Cancer arises from when cell survival and
proliferation are favored over cell death, resulting in a
disequilibrium (1). Traditional
cancer treatments include chemotherapy, radiation and surgery;
however, these therapies have limitations and a risk of cancer
recurrence remains following the treatments (1). It has been reported that the recurrence
rate of non-small cell lung cancer (NSCLC) is 30–50% (2). Furthermore, although chemotherapy and
radiation are able to effectively control the mitosis of tumor
cells, they also cause harm to normal tissues. In previous years,
researchers have reported that cancer is associated with
deficiencies of the immune system. In this regard, researchers have
been prompted to consider immunotherapy as a potential approach for
the treatment of cancer (3). To date,
substantial research data have indicated the effectiveness of
immunotherapy (2). Survivin is highly
expressed in cancer cells (Table I),
whereas it is expressed at a low level in normal adult tissues that
have terminated proliferation (3).
Survivin is considered as a breakthrough target in this approach
and many therapeutic strategies, including small-molecule
inhibitors and molecular antagonists, have been developed (3). Although low levels of survivin are
expressed in terminally differentiated tissues, it is abundantly
expressed in proliferating adult tissues; therefore, it is
essential to investigate the potential for toxicity during therapy
and to reduce the occurrence of adverse side effects (4,5).
Unfortunately, survivin has no known catalytic activity, making it
challenging to target (6).
Survivin, also called baculoviral inhibitor of
apoptosis repeat-containing 5, is a member of the inhibitor of
apoptosis protein family (IAP), which also includes X-linked
inhibitor of apoptosis (XIAP), cIAP1, cIAP2, NOD-like receptor
family apoptosis inhibitory protein, livin, IAP-like protein 2 and
baculovirus inhibitor of apoptosis protein repeat (BIR) containing
ubiquitin-conjugating enzyme, isoform C (7,8). Survivin
is a 142-amino acid, 16.5-kDa protein encoded by a single gene
located on human chromosome 17q25, consisting of an N-terminal
Zn2+-binding BIR domain linked to a 65A° amphipathic
C-terminal α-helix, as well as 3 introns and 4 exons (3,9–11). Heat shock protein 90 (HSP90) maintains
the stability and folding of multiple bioenergetic effectors of
survivin (12). Unlike other IAP
members, survivin is highly expressed in the majority of neoplasms,
whereas it is rarely expressed in normal adult tissues (13). Increased levels of survivin
effectively inhibit apoptosis (14–17), and
so survivin overexpression has an impact on the abnormal
proliferation of various cancer cells. According to a previous
study, survivin overexpression is associated with reduced
expression of the cell adhesion molecules cluster of
differentiation CD31 and CD44 in certain cells, which enable them
to avoid contact inhibition and undergo abnormal proliferation
(18). Furthermore, survivin has been
reported to be overexpressed in gastric cancer cells during
chemotherapy, suggesting that survivin may be responsible for
chemoresistance in gastric cancer (19–21). In
addition, a previous study demonstrated that survivin expression
was enhanced in recurrent glioblastoma multiforme (GBM) tumors
(22). Survivin may also be
associated with cell-damaging processes, including transfection
(22). Survivin expression has been
demonstrated to be associated with p53 expression, which may be
induced by cell damage (5). Together,
the reported properties of survivin make it an effective prognostic
index for postsurgical patients with GBM (22) and a notable tumor marker (23). Survivin overexpression affects cancer
cell proliferation via a number of routes and researchers have
developed corresponding survivin-targeted therapies, as discussed
below.
It has previously been reported that the major
function of survivin is to cell division, rather than to act as a
direct inhibitor of apoptosis (9).
Survivin serves an essential role as a chromosomal passenger
protein (CPP) and as a regulator of microtubule dynamics, ensuring
that chromosomes occupy the correct positions during mitosis for
accurate cell division (9). Survivin
levels increase in G1 phase and reach a peak in G2M phase (9,24).
Decreased transcription rates induce the upregulation of survivin
in the G2/M phase (25). Survivin
affects centrosomes and microtubules during metaphase and anaphase,
stabilizing and ensuring the separation of sister chromatids
(9). In addition, survivin is
responsible for regulating chromosome congression and the
progression of mitosis, along with microtubule dynamics and
elevated microtubule stability (26).
It has previously been reported that survivin interacts with both
Aurora-B and inner centrome protein to form a CPP complex, which is
vital to cytokinesis (27).
Elimination of survivin results in deficient mitosis and the
activation of spindle checkpoints regulated by the tumor suppressor
protein p53, ultimately resulting in apoptosis of the dividing
cells (28,29). Survivin silencing leads to DNA
double-stranded breaks (DSBs) in cancer cells and functionally
reduces homologous recombination (30). Even though it serves a critical role
in regulating CPP targeting, survivin is not the only protein in
the CPP family that contributes to the stability of the structure
(23).
Survivin is a potent anti-apoptosis factor and is
inversely mediated by p53 at the mRNA and protein levels (31,32).
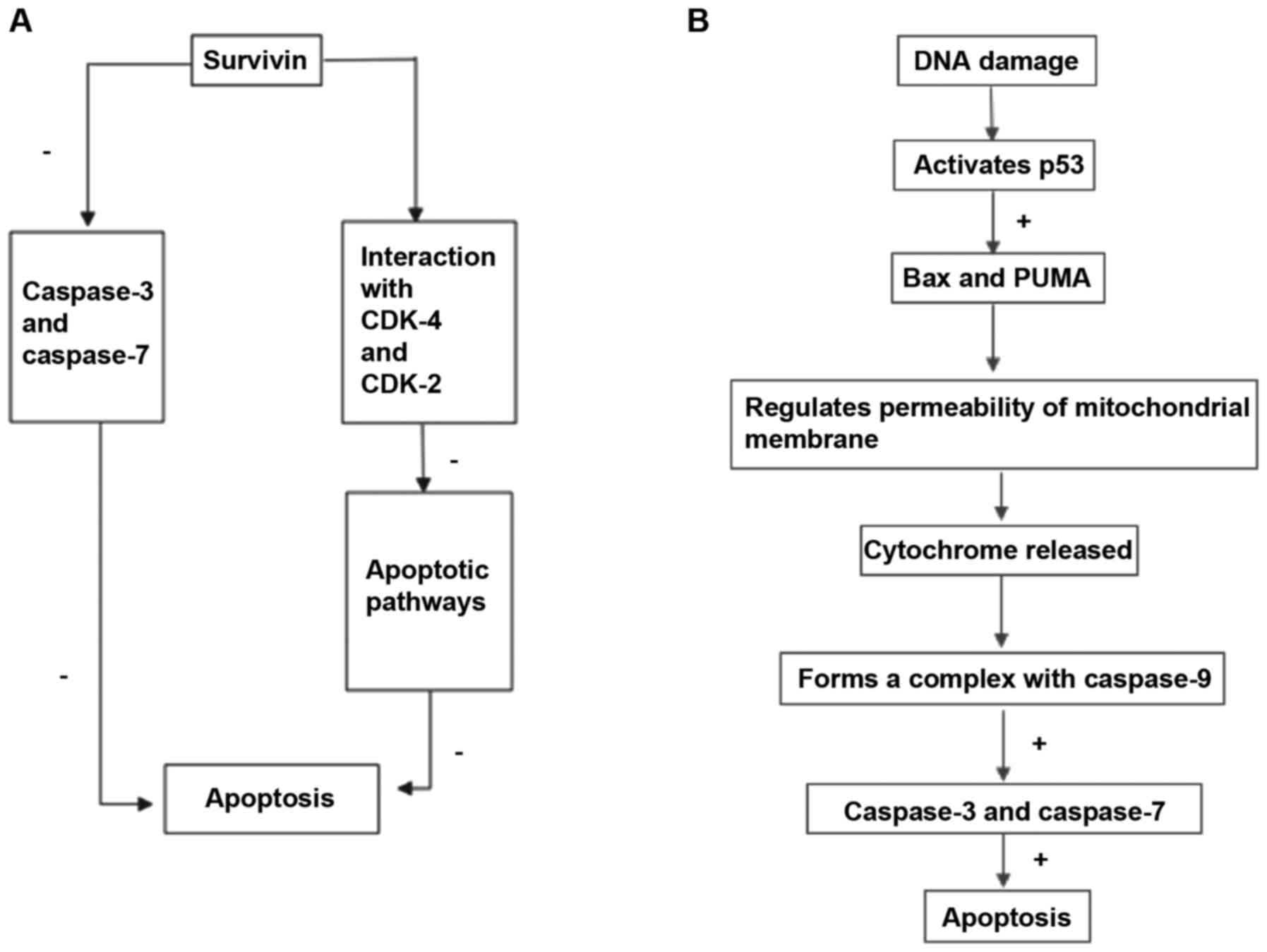
Survivin suppresses programmed cell death in two ways (Fig. 1); firstly, it directly suppresses the
activities of terminal effector enzymes caspase-3 and caspase-7 to
resist cell apoptosis induced by specific stimuli (33). Secondly, interactions between survivin
and the cyclin-dependent kinase (CDK)-4 and CDK-2 suppress
apoptotic signaling pathways (33).
Survivin overexpression suppresses the extrinsic and intrinsic
apoptosis pathways (23) and
apoptosis is stimulated when survivin is depleted in human cells
(34). During cell apoptosis, DNA
damage activates p53, which stimulates the transcription of B-cell
lymphoma 2-associated X protein Bax and p53 upregulated modulator
of apoptosis (35). Subsequently, the
gene products regulate the permeability of the mitochondrial
membrane and cytochrome release. Cytochrome c binds with
apoptotic protease activating factor-1, forming a complex with
caspase-9; this complex activates caspase-3 and caspase-7 which in
turn activate apoptosis (35).
Through binding with the cofactor XIAP, survivin suppresses the
activity of caspase-9, functioning as an anti-apoptosis factor
(35). Functional inhibition of
survivin using small interfering (si)RNA and ribozymes may
therefore be used to enhance tumor cell sensitivity to existing
pharmacological agents (35).
Wheatley (36) confirmed that the
C-terminus of survivin is essential for cell division, whereas the
N-terminus of survivin serves a role in apoptosis. Although a dual
role of survivin in apoptosis inhibition and spindle dynamics
regulation has been reported (26),
further studies are required to improve our understanding of the
connection between the two roles of survivin.
Survivin is undetectable in the majority of
non-proliferating, fully differentiated cells, except for
CD34+ hematopoietic stem cells, placental cells and
basal cells of the colonic epithelium and thymus (37). Survivin is highly expressed in a
number of cancers, including lung, breast, colon, brain, gastric,
esophageal, pancreatic, liver, uterine and ovarian cancer cells
(37). The unique properties of
survivin make it a useful molecule for studying the potential
biology of tumorigenesis and provide a basis for modifying and
constructing molecules that specifically target and suppress cancer
cells (37). In tumor cells, survivin
accumulates and localizes to the mitochondria (16), enhancing cell resistance to apoptosis
(38) and impacting organelle
bioenergy (39). In this way,
survivin functions as a potential cancer driver. Survivin enhances
the survival of cancer cells as part of several molecular networks
associated with major apoptotic regulators, including caspases,
XIAP and the endogenous survivin inhibitor second
mitochondria-derived activator of caspases (38,40). DNA
DSBs are a common challenge for cancer cells, the fate of which
depend largely on their ability to perform DSB repair, which in
turn depends on homologous recombination and non-homologous end
joining (30). It has been reported
that survivin elimination may impair DNA repair via homologous
recombination (30). According to a
previous study, survivin is vital for efficient DNA repair, as the
elimination of survivin results in reduced expression of several
major regulators of DNA repair and impairs gene expression
essential to repair onset. Survivin silencing also resulted in DNA
DSBs in breast cancer cells and a reduction in homologous
recombination (30). Furthermore,
survivin inhibition has been reported to initiate the p53 response
and enhance the vulnerability of cells to poly ADP-ribose
polymerase inhibition (30).
According to other research, patients with higher survivin levels
in tumor tissues are at increased risk of relapse and
chemoresistance (37).
YM155 is a small-molecule survivin suppressor that
distinctly interacts with the survivin core promoter region of 269
base pairs, specifically inhibiting the expression of survivin
(4,53). YM155 has effects on gene expression
and phosphorylation (54). A certain
study demonstrated that YM155 effectively inhibited the expression
of survivin mRNA in SGC-7901 and MKN-28 cells in a dose-dependent
manner (55). YM155 inhibits survivin
expression by interfering with the binding of Sp1 and survivin
promoter (56). YM155 has been
evaluated in phase II clinical trials for breast cancer (57), melanoma (58) and NSCLC (59). Furthermore, a number of studies have
reported that YM155 is able to effectively inhibit survivin
expression and induce the apoptosis of human cancer cells (60), as well as promoting the expansion of
CD44+ CSCs (55). It has
been confirmed that YM155 is able to overcome drug resistance in
tumors when used with other chemotherapeutical agents; for example,
YM155 reversed rapamycin resistance in rapamycin-resistant renal
cell carcinoma (61). It has also
been reported that YM155 is able to inhibit the progression of
gastric cancer cells. Notably, it was demonstrated that gastric
cancer SGC-7901 cells treated with YM155 formed smaller and fewer
colonies compared with a control group (55). These results indicate that YM155
suppresses anchorage-dependent and anchorage-independent
proliferation in gastric cancer cells (55). It has been observed that YM155
exhibits potent antiproliferative effects against human leukemia
cell lines in a dose-dependent manner (55). Furthermore, it has been demonstrated
that activation of caspase-8, an important protein associated with
the extrinsic apoptosis pathway, occurs in cell lines treated with
YM155 (62). Rivadeneira et al
(63) demonstrated that YM155 is able
to disrupt mitochondrial bioenergetics and thus activate tumor
suppressor mechanisms involving AMP-activated protein kinase
activation or mammalian target of rapamycin inhibition. There may
be other mechanisms by which YM155 inhibits cancer cell progression
(54). According to Chang et
al (54), YM155 activates the DNA
damage pathway. Following 24-h treatment with 100 nM dasatinib DNA
damage was significantly increased in the presence of YM155. This
study confirmed that YM155 is able to activate the DNA damage
response pathways via S phase arrest, which elevates p53,
checkpoint kinase 2 and H2AX phosphorylation, eventually resulting
in apoptosis (54). An increasing
number of studies have suggested that YM155 may have more
off-target effects that result in cell death, including inhibition
of Mcl expression and direct DNA damage (62,64,65). It
has also been reported that YM155 may induce autophagy-dependent
DNA damage in breast carcinoma via a survivin-XIAP-dependent
mechanism (66). YM155 inhibits
survivin and also mediates the expression of major genes, including
death receptor signaling and tumor necrosis factor receptor 1
signaling factors, that serve a role in apoptosis induction via the
extrinsic apoptotic pathway (67).
YM155 may have potential as an effective inhibitor of nuclear
factor-κB and its downstream target gene matrix
metalloproteinase-9, which in turn inhibits the growth, invasion
and metastasis of survivin-enriched oral squamous cell carcinoma
cells (68). Furthermore, YM155 does
not affect normal tissues (55); in
in phase I studies, YM155 was demonstrated to be tolerable in
patients with advanced cancer, as well as exhibiting anti-tumor
activity in patients with non-Hodgkin's lymphoma and hereditary
papillary renal carcinoma (69,70). YM155
has been investigated as a single-agent first-line treatment in 34
patients with metastatic melanoma in a phase II study (71). Of these patients, one exhibited a
complete response, one exhibited a partial response and 11 retained
stable disease (71). A clinical
trial, in which the efficacy of YM155 as a single agent or in
combination with either immunotherapy or cytotoxic chemotherapy was
investigated, confirmed that the drug is fairly tolerable under
such conditions (72). However, the
response has been minimal. Chang et al (54) suggested that patients need to be
pre-selected for YM155 sensitivity to guarantee beneficial
outcomes. Their findings confirmed that YM155 is an ideal candidate
drug for therapeutic regimens when administered to a certain
subgroup of patients (54). Further
study is required to identify the underlying mechanism of selective
sensitivity to YM155 in cancer cells.
LY2181308 is a novel second-generation 18-mer
antisense oligonucleotide (ASO). LY2181308 is able to bind to human
survivin mRNA and suppress translation, restoring the apoptotic
pathway in cancer cells (73). In
preclinical models, LY2181308 has exhibited antitumor activities
when combined with docetaxel, which is one of several
chemotherapeutic options for patients with advanced metastatic
NSCLC who have no responded to first-line treatment (74,75).
Compared with phosphorothioates, second-generation ASOs exhibit a
higher level of stability, an improved pharmacokinetic profile,
increased potency and reduced toxicity (76). However, many patients have exhibited
flu-like symptoms in studies involving LY2181308 (77,78). In a
phase I study involving 14 patients with malignant solid tumors,
flu-like syndrome, prolonged prothrombin time-international
normalized ratio, thrombocytopenia and fatigue were common
reversible grade 1/2 toxicities (78). These results indicated that LY2181308
monotherapy is tolerable at doses up to 750 mg; however, the
efficacy of LY2181308 in combination with other toxic therapeutic
agents requires further study (78).
A pharmacodynamics study was performed for 34 patients with
advanced or metastatic malignancies, 22 of whom were available for
pre- and post-treatment biopsies (79). Immunohistochemistry revealed a
reduction in survivin levels in the nucleus and cytoplasm of 11/17
and 5/14 evaluable pairs, respectively. Gene expression analysis
also indicated that there was a 20–50% reduction in survivin
expression in 11/15 of the evaluable pairs. In addition, analysis
of fresh tumor tissues revealed that 2/3 patients with NSCLC
exhibited a near-complete elimination of survivin-positive cells
along with an elevation in the fraction of cells with a sub-G1 DNA
content, which is consistent with cell death (79).
Shepherdin is a small molecule peptidomimetic
inhibitor of only 5 amino acids in length (80). It functions as an antagonist of the
survivin-HSP complex and is now under early-stage clinical
development (80). HSP90 binds to
substrate proteins that are in a near-native state, contributing to
the stability of survivin; it has been postulated that the
ATP-bound state of HSP90 binds stably to substrate polypeptides,
held by an internally dimerized clamp (80). ATP hydrolysis facilitates the release
of the substrate, leading to conformational changes in HSP90
(81). Shepherdin is able to
effectively counteract the binding of HSP90 with survivin (80). Therefore, it functions as an HSP90
global inhibitor via competition inhibition with ATP (80).
RNA interference by siRNA may be used to reduce the
expression of a target gene in a sequence-specific manner via
degradation of the corresponding mRNA (82–85). siRNA
molecules are 19–21 nt in length and have a molecular weight of
13–15 kDa with 38–46 negative charges (86). siRNA-induced gene silencing is highly
efficient and specific to target genes, and so has applications in
cancer treatment (87,88). Unmodified siRNA is problematic and
modifying siRNAs may impair their activity, which makes the
development of siRNA-based agents difficult (89,90).
Furthermore, siRNAs are not taken up by the majority of mammalian
cells in a way that maintains their activity (91). Recent progress in the structural
modification of sticky siRNA includes hybridization reactions with
sticky siRNA and chemical polymerization of sticky siRNA (89). However, building clinically successful
siRNA-based structures remains challenging (89). A lack of effective siRNA delivery into
target cells is the main issue preventing the clinical use of siRNA
therapeutics. The cell trafficking pathways of siRNA are not well
understood and so cannot inform pharmacological development
(92,93). These factors suggest that siRNA should
be studied further to elucidate its potential as a therapeutic
agent.
The role of IAPs in cellular homeostasis has been
widely investigated in the past decades. Of the IAP family, the
survivin protein serves several roles in various processes related
to the survival of cells. Survivin is highly expressed in a number
of types of tumor and is associated with the proliferation and
invasion of cancer cells, radiation and chemotherapy resistance and
poor prognosis. Furthermore, survivin is highly expressed in tumor
cells while it is expressed at low levels in normal, terminally
differentiated cells. Survivin may serve roles in cell survival by
affecting complex intracellular signaling, stabilizing mitosis and
facilitating cellular adaptation. These properties make survivin a
potential therapeutic target for the treatment of cancer. Further
studies are required to identify other signaling pathways through
which it functions, as its other effects in tumor cells.
Elucidating the mechanisms by which survivin regulates cell growth
may assist in the development of therapeutic approaches in
pre-clinical settings. Recent progress in this field includes the
discovery of transcriptional repressors, mRNA inhibitors, small
molecule survivin inhibitors and immunotherapy as potential
treatments. However, these approaches are flawed and may not be
suitable for use in clinical settings; as such, further
investigation is required to better understand survivin.
The authors are thankful for the assistance provided
by Fang Chen from Linyi People's Hospital (Linyi, China).
No funding was received.
Not applicable.
HL designed the structure of the paper and advised
DL and CH during writing. DL and CH wrote the majority of the
contents in this manuscript. All authors read and approved the
final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Mobahat M, Narendran A and Riabowol K:
Survivin as a preferential target for cancer therapy. Int J Mol
Sci. 15:2494–2516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
al-Kattan K, Sepsas E, Fountain SW and
Townsend ER: Disease recurrence after resection for stage I lung
cancer. Eur J Cardiothorac Surg. 12:380–384. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iwasa T, Okamoto I, Suzuki M, Nakahara T,
Yamanaka K, Hatashita E, Yamada Y, Fukuoka M, Ono K and Nakagawa K:
Radiosensitizing effect of YM155, a novel small-molecule survivin
suppressant, in non-small cell lung cancer cell lines. Clin Cancer
Res. 14:6496–6504. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li WL, Lee MR and Cho MY: The small
molecule survivin inhibitor YM155 may be an effective treatment
modality for colon cancer through increasing apoptosis. Biochem
Biophys Res Commun. 471:309–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hunter AM, LaCasse EC and Korneluk RG: The
inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis.
12:1543–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schimmer AD: Inhibitor of apoptosis
proteins: Translating basic knowledge into clinical practice.
Cancer Res. 64:7183–7190. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li F and Altieri DC: The cancer
antiapoptosis mouse survivin gene: Characterization of locus and
transcriptional requirements of basal and cell cycle-dependent
expression. Cancer Res. 59:3143–3151. 1999.PubMed/NCBI
|
|
11
|
Chantalat L, Skoufias DA, Kleman JP, Jung
B, Dideberg O and Margolis RL: Crystal structure of human survivin
reveals a bow tie-shaped dimer with two unusual alpha-helical
extensions. Mol Cell. 6:183–189. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chae YC, Angelin A, Lisanti S, Kossenkov
AV, Speicher KD, Wang H, Powers JF, Tischler AS, Pacak K, Fliedner
S, et al: Landscape of the mitochondrial Hsp90 metabolome in
tumours. Nat Commun. 4:21392013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheung CH, Huang CC, Tsai FY, Lee JY,
Cheng SM, Chang YC, Huang YC, Chen SH and Chang JY: Survivin -
biology and potential as a therapeutic target in oncology. Onco
Targets Ther. 6:1453–1462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tazo Y, Hara A, Onda T and Saegusa M:
Bifunctional roles of survivin-Delta Ex3 and survivin-2B for
susceptibility to apoptosis in endometrial carcinomas. J Cancer Res
Clin. 140:2027–2037. 2014. View Article : Google Scholar
|
|
15
|
Liu YB, Gao X, Deeb D, Brigolin C, Zhang
Y, Shaw J, Pindolia K and Gautam SC: Ubiquitin-proteasomal
degradation of antiapoptotic survivin facilitates induction of
apoptosis in prostate cancer cells by pristimerin. Int J Oncol.
45:1735–1741. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dohi T, Beltrami E, Wall NR, Plescia J and
Altieri DC: Mitochondrial survivin inhibits apoptosis and promotes
tumorigenesis. J Clin Invest. 114:1117–1127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altieri DC: Survivin and IAP proteins in
cell-death mechanisms. Biochem J. 430:199–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuneki M and Madri JA: CD44 regulation of
endothelial cell proliferation and apoptosis via modulation of CD31
and VE-cadherin expression. J Biol Chem. 289:5357–5370. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ikeguchi M, Liu J and Kaibara N:
Expression of survivin mRNA and protein in gastric cancer cell line
(MKN-45) during cisplatin treatment. Apoptosis. 7:23–29. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng WE, Chen H, Yuan SF, Wu LL, Zhang W,
Sun HY and Chen WJ: Overexpression of βIII-tubulin and survivin
associated with drug resistance to docetaxel-based chemotherapy in
advanced gastric cancer. J BUON. 17:284–290. 2012.PubMed/NCBI
|
|
21
|
Shen X, Zheng JY, Shi H, Zhang Z and Wang
WZ: Survivin knockdown enhances gastric cancer cell sensitivity to
radiation and chemotherapy in vitro and in nude mice. Am J Med Sci.
344:52–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guvenc H, Pavlyukov MS, Joshi K, Kurt H,
Banasavadi-Siddegowda YK, Mao P, Hong C, Yamada R, Kwon CH, Bhasin
D, et al: Impairment of glioma stem cell survival and growth by a
novel inhibitor for Survivin-Ran protein complex. Clin Cancer Res.
19:631–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garg H, Suri P, Gupta JC, Talwar GP and
Dubey S: Survivin: A unique target for tumor therapy. Cancer Cell
Int. 16:492016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lens SM, Wolthuis RM, Klompmaker R, Kauw
J, Agami R, Brummelkamp T, Kops G and Medema RH: Survivin is
required for a sustained spindle checkpoint arrest in response to
lack of tension. EMBO J. 22:2934–2947. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao J, Tenev T, Martins LM, Downward J
and Lemoine NR: The ubiquitin-proteasome pathway regulates survivin
degradation in a cell cycle-dependent manner. J Cell Sci.
113:4363–4371. 2000.PubMed/NCBI
|
|
26
|
Giodini A, Kallio MJ, Wall NR, Gorbsky GJ,
Tognin S, Marchisio PC, Symons M and Altieri DC: Regulation of
microtubule stability and mitotic progression by survivin. Cancer
Res. 62:2462–2467. 2002.PubMed/NCBI
|
|
27
|
Fangusaro JR, Caldas H, Jiang Y and Altura
RA: Survivin: An inhibitor of apoptosis in pediatric cancer.
Pediatr Blood Cancer. 47:4–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Altieri DC: Survivin in apoptosis control
and cell cycle regulation in cancer. Prog Cell Cycle Res.
5:447–452. 2003.PubMed/NCBI
|
|
29
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Véquaud E, Desplanques G, Jézéquel P, Juin
P and Barillé-Nion S: Survivin contributes to DNA repair by
homologous recombination in breast cancer cells. Breast Cancer Res
Treat. 155:53–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mirza A, McGuirk M, Hockenberry TN, Wu Q,
Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, et al:
Human survivin is negatively regulated by wild-type p53 and
participates in p53-dependent apoptotic pathway. Oncogene.
21:2613–2622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang M, Li B, Liu J and Sun H: Protection
effect of survivin protein overexpression on acute myocardial
infarction in rats. Int J Clin Exp Med. 8:12995–13000.
2015.PubMed/NCBI
|
|
33
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
34
|
Li F, Ackermann EJ, Bennett CF, Rothermel
AL, Plescia J, Tognin S, Villa A, Marchisio PC and Altieri DC:
Pleiotropic cell-division defects and apoptosis induced by
interference with survivin function. Nat Cell Biol. 1:461–466.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wheatley SP: The functional repertoire of
survivin's tails. Cell Cycle. 14:261–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dohi T, Xia F and Altieri DC:
Compartmentalized phosphorylation of IAP by protein kinase A
regulates cytoprotection. Mol Cell. 27:17–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hagenbuchner J, Kuznetsov AV, Obexer P and
Ausserlechner MJ: BIRC5/survivin enhances aerobic glycolysis and
drug resistance by altered regulation of the mitochondrial
fusion/fission machinery. Oncogene. 32:4748–4757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Song Z, Yao X and Wu M: Direct interaction
between survivin and Smac/DIABLO is essential for the
anti-apoptotic activity of survivin during taxol-induced apoptosis.
J Biol Chem. 278:23130–23140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schulenburg A, Blatt K, Cerny-Reiterer S,
Sadovnik I, Herrmann H, Marian B, Grunt TW, Zielinski CC and Valent
P: Cancer stem cells in basic science and in translational
oncology: Can we translate into clinical application? J Hematol
Oncol. 8:162015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stoica G, Lungu G, Martini-Stoica H,
Waghela S, Levine J and Smith R III: Identification of cancer stem
cells in dog glioblastoma. Vet Pathol. 46:391–406. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hensley ML, Schuchter LM, Lindley C,
Meropol NJ, Cohen GI, Broder G, Gradishar WJ, Green DM, Langdon RJ
Jr, Mitchell RB, et al: American Society of Clinical Oncology
clinical practice guidelines for the use of chemotherapy and
radiotherapy protectants. J Clin Oncol. 17:3333–3355. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Walker MD, Alexander E Jr, Hunt WE,
MacCarty CS, Mahaley MS Jr, Mealey J Jr, Norrell HA, Owens G,
Ransohoff J, Wilson CB, et al: Evaluation of BCNU and/or
radiotherapy in the treatment of anaplastic gliomas. A cooperative
clinical trial. J Neurosurg. 49:333–343. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dick JE: Looking ahead in cancer stem cell
research. Nat Biotechnol. 27:44–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem
cells in glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lin L, Fuchs J, Li C, Olson V, Bekaii-Saab
T and Lin J: STAT3 signaling pathway is necessary for cell survival
and tumorsphere forming capacity in
ALDH+/CD133+ stem cell-like human colon
cancer cells. Biochem Biophys Res Commun. 416:246–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vermeulen L, De Sousa E, Melo F, van der
Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M,
Merz C, Rodermond H, et al: Wnt activity defines colon cancer stem
cells and is regulated by the microenvironment. Nat Cell Biol.
12:468–476. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mao J, Fan S, Ma W, Fan P, Wang B, Zhang
J, Wang H, Tang B, Zhang Q, Yu X, et al: Roles of Wnt/β-catenin
signaling in the gastric cancer stem cells proliferation and
salinomycin treatment. Cell Death Dis. 5:e10392014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cai C and Zhu X: The Wnt/β-catenin pathway
regulates self-renewal of cancer stem-like cells in human gastric
cancer. Mol Med Rep. 5:1191–1196. 2012.PubMed/NCBI
|
|
51
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Blagosklonny MV: Cancer stem cell and
cancer stemloids: From biology to therapy. Cancer Biol Ther.
6:1684–1690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nakahara T, Kita A, Yamanaka K, Mori M,
Amino N, Takeuchi M, Tominaga F, Kinoyama I, Matsuhisa A, Kudou M
and Sasamata M: Broad spectrum and potent antitumor activities of
YM155, a novel small-molecule survivin suppressant, in a wide
variety of human cancer cell lines and xenograft models. Cancer
Sci. 102:614–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chang BH, Johnson K, LaTocha D, Rowley JS,
Bryant J, Burke R, Smith RL, Loriaux M, Müschen M, Mullighan C, et
al: YM155 potently kills acute lymphoblastic leukemia cells through
activation of the DNA damage pathway. J Hematol Oncol. 8:392015.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cheng XJ, Lin JC, Ding YF, Zhu L, Ye J and
Tu SP: Survivin inhibitor YM155 suppresses gastric cancer xenograft
growth in mice without affecting normal tissues. Oncotarget.
7:7096–7109. 2016.PubMed/NCBI
|
|
56
|
Cheng Q, Ling X, Haller A, Nakahara T,
Yamanaka K, Kita A, Koutoku H, Takeuchi M, Brattain MG and Li F:
Suppression of survivin promoter activity by YM155 involves
disruption of Sp1-DNA interaction in the survivin core promoter.
Int J Biochem Mol Biol. 3:179–197. 2012.PubMed/NCBI
|
|
57
|
Clemens MR, Gladkov OA, Gartner E,
Vladimirov V, Crown J, Steinberg J, Jie F and Keating A: Phase II,
multicenter, open-label, randomized study of YM155 plus docetaxel
as first-line treatment in patients with HER2-negative metastatic
breast cancer. Breast Cancer Res Treat. 149:171–179. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kudchadkar R, Ernst S, Chmielowski B,
Redman BG, Steinberg J, Keating A, Jie F, Chen C, Gonzalez R and
Weber J: A phase 2, multicenter, open-label study of sepantronium
bromide (YM155) plus docetaxel in patients with stage III
(unresectable) or stage IV melanoma. Cancer Med. 4:643–650. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kelly RJ, Thomas A, Rajan A, Chun G,
Lopez-Chavez A, Szabo E, Spencer S, Carter CA, Guha U, Khozin S, et
al: A phase I/II study of sepantronium bromide (YM155, survivin
suppressor) with paclitaxel and carboplatin in patients with
advanced non-small-cell lung cancer. Ann Oncol. 24:2601–2606. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Nakahara T, Kita A, Yamanaka K, Mori M,
Amino N, Takeuchi M, Tominaga F, Hatakeyama S, Kinoyama I,
Matsuhisa A, et al: YM155, a novel small-molecule survivin
suppressant, induces regression of established human
hormone-refractory prostate tumor xenografts. Cancer Res.
67:8014–8021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Koike H, Nitta T, Sekine Y, Arai S, Furuya
Y, Nomura M, Matsui H, Shibata Y, Ito K, Oyama T and Suzuki K:
YM155 reverses rapamycin resistance in renal cancer by decreasing
survivin. J Cancer Res Clin Oncol. 140:1705–1713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Feng W, Yoshida A and Ueda T: YM155
induces caspase-8 dependent apoptosis through downregulation of
survivin and Mcl-1 in human leukemia cells. Biochem Biophys Res
Commun. 435:52–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rivadeneira DB, Caino MC, Seo JH, Angelin
A, Wallace DC, Languino LR and Altieri DC: Survivin promotes
oxidative phosphorylation, subcellular mitochondrial repositioning,
and tumor cell invasion. Sci Signal. 8:ra802015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Glaros TG, Stockwin LH, Mullendore ME,
Smith B, Morrison BL and Newton DL: The ‘survivin suppressants’ NSC
80467 and YM155 induce a DNA damage response. Cancer Chemother
Pharmacol. 70:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tang H, Shao H, Yu C and Hou J: Mcl-1
downregulation by YM155 contributes to its synergistic anti-tumor
activities with ABT-263. Biochem Pharmacol. 82:1066–1072. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cheng SM, Chang YC, Liu CY, Lee JY, Chan
HH, Kuo CW, Lin KY, Tsai SL, Chen SH, Li CF, et al: YM155
down-regulates survivin and XIAP, modulates autophagy and induces
autophagy-dependent DNA damage in breast cancer cells. Br J
Pharmacol. 172:214–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tao YF, Lu J, Du XJ, Sun LC, Zhao X, Peng
L, Cao L, Xiao PF, Pang L, Wu D, et al: Survivin selective
inhibitor YM155 induce apoptosis in SK-NEP-1 Wilms tumor cells. BMC
Cancer. 12:6192012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang W, Liu Y, Li YF, Yue Y, Yang X and
Peng L: Targeting of survivin pathways by YM155 inhibits cell death
and invasion in oral squamous cell carcinoma cells. Cell Physiol
Biochem. 38:2426–2437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Tolcher AW, Mita A, Lewis LD, Garrett CR,
Till E, Daud AI, Patnaik A, Papadopoulos K, Takimoto C, Bartels P,
et al: Phase I and pharmacokinetic study of YM155, a small-molecule
inhibitor of survivin. J Clin Oncol. 26:5198–5203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lewis KD, Samlowski W, Ward J, Catlett J,
Cranmer L, Kirkwood J, Lawson D, Whitman E and Gonzalez R: A
multi-center phase II evaluation of the small molecule survivin
suppressor YM155 in patients with unresectable stage III or IV
melanoma. Invest New Drugs. 29:161–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Alexandrescu DT, Gonzales R, Lewis K,
Samlowski W, Cranmer L, Catlett J, Kirkwood J, Whitman E, Lawson D,
Bartels P, et al: A phase II study of YM155 administered as 168
hour continuous infusion in stage IV and unresectable stage III
melanoma. Mol Cancer Ther. 6:3385S. 2007.
|
|
72
|
Rauch A, Hennig D, Schäfer C, Wirth M,
Marx C, Heinzel T, Schneider G and Krämer OH: Survivin and YM155:
How faithful is the liaison? Biochim Biophys Acta. 1845:202–220.
2014.PubMed/NCBI
|
|
73
|
Ansell SM, Arendt BK, Grote DM, Jelinek
DF, Novak AJ, Wellik LE, Remstein ED, Bennett CF and Fielding A:
Inhibition of survivin expression suppresses the growth of
aggressive non-Hodgkin's lymphoma. Leukemia. 18:616–623. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, De Marinis F, von Pawel J, Gatzemeier U, Tsao TC, Pless M,
Muller T, et al: Randomized phase III trial of pemetrexed versus
docetaxel in patients with non-small-cell lung cancer previously
treated with chemotherapy. J Clin Oncol. 22:1589–1597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Carrasco RA, Stamm NB, Marcusson E,
Sandusky G, Iversen P and Patel BK: Antisense inhibition of
survivin expression as a cancer therapeutic. Mol Cancer Ther.
10:221–232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Henry SP, Geary RS, Yu R and Levin AA:
Drug properties of second-generation antisense oligonucleotides:
How do they measure up to their predecessors? Curr Opin Investig
Drugs. 2:1444–1449. 2001.PubMed/NCBI
|
|
77
|
Talbot DC, Ranson M, Davies J, Lahn M,
Callies S, André V, Kadam S, Burgess M, Slapak C, Olsen AL, et al:
Tumor survivin is downregulated by the antisense oligonucleotide
LY2181308: A proof-of-concept, first-in-human dose study. Clin
Cancer Res. 16:6150–6158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tanioka M1, Nokihara H, Yamamoto N, Yamada
Y, Yamada K, Goto Y, Fujimoto T, Sekiguchi R, Uenaka K, Callies S
and Tamura T: Phase I study of LY2181308, an antisense
oligonucleotide against survivin, in patients with advanced solid
tumors. Cancer Chemother Pharmacol. 68:505–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Talbot DC, Davies J, Olsen A, Andre V,
Lahn M, Powell E, Kadam S, de Bono J, McHugh P and Ranson M:
Pharmacodynamic (PD) evaluation of LYPharmacodynamic (PD)
evaluation of LY2181308 in patients with metastatic malignancies. J
Clin Oncol. 27:35072009.
|
|
80
|
Plescia J, Salz W, Xia F, Pennati M,
Zaffaroni N, Daidone MG, Meli M, Dohi T, Fortugno P, Nefedova Y, et
al: Rational design of shepherdin, a novel anticancer agent. Cancer
Cell. 7:457–468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Young JC, Moarefi I and Hartl FU: Hsp90: A
specialized but essential protein-folding tool. J Cell Biol.
154:267–273. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hendruschk S, Wiedemuth R, Aigner A,
Töpfer K, Cartellieri M, Martin D, Kirsch M, Ikonomidou C,
Schackert G and Temme A: RNA interference targeting survivin exerts
antitumoral effects in vitro and in established glioma xenografts
in vivo. Neuro-Oncol. 13:1074–1089. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Yang Z, Yu B, Zhu J, Huang X, Xie J, Xu S,
Yang X, Wang X, Yung BC, Lee LJ, et al: A microfluidic method to
synthesize transferrin-lipid nanoparticles loaded with siRNA
LOR-1284 for therapy of acute myeloid leukemia. Nanoscale.
6:9742–9751. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Boukany PE, Morss A, Liao WC, Henslee B,
Jung H, Zhang X, Yu B, Wang X, Wu Y, Li L, et al: Nanochannel
electroporation delivers precise amounts of biomolecules into
living cells. Nat Nanotechnol. 6:747–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wen Y and Meng WS: Recent in vivo
evidences of particle-based delivery of small-interfering RNA
(siRNA) into solid tumors. J Pharm Innov. 9:158–173. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Devi GR: siRNA-based approaches in cancer
therapy. Cancer Gene Ther. 13:819–829. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Seth S, Matsui Y, Fosnaugh K, Liu Y, Vaish
N, Adami R, Harvie P, Johns R, Severson G, Brown T, et al:
RNAi-based therapeutics targeting survivin and PLK1 for treatment
of bladder cancer. Mol Ther. 19:928–935. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lee SJ, Son S, Yhee JY, Choi K, Kwon IC,
Kim SH and Kim K: Structural modification of siRNA for efficient
gene silencing. Biotechnol Adv. 31:491–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zheng J, Zhang L, Zhang J, Wang X, Ye K,
Xi Z, Du Q and Liang Z: Single modification at position 14 of siRNA
strand abolishes its gene-silencing activity by decreasing both
RISC loading and target degradation. FASEB J. 27:4017–4026. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dykxhoorn DM and Lieberman J: Knocking
down disease with siRNAs. Cell. 126:231–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sahay G, Querbes W, Alabi C, Eltoukhy A,
Sarkar S, Zurenko C, Karagiannis E, Love K, Chen D, Zoncu R, et al:
Efficiency of siRNA delivery by lipid nanoparticles is limited by
endocytic recycling. Nat Biotechnol. 31:653–658. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Perez AP, Cosaka ML, Romero EL and Morilla
MJ: Uptake and intracellular traffic of siRNA dendriplexes in
glioblastoma cells and macrophages. Int J Nanomedicine.
6:2715–2728. 2011.PubMed/NCBI
|
|
94
|
Mamori S, Matsushima M, Matsuura T and
Tajiri H: Survivin is expressed in early hepatocellular carcinoma
and surrounding hepatitis tissue. Mol Med Rep. 2:911–915. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shariat SF, Lotan Y, Saboorian H, Khoddami
SM, Roehrborn CG, Slawin KM and Ashfaq R: Survivin expression is
associated with features of biologically aggressive prostate
carcinoma. Cancer. 100:751–757. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ferrandina G, Legge F, Martinelli E,
Ranelletti FO, Zannoni GF, Lauriola L, Gessi M, Gallotta V and
Scambia G: Survivin expression in ovarian cancer and its
correlation with clinico-pathological, surgical and
apoptosis-related parameters. Br J Cancer. 92:271–277. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Shinohara ET, Gonzalez A, Massion PP, Chen
H, Li M, Freyer AS, Olson SJ, Andersen JJ, Shyr Y, Carbone DP, et
al: Nuclear survivin predicts recurrence and poor survival in
patients with resected nonsmall cell lung carcinoma. Cancer.
103:1685–1692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nasu S, Yagihashi A, Izawa A, Saito K,
Asanuma K, Nakamura M, Kobayashi D, Okazaki M and Watanabe N:
Survivin mRNA expression in patients with breast cancer. Anticancer
Res. 22:1839–1843. 2002.PubMed/NCBI
|
|
99
|
Yu J, Leung WK, Ebert MP, Ng EK, Go MY,
Wang HB, Chung SC, Malfertheiner P and Sung JJ: Increased
expression of survivin in gastric cancer patients and in first
degree relatives. Br J Cancer. 87:91–97. 2002. View Article : Google Scholar : PubMed/NCBI
|