Introduction
Total knee arthroplasty (TKA) is one of the most
common orthopedic procedures and is an effective treatment for
patients with severe osteoarthritis of the knees that allows pain
relief and improved function and quality of life (1). However, infection is a common
complication of the implementation of total knee prosthetics, and
the current incidence of prosthetic knee infection is 1–3%
(2). The most frequent microorganism
involved is Staphylococcus aureus (3). Staphylococcus epidermidis was
commonly described as relatively innocuous, but has been recognized
as an important opportunistic pathogen, being the most frequent
cause of device-associated infections in hospital (4). Overall, 20–36% infections in TKA are
caused by methicillin-resistant staphylococci, with
methicillin-resistant Staphylococcus aureus and
methicillin-resistant S. epidermidis being increased
(5,6)
It has been reported that the treatment success rate is only ~18%
when the associated bacteria is multi-drug resistant (6,7). The
present case study described a case of S. epidermidis
infection, which developed following cemented TKA. S.
epidermidis infection led to acute arrest of hematopoiesis
(AAH) in the present case. AAH, also known as aplastic crisis, is
the temporary cessation of red cell production. A previous study
reported that infection may trigger the process of AAH, and
exposure to certain drugs may be the possible etiology (8). The patient presented with a high fever,
pallor and tiredness. Routine blood test revealed neutropenia and
anemia and S. epidermidis was detected in the peripheral
blood and bone marrow. The aim of the present study was to describe
a case of TKA that resulted in infection with S. epidermidis
infection and subsequent development of AHH. Its objective is to
enable physicians to be aware of AAH, a rare complication of
patients infected with S. epidermidis. To the best of our
knowledge, this is the first such case reported in the
literature.
Case report
A 74-year-old Chinese female patient was referred to
the Department of Orthopedics at the China-Japan Union Hospital
Affiliated to Jilin University (Changchun, China) on June the
13th, 2014. The patient presented with fever, restricted
movement, pain and swelling of the knee, which developed 4 months
after left TKA surgery due to gonarthrosis. The patient was
receiving regular medical treatment for hypertension for 5 years
(oral administration 30 mg nifedipine once/day; Bayer AG,
Leverkusen, Germany) and had previously undergone a right TKA due
to gonarthrosis 4 years prior to the present admission.
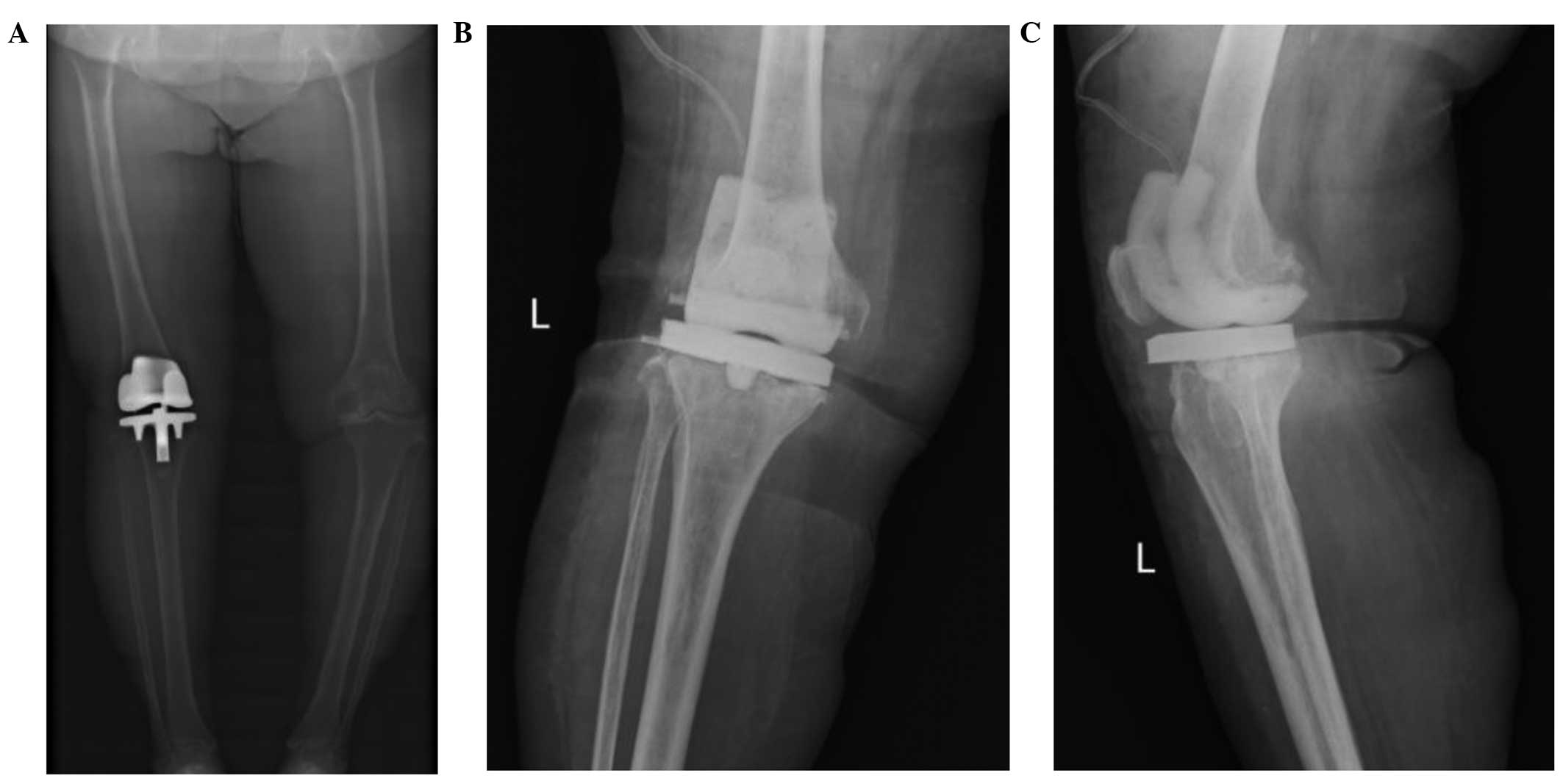
Preoperative X-ray scans of the patient's left knee are shown in
Fig. 1A. Following admission, the
left knee was aspirated and the fluid was sent for culture. Prior
to the culture results, the patient was administered vancomycin
(intravenous administration 1 g/12 h for 18 days; Elli Lilly and
Company, Indianapolis, IN, USA) via intravenous and intra-articular
injection. Several germicultures of the joint fluid were negative.
Routine blood tests and analysis of the renal and liver function
were normal upon admission. However, additional testing revealed an
elevated C-reactive protein level (129 mg/l; normal range, 0–8
mg/l) and erythrocyte sedimentation rate (64 mm/h). X-ray scans
were then performed following implantation of a drainage tube into
the articular cavity (Fig. 1B and
C). As no improvement was observed following antibiotic therapy
and drainage, the components of the prosthetic knee were removed.
The temperature of the patient was reduced to <38°C during the
first postoperative week; however, the patient presented with an
increased fever (body temperature, 39.8°C) once again in the second
postoperative week. At 3 weeks postoperatively, routine blood tests
demonstrated a reduced leucocyte count and anemia: White blood cell
(WBC) count, 0.5×109/l (granulocytes, 7%; lymphocytes,
88%; and monocytes, 5%; normal range, 4–10×109/l); red
blood cell count, 2.52×1012/l (normal range,
3.5–5×1012/l); hemoglobin concentration, 7.8 g/dl
(normal range, 97 g/l); hematocrit, 22.4% (normal range,
0.35–0.47%); mean corpuscular volume, 88.6 fl (normal range, 80–100
fl); and platelet count, 143×109/l (normal range,
100–300×109/l). The reticulocyte count had decreased to
0% (normal range, 0.5–1.5%). A subsequent bone marrow smear
demonstrated marked diminution of hematopoietic cells (Fig. 2). Megakaryocytes were not detected.
Predominantly, non-hematopoietic cells, were detected, including
stromal cells, plasma cells, macrophages and infiltrate of lymphoid
cells, while giant pronormoblasts were clearly visible. The results
of the peripheral blood and bone marrow cultures were compatible
with the diagnosis of AAH (9). The
patient was subsequently transferred to the Department of
Hematology and Oncology at the China-Japan Union Hospital
Affiliated to Jilin University on July the 25th, 2015.
Blood and bone marrow cultures detected the growth of S.
epidermidis (methicillin-resistant strain), which was
subsequently demonstrated to be susceptible to vancomycin and
tigecycline (Pfizer Inc., New York, NY, USA). According to the
results of the antibiotic sensitivity test, the patient was treated
with vancomycin and a supportive treatment, which consisted of
nutritional support (intravenous administration of 10 g albumin/day
for 5 days; Octapharma AG, Lachen, Switzerland), defervescence
(oral administration of 500 mg acetaminophen when fever is
>38.5°C; Beijing Shuguang Pharmaceutical Industrial Co., Ltd.,
Beijing, China), and bone marrow hematopoiesis stimulation
(hypodermic injection of 300 µg G-CSF once/day for 12 days;
Changchun Jinsai Pharmaceutical Co. Ltd., Jilin, China). However,
the symptoms persisted after 1 week of treatment and the WBC count
had reduced to 0.1×109 cells/l. Furthermore, the patient
exhibited left-sided heart failure and brain natriuretic peptide
(BNP) levels increased to 35,000 pg/ml; therefore, the patient was
administered cardiotonic (three intravenous bolus injections of 200
mg cedilanid; Xudong Haipu Pharmaceutical Co., Ltd, Shanghai,
China) and diuretics (eight intravenous bolus injections of 20 mg
lasix; Nantong Jinghua Pharmaceutical Co., Ltd., Nantong, China).
The patient also presented with a cough, without expectoration. The
results of the (1→3)-β-D-glucanemia test (10) indicated a level of
(1→3)-β-D-glucanemia was 1,509.1 pg/ml, which was markedly elevated
(normal range, 100.5–151.5 pg/ml). Therefore, the patient was
diagnosed with a deep mycotic infection and administered vancomycin
(intravenous administration of 1 g/12 h for 17 days; Eli Lilly and
Company), meropenem (intravenous administration of 0.5 g/8 h for 8
days; Sumitomo Dainippon Pharma Co., Ltd., Tokyo, Japan) and
voriconazole (02 g/12 h for 10 days; Pfizer, Inc.) triple
anti-inflammatory treatment. After 1 week, the patient's
temperature and reticulocyte count returned to normal, while the
WBC count increased to 15×109 cells/l; therefore,
meropenem and voriconazole treatment was stopped. However, the
left-sided heart failure persisted following treatment and the BNP
levels remained elevated. Renal failure was subsequently detected,
since the serum creatinine level was 229 umol/l and blood urea
nitrogen (BUN) was 21.5 mmol/l. Reexamination of the blood cultures
demonstrated the growth of S. epidermidis
(methicillin-resistant); therefore, antibiotic treatment was
amended from vancomycin to tigecycline (intravenous administration
of 100 mg/day on day 1 then 50 mg/12 h on days 2 and 3; Pfizer,
Inc.). However, renal failure persisted and several days later the
patient presented with anuria as the renal function continued to
deteriorate (serum creatinine, 520 umol/l; BUN, 40.8 mmol/l; and
serum potassium, 5.7 mmol/l). The patient succumbed to infection
with S. epidermidis and the secondary renal and heart
failure 25 days after admission to the Department of Hematology and
Oncology.
Discussion
AAH is defined as a transient episode of pure red
cell aplasia characterized by the absence of reticulocytes in the
peripheral blood, the absence of erythroid precursors and elevated
giant pronormoblasts in the bone marrow (11–13). It
has previously been reported that infection or drug exposure may
trigger AAH (8). AAH predominantly
occurs in patients with hemolytic anemia and, as it is a
self-limiting disease, aplasia usually persists for 5–10 days
(12,13). Routine blood tests typically
demonstrate anemia and a decreased reticulocyte count, and although
pancytopenia does not usually occur, mild leukopenia and/or
thrombocytopenia are occasionally detected (11). In the present patient, the complete
blood count demonstrated neutropenia and moderate anemia. Although
the hematopoietic activity was recovered, the patient eventually
succumbed to infection with S. epidermidis and the secondary
renal and heart failure.
The most common cause of severe aplastic crisis for
patients with chronic hemolytic anemia is infection with parvovirus
B19 (11,14). However, rare cases of severe aplastic
anemia due to acute parvovirus B19 infection have been reported in
previously healthy individuals (15,16). In
addition to parvovirus B19, there are various viral infections that
contribute to AAH, including the Epstein Barr virus,
cytomegalovirus, HIV and rubella (17,18).
Additional triggering agents in transient aplastic crises include
pharmacological agents (such as methotrexate) and irradiation
(18,19). Patients typically present with
increased fatigue, pallor, activity intolerance or shortness of
breath. For the vast majority of patients with acute aplastic
anemia, only supportive and symptomatic treatment can be offered.
Transfusion of red blood cells (RBC) is required to relieve the
symptoms of anemia for certain patients.
The present study described a case of AAH caused by
S. epidermidis infection which appeared following the
application of cemented TKA. Periprosthetic joint infection (PJI)
is one of the most severe complications following TKA. This is a
difficult issue for orthopaedic surgeons, as well as for patients
and their families, due to its challenging management and the
impact on the patients' quality of life (3,20).
Epidemiologic studies investigating various trends
in the microbiological profile of PJI between two referral centers
in Europe and the United States demonstrated that PJI caused by
methicillin-resistant S. aureus is becoming increasingly
prevalent (21,22). In the present case, growth of
methicillin-resistant S. epidermidis was detected in blood
and bone marrow cultures; however, a germiculture of joint fluid
remained negative following various repeats. Biofilm formation may
explain the phenomenon of culture-negative PJI. Novel methods,
including DL-dithiothreitol and polymerase chain reaction using
various probes, are being developed in order to improve the ability
of microbiological culture in detecting the pathogenic causes of
PJI (23,24).
Wu et al (25)
conducted a case-control study in order to investigate the effects
of patient factors on the risk of PJI in a Chinese population. The
results demonstrated that an increased risk of PJI in this Chinese
population was associated with: Diabetes; age of 65–75 years;
body-mass index of ≥28 kg/m2; a history of alcohol
abuse; and residence in rural areas. For the present patient, old
age and obesity may have been influential risk factors.
The present patient was not receiving any medication
that could trigger transient aplastic crises, and had no history of
irradiation therapy. Therefore, it is hypothesized that the cause
of AAH was infection with S. epidermidis. The pathogenesis
of acute aplastic anemia induced by S. epidermidis infection
is yet to be elucidated.
In conclusion, the present study described a case of
AAH induced by S. epidermidis infection, which developed
following cemented TKA. AAH induced by methicillin-resistant S.
epidermidis infection is a rare and life-threatening
complication. The present study increased our knowledge of this
rare disease and its characteristics, which will enable physicians
to be aware of the development of AAH as a rare complication of
S. epidermidis infection. Further studies are required in
order to elucidate the exact pathogenesis of AAH.
References
|
1
|
Vahedian-Ardakani M, Mortazavi S and
Farzan M: Total knee arthroplasty: Does the tibial medial side
defect affect outcome? Acta Med Iran. 8:462–465. 2015.
|
|
2
|
Soriano A, Bori G, García-Ramiro S,
Martinez-Pastor JC, Miana T, Codina C, Maculé F, Basora M, Martínez
JA, Riba J, et al: Timing of antibiotic prophylaxis for primary
total knee arthroplasty performed during ischemia. Clin Infect Dis.
46:1009–1014. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martínez-Pastor JC, Maculé-Beneyto F and
Suso-Vergara S: Acute infection in total knee arthroplasty:
Diagnosis and treatment. Open Orthop J. 7:197–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Otto M: Molecular basis of
Staphylococcus epidermidis infections. Semin Immunopathol.
34:201–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peersman G, Laskin R, Davis J and Peterson
M: Infection in total knee replacement: A retrospective review of
6489 total knee replacements. Clin Orthop Relat Res. 392:15–23.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joshy S, Gogi N, Thomas B, Mahale A and
Singh BK: Delayed onset of deep infection after total knee
arthroplasty: Comparison based on the infecting organism. J Orthop
Surg (Hong Kong). 15:154–158. 2007.PubMed/NCBI
|
|
7
|
Kilgus DJ, Howe DJ and Strang A: Results
of periprosthetic hip and knee infections caused by resistant
bacteria. Clin Orthop Relat Res. 404:116–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee JH, Lee JH, Shin YR, Lee JS, Kim WK,
Chi HS, Park CJ and Lee KH: Spontaneous remission of aplastic
anemia: A retrospective analysis. Haematologica. 86:928–933.
2001.PubMed/NCBI
|
|
9
|
Yan ZS, Zhang L, Wang HJ, Zhou K, Li JP,
Huang ZD, Wan L, Shang L, Bao XL and Zhang FK: Acute arrest of
hemopoiesis mimics aplastic anemia: 23 cases report. Zhonghua Xue
Ye Xue Za Zhi. 28:750–753. 2007.(In Chinese). PubMed/NCBI
|
|
10
|
Marty FM and Koo S: Med Mycol. Role of
(1->3)-beta-D-glucan in the diagnosis of invasive aspergillosis.
Med Mycol 47 Suppl. 1:S233–S240. 2009. View Article : Google Scholar
|
|
11
|
Borsato ML, Bruniera P, Cusato MP, Spewien
KE, Durigon EL and Toporovski J: Aplastic crisis in sickle cell
anemia induced by parvovírus B19. J Pediatr (Rio J). 76:458–460.
2000.(In Portuguese). View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ilesanmi OO: Pathological basis of
symptoms and crises in sickle cell disorder: Implications for
counseling and psychotherapy. Hematol Rep. 26:e22010. View Article : Google Scholar
|
|
13
|
Cauff BE and Quinn CT: Transient
parvovirus-associated hypoplasia of multiple peripheral blood cell
lines in children with chronichemolytic anemia. Pediatr Blood
Cancer. 50:861–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carzavec D, Gaćina P, Vasilj A and Katović
SK: Aplastic crisis induced by human parvovirus B19 as an initial
presentation of hereditary spherocytosis. Coll Antropol.
34:619–621. 2010.PubMed/NCBI
|
|
15
|
Al-Abdwani RM, Khamis FA, Balkhair A,
Sacharia M and Wali YA: A child with human parvovirus B19 infection
induced aplastic anemia and acute hepatitis: Effectiveness of
immunosuppressive therapy. Pediatr Hematol Oncol. 25:699–703. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaptan K, Beyan C, Ural AU, Ustün C, Cetin
T, Avcu F, Kubar A, Aliş M and Yalçin A: Successful treatment of
severe aplastic anemia associated with human parvovirus B19 and
Epstein-Barr virus in a healthy subject with allo-BMT. Am J
Hematol. 67:252–255. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leoz Gordillo I and Suárez Pérez E:
Aplastic crisis due to Parvovirus B19 and Epstein-Barr virus in a
patient with hereditary spherocytosis. An Pediatr (Barc).
82:e102–e107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rajput R, Sehgal A, Jain D, Sen R and
Gupta A: Acute parvovirus B19 infection leading to severe aplastic
anemia in a previously healthy adult female. Indian J Hematol Blood
Transfus. 28:123–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Yan and Zhu Shuqing: Acute arrest of
hematopoiesis due to oral methotrexate in a patient with uremia.
Adverse Drug Reactions J. 12:351–352. 2010.
|
|
20
|
Alijanipour P and Parvizi J: Infection
post-total knee replacement: Current concepts. Curr Rev
Musculoskelet Med. 7:96–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bjerke-Kroll BT, Christ AB, McLawhorn AS,
Sculco PK, Jules-Elysée KM and Sculco TP: Periprosthetic joint
infections treated with two-stage revision over 14 years: An
evolving microbiology profile. J Arthroplasty. 29:877–882. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aggarwal VK, Bakhshi H, Ecker NU, Parvizi
J, Gehrke T and Kendoff D: Organism profile in periprosthetic joint
infection: Pathogens differ at two arthroplasty infection referral
centers in Europe and in the United States. J Knee Surg.
27:399–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Drago L, Signori V, De Vecchi E, Vassena
C, Palazzi E, Cappelletti L, Romanò D and Romanò CL: Use of
dithiothreitol to improve the diagnosis of prosthetic joint
infections. J Orthop Res. 31:1694–1699. 2013.PubMed/NCBI
|
|
24
|
Rak M, Barlič-Maganja D, Kavčič M, Trebše
R and Cőr A: Comparison of molecular and culture method in
diagnosis of prosthetic joint infection. FEMS Microbiol Lett.
343:42–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu C, Qu X, Liu F, Li H, Mao Y and Zhu Z:
Risk factors for periprosthetic joint infection after total hip ar
oplasty and total knee arthroplasty in Chinese patients. PLoS One.
9:e953002014. View Article : Google Scholar : PubMed/NCBI
|