the detection of prostate
cancer: A systematic review. World J Urol. 33:1651–1659. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Good DW, Stewart GD, Hammer S, Scanlan P,
Shu W, Phipps S, Reuben R and McNeill AS: Elasticity as a biomarker
for prostate cancer: A systematic review. BJU Int. 113:523–534.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aigner F, Pallwein L, Schocke M, Lebovici
A, Junker D, Schäfer G, Mikuz G, Pedross F, Horninger W, Jaschke W,
et al: Comparison of real-time sonoelastography with T2-weighted
endorectal magnetic resonance imaging for prostate cancer
detection. J Ultrasound Med. 30:643–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correas JM, Tissier AM, Khairoune A,
Khoury G, Eiss D and Hélénon O: Ultrasound elastography of the
prostate: State of the art. Diagn Interv Imaging. 94:551–560. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nelson ED, Slotoroff CB, Gomella LG and
Halpern EJ: Targeted biopsy of the prostate: The impact of color
Doppler imaging and elastography on prostate cancer detection and
Gleason score. Urology. 70:1136–1140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ophir J, Garra B, Kallel F, Konofagou E,
Krouskop T, Righetti R and Varghese T: Elastographic imaging.
Ultrasound Med Biol. 26 Suppl 1:S23–S29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitri FG, Urban MW, Fatemi M and Greenleaf
JF: Shear wave dispersion ultrasonic vibrometry for measuring
prostate shear stiffness and viscosity: An in vitro pilot study.
IEEE Trans Biomed Eng. 58:235–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woo S, Kim SY, Lee MS, Cho JY and Kim SH:
Shear wave elastography assessment in the prostate: An
intraobserver reproducibility study. Clin Imaging. 39:484–487.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barr RG, Memo R and Schaub CR: Shear wave
ultrasound elastography of the prostate: Initial results.
Ultrasound Q. 28:13–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harnden P, Naylor B, Shelley MD, Clements
H, Coles B and Mason MD: The clinical management of patients with a
small volume of prostatic cancer on biopsy: What are the risks of
progression? A systematic review and meta-analysis. Cancer.
112:971–981. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vilanova JC, Barceló-Vidal C, Comet J,
Boada M, Barceló J, Ferrer J and Albanell J: Usefulness of
prebiopsy multifunctional and morphologic MRI combined with
free-to-total prostate-specific antigen ratio in the detection of
prostate cancer. AJR Am J Roentgenol. 196:W715–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Delongchamps NB, Rouanne M, Flam T, Beuvon
F, Liberatore M, Zerbib M and Cornud F: Multiparametric magnetic
resonance imaging for the detection and localization of prostate
cancer: Combination of T2-weighted, dynamic contrast-enhanced and
diffusion-weighted imaging. BJU Int. 107:1411–1418. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barentsz JO, Richenberg J, Clements R,
Choyke P, Verma S, Villeirs G, Rouviere O, Logager V and Fütterer
JJ; European Society of Urogenital Radiology, : ESUR prostate MR
guidelines 2012. Eur Radiol. 22:746–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Röthke M, Blondin D, Schlemmer HP and
Franiel T: PI-RADS classification: Structured reporting for MRI of
the prostate. Rofo. 185:253–261. 2013.(In German). PubMed/NCBI
|
|
15
|
Hamoen EHJ, de Rooij M, Witjes JA,
Barentsz JO and Rovers MM: Use of the Prostate Imaging Reporting
and Data System (PI-RADS) for Prostate Cancer Detection with
Multiparametric Magnetic Resonance Imaging: A Diagnostic
Meta-analysis. Eur Urol. 67:1112–1121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oto A, Kayhan A, Jiang Y, Tretiakova M,
Yang C, Antic T, Dahi F, Shalhav AL, Karczmar G and Stadler WM:
Prostate cancer: Differentiation of central gland cancer from
benign prostatic hyperplasia by using diffusion-weighted and
dynamic contrast-enhanced MR imaging. Radiology. 257:715–723. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shoji S, Hiraiwa S, Ogawa T, Kawakami M,
Nakano M, Hashida K, Sato Y, Hasebe T, Uchida T and Tajiri T:
Accuracy of real-time magnetic resonance imaging-transrectal
ultrasound fusion image-guided transperineal target biopsy with
needle tracking with a mechanical position-encoded stepper in
detecting significant prostate cancer in biopsy-naïve men. Int J
Urol. 24:288–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gleason DF, Mellinger GT, Arduino LJ,
Bailar JC III, Becker LE III, Berman HI III, Bischoff AJ, Byar DP,
Blackard CE, Doe RP, et al: Prediction of prognosis for prostatic
adenocarcinoma by combined histological grading and clinical
staging. J Urol. 111:58–64. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmad S, Cao R, Varghese T, Bidaut L and
Nabi G: Transrectal quantitative shear wave elastography in the
detection and characterisation of prostate cancer. Surg Endosc.
27:3280–3287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Correas JM, Tissier AM, Khairoune A,
Vassiliu V, Méjean A, Hélénon O, Memo R and Barr RG: Prostate
cancer: Diagnostic performance of real-time shear-wave
elastography. Radiology. 275:280–289. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Woo S, Kim SY, Cho JY and Kim SH: Shear
wave elastography for detection of prostate cancer: A preliminary
study. Korean J Radiol. 15:346–355. 2014. View Article : Google Scholar : PubMed/NCBI
|