Introduction
Ionizing radiation causes biological effects, such
as cell death and chromosomal aberrations, on cells. There are many
evidences that ionizing radiation affects, not only the irradiated
cells, but also the non-irradiated neighboring cells (1–3). Such
response is known as radiation-induced non-targeted effects, which
includes genomic instability and radiation-induced bystander
effects. Genomic instability is characterized by effects such as
delayed gene mutation and chromosomal aberrations that occur in the
progeny of irradiated cells (3). In
radiation-induced bystander effects, it has been suggested that the
irradiated cells transmit signals to the non-irradiated cells via
gap junctions or soluble factors (such as cytokines and growth
factors) (1,2). To investigate the soluble
factor-mediated bystander effects in vitro, non-irradiated
cells were co-cultured with irradiated cells or cultured in
irradiated cell conditioned medium (ICCM). It has been reported
that non-irradiated cells co-cultured with irradiated cells or
treated with ICCM undergo various biological responses, such as DNA
double-strand breaks, decrease in clonogenic cell survival, and
cell death, similar to irradiated cells (1).
The nuclear factor erythroid 2-related factor 2
(Nrf2) is a transcription factor, which plays an important role in
cellular defense against oxidative stress (4). In response to oxidative stresses, such
as reactive oxygen species (ROS), Nrf2 rapidly translocates to the
nucleus and induces the expression of various antioxidant genes,
such as heme oxygenase-1. Recent studies have demonstrated the
over-activation of Nrf2 by the somatic mutation of Nrf2 or
its inhibitor Keap1 in various types of cancer (5,6). For
example, Nrf2 promotes the proliferation and metastasis of lung
cancer and oesophageal cancer cells (7,8). Moreover,
the over-activation of Nrf2 leads to resistance toward
chemotherapeutic agents (7,9).
Low linear energy transfer radiations, such as
X-rays, cause biological damage through ROS production (10,11).
Nrf2-mediated cellular defense is involved in the cellular response
to ionizing radiation (12–14). Furthermore, it has been reported that
Nrf2 downregulation by shRNA and its inhibition using a small
molecular weight compound 4-(2-cyclohexylethoxy)aniline enhance the
sensitivity to ionizing radiation (15,16). These
results indicate that Nrf2 is a useful target to improve the
efficacy of cancer radiotherapy. However, it remains unknown
whether a modification of the radiosensitivity by Nrf2 knockdown
affects the property of ICCM.
In this study, we hypothesized than the upregulation
of radiosensitivity by Nrf2 inhibition alters the ICCM-mediated
effects on non-irradiated cells. To test this hypothesis, we
transfected siRNA against Nrf2 into A549 human lung cancer cells,
which constitutively overexpress Nrf2 because they have a somatic
mutation in Keap1 (5). We then
investigated whether the effects of ICCM from A549 cells on cell
growth and cell death induction vary depending on the Nrf2
knockdown.
Materials and methods
Reagents
Propidium iodide (PI) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Anti-Nrf2 antibody (cat. no.
sc-13032) was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Anti-β-actin antibody (cat. no. 4967) and
anti-rabbit horseradish peroxidase (HRP)-linked IgG antibody (cat.
no. 7074) were purchased from Cell Signaling Technology Japan, K.K.
(Tokyo, Japan). Ambion's Silencer® Select Pre-designed
siRNA against the gene encoding Nrf2 (ID: s9492) and
Silencer® Select Negative Control 1 siRNA were purchased
from Life Technologies Corporation; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA).
Cell culture
The A549 lung cancer cell line was purchased from
the American Type Culture Collection (Manassas, VA, USA). A549
cells were maintained at 37°C in a humidified 5% CO2
atmosphere and cultured in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.) and 10% heat-inactivated
FBS (Japan Bioserum Co., Ltd., Nagoya, Japan).
siRNA transfection
A549 cells were transfected with target or control
siRNA using Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.), as previously reported (17). The final concentration of siRNAs in
the medium was 10 nM. After incubating the cells with the medium
containing siRNAs for 48 h, transfected cells were collected and
used for subsequent analyses.
In vitro irradiation
The cells were irradiated (150 kVp, 20 mA, 0.5-mm Al
and 0.3-mm Cu filters) using an X-ray generator (MBR-1520R-3;
Hitachi Medical Corporation, Tokyo, Japan) at a distance of 45 cm
from the focus and a dose rate of 1.00–1.05 Gy/min.
Clonogenic survival assay
To examine the radiosensitivity, the cells were
seeded on 60-mm diameter culture dishes (Iwaki, Chiba, Japan) and
cultured overnight. After culturing for 6 h, the cells were exposed
to X-ray radiation and incubated for the next 8–11 days. Next, the
cells were fixed with methanol and stained with Giemsa solution
(Wako Pure Chemical Industries, Ltd., Osaka, Japan). Colonies
containing >50 cells were counted.
Sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and western blot analysis
Protein preparation and determination of the protein
concentration were performed as reported previously (18). SDS-PAGE and western blot analysis were
performed as reported previously (19). The following concentrations of primary
antibodies were used: Anti-Nrf2 antibody (dilution, 1:3,000) and
anti-actin antibody (dilution, 1:4,000). The secondary antibody
used was HRP-linked anti-rabbit IgG antibody (dilution, 1:10,000).
Antigens were visualized using the ECL Prime Western Blotting
Detection System (GE Healthcare Life Sciences, Chalfont, UK). Blots
were stripped using a commercially available stripping solution
(Wako Pure Chemical Industries, Ltd.).
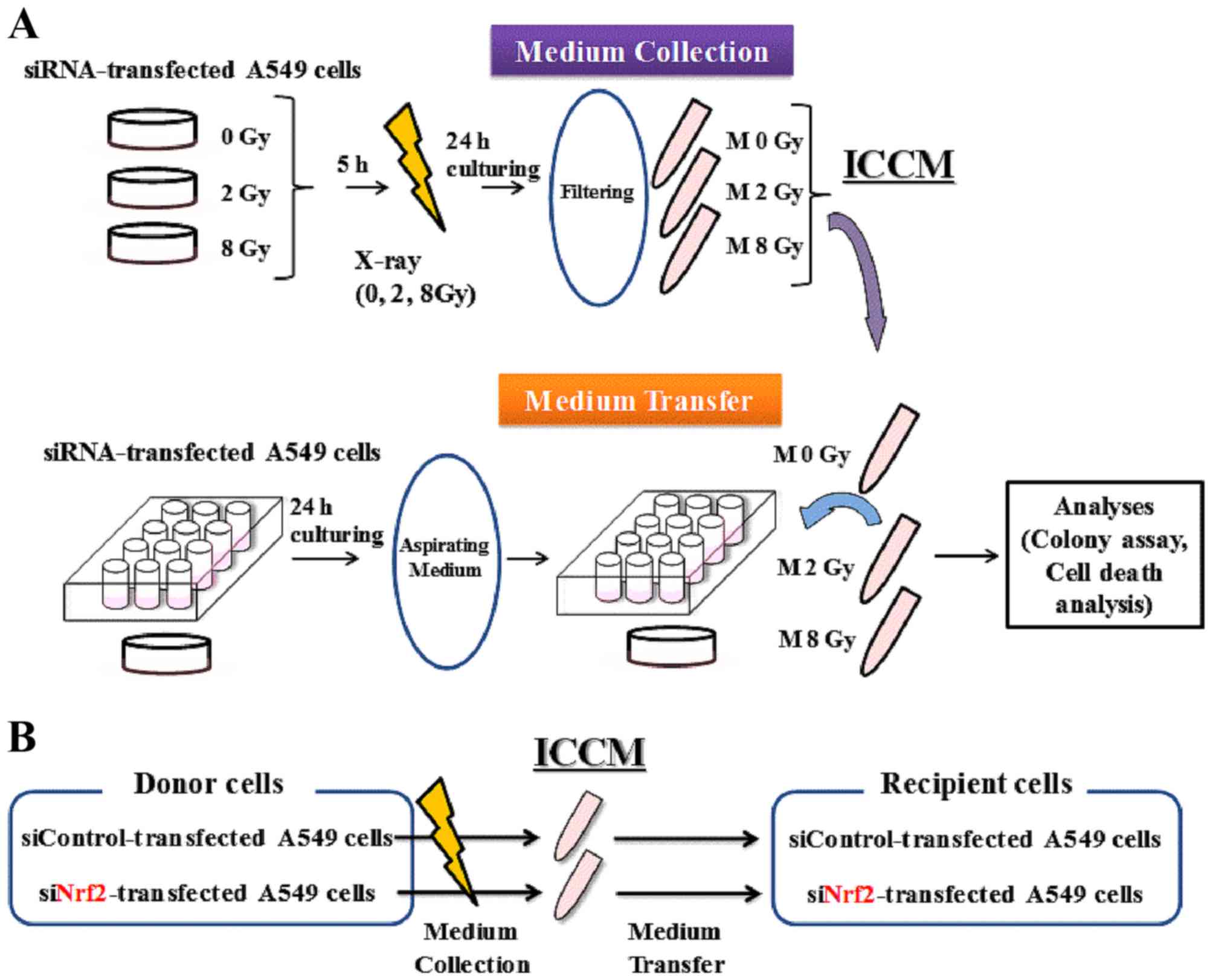
Medium transfer experiments
The schematic for medium transfer experiments is
shown in Fig. 1. Approximately
2.4×105 transfected cells were seeded onto 35-mm culture
dishes and cultured for 5 h to promote their adherence to the dish.
The cells were then exposed to X-rays, cultured for 24 h, and the
cell conditioned medium was then collected by centrifugation (1,000
rpm for 5 min at room temperature). After centrifugation, the
supernatant was collected and filtered using a 0.45-mm syringe
filter (2053-025; Iwaki) to remove cells and debris. The filtrated
cell conditioned medium (hereafter referred to as ICCM) was used
for subsequent experiments.
One day before collecting the ICCM, approximately
6.0×104 cells were seeded onto 35-mm culture dishes for
cell death analysis, or in a 12-well plate (BD Falcon) (100 or 120
cells) for colony assay, and cultured overnight to allow for their
adherence to the dish. On the following day, the medium was
aspirated and ICCM was added to the 35-mm culture dishes or to the
12-well plate. After 3 days of culturing, the cells that were
seeded in the 35-mm culture dishes were collected using 0.1%
trypsin-ethylene diamine tetraacetic acid (Gibco; Thermo Fisher
Scientific, Inc.), and the number of viable cells was counted using
the trypan blue dye exclusion assay. Finally, the harvested cells
were used to perform the cell death analysis.
The cells that were seeded in the 12-well plates for
colony assay were incubated for 8–10 days. The cells were then
fixed with methanol and stained with Giemsa solution. Colonies
containing >50 cells were counted.
Cell death analysis
Cell death was analyzed using Annexin V-FITC
(BioLegend, Inc., San Diego, CA, USA), PI, and Annexin V binding
buffer (BioLegend, Inc.), as reported previously (20). Stained cells were analyzed by
performing flow cytometry (Cytomics FC500; Beckman Coulter, Inc.,
Brea, CA, USA). In the Annexin V/PI quadrant gating, Annexin
V−/PI−, Annexin V+/PI−,
and Annexin V+/PI+ were used to identify the
fraction of viable cells, early apoptotic cells, and late
apoptotic/necrotic cells, respectively.
Statistical analysis
Data are presented as mean ± standard deviation.
Comparisons between control and experimental groups were performed
using a two-sided Student's t-test or a two-sided Mann-Whitney
U-test depending on the data distribution. Non-parametric multiple
data were analyzed using the Kruskal-Wallis test followed by the
Steel test. Differences were considered significant at P<0.05.
All statistical analyses were performed using Excel 2016 software
(Microsoft Corporation, Redmond, WA, USA), with an add-on software
Statcel 4 (The Publisher OMS Ltd., Tokyo, Japan).
Results and Discussion
We investigated whether the effects of ICCM from
A549 cells on growth and death induction vary depending on the
regulation of radiosensitivity by Nrf2 knockdown.
To this aim, we first transfected A549 cells with
siRNA against Nrf2, which led to a decreased Nrf2 protein
expression in these cells (Fig. 2A).
Furthermore, the radiosensitivity of the Nrf2 knockdown cells was
significantly higher than that of the control cells (Fig. 2B). Consistent with previous reports
(14–16), our results indicate that Nrf2
regulates the radiosensitivity of cancer cells.
Next, we investigated the effects of ICCM on the
growth and death induction in non-irradiated cells. The
relationship between ICCM donor and recipient cells is shown in
Fig. 1B. When non-irradiated A549
cells that were transfected with control siRNA were treated with
ICCM, no significant difference was observed in the cell growth
(estimated using the trypan blue dye exclusion and colony assays)
between ICCM from non-irradiated cells and that from 2 or 8
Gy-irradiated cells (Fig. 3A and B).
Furthermore, we did not observe any significant difference in the
proportion of Annexin V+ dead cells upon treatment with
ICCM (Fig. 3C). Next, we performed
similar experiments using the Nrf2 knockdown A549 cells and found
that the cell growth and clonogenic potential were significantly
lower for Nrf2 knockdown cells treated with non-irradiated Nrf2
knockdown cell conditioned medium than for those treated with
control siRNA (Fig. 3A and B). When
the medium transfer experiments were performed using ICCM from Nrf2
knockdown cells, no significant difference was observed in the cell
growth, clonogenic potential, and proportion of Annexin
V+ dead cells (Fig. 3A-C).
Taken together, these results suggest that although Nrf2 knockdown
affects the cell growth of A549 cells, it does not alter the
effects of ICCM on the cell growth and cell death induction. Yang
et al have reported that the cell conditioned medium from
irradiated A549 cells causes cytotoxicity in the non-irradiated
A549 cells (21); however, we could
not observe the cytotoxic effects of ICCM from A549 cells. It has
been reported that bystander effects by photon-irradiation are
strongly influenced by radiation dose (1). While we used ICCM from A549 cells
exposed to 2 or 8 Gy X-ray, Yang et al used ICCM from A549
cells exposed to 0.5–2 Gy X-ray. However, because ICCM from 2 Gy
irradiation decreased the clonogenic cell survival of
non-irradiated cells (21), it seems
that the discrepancy between our results and their results is due
to another factor and not the radiation dose. Suzuki has reported
that the decrease in clonogenic cell survival caused by bystander
effects varies depending on the radiation quality, such as the
types of ions (22). While Yang et
al used 6 MV X-ray radiation for their study, we used 150 kVp
X-ray (21). Thus, the difference in
the energy of X-ray likely explains the discrepancy between our
results and those of Yang et al (21).
Howe et al have previously investigated the
relationship between intrinsic radiosensitivity and bystander
effects (23). They treated HaCaT
human skin cells with ICCM from irradiated lymphocytes obtained
from healthy individuals and colorectal carcinoma patients and
investigated the bystander effects of ICCM using a viability test.
They also examined the intrinsic radiosensitivity of the irradiated
lymphocytes and investigated the relationship between
radiosensitivity and bystander effects. They found no significant
relationship between the radiation-induced intrinsic sensitivity
and bystander effects. In line with their report, the modification
of radiosensitivity by Nrf2 knockdown did not alter the effects of
ICCM on cell growth and cell death induction in our study.
Therefore, it is unlikely that cellular radiosensitivity determines
the cytotoxic effects of ICCM.
In conclusion, the present findings suggest that
Nrf2 knockdown enhanced the radiosensitivity of A549 cells, but it
did not alter the effects of ICCM on cell growth. It is concerning
that the radiation-induced non-targeted effects, such as
chromosomal aberrations, are related to the risk of carcinogenesis
in normal cells. Therefore, considering combined therapy comprising
Nrf2-targeted cancer therapy and radiation therapy, future studies
to investigate whether the radiation-induced bystander effects
against normal cells vary depending on Nrf2 inhibition knockdown
are needed.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This study was supported by JSPS KAKENHI (grant no.
JP15K09985).
Availability of data and material
All data generated or analyzed in this study are
included in this article.
Authors' contributions
HY initiated the research. HY, KM, and MN performed
experiments, collected data, and analyzed data. HY and IK wrote,
reviewed, and revised manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of
interest regarding the publication of this study.
Glossary
Abbreviations
Abbreviations:
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
ICCM
|
irradiated cell conditioned medium
|
|
ROS
|
reactive oxygen species
|
|
PI
|
propidium iodide
|
|
HRP
|
horseradish peroxidase
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
gel electrophoresis
|
|
SD
|
standard deviation
|
References
|
1
|
Tomita M and Maeda M: Mechanisms and
biological importance of photon-induced bystander responses: Do
they have an impact on low-dose radiation responses. J Radiat Res
(Tokyo). 56:205–219. 2015. View Article : Google Scholar
|
|
2
|
Hamada N, Maeda M, Otsuka K and Tomita M:
Signaling pathways underpinning the manifestations of ionizing
radiation-induced bystander effects. Curr Mol Pharmacol. 4:79–95.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kadhim MA and Hill MA: Non-targeted
effects of radiation exposure: Recent advances and implications.
Radiat Prot Dosimetry. 166:118–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Itoh K, Tong KI and Yamamoto M: Molecular
mechanism activating Nrf2-Keap1 pathway in regulation of adaptive
response to electrophiles. Free Radic Biol Med. 36:1208–1213. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh A, Misra V, Thimmulappa RK, Lee H,
Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E,
et al: Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung
cancer. PLoS Med. 3:e4202006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Na HK and Surh YJ: Oncogenic potential of
Nrf2 and its principal target protein heme oxygenase-1. Free Radic
Biol Med. 67:353–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohta T, Iijima K, Miyamoto M, Nakahara I,
Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T,
et al: Loss of Keap1 function activates Nrf2 and provides
advantages for lung cancer cell growth. Cancer Res. 68:1303–1309.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kitano Y, Baba Y, Nakagawa S, Miyake K,
Iwatsuki M, Ishimoto T, Yamashita YI, Yoshida N, Watanabe M, Nakao
M and Baba H: Nrf2 promotes oesophageal cancer cell proliferation
via metabolic reprogramming and detoxification of reactive oxygen
species. J Pathol. 244:346–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong Y, Zhang F, Sun Z, Zhou W, Li ZY,
You QD, Guo QL and Hu R: Drug resistance associates with activation
of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by
down-regulating Nrf2-mediated cellular defense response. Mol
Carcinog. 52:824–834. 2013.PubMed/NCBI
|
|
10
|
Mikkelsen RB and Wardman P: Biological
chemistry of reactive oxygen and nitrogen and radiation-induced
signal transduction mechanisms. Oncogene. 22:5734–5754. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY,
Kim CH, Park HG, Han SI and Kang HS: Induction of metastasis,
cancer stem cell phenotype, and oncogenic metabolism in cancer
cells by ionizing radiation. Mol Cancer. 16:102017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsukimoto M, Tamaishi N, Homma T and
Kojima S: Low-dose gamma-ray irradiation induces translocation of
Nrf2 into nuclear in mouse macrophage RAW264.7 cells. J Radiat Res
(Tokyo). 51:349–353. 2010. View Article : Google Scholar
|
|
13
|
McDonald JT, Kim K, Norris AJ, Vlashi E,
Phillips TM, Lagadec C, Della Donna L, Ratikan J, Szelag H, Hlatky
L and McBride WH: Ionizing radiation activates the Nrf2 antioxidant
response. Cancer Res. 70:8886–8895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshino H, Kiminarita T, Matsushita Y and
Kashiwakura I: Response of the Nrf2 protection system in human
monocytic cells after ionising irradiation. Radiat Prot Dosimetry.
152:104–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh A, Bodas M, Wakabayashi N, Bunz F
and Biswal S: Gain of Nrf2 function in non-small-cell lung cancer
cells confers radioresistance. Antioxid Redox Signal. 13:1627–1637.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee S, Lim MJ, Kim MH, Yu CH, Yun YS, Ahn
J and Song JY: An effective strategy for increasing the
radiosensitivity of human lung cancer cells by blocking
Nrf2-dependent antioxidant responses. Free Radic Biol Med.
53:807–816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshino H, Iwabuchi M, Kazama Y and
Kashiwakura I: Effects of retinoic acid-inducible gene-I-like
receptors activations and ionizing radiation cotreatment on
cytotoxicity against human non-small cell lung cancer in
vitro. Oncol Lett. 15:4697–4705. 2018.PubMed/NCBI
|
|
18
|
Yoshino H, Kumai Y and Kashiwakura I:
Effects of endoplasmic reticulum stress on apoptosis induction in
radioresistant macrophages. Mol Med Rep. 15:2867–2872. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yoshino H, Saitoh T, Kozakai M and
Kashiwakura I: Effects of ionizing radiation on retinoic
acid-inducible gene-I-like receptors. Biomed Rep. 3:59–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukushi S, Yoshino H, Yoshizawa A and
Kashiwakura I: p53-independent structure-activity relationships of
3-ring mesogenic compounds' activity as cytotoxic effects against
human non-small cell lung cancer lines. BMC Cancer. 16:5212016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang S, Xu J, Shao W, Geng C, Li J, Guo F,
Miao H, Shen W, Ye T, Liu Y, et al: Radiation-induced bystander
effects in A549 cells exposed to 6 MV X-rays. Cell Biochem Biophys.
72:877–882. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki M: Significance of
radiation-induced bystander effects in radiation therapy. Igaku
Butsuri. 34:70–78. 2014.(In Japanese). PubMed/NCBI
|
|
23
|
Howe O, O'Sullivan J, Nolan B, Vaughan J,
Gorman S, Clarke C, McClean B and Lyng FM: Do radiation-induced
bystander effects correlate to the intrinsic radiosensitivity of
individuals and have clinical significance? Radiat Res.
171:521–529. 2009. View
Article : Google Scholar : PubMed/NCBI
|