Introduction
In recent years, research associated with forensic
medicine has at times been regarded as insufficient and of poor
quality, particularly when parameters such as journal impact
factors and external findings are taken into account. Forensic
medicine involves different tasks as compared with clinical
medicine (1). Estimation of the
postmortem interval (PMI) is one of the most important tasks in
forensic medicine (2). In the last
60 years, numerous methods have been proposed for the determination
of the time since death by chemical means (3). Previous contributions have reviewed
the historical background of this discipline and important
postmortem processes, and have discussed the scientific basis
underlying attempts to determine the time interval since death
(4). Biochemical markers that help
to evaluate the time since death have been investigated. These
include protein fractions, urea, creatinine, glucose, iron,
potassium, calcium, enzymes, immunohistochemical detection of
insulin in pancreatic β-cells, the myo-albumin fraction and the
level of strontium-90 calcium analogs (2,5–13).
Using medical techniques such as measurement of body temperature,
analyzing the liver, or assessing rigor mortis, the time since
death can be accurately measured only for the first two or three
days after death (4). The number
of studies estimating the PMI has a reverse correlation with its
importance and value in practice (14). Therefore, research on estimating
the PMI is most important.
High mobility group box-1 (HMGB1) is primarily a
nuclear protein present in many eukaryotic cells and has a highly
conserved amino-acid sequence among species. HMGB1 appears to have
two distinct functions in cellular systems. First, it acts as an
intracellular regulator of transcription, having a crucial role in
the maintenance of DNA function. Second, HMGB1 translocates to the
outside of the nucleus in all eukaryotic cells upon necrosis, and
is released from macrophages through activation by
lipopolysaccharides (LPS), tumor necrosis factor (TNF)-α,
interleukin (IL)-1 and interferon (IFN)-γ (15,16).
Studies demonstrating a role for HMGB1 in the PMI
are lacking. Postmortem change induces necrosis, and necrotic cells
release HMGB1 (16), so detection
of serum HMGB1 exuded from corpus necrotic tissue may be related to
PMI. In the present study, the postmortem change in serum HMGB1 in
the bodies of dead rats at three environmental temperatures was
detected. The aim of our study was to investigate the potential use
of serum HMGB1 as an estimation of the PMI. Serum HMGB1 during the
postmortem period was analyzed by enzyme-linked immunosorbent assay
(ELISA).
Materials and methods
Animal protocol
Animal procedures were conducted with the approval
of the Animal Care Committee of the Ethics Board of the Institute
of Laboratory Animal Sciences of Kagoshima University (Kagoshima,
Japan).
Ninety male Wistar rats (weight, 230–260 g; age, 8
weeks) were purchased from Japan SLC Inc. (Tokyo, Japan). They were
maintained on a 12-h light/dark cycle with free access to food and
water. Rats were anesthetized with chloral hydrate (400 mg/kg body
weight, i.p.). They were sacrificed by cervical dislocation and
stored at 4, 14 and 24°C. Room temperature was set at 4 or 24°C,
and a water bath (Thermominder SD, Taitec, Saitama, Japan) was set
at 14°C for 7 days postmortem. At 0, 0.25, 0.5, 1, 2, 3, 4, 5, 6
and 7 days postmortem, blood was removed from each dead body.
Rat samples
Blood samples were collected from the heart and
great vessels at autopsy. Samples were centrifuged immediately for
5 min at 5000 rpm. Serum was stored at −80°C until analysis.
HMGB1 ELISA
The concentration of HMGB1 in serum was measured
using an ELISA kit (Shino-Test Corp., Kanagawa, Japan).
Statistical analysis
Statistical analysis was carried out using the
Student’s t-test. P<0.05 was considered significant.
Results
Postmortem change induces necrosis, and necrotic
cells release HMGB1 (16). In the
present study, the postmortem change in serum HMGB1 in the bodies
of dead rats at three environmental temperatures was detected. At
24°C, the serum HMGB1 level reached a peak on the second day with a
time-dependent decrease in the following days whereas, at 14°C the
level reached a peak at day 3 with a plateau in the following days
(Fig. 1). The HMGBi level showed a
time-dependent increase at 4°C. The standard deviation (SD) of
HMGB1 levels was very small; reproducibility was probably high. The
increase and decrease in HMGB1 levels at postmortem time was
extemely large.
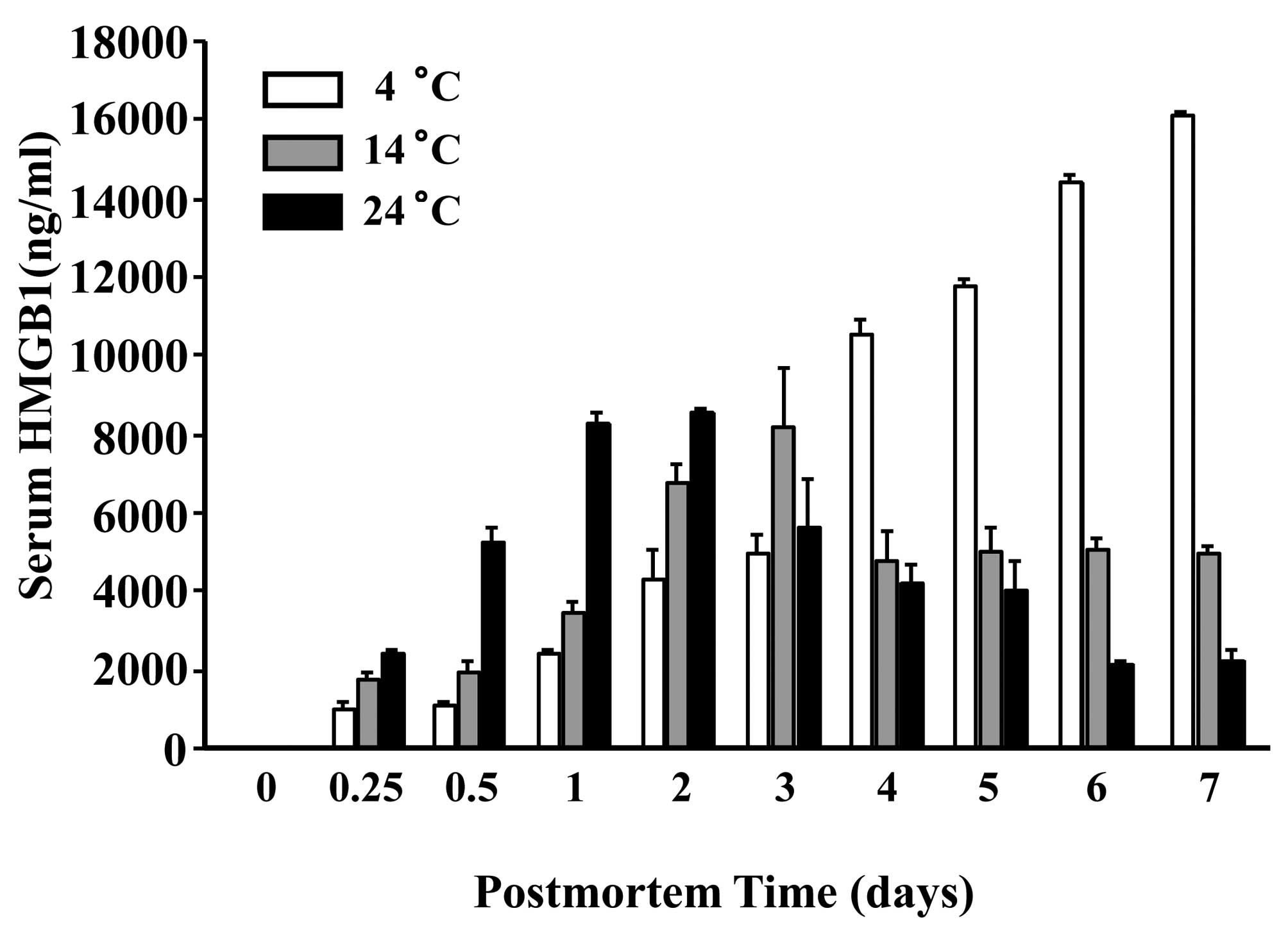 | Figure 1.HMGB1 concentration in the serum of
dead Wistar rats. Ninety male Wistar rats were sacrificed and
stored at 4, 14 and 24°C. At 0, 0.25, 0.5, 1, 2, 3, 4, 5, 6 and 7
days postmortem, blood samples were collected from each body. The
serum HMGB1 concentration was measured by ELISA. At 24°C, the serum
HMGB1 level reached a peak on the second day with time-dependent
decrease in the following days whereas, at 14°C, the level reached
a peak on the third day and plateaued in the following days. The
HMGB1 level showed a time-dependent increase at 4°C (n=3 for each
group). |
Discussion
Assessment of the PMI by HMGB1 measurement is a
useful method. Serum samples of only 10 μl are required, and organs
do not need to be homogenized. HMGB1 levels can be obtained in just
two days using ELISA. The HMGB1 level in a dead body can be used to
estimate the PMI. Postmortem analysis of HMGB1 levels could provide
an insight into the biochemical changes that occur after death, and
could also present potential indicators for assessment of the
PMI.
In live humans, the HMGB1 concentration in blood
cells was very low in our preliminary data. In the experiment,
blood samples were collected from live humans (n=3). Samples were
kept up to 7 days at 24°C. The serum HMGB1 level was detected at 0,
1, 2, 3, 4, 5, 6 and 7 days using ELISA. The HMGB1 level showed a
time-dependent increase up to 7 days; the HMGB1 concentration in
blood cells was very low (median, 6.24 ng/ml).
The high level of HMGB1 in the bodies of dead rats
may have been exuded from necrotic cells in the tissue of the heart
and the great vessels, and not released only from blood cells.
HMGB1 has been identified to be a potent
pro-inflammatory and cytotoxic cytokine (17). HMGB1 has a critical role in sepsis,
cancer, disseminated intravascular coagulation, rheumatoid
arthritis, cerebral infarction, myocardial infarction,
periodontitis and xenotransplantation (18–27).
The serum HMGB1 level is elevated in human patients with various
diseases. In previous studies, serum HMGB1 levels were elevated in
patients with myocardial ischemia (median, 159 ng/ml), cerebral
ischemia (median, 218 ng/ml), sepsis (median, 83.7 ng/ml) and
pancreatitis (median, 5.4 ng/ml) (19,28,29).
The maximal serum HMGB1 level is approximately 200 ng/ml. The level
of serum HMGB1 in dead rats is very high, suggesting that the cause
of death may not influence postmortem serum HMGB1 levels when
estimating the PMI.
Body temperature was not measured in the present
study, but postmortem temperature has been reported in several
publications (30–32).
In recent years, research associated with forensic
medicine has sometimes been regarded as insufficient and of poor
quality (1). Forensic medicine has
been removed as an academic discipline from universities in some
countries (33). Yet, forensic
medicine has made advances. In addition to traditional invasive
‘body-opening’ autopsy of postmortem investigation in humans,
virtual ‘body non-opening’ autopsy has been conducted using CT and
MRI (34,35). HMGB1 analysis may be a new method
in forensic medicine. The data in the present study demonstrate
that this technique may be a major advance in the determination of
the time since death, providing reliable semi-quantitative
biochemical markers from blood samples as opposed to estimates such
as those based on direct measurement of temperature. HMGB1 is a new
postmortem marker and could be a tool for the estimation of the PMI
in the short- and long-term. Further investigations into the timing
and physical factors that affect postmortem levels of HMGB1 in
different tissues are essential, but detection of HMGB1 permits the
development of techniques for the precise determination of the PMI.
Upon further validation, this method could be used in combination
with established methods to improve estimation of the PMI.
Acknowledgements
We thank N. Uto, T. Nagasato and T.
Morizono for their excellent technical assistance.
References
|
1.
|
Madea B, Saukko P and Musshoff F: Tasks of
research in forensic medicine – different study types in clinical
research and forensic medicine. Forensic Sci Int. 165:92–97.
2007.
|
|
2.
|
Thaik-Oo M, Tanaka E, Tsuchiya T, et al:
Estimation of postmortem interval from hypoxic inducible levels of
vascular endothelial growth factor. J Forensic Sci. 47:186–189.
2002.PubMed/NCBI
|
|
3.
|
Madea B: Is there recent progress in the
estimation of the postmortem interval by means of thanatochemistry?
Forensic Sci Int. 151:139–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Amendt J, Krettek R and Zehner R: Forensic
entomology. Naturwissenschaften. 91:51–65. 2004. View Article : Google Scholar
|
|
5.
|
Gallois-Montbrun FG, Barres DR and Durigon
M: Postmortem interval estimation by biochemical determination in
birds muscle. Forensic Sci Int. 37:189–192. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gos T and Raszeja S: Postmortem activity
of lactate and malate dehydrogenase in human liver in relation to
time after death. Int J Legal Med. 106:25–29. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Kang S, Kassam N, Gauthier ML and O’Day
DH: Post-mortem changes in calmodulin binding proteins in muscle
and lung. Forensic Sci Int. 131:140–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Mittmeyer HJ: [Investigations to determine
the time of death, late post mortem, by means of electrophoresis of
inner organs (author’s translation)]. Z Rechtsmed. 84:47–56.
1979.
|
|
9.
|
Mittmeyer HJ: [Determination of the
myo-albumin content. A possibility to determine the hour of death
(author’s translation)]. Z Rechtsmed. 84:233–237. 1980.
|
|
10.
|
Mittmeyer HJ and Strebel KH: [Experimental
examinations on forensic determination of time of death by
electrofocusing of soluble muscle protein (author’s translation)].
Z Rechtsmed. 85:235–240. 1980.
|
|
11.
|
Neis P, Hille R, Paschke M, et al:
Strontium90 for determination of time since death. Forensic Sci
Int. 99:47–51. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sabucedo AJ and Furton KG: Estimation of
postmortem interval using the protein marker cardiac Troponin I.
Forensic Sci Int. 134:11–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wehner F, Wehner HD, Schieffer MC and
Subke J: Delimitation of the time of death by immunohistochemical
detection of insulin in pancreatic beta-cells. Forensic Sci Int.
105:161–169. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Henssge C and Madea B: Estimation of the
time since death. Forensic Sci Int. 165:182–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ito T, Kawahara K, Nakamura T, et al:
High-mobility group box 1 protein promotes development of
microvascular thrombosis in rats. J Thromb Haemost. 5:109–116.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Kawahara K, Tancharoen S, Hashiguchi T, et
al: Inhibition of HMGB1 by deep ocean water attenuates
endotoxin-induced sepsis. Med Hypotheses. 68:1429–1430. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Dumitriu IE, Baruah P, Manfredi AA,
Bianchi ME and Rovere-Querini P: HMGB1: guiding immunity from
within. Trends Immunol. 26:381–387. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Goldstein RS, Gallowitsch-Puerta M, Yang
L, et al: Elevated high-mobility group box 1 levels in patients
with cerebral and myocardial ischemia. Shock. 25:571–574. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Inoue K, Kawahara K, Biswas KK, et al:
HMGB1 expression by activated vascular smooth muscle cells in
advanced human atherosclerosis plaques. Cardiovasc Pathol.
16:136–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kawahara K, Setoyama K, Kikuchi K, et al:
HMGB1 release in co-cultures of porcine endothelial and human T
cells. Xenotransplantation. 14:636–641. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kikuchi K, Kawahara KI, Biswas KK, et al:
Minocycline attenuates both OGD-induced HMGB1 release and
HMGB1-induced cell death in ischemic neuronal injury in PC12 cells.
Biochem Biophys Res Commun. 385:132–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kikuchi K, Kawahara KI, Tancharoen S, et
al: The free-radical scavenger edaravone rescues rats from cerebral
infarction by attenuating the release of high-mobility group box-1
in neuronal cells. J Pharmacol Exp Ther. 329:865–874. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lotze MT and Tracey KJ: High-mobility
group box 1 protein (HMGB1): nuclear weapon in the immune arsenal.
Nat Rev Immunol. 5:331–342. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Morimoto Y, Kawahara KI, Tancharoen S, et
al: Tumor necrosis factor-alpha stimulates gingival epithelial
cells to release high mobility-group box 1. J Periodontal Res.
43:76–83. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Taniguchi N, Kawahara K, Yone K, et al:
High mobility group box chromosomal protein 1 plays a role in the
pathogenesis of rheumatoid arthritis as a novel cytokine. Arthritis
Rheum. 48:971–981. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Ulloa L and Messmer D: High-mobility group
box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev.
17:189–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Wang H, Bloom O, Zhang M, et al: HMG-1 as
a late mediator of endotoxin lethality in mice. Science.
285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yasuda T, Ueda T, Takeyama Y, et al:
Significant increase of serum high-mobility group box chromosomal
protein 1 levels in patients with severe acute pancreatitis.
Pancreas. 33:359–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Henssge C: Concerning the paper by Mall
et al, entitled ‘Temperature-based death time estimation
with only partially environment conditions’ (Int J Legal Med (2005)
119: 185–194). Int J Legal Med. 121:822007.PubMed/NCBI
|
|
31.
|
Henssge C, Althaus L, Bolt J, et al:
Experiences with a compound method for estimating the time since
death. I. Rectal temperature nomogram for time since death. Int J
Legal Med. 113:303–319. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Mall G, Eckl M, Sinicina I, Peschel O and
Hubig M: Temperature-based death time estimation with only
partially known environmental conditions. Int J Legal Med.
119:185–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Vanezis P: Forensic medicine: past,
present and future. Lancet. 364:8–9. 2004. View Article : Google Scholar
|
|
34.
|
Dirnhofer R, Jackowski C, Vock P, Potter K
and Thali MJ: VIRTOPSY: minimally invasive, imaging-guided virtual
autopsy. Radiographics. 26:1305–1333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hayakawa M, Yamamoto S, Motani H, Yajima
D, Sato Y and Iwase H: Does imaging technology overcome problems of
conventional postmortem examination? A trial of computed tomography
imaging for postmortem examination. Int J Legal Med. 120:24–26.
2006. View Article : Google Scholar
|















