Introduction
Virtual autopsies based on computed tomography (CT)
and magnetic resonance imaging (MRI) are now used in addition to
the traditional ‘body-opening’ autopsies to determine the cause of
death in humans (1–5).
Pulmonary thromboembolism (PTE) is a cause of sudden
death that is often difficult to diagnose with conventional
imaging. Contrast-enhanced CT (CECT) is often used to diagnose PTE
in living patients (6,7), but no studies have established a role
for post mortem CECT.
Here, we describe a case of PTE diagnosed by post
mortem CECT in a patient who died as a result of post-operative
cardiopulmonary arrest.
Case report
A 77-year-old woman suffering from dural
arteriovenous fistulae was treated by transfemoral transvenous
embolization. She had a cardiopulmonary arrest after getting out of
bed on the first post-operative day. Despite receiving prompt
cardiopulmonary resuscitation, the patient died.
Post mortem echocardiography showed mild
enlargements of her right atrium and right ventricle, but no
intracardiac thrombus. We obtained permission from the patient’s
family for a post mortem CT to determine the cause of death. We did
not identify any specific lesion on a plain, whole-body,
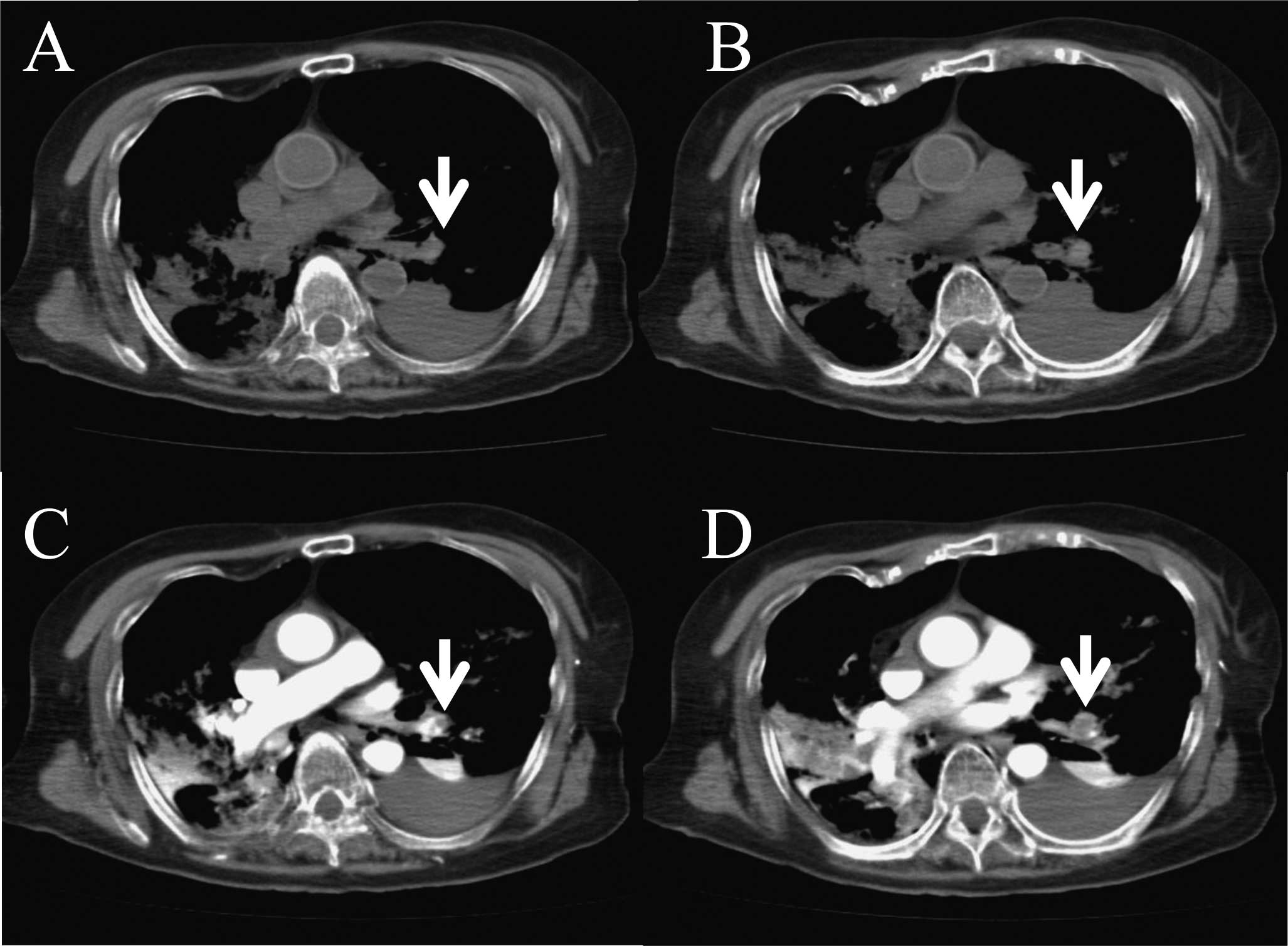
64-multidetector row CT scan (Aquilion 64; Toshiba Medical Systems,
Tokyo, Japan) (Fig. 1A and B). We
then performed cardiac compressions at a rate of 70/min for 4 min
while administering 100 ml of nonionic contrast material (iopamidol
370 mg I/ml, Iopamiron 370; Bayer Yakuhin, Osaka, Japan) via a
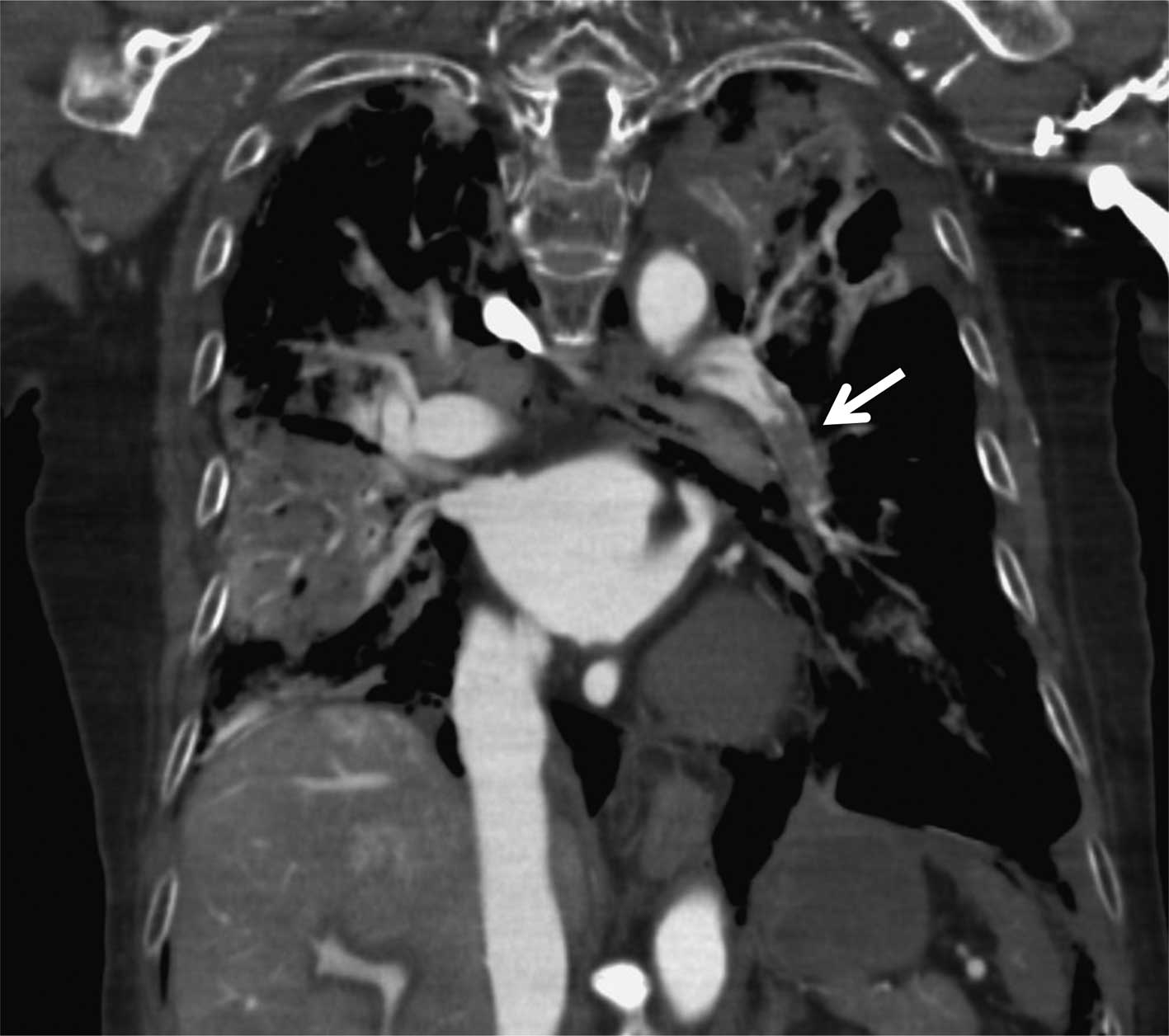
peripheral vessel at 0.5 ml/sec. A subsequent post mortem CECT of
the thorax showed filling defects characteristic of a large
pulmonary thrombus in the left lower pulmonary artery (Figs. 1C, D and 2). Her D-dimer was also elevated at 149.2
μg/ml. Therefore, we diagnosed PTE as the cause of death.
Discussion
PTE is a relatively common cardiovascular emergency
that is difficult to diagnose and is frequently missed (6,7).
Acute occlusion of the pulmonary arterial bed may cause
life-threatening complications, and high-risk PTE has a short-term
mortality rate of more than 15% (7–9).
Patient-related predisposing factors for PTE include increased age,
a past history of venous thromboembolism (VTE), active cancer,
neurological disease with extremity paresis, medical disorders
causing prolonged bed rest, such as heart and respiratory failure,
congenital or acquired thrombophilia, hormone replacement therapy
and oral contraceptive therapy (7). Short-term immobilization also
increases the risk of VTE (6).
Although these risk factors are well established, many cases of PTE
still go unrecognized and untreated (6). The prevalence of PTE at autopsy is
approximately 12–15% in hospitalized patients and has not changed
over the last three decades (10).
The rate of undiagnosed PTE in patients at post mortem has not
diminished either, even in individuals who die from massive or
sub-massive PTE (6,11). In autopsy studies, the prevalence
of unsuspected PTE, either fatal or contributing to death, ranges
from 3 to 8% (6,10,12).
The incidence of VTE increases exponentially with age, as do the
rates of idiopathic and secondary PTE (13,14).
The mean age of patients with PTE is 62 years and approximately 65%
are 60 years of age or older. Eight-fold higher rates of PTE are
observed in patients over 80 years, compared to those younger than
50 years (15). PTE has a wide
range of clinical presentations including dyspnea, chest pain,
syncope, hypotension and shock (6,7).
First-line diagnostic tests, such as ECG, chest X-ray and blood-gas
analysis, are indicated to assess the clinical probability of PTE
and the general condition of the patient (6). A negative D-dimer result safely
excludes the diagnosis in patients with a low or moderate clinical
probability of PTE (6,7). However, D-dimer has very high
sensitivity but low specificity, so a positive result requires
imaging to confirm the diagnosis. Specific diagnostic imaging
techniques for PTE include plain chest radiography,
echocardiography, ventilation-perfusion scintigraphy, CT, MRI and
pulmonary angiography (6,7). In recent years, technical advances in
CT have prompted interest in this technique for the diagnosis of
PTE (6,7). However, two systematic overviews of
the performance of single detector spiral CT in suspected PTE
reported wide variations in sensitivity (53–100%) and specificity
(73–100%) (7).
Invasive ‘body-opening’ autopsy is the traditional
post mortem investigation in humans (1). Modern cross-sectional imaging
techniques, however, can supplement and even partially replace
traditional autopsy (1–3,5).
Conventional autopsies, which are often rejected by family members
or certain religious groups, may eventually be replaced by
noninvasive imaging (1). CT is the
imaging modality of choice for two- and three-dimensional
documentation and detects fractures, pathological gas collections
(air embolism, subcutaneous emphysema after trauma, hyperbaric
trauma and decomposition effects) and gross tissue injuries
(1). The documentation and
analysis of post mortem findings with CT and MRI and
post-processing techniques (‘virtopsy’ or ‘autopsy imaging’) is
investigator-independent, objective and noninvasive, and should
lead to qualitative improvements in pathologic investigation
(1,3). Potential applications for this
technique include the assessment of morbidity and mortality in the
general population and the routine screening of bodies prior to
burial (1). A transdisciplinary
research project, virtopsy, is dedicated to increase the use of
modern imaging techniques in forensic medicine and pathology to
augment current examination techniques and offer alternative
methods (16).
In conclusion, our patient died suddenly in the
hospital the day after her surgery. Although a previous study using
post mortem CT reported a high success rate in detecting causes of
sudden death (17), we did not
find any lesion on a plain CT or CECT without cardiac compression
in our patient. However, a post mortem CECT conducted after cardiac
compression confirmed PTE as the cause of her sudden death.
Acknowledgements
We thank T. Fujimura and S. Sueyoshi
for the excellent technical assistance.
References
|
1.
|
Dirnhofer R, Jackowski C, Vock P, Potter K
and Thali MJ: VIRTOPSY: minimally invasive, imaging-guided virtual
autopsy. Radiographics. 26:1305–1333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hayakawa M, Yamamoto S, Motani H, Yajima
D, Sato Y and Iwase H: Does imaging technology overcome problems of
conventional postmortem examination? A trial of computed tomography
imaging for postmortem examination. Int J Legal Med. 120:24–26.
2006. View Article : Google Scholar
|
|
3.
|
Ikeda G, Yamamoto R, Suzuki M, Ishikawa H,
Kikuchi K and Shiotani S: Postmortem computed tomography and
magnetic resonance imaging in a case of terminal-stage small cell
lung cancer: an experience of autopsy imaging in tumor-related
death. Radiat Med. 25:84–87. 2007. View Article : Google Scholar
|
|
4.
|
Kikuchi K, Kawahara KI, Biswas KK, et al:
HMGB1: A new marker for estimation of the post mortem interval. Exp
Ther Med. 1:109–111. 2010.PubMed/NCBI
|
|
5.
|
Mitka M: CT, MRI scans offer new tools for
autopsy. Jama. 298:392–393. 2007.PubMed/NCBI
|
|
6.
|
Task Force on Pulmonary Embolism, European
Society of Cardiology: Guidelines on diagnosis and management of
acute pulmonary embolism. Eur J Heart. 21:1301–1336. 2000.
View Article : Google Scholar
|
|
7.
|
Torbicki A, Perrier A, Konstantinides S,
et al: Guidelines on the diagnosis and management of acute
pulmonary embolism: the Task Force for the Diagnosis and Management
of Acute Pulmonary Embolism of the European Society of Cardiology
(ESC). Eur J Heart. 29:2276–2315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Goldhaber SZ, Visani L and de Rosa M:
Acute pulmonary embolism: clinical outcomes in the International
Cooperative Pulmonary Embolism Registry (ICOPER). Lancet.
353:1386–1389. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kasper W, Konstantinides S, Geibel A, et
al: Management strategies and determinants of outcome in acute
major pulmonary embolism: results of a multicenter registry. J Am
Coll Cardiol. 30:1165–1171. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Stein PD and Henry JW: Prevalence of acute
pulmonary embolism among patients in a general hospital and at
autopsy. Chest. 108:978–981. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Mandelli V, Schmid C, Zogno C and Morpurgo
M: ‘False negatives’ and ‘false positives’ in acute pulmonary
embolism: a clinical-postmortem comparison. Cardiologia.
42:205–210. 1997.
|
|
12.
|
Rubinstein I, Murray D and Hoffstein V:
Fatal pulmonary emboli in hospitalized patients. An autopsy study.
Arch Intern Med. 148:1425–1426. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Nordstrom M and Lindblad B:
Autopsy-verified venous thromboembolism within a defined urban
population – the city of Malmo, Sweden. APMIS. 106:378–384.
1998.PubMed/NCBI
|
|
14.
|
Oger E: Incidence of venous
thromboembolism: a community-based study in Western France.
EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne
Occidentale. Thromb Haemost. 83:657–660. 2000.
|
|
15.
|
Hansson PO, Welin L, Tibblin G and
Eriksson H: Deep vein thrombosis and pulmonary embolism in the
general population. ‘The Study of Men Born in 1913’. Arch Intern
Med. 157:1665–1670. 1997.
|
|
16.
|
Bolliger SA, Thali MJ, Ross S, Buck U,
Naether S and Vock P: Virtual autopsy using imaging: bridging
radiologic and forensic sciences. A review of the Virtopsy and
similar projects. Eur Radiol. 18:273–282. 2008. View Article : Google Scholar
|
|
17.
|
Oyake Y, Aoki T, Shiotani S, et al:
Postmortem computed tomography for detecting causes of sudden death
in infants and children: retrospective review of cases. Radiat Med.
24:493–502. 2006. View Article : Google Scholar
|