Introduction
Immune responses to infection or injury are causes
of systemic or local inflammation, respectively. Inflammation is a
complex biological response leading to numerous diseases, including
rheumatoid arthritis, chronic asthma, multiple sclerosis,
inflammatory bowel disease and psoriasis (1). Inflammatory bowel disease and
ulcerative colitis in particular are chronic debilitating diseases
that affect millions of people worldwide. Furthermore,
Drosophila melanogaster is a well-established model organism
for studying various diseases, including inflammatory bowel
diseases (2). Intestinal stem cells
(ISCs) have been identified in Drosophila midgut and
hindgut, which are equivalent to mammalian intestine and colon,
respectively (3). In order to
maintain gut homeostasis, intestinal epithelial cells turn over
rapidly following damage from ingested pathogens, chemicals and
toxic compounds. In the Drosophila midgut, cell turnover is
functionally equivalent to that occurring in the mammalian small
intestine. An ISC divides into a new ISC and a post-mitotic
enteroblast (EB), which differentiates into an absorptive
enterocyte or a secretory enteroendocrine cell (4). In addition, gut cell turnover is
regulated by a balance between cell death and stem cell
proliferation (5).
In the Drosophila gut, the immune response
primarily relies on the local production of microbicidal reactive
oxygen species (ROS) and the release of antimicrobial peptides
(AMPs) (6). The production of ROS in
the gut by the nicotinamide adenine dinucleotide phosphate oxidase
Duox provides an efficient barrier against the majority of ingested
microbes (7). However, the excessive
accumulation of ROS can disrupt mitochondrial DNA, protein
oxidation and lipid peroxidation, which results in impaired
function of the mitochondria and metabolism (8). Furthermore, the local production of
AMPs are important in the inducible defense mechanisms in the gut.
AMPs are triggered by the Imd pathway through the recognition of
Gram-negative peptidoglycan (9).
Traditional, medicinal plants are globally used and
have rapidly grown in economic importance. Intrinsically active
compounds are well-known for their anti-oxidant, anti-tumor,
anti-viral and anti-inflammatory activities, and for improving
immunity in general (10–12).
In the present study, Drosophila were used as
a model organism in order to identify the protective effects of 50
different traditional medicinal plant extracts that are known to
have curative or beneficial effects on the symptoms of various
disorders in China. Investigating these medicinal plants,
particularly the aqueous extracts of four species (C.
pilosula, S. lappa, I. cylindrical var.
major and M. toosendan), may help clinical
researchers to improve their understanding of the complex roles of
medicinal plants in gut disorders, including inflammatory bowel
disease.
Materials and methods
Drosophila stocks
Drosophila melanogaster strains were cultured
on a standard cornmeal-yeast medium at 25°C and 60% humidity under
a 12-h light/dark cycle. W1118 was purchased from the
Bloomington Drosophila stock center (Bloomington, IN, USA),
and esg-Gal4 UAS-green fluorescent protein (GFP) antibodies was a
gift from Dr Rongwen Xi (National Institute of Biological Sciences,
Beijing, China).
Aqueous extracts of traditional
medicinal plants and preparation of growth media
A total of 50 different traditional medicinal plants
were purchased from the Renmintongtai Pharmacy (Harbin, China).
Aqueous plant extracts were obtained as previously described
(11). A total of 50 types of
traditional medicinal plants (20 g) were immersed in deionized
water (200 ml; yield, ~5–14%) overnight at 25°C. The aqueous
extraction was boiled for 3 h, and the extraction process was
repeated twice. The total extracts were mixed and concentrated to
100 ml. Flies fed a standard cornmeal-yeast medium were used as the
control group. Flies fed the standard medium containing extracts of
the medicinal plants served as the experimental groups. The final
concentrations of the extracts ranged between 1.25 and 10% (w/v)
(Table I).
 | Table I.Fifty different traditional medicinal
plants, plant parts and final concentrations (w/v) for screening in
gut inflammation. |
Table I.
Fifty different traditional medicinal
plants, plant parts and final concentrations (w/v) for screening in
gut inflammation.
| Latin name | Plant part |
|---|
| Taxillus
chinensis (DC) Danser | Stem |
| Raphanus
sativus L. | Seed |
| Acorus
tatarinowii Schott | Rootstalk |
| Rheum
officinale Baill. | Root and
rootstalk |
| Peucedanum
praeruptorum Dunn | Root |
| Trichosanthes
kirilowii Maxim. | Fruit |
| Codonopsis
pilosula (Franch.) Nannf | Root |
| Fructus
liquidambaris | Fruit |
| Aconitum
kusnezoffii Reichb. | Root |
| Cinnamomun
cassia Presl. | Bark |
| Quisqualis
indica L. | Fruit |
| Polygonum
multiflorum Thunb. | Root |
| Stellaria
dichatoma L.var.lanceolata Bge. | Root |
| Achyranthes
Bidentata Bl. | Root |
| Saussurea
lappa (Decne.) C.B.Clarke | Root |
| Pollen
typhae | Pollen |
| Dianthus
superbus L. | The whole |
| Leonurus
heterophyllus Sweet | The whole |
| Panax
notoginseng (Burk) F. H. Chen | Rootstalk |
| Imperata
cylindrica Beauv. var. major (Nees) C. E. Hubb. | Rootstalk |
| Ophiopogon
japonicns (Thumb.) Ker-Gawl. | Root |
| Allium
macrostemon Bunge | Stem |
| Salvia
miltiorrhiza Bunge | Root and
rootstalk |
| Artemisia
capillaris Thunb. | Whole plant |
| Aconitum
carmichaeli Debx. | Root |
| Caesalpinina
sappan L. | Heartwood |
| Melia
toosendan Sied.Et Zucc. | Fruit |
| Uncaria
rhynchophylla (Miq.) Jacks | Stem |
| Lithospermum
erythrorhizon Sieb. et Zucc. | Root |
| Spatholobus
suberectus Dunn | Stem |
| Stephania
tetrandra S.Moore | Root |
| Cyathula
officinalis Kuan | Root |
| Pyrrosia
lingua (Thunb.) Farwell | Leaf |
| Alpinia
katsumadai Hayata | Seed |
| Dalbergia
odorifera T.chen | Trunk and root |
| Carthamus
tinctorius L. | Flower |
| Lilium
brownii var.viridulum Baker. | Leaf |
| Ligusticum
chuanxiong Hort. | Rootstalk |
| Cyperus
rotundus L. | Rootstalk |
| Pbyporus
umbellatus (pers.) Fries | Sclerotium |
| Chrysanthemum
monfolium Ramat. | Flower |
| Sophora
flavescens Ait | Root |
| Curcuma
phaeocaulis Valeton | Rootstalk |
| Cynanchum
glaucescens (Decne.) Hand.-Mazz | Rootand
rootstalk |
| Curcuma
aromatica Salisb. | Root |
| Acanthopanax
gracilistylus W.W.smith | Bark |
| Drynaria
fortunei (Kunze) J.Sm | Rootstalk |
| Lygodium
japonicum (Thunb) Sw. | Whole plant |
| Sanguisorba
officinalis L. | Root and
rootstalk |
| Stemona
japonica (Blume) Miq. | Root |
Feeding experiments
The 4- to 5-day-old adult flies were used for the
feeding experiments, with each vial containing 15 males and 15
females. Following a 2 h fast in an empty vial, flies were
transferred into a vial with five layers of filter paper hydrated
with 5% sucrose (w/v) with toxic compounds, containing 0.4 M NaCl,
0.6% SDS or 4% DSS. Filter papers were changed every day, and the
number of living flies was recorded at each transfer for 6 or 8
days.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Due to their larger size, female flies were used for
gut dissection. The survival and gut cell development were similar
in both females and males (3). Adult
females were treated with 1% SDS for 0, 4 or 16 h. In addition, the
total RNA was extracted from 25–30 dissected guts (without
Malpighian tubules) using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and cDNA was synthesized via
RT using M-MLV reverse transcriptase, RNase H minus and a point
mutant kit (Promega Corporation, Madison, WI, USA). qPCR was
performed in a total reaction volume of 20 µl with 3 µl DDW, 3 µl
PCR primer, 10 µl master mix (2X) and 5 µl template cDNA.
Lightcycler 480 SYBR Green I Master Mix was used (Roche
Diagnostics, Basel, Switzerland). qPCR thermal cycling conditions
were as follows: 95°C for 5 min, followed by 40 cycles of 95°C for
10 sec, 55°C for 10 sec and 72°C for 10 sec, and one melting curve
cycle of 95°C for 5 sec, 65°C for 1 min and continuous 97°C,
followed by 40°C for 10 sec. Results were normalized to the level
of RpL32 mRNA in each sample from two independent experiments using
LightCycler 480 software version 1.5 (Roche Diagnostics). Primer
sequences are depicted in Table
II.
 | Table II.Primer sequences used for polymerase
chain reaction analyses. |
Table II.
Primer sequences used for polymerase
chain reaction analyses.
| Target gene | Forward (5′ to
3′) | Reverse (5′ to
3′) |
|---|
| Dpt |
ATGCAGTTCACCATTGCCGTC |
TCCAGCTCGGTTCTGAGTTG |
| Mtk |
GCATCAATCAATTCCCGCCACC |
CGGCCTCGTATCGAAAATGGG |
| AttA |
AGGTTCCTTAACCTCCAATC |
CATGACCAGCATTGTTGTAG |
| CecC |
GATGAGCCTTTAATGTCC |
TGTAAGCTAGTTTATTTCTA |
| Dro3 |
TCCACGCTGCAGAGCAC |
CTAATGGAGGCCAACACTGTT |
| Dfn |
CGCTTTTGCTCTGCTTGCTTGC |
TAGGTCGCATGTGGCTCGCTTC |
| rp49 |
AGTCGGATCGATATGCTAAGCTGT |
TAACCGATGTTGGGCATCAGATACT |
Immunostaining
Dead cells were detected by 7-aminoactinomycin D
(7-AAD; Invitrogen; Thermo Fisher Scientific, Inc.); gut imaging
and staining were performed as described previously (11). Briefly, guts of adult females were
dissected in cold phosphate-buffered saline (PBS), incubated in
7-AAD (5 µg/ml in PBS) for 30 min at room temperature, and washed
with PBS three times. For immunostaining, dissected guts of female
flies were fixed in 4% paraformaldehyde for 30 min at room
temperature. Samples were blocked with 5% goat serum in PBS-Tween
20 (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) for 30 min
followed by incubation with polyclonal anti-GFP antibodies
synthesized in our laboratory (1:200) overnight at 4°C. Following
washing four times with PBS with Tween 20, samples were incubated
with anti-rat IgG-fluorescein isothiocyanate secondary antibody
(1:200; F1763; Sigma-Aldrich; Merck Millipore) for 2 h at room
temperature adn subsequently stained with
4′,6-diamidino-2-phenylindole (Sigma-Aldrich; Merck Millipore) for
10 min. Finally, the guts were mounted in 70% glycerol and imaged
with an Axioskop 2 plus microscope (Zeiss AG, Oberkochen, Germany).
All the data are representative of three independent experiments.
The number of dead cells, intestinal stem cells and enteroblasts in
the Drosophila gut was quantified using ImageJ software (V1.47;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using a
two-tailed unpaired Student's t-test with Prism Prism 6 software
(GraphPad Software, Inc., La Jolla, CA, USA). P<0.005 was
considered to indicate a statistically significant difference.
Error bars indicate the mean ± standard error of the mean.
Results
Medicinal plant extracts improve
survival rates in vivo
The intestinal epithelium is susceptible to damage
caused by pathogens, oxidative stress and toxic compounds. Foods
containing SDS or NaCl could cause injury to the intestines and
result in a melanotic phenotype in Drosophila (13). To screen for protective activities of
traditional medicinal plants, flies were fed a standard cornmeal
medium supplemented with (experimental groups) or without (control
group) aqueous extracts of the medicinal plants. Adult flies from
each of the culture conditions were orally treated with the
inflammatory reagent SDS or NaCl. Initially, a vial containing 30
adult flies from each culture condition was treated with 0.6% SDS,
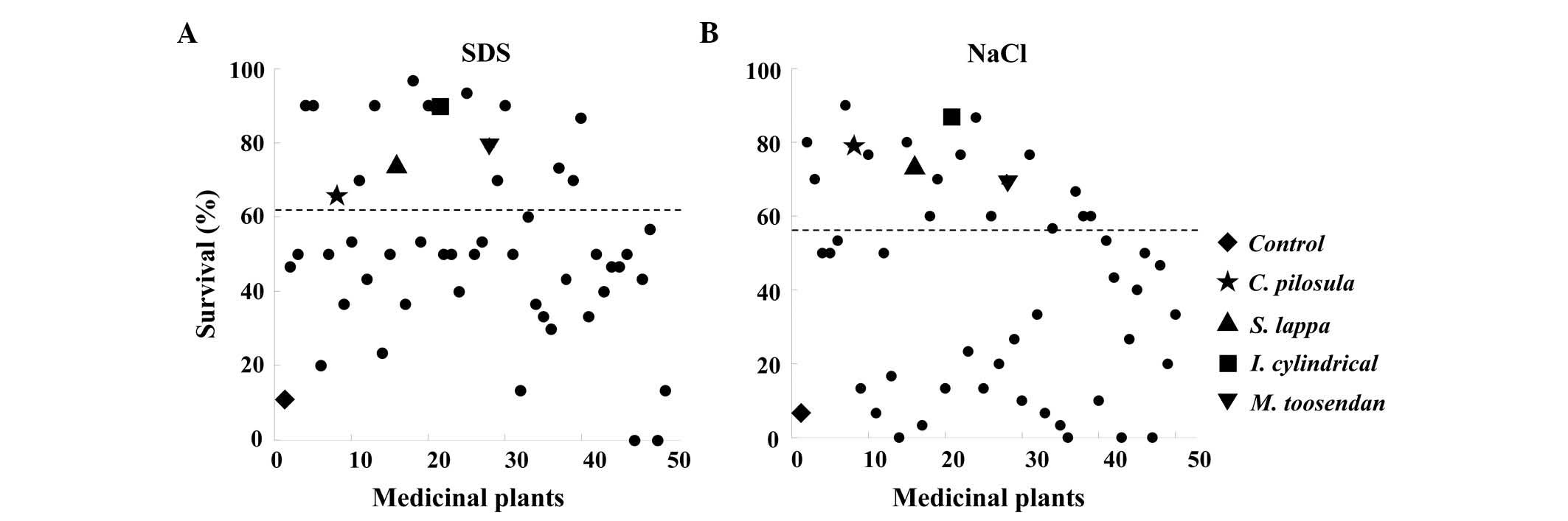
and the survival rate was assessed over 6 days (Table III). The control group revealed
>88% mortality, however, a number of flies in the experimental
groups appeared to have an increased survival rate. Out of 50
different medicinal plant extracts, 16 species increased the
survival rate by >50% compared with the control group (Fig. 1A). In addition, following treatment
with 0.4 M NaCl, 18 species increased in survival rate by 50%
compared with the control (Fig. 1B
and Table IV).
 | Table III.Survival rate of control and
experimental groups that were treated with 0.6% sodium dodecyl
sulfate. |
Table III.
Survival rate of control and
experimental groups that were treated with 0.6% sodium dodecyl
sulfate.
| Group | D0 | D1 | D2 | D3 | D4 | D5 | D6 |
|---|
| Control | 100.0 | 100.0 | 98.5 | 85.7 | 52.8 | 28.8 | 11.2 |
| Taxillus
chinensis (DC) Danser | 100.0 | 100.0 | 96.7 | 96.7 | 96.7 | 73.3 | 46.7 |
| Raphanus
sativus L. | 100.0 | 100.0 | 100.0 | 100.0 | 90.0 | 73.3 | 50.0 |
| Acorus
tatarinowii Schott | 100.0 | 100.0 | 100.0 | 93.3 | 93.3 | 90.0 | 90.0 |
| Rheum
officinale Baill. | 100.0 | 100.0 | 100.0 | 93.3 | 93.3 | 90.0 | 90.0 |
| Peucedanum
praeruptorum Dunn | 100.0 | 100.0 | 90.0 | 66.7 | 53.3 | 43.3 | 20.0 |
| Trichosanthes
kirilowii Maxim. | 100.0 | 100.0 | 100.0 | 90.0 | 80.0 | 66.7 | 50.0 |
| Codonopsis
pilosula (Franch.) Nannf | 100.0 | 100.0 | 96.7 | 96.7 | 96.7 | 80.0 | 66.7 |
| Fructus
liquidambaris | 100.0 | 100.0 | 100.0 | 93.3 | 63.3 | 63.3 | 36.7 |
| Aconitum
kusnezoffii Reichb. | 100.0 | 100.0 | 100.0 | 93.3 | 76.7 | 76.7 | 53.3 |
| Cinnamomun
cassia Presl. | 100.0 | 96.7 | 93.3 | 93.3 | 93.3 | 83.3 | 70.0 |
| Quisqualis
indica L. | 100.0 | 100.0 | 93.3 | 93.3 | 76.7 | 56.7 | 43.3 |
| Polygonum
multiflorum Thunb. | 100.0 | 100.0 | 100.0 | 100.0 | 96.7 | 96.7 | 90.0 |
| Stellaria
dichatoma L.var.lanceolata Bge. | 100.0 | 93.3 | 73.3 | 56.7 | 40.0 | 30.0 | 23.3 |
| Achyranthes
Bidentata Bl. | 100.0 | 96.7 | 96.7 | 96.7 | 90.0 | 70.0 | 50.0 |
| Saussurea
lappa (Decne.) C.B.Clarke | 100.0 | 93.3 | 90.0 | 90.0 | 90.0 | 86.7 | 73.3 |
| Pollen
typhae | 100.0 | 100.0 | 96.7 | 90.0 | 73.3 | 43.3 | 36.7 |
| Dianthus
superbus L. | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 96.7 | 96.7 |
| Leonurus
heterophyllus Sweet | 100.0 | 100.0 | 100.0 | 86.7 | 80.0 | 60.0 | 53.3 |
| Panax
notoginseng (Burk) F. H. Chen | 100.0 | 100.0 | 100.0 | 96.7 | 96.7 | 93.3 | 90.0 |
| Imperata
cylindrica Beauv. var. major (Nees) C. E.Hubb. | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 89.9 |
| Ophiopogon
japonicns (Thumb.) Ker-Gawl. | 100.0 | 100.0 | 100.0 | 93.3 | 90.0 | 66.7 | 50.0 |
| Allium
macrostemon Bunge | 100.0 | 100.0 | 96.7 | 93.3 | 86.7 | 63.3 | 50.0 |
| Salvia
miltiorrhiza Bunge | 100.0 | 96.7 | 96.7 | 90.0 | 80.0 | 50.0 | 40.0 |
| Artemisia
capillaris Thunb. | 100.0 | 96.7 | 96.7 | 96.7 | 93.3 | 93.3 | 93.3 |
| Aconitum
carmichaeli Debx. | 100.0 | 86.7 | 83.3 | 83.3 | 76.7 | 73.3 | 50.0 |
| Caesalpinina
sappan L. | 100.0 | 100.0 | 100.0 | 96.7 | 66.7 | 53.3 | 53.3 |
| Melia
toosendan Sied.Et Zucc. | 100.0 | 96.7 | 93.3 | 93.3 | 93.3 | 93.3 | 80.0 |
| Uncaria
rhynchophylla (Miq.) Jacks | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 93.3 | 70.0 |
| Lithospermum
erythrorhizon Sieb. et Zucc. | 100.0 | 100.0 | 100.0 | 96.7 | 96.7 | 96.7 | 90.0 |
| Spatholobus
suberectus Dunn | 100.0 | 100.0 | 96.7 | 96.7 | 80.0 | 76.7 | 50.0 |
| Stephania
tetrandra S.Moore | 100.0 | 100.0 | 93.3 | 90.0 | 33.3 | 26.7 | 13.3 |
| Cyathula
officinalis Kuan | 100.0 | 100.0 | 96.7 | 96.7 | 90.0 | 86.7 | 60.0 |
| Pyrrosia
lingua (Thunb.) Farwell | 100.0 | 100.0 | 100.0 | 100.0 | 80.0 | 56.7 | 36.7 |
| Alpinia
katsumadai Hayata | 100.0 | 100.0 | 93.3 | 80.0 | 50.0 | 40.0 | 33.3 |
| Dalbergia
odorifera T.chen | 100.0 | 100.0 | 100.0 | 96.7 | 66.7 | 46.7 | 30.0 |
| Carthamus
tinctorius L. | 100.0 | 100.0 | 100.0 | 100.0 | 93.3 | 90.0 | 73.3 |
| Lilium
brownii var.viridulum Baker. | 100.0 | 100.0 | 86.7 | 86.7 | 76.7 | 56.7 | 43.3 |
| Ligusticum
chuanxiong Hort. | 100.0 | 100.0 | 100.0 | 96.7 | 96.7 | 96.7 | 70.0 |
| Cyperus
rotundus L. | 100.0 | 100.0 | 100.0 | 100.0 | 86.7 | 86.7 | 86.7 |
| Pbyporus
umbellatus (pers.) Fries | 100.0 | 100.0 | 100.0 | 96.7 | 66.7 | 46.7 | 33.3 |
| Chrysanthemum
monfolium Ramat. | 100.0 | 93.3 | 93.3 | 93.3 | 73.3 | 53.3 | 50.0 |
| Sophora
flavescens Ait | 100.0 | 100.0 | 100.0 | 93.3 | 86.7 | 66.7 | 40.0 |
| Curcuma
phaeocaulis Valeton | 100.0 | 100.0 | 86.7 | 83.3 | 60.0 | 60.0 | 46.7 |
| Cynanchum
glaucescens (Decne.) Hand.-Mazz | 100.0 | 100.0 | 100.0 | 93.3 | 86.7 | 60.0 | 46.7 |
| Curcuma
aromatica Salisb. | 100.0 | 93.3 | 90.0 | 73.3 | 56.7 | 53.3 | 50.0 |
| Acanthopanax
gracilistylus W.W.smith | 100.0 | 96.7 | 83.3 | 53.3 | 16.7 | 0.0 | 0.0 |
| Drynaria
fortunei (Kunze) J.Sm | 100.0 | 96.7 | 96.7 | 93.3 | 63.3 | 53.3 | 43.3 |
| Lygodium
japonicum (Thunb) Sw. | 100.0 | 100.0 | 100.0 | 93.3 | 83.3 | 60.0 | 56.7 |
| Sanguisorba
officinalis L. | 100.0 | 100.0 | 93.3 | 80.0 | 40.0 | 3.3 | 0.0 |
| Stemona
japonica (Blume) Miq. | 100.0 | 96.7 | 73.3 | 56.7 | 33.3 | 33.3 | 13.3 |
 | Table IV.Survival rate of control and
experimental groups that were treated with 0.4 M NaCl. |
Table IV.
Survival rate of control and
experimental groups that were treated with 0.4 M NaCl.
| Group | D0 | D1 | D2 | D3 | D4 | D5 | D6 |
|---|
| Control | 100.0 | 99.5 | 98.2 | 89.5 | 54.4 | 23.5 | 7.2 |
| Taxillus
chinensis (DC) Danser | 100.0 | 96.7 | 96.7 | 96.7 | 93.3 | 93.3 | 80.0 |
| Raphanus
sativus L. | 100.0 | 96.7 | 96.7 | 96.7 | 90.0 | 90.0 | 70.0 |
| Acorus
tatarinowii Schott | 100.0 | 96.7 | 86.7 | 80.0 | 73.3 | 63.3 | 50.0 |
| Rheum
officinale Baill. | 100.0 | 96.7 | 86.7 | 80.0 | 73.3 | 63.3 | 50.0 |
| Peucedanum
praeruptorum Dunn | 100.0 | 96.7 | 86.7 | 83.3 | 83.3 | 63.3 | 53.3 |
| Trichosanthes
kirilowii Maxim. | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 93.3 | 90.0 |
| Codonopsis
pilosula (Franch.) Nannf | 100.0 | 100.0 | 100.0 | 96.7 | 96.7 | 90.0 | 80.0 |
| Fructus
liquidambaris | 100.0 | 100.0 | 96.7 | 96.7 | 73.3 | 46.7 | 13.3 |
| Aconitum
kusnezoffii Reichb. | 100.0 | 96.7 | 96.7 | 93.3 | 86.7 | 86.7 | 76.7 |
| Cinnamomun
cassia Presl. | 100.0 | 100.0 | 93.3 | 86.7 | 50.0 | 20.0 | 6.7 |
| Quisqualis
indica L. | 100.0 | 100.0 | 93.3 | 86.7 | 73.3 | 63.3 | 50.0 |
| Polygonum
multiflorum Thunb. | 100.0 | 100.0 | 100.0 | 93.3 | 83.3 | 46.7 | 16.7 |
| Stellaria
dichatoma L.var.lanceolata Bge. | 100.0 | 90.0 | 50.0 | 10.0 | 0.0 | 0.0 | 0.0 |
| Achyranthes
Bidentata Bl. | 100.0 | 100.0 | 96.7 | 96.7 | 90.0 | 86.7 | 80.0 |
| Saussurea
lappa (Decne.) C.B.Clarke | 100.0 | 100.0 | 96.7 | 96.7 | 90.0 | 90.0 | 73.3 |
| Pollen
typhae | 100.0 | 96.7 | 90.0 | 76.7 | 40.0 | 13.3 | 3.3 |
| Dianthus
superbus L. | 100.0 | 100.0 | 96.7 | 96.7 | 80.0 | 76.7 | 60.0 |
| Leonurus
heterophyllus Sweet | 100.0 | 100.0 | 100.0 | 90.0 | 86.7 | 76.7 | 70.0 |
| Panax
notoginseng (Burk) F. H. Chen | 100.0 | 96.7 | 96.7 | 93.3 | 73.3 | 43.3 | 13.3 |
| Imperata
cylindrica Beauv. var. major (Nees) C. E. Hubb. | 100.0 | 93.3 | 93.3 | 93.3 | 93.3 | 93.3 | 86.7 |
| Ophiopogon
japonicns (Thumb.) Ker-Gawl. | 100.0 | 100.0 | 96.7 | 96.7 | 93.3 | 76.7 | 76.7 |
| Allium
macrostemon Bunge | 100.0 | 100.0 | 100.0 | 90.0 | 73.3 | 50.0 | 23.3 |
| Salvia
miltiorrhiza Bunge | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 96.7 | 86.7 |
| Artemisia
capillaris Thunb. | 100.0 | 100.0 | 96.7 | 83.3 | 80.0 | 43.3 | 13.3 |
| Aconitum
carmichaeli Debx. | 100.0 | 100.0 | 93.3 | 93.3 | 86.7 | 86.7 | 60.0 |
| Caesalpinina
sappan L. | 100.0 | 100.0 | 100.0 | 93.3 | 73.3 | 53.3 | 20.0 |
| Melia
toosendan Sied.Et Zucc. | 100.0 | 96.7 | 86.7 | 86.7 | 83.3 | 70.0 | 70.0 |
| Uncaria
rhynchophylla (Miq.) Jacks | 100.0 | 100.0 | 100.0 | 96.7 | 93.3 | 73.3 | 26.7 |
| Lithospermum
erythrorhizon Sieb. et Zucc. | 100.0 | 96.7 | 93.3 | 83.3 | 66.7 | 23.3 | 10.0 |
| Spatholobus
suberectus Dunn | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 76.7 |
| Stephania
tetrandra S.Moore | 100.0 | 100.0 | 90.0 | 86.7 | 70.0 | 50.0 | 33.3 |
| Cyathula
officinalis Kuan | 100.0 | 100.0 | 96.7 | 76.7 | 56.7 | 26.7 | 6.7 |
| Pyrrosia
lingua (Thunb.) Farwell | 100.0 | 100.0 | 100.0 | 100.0 | 90.0 | 76.7 | 56.7 |
| Alpinia
katsumadai Hayata | 100.0 | 100.0 | 100.0 | 96.7 | 80.0 | 36.7 | 3.3 |
| Dalbergia
odorifera T.chen | 100.0 | 100.0 | 100.0 | 100.0 | 50.0 | 16.7 | 0.0 |
| Carthamus
tinctorius L. | 100.0 | 100.0 | 100.0 | 100.0 | 93.3 | 90.0 | 66.7 |
| Lilium
brownii var.viridulum Baker. | 100.0 | 100.0 | 100.0 | 96.7 | 93.3 | 76.7 | 60.0 |
| Ligusticum
chuanxiong Hort. | 100.0 | 96.7 | 96.7 | 96.7 | 96.7 | 76.7 | 60.0 |
| Cyperus
rotundus L. | 100.0 | 100.0 | 100.0 | 96.7 | 66.7 | 23.3 | 10.0 |
| Pbyporus
umbellatus (pers.) Fries | 100.0 | 100.0 | 96.7 | 96.7 | 93.3 | 80.0 | 53.3 |
| Chrysanthemum
monfolium Ramat. | 100.0 | 100.0 | 100.0 | 100.0 | 96.7 | 86.7 | 43.3 |
| Sophora
flavescens Ait | 100.0 | 100.0 | 93.3 | 80.0 | 36.7 | 0.0 | 0.0 |
| Curcuma
phaeocaulis Valeton | 100.0 | 100.0 | 93.3 | 90.0 | 70.0 | 60.0 | 26.7 |
| Cynanchum
glaucescens (Decne.) Hand.-Mazz | 100.0 | 96.7 | 93.3 | 80.0 | 73.3 | 50.0 | 40.0 |
| Curcuma
aromatica Salisb. | 100.0 | 100.0 | 93.3 | 86.7 | 76.7 | 73.3 | 50.0 |
| Acanthopanax
gracilistylus W.W.smith | 100.0 | 83.3 | 20.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Drynaria
fortunei (Kunze) J.Sm | 100.0 | 96.7 | 86.7 | 86.7 | 73.3 | 66.7 | 46.7 |
| Lygodium
japonicum (Thunb) Sw. | 100.0 | 100.0 | 93.3 | 90.0 | 63.3 | 30.0 | 20.0 |
| Sanguisorba
officinalis L. | 100.0 | 100.0 | 100.0 | 100.0 | 73.3 | 53.3 | 33.3 |
| Stemona
japonica (Blume) Miq. | 100.0 | 100.0 | 96.7 | 76.7 | 40.0 | 13.3 | 3.3 |
In other experiments, four plant extracts that
revealed a higher fly survival rate following treatment with SDS or
NaCl, including Codonopsis pilosula (Franch.) Nannf (C.
pilosula), Saussurea lappa (Decne.) C.B.Clarke (S.
lappa), Imperata cylindrica Beauv.var.major
(Nees) C.E.Hubb. (I. cylindrical var. major) and
Melia toosendan Sied.Et Zucc. (M. toosendan), were
selected for use as test extracts. Following treatment with SDS for
6 days, the survival rates of the experimental groups were 94.4
(P<0.001), 92.1 (P<0.001), 92.1 (P<0.001) and 76.6%
(P<0.005), respectively, which were significantly higher
compared with the survival rate of the control group (11.17%;
Fig. 2A). Similarly, the four
experimental groups demonstrated significantly increased survival
rates [84.4 (P<0.001), 66.6 (P<0.005), 57.7 (P<0.001) and
65.5% (P<0.001), respectively] following treatment with 0.4 M
NaCl (Fig. 2B). To confirm the
protective effects of the four medicinal plants, another
inflammatory reagent was analyzed, DSS, which interferes with the
intestinal barrier function and stimulates local and systemic
inflammation, causing similar tissue damage in the gut of an adult
Drosophila (14,15). As shown in Fig. 2C, increased survival rates of 35.5,
60, 51.1 and 61.1%, respectively, were observed for extracts of
these medicinal plants compared with the control group (1.1%).
These results indicate that extracts of C.
pilosula, S. lappa, I. cylindrical var. major and M.
toosendan are able to increase the Drosophila survival
rate following exposure to toxic compounds.
AMP levels increase following medicinal plant
extract treatment. The four different medicinal plants C.
pilosula, S. lappa, I. cylindrical var. major and M.
toosendan have a strong protective effect against SDS-induced
gut damage, therefore, the pharmacological functions against SDS
damage were analyzed. AMP-mediated defenses are capable of
enhancing the stress response in adult flies and are regulated by
the Imd pathway (16). In order to
determine whether extracts of these four medicinal plants can
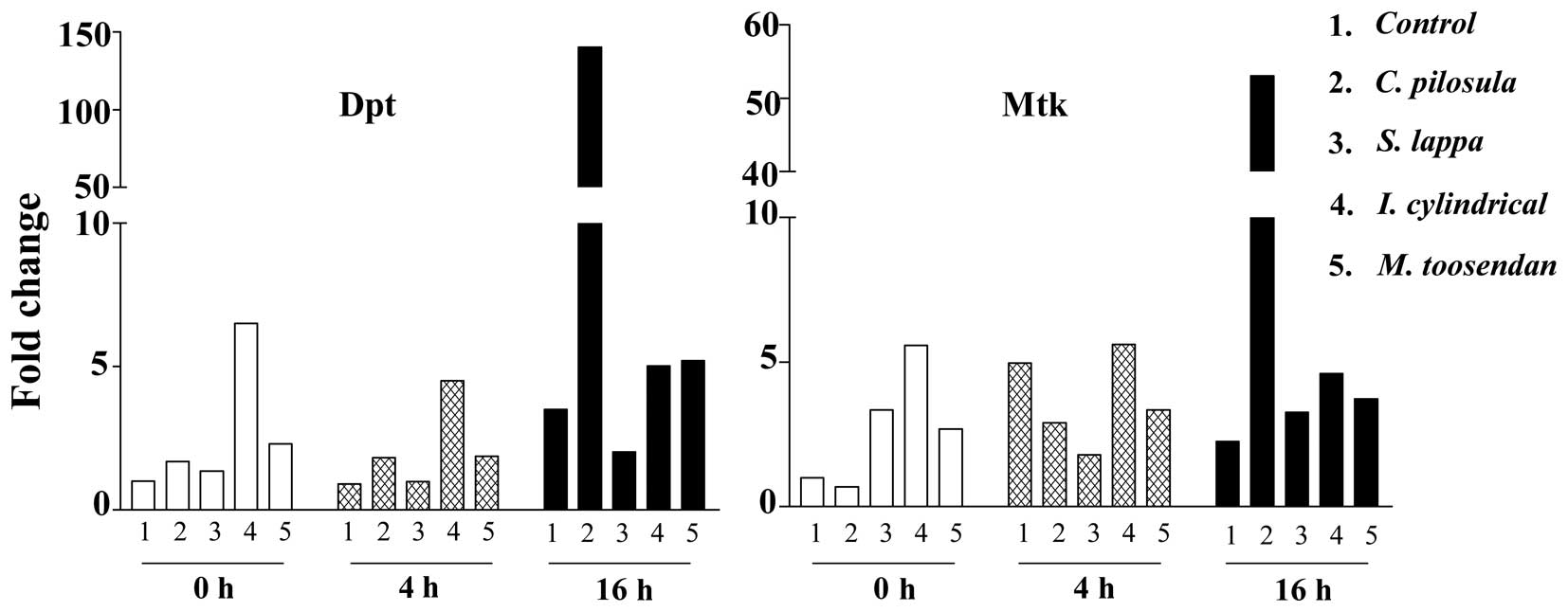
reduce Drosophila intestinal damage, AMP levels were
analyzed (Dpt, Diptericin; Mtk, Metchnikowin) using qPCR. As shown
in Fig. 3, slightly increased AMP
levels in the experimental groups were observed compared with the
controls. In addition, Dpt and Mtk RNA levels were increased in the
C. pilosula feeding group 16 h after SDS treatment, with
40-and 23.5-fold increases, respectively, compared with the control
group. The extracts of S. lappa, I. cylindrical var.
major and M. toosendan did not significantly affect
the AMP levels in the Drosophila gut. Furthermore, the RNA
levels of other AMPs (AttA, AttacinA; CecC, Cecropin C; Dro3,
Dromycin-like peptides 3; Dfn, Defencin) were similar between
groups (data not shown). These results indicate that extracts of
C. pilosula can induce high levels of Dpt and Mtk 16 h after
treatment with SDS in the Drosophila gut.
Medicinal plant extracts do not
increase SDS-induced ISC proliferation in the midgut
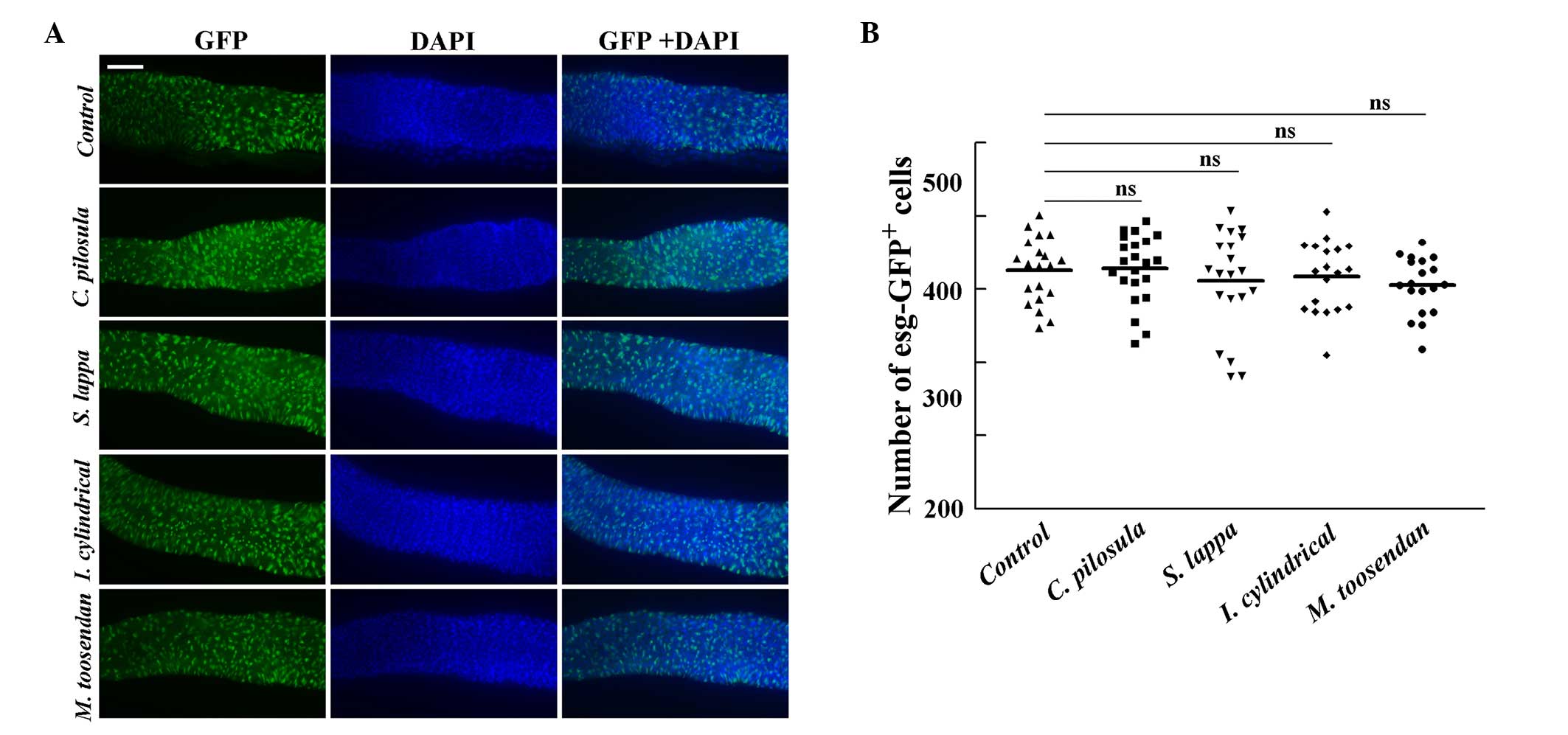
Following ingestion of toxic compounds, including
SDS or DSS, Drosophila ISCs increase their rate of
proliferation in response to tissue damage (14). To analyze the protective effects of
the four different medicinal plant extracts, the esg-Gal4 UAS-GFP
marker (for ISCs and EBs) was used to assess adult flies following
treatment with 0.6% SDS. Furthermore, the numbers of ISCs and EBs
were not significantly different between groups (Fig. 4). This result indicates that these
medicinal plant extracts do not induce stem cell proliferation in
the Drosophila midgut in response to SDS.
Medicinal plant extracts are able to
reduce SDS-induced cell death
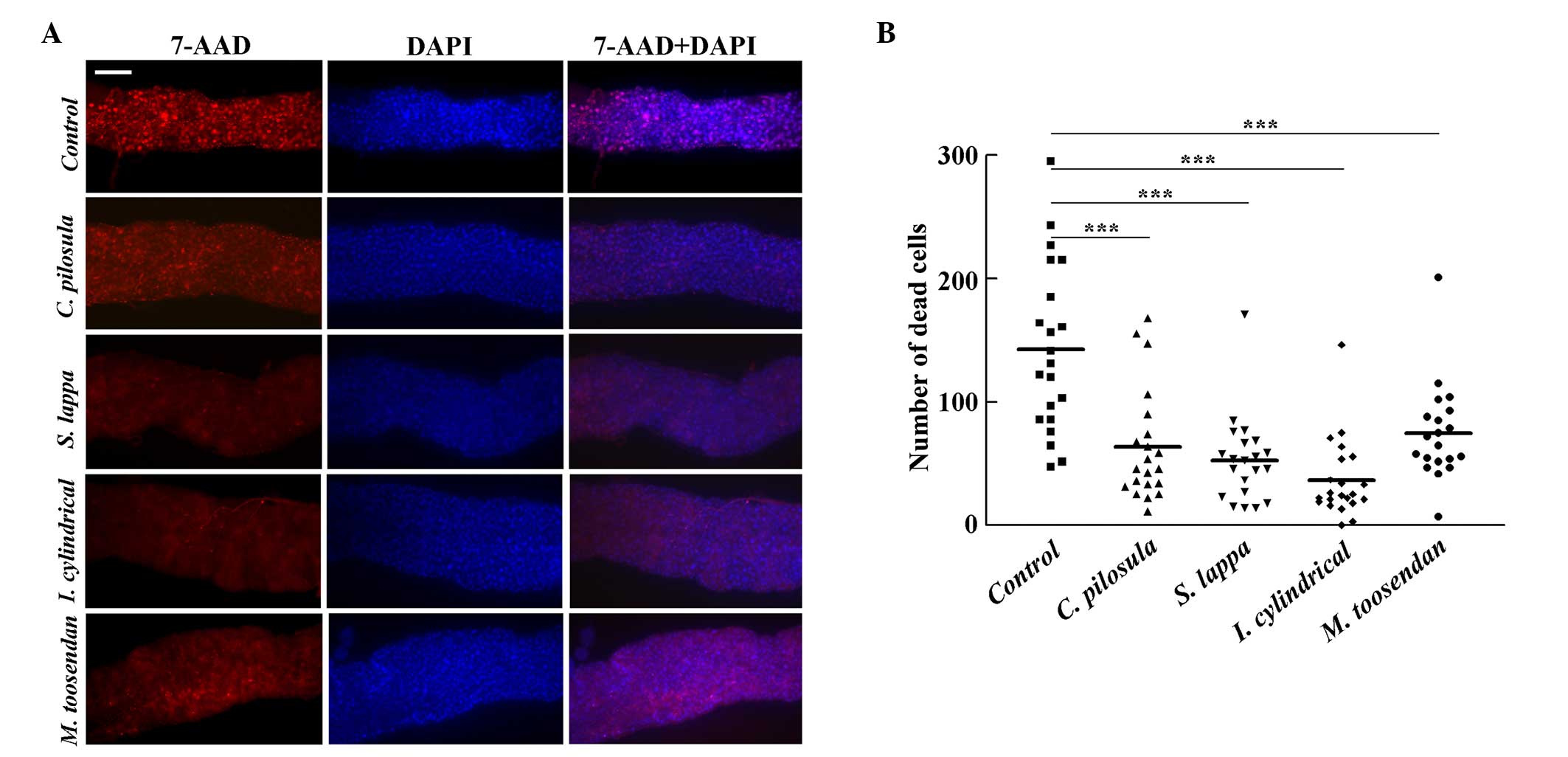
In the Drosophila midgut, exposure to toxic
compounds can increase apoptosis of epithelial cells (11). To determine whether the increased
survival rate of adult flies resulted from decreased cell death in
response to SDS, adult flies were treated with 0.6% SDS for 96 h. A
larger number of dead epithelial cells were observed in the control
group, however, flies fed with extracts of C. Zpilosula, S.
lappa, I. cylindrical var. major and M. toosendan
demonstrated significantly reduced 7-AAD signals (46.3, 38.2, 26.5
and 54.4%) compared with the control flies, respectively
(P<0.001; Fig. 5). This result
indicates that extracts of C. pilosula, S. lappa, I.
cylindrical var. major and M. toosendan can
increase epithelial cell viability following toxic compound
treatment.
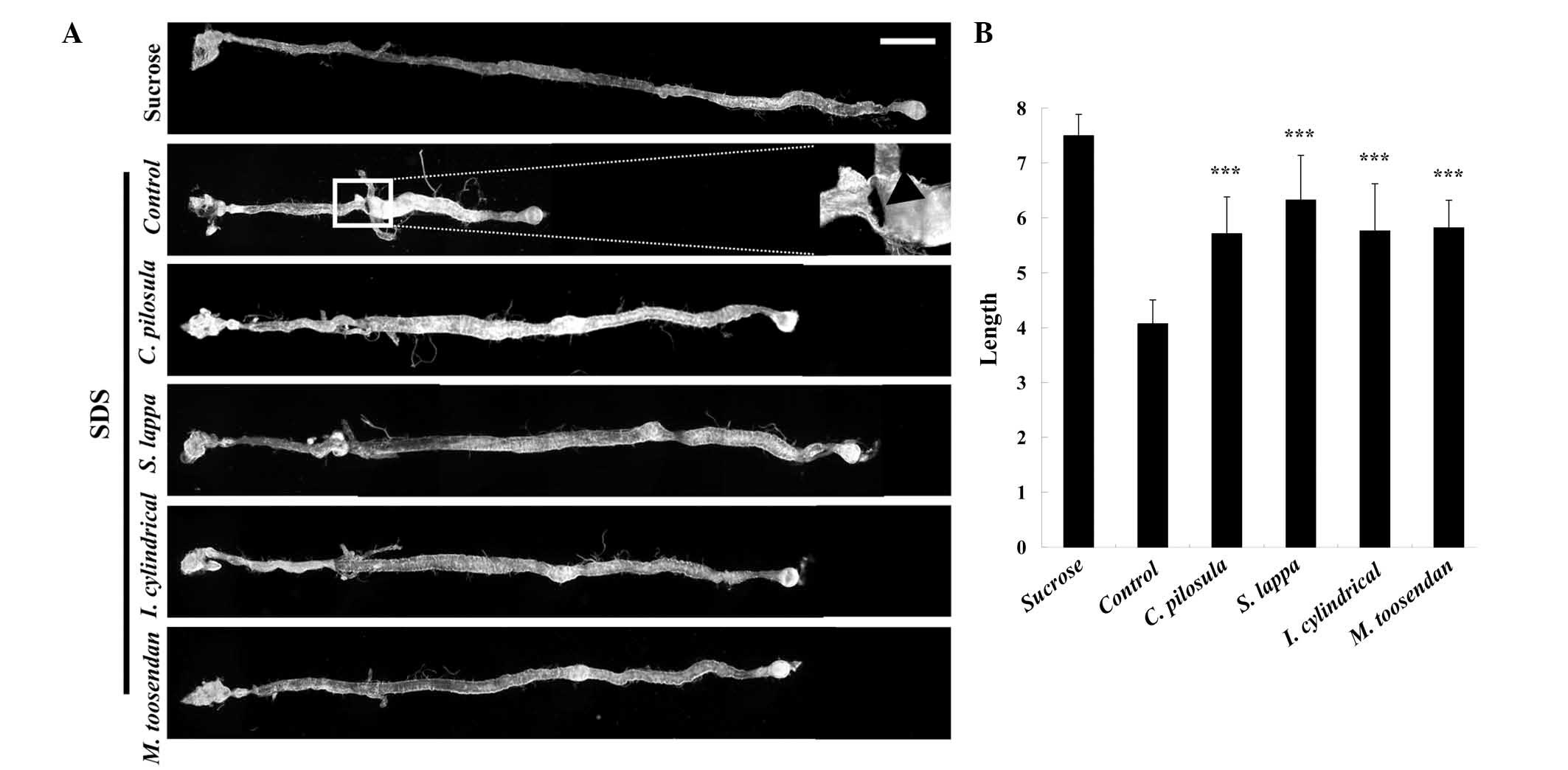
Medicinal plant extracts have
protective effects against SDS-induced gut damage and morphological
changes
It has previously been reported that SDS is able to
induce melanotic tumors and morphological changes in the
Drosophila gut (11).
Following treatment with 0.6% SDS for 4 days, the guts of control
flies appeared shorter than that of the group that was fed with
sucrose. Furthermore, melanotic tumors were observed in the
posterior midguts of control flies (Fig.
6A). However, the gut length of the C. pilosula-, S. lappa-,
I. cylindrical var. major and M. toosendan
extract-fed groups revealed significantly increased gut lengths
compared with the control group, similar to the sucrose fed groups
(P<0.001; Fig. 6A and B). In
addition, no melanotic masses were observed in the C. pilosula-,
S. lappa-, I. cylindrical var. major- and M.
toosendan extract fed groups (Fig.
6A).
Discussion
Traditional medicinal plants have been effectively
used with few side effects and over a long period of time (17). However, due to the large number of
diverse plant species and complex multicomponent systems, the
active components and pharmacological functions of numerous of
these plants have not been defined. Therefore, the use of these
plants as sources of novel drugs must still be explored.
In order to screen the protective effects of
medicinal plant extracts in vivo, Drosophila were
used as a model organism, and adult flies were treated with toxic
compounds. Of 50 different medicinal plant extracts, 8 and 9
species significantly increased the survival rates >70% compared
with the controls following treatment with SDS or NaCl,
respectively (Tables III and
IV). Among these extracts, however,
a protective effect against SDS or NaCl was not identified.
Furthermore, P. multiflorum Thunb., P. notoginseng
(Burk) F. H. Chen, L. erythrorhizon Sieb. et Zucc. and C.
rotundus L. protect against SDS-induced gut damage but do not
increase the survival rate following NaCl treatment. This
observation suggests that distinct mechanisms exist for these
functions.
Medicinal plants that have broad protective effects
against SDS and NaCl were selected for further investigation.
Extracts of C. pilosula, S. lappa, I. cylindrical var.
major and M. toosendan were used to examine their
protective properties in the Drosophila intestine (Figs. 1 and 2). Furthermore, C. pilosula can be
used to invigorate the function of the spleen, which is beneficial
to the liver and has anti-tumor, anti-oxidant and antimicrobial
properties (18–21). Its primary constituents include
polysaccharides, saponins, sesquiterpenes, polyphenolic glycosides,
alkaloids, polyacetylenes, essential oils and phytosteroids
(22). S. lappa is a
traditional herbal medicine that has been used to treat asthma,
inflammation, rheumatism, coughs, tuberculosis and numerous other
diseases (23). It contains numerous
sesquiterpene lactones, flavonoids, lignans, phenyl propanoids,
alkaloids, triterpenes and phytosterols (24). I. cylindrical var.
major is commonly used as a diuretic and is an
anti-inflammatory agent in traditional Chinese medicine (25) that exhibits diverse pharmacological
activities, including cytotoxicity, neuroprotection and
vasodilation (26). However, its
active compounds remain unclear. Furthermore, M. toosendan
has been widely used for the treatment of malaria, stomach aches
caused by round worms or as an anti-helminthic, antiseptic and
anti-inflammatory analgesic. In addition, it primarily contains
limonoids, toosen-danin and triterpenoid derivatives (27).
Although the medicinal plants used in the present
study have been previously explored, the majority of the results
were limited to in vitro studies, with only a few
researchers investigating their pharmacological roles in
vivo (28,29). To the best of our knowledge, there
are no references with regard to their protective effects in gut
immunity. In the present study, high survival rates were observed
in the experimental groups following treatment with toxic
compounds. The previous studies indicated that following ingestion
of pathogenic or toxic compounds, the proliferation of ISCs
increased to replace dead cells, which was required for tissue
homeostasis (14). Following
treatment with SDS, large numbers of 7-AAD-stained cells were
detected in the control group, however, only a few dead cells were
observed in the groups fed with plant extracts (Fig. 5). These plant extracts decreased
epithelial cell damage and melanotic tumor formation, protected the
gut morphology and significantly improved the survival rates of
adult flies following toxic compound treatment. However, there were
no differences between groups with regard to stem cell
proliferation (Fig. 4). In addition,
only extracts of C. pilosula significantly increased AMP
levels following treatment with SDS for 16 h, whereas extracts of
S. lappa, I. cylindrical var. major and M.
toosendan were observed similar to the controls (Fig. 3). The correlation between gut
microbiota and the host immune system is important in the health of
an organism, and the dysregulation of this balance can lead to
chronic inflammation and initiate tumor formation (30,31). The
extracts of S. lappa, I. cylindrical var. major and
M. toosendan may contribute to the basal host immune system
in the Drosophila intestine.
In summary, the present study provides a foundation
for the effective screening of a large number of pharmacological
functions from traditional medicinal plant extracts. The present
study demonstrated that extracts of four different traditional
medicinal plants (C. pilosula, S. lappa, I. cylindrical var.
major and M. toosendan) have protective effects
against gut disorders in Drosophila. These results may
provide a pharmacological basis for the treatment of inflammatory
bowel diseases in humans.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31270923) and the
Fundamental Research Funds for the Central Universities (grant nos.
DL13EA08-01 and DL12EA-02).
References
|
1
|
Gautam R and Jachak SM: Recent
developments in anti-inflammatory natural products. Med Res Rev.
29:767–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee KA and Lee WJ: Drosophila as a model
for intestinal dysbiosis and chronic inflammatory diseases. Dev
Comp Immunol. 42:102–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chatterjee M and Ip YT: Pathogenic
stimulation of intestinal stem cell response in Drosophila. J Cell
Physiol. 220:664–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Micchelli CA and Perrimon N: Evidence that
stem cells reside in the adult Drosophila midgut epithelium.
Nature. 439:475–479. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang H, Patel PH, Kohlmaier A, Grenley
MO, McEwen DG and Edgar BA: Cytokine/Jak/Stat signaling mediates
regeneration and homeostasis in the Drosophila midgut. Cell.
137:1343–1355. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ha EM, Oh CT, Bae YS and Lee WJ: A direct
role for dual oxidase in Drosophila gut immunity. Science.
310:847–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryu JH, Ha EM, Oh CT, Seol JH, Brey PT,
Jin I, Lee DG, Kim J, Lee D and Lee WJ: An essential complementary
role of NF-kappaB pathway to microbicidal oxidants in Drosophila
gut immunity. EMBO J. 25:3693–3701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lenaz G: Role of mitochondria in oxidative
stress and ageing. Biochim Biophys Acta. 1366:53–67. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liehl P, Blight M, Vodovar N, Boccard F
and Lemaitre B: Prevalence of local immune response against oral
infection in a Drosophila/Pseudomonas infection model. PLoS Pathog.
2:e562006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Y, Wang S, Wu X, Zhang J, Chen R, Chen
M and Wang Y: Chinese herbal medicine-derived compounds for cancer
therapy: A focus on hepatocellular carcinoma. J Ethnopharmacol.
149:601–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Luo Q and Jin LH: Acanthopanax
senticosus extracts have a protective effect on Drosophila gut
immunity. J Ethnopharmacol. 146:257–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steele ML, Truong J, Govindaraghavan S,
Ooi L, Sucher NJ and Münch G: Cytoprotective properties of
traditional chinese medicinal herbal extracts in hydrogen peroxide
challenged human U373 astroglia cells. Neurochem Int. 62:522–529.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seisenbacher G, Hafen E and Stocker H:
MK2-dependent p38b signalling protects Drosophila hindgut
enterocytes against JNK-induced apoptosis under chronic stress.
PLoS Genet. 7:e10021682011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Amcheslavsky A, Jiang J and Ip YT: Tissue
damage-induced intestinal stem cell division in Drosophila. Cell
Stem Cell. 4:49–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawada M, Arihiro A and Mizoguchi E:
Insights from advances in research of chemically induced
experimental models of human inflammatory bowel disease. World J
Gastroenterol. 13:5581–5593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buchon N, Broderick NA, Poidevin M,
Pradervand S and Lemaitre B: Drosophila intestinal response to
bacterial infection: Activation of host defense and stem cell
proliferation. Cell Host Microbe. 5:200–211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu L, Qin Y, Wang Q, Zhang L, Liu Y, Wang
T, Huang L, Wu L and Xiong H: The efficacy and safety of Chinese
herbal medicine, Rhodiola formulation in treating ischemic heart
disease: A systematic review and meta-analysis of randomized
controlled trials. Complement Ther Med. 22:814–825. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang Su New Medical University, .
Dictionary of Chinese Traditional Medicine. 2:18371996.
|
|
19
|
Ng TB, Liu F and Wang HX: The antioxidant
effects of aqueous and organic extracts of Panax quinquefolium,
Panax notoginseng, Codonopsis pilosula, Pseudostellaria
heterophylla and Glehnia littoralis. J Ethnopharmacol. 93:285–288.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh B, Song H, Liu X, Hardy M, Liu GZ,
Vinjamury SP and Martironsian CD: Dangshen (Codonopsis pilosula)
and Bai guo (Gingko biloba) enhance learning and memory. Altern
Ther Health Med. 10:52–56. 2004.PubMed/NCBI
|
|
21
|
Xin T, Zhang F, Jiang Q, Chen C, Huang D,
Li Y, Shen W, Jin Y and Sui G: The inhibitory effect of a
polysaccharide from Codonopsis pilosula on tumor growth and
metastasis in vitro. Int J Biol Macromol. 51:788–793. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang ZT, Ma JY, Tu PF and Ng TB:
Chemotaxonomic study of Codonopsis (Family Campanulaceae) and its
related Genera. Biochem Syst Ecol. 23:809–812. 1995. View Article : Google Scholar
|
|
23
|
Choi HG, Lee DS, Li B, Choi YH, Lee SH and
Kim YC: Santamarin, a sesquiterpene lactone isolated from Saussurea
lappa, represses LPS-induced inflammatory responses via expression
of heme oxygenase-1 in murine macrophage cells. Int
Immunopharmacol. 13:271–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Ma L, Wu F and Chen R: Chemical
constituents from a portion of ethanolic extract of Saussurea lappa
roots. Zhongguo Zhong Yao Za Zhi. 37:1232–1236. 2012.(In Chinese).
PubMed/NCBI
|
|
25
|
Pharmacopeia Committee of P.R. China, .
Pharmacopoeia of People's Republic of China. China Medical Science
and Technology Press; Beijing: pp. 992010
|
|
26
|
Yoon JS, Lee MK, Sung SH and Kim YC:
Neuroprotective 2-(2-phenylethyl) chromones of Imperata cylindrica.
J Nat Prod. 69:290–291. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie F, Zhang M, Zhang CF, Wang ZT, Yu BY
and Kou JP: Anti-inflammatory and analgesic activities of ethanolic
extract and two limonoids from Melia toosendan fruit. J
Ethnopharmacol. 117:463–466. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin QY, Jin LJ, Cao ZH, Lu YN, Xue HY and
Xu YP: Acanthopanax senticosus suppresses reactive oxygen species
production by mouse peritoneal macrophages in vitro and in vivo.
Phytother Res. 22:740–745. 2009. View Article : Google Scholar
|
|
29
|
Zhang S, Qi Y, Xu YW, Han X, Peng JY, Liu
KX and Sun CK: Protective effect of flavonoid-rich extract from
Rosa laevigata Michx on cerebral ischemia-reperfusion injury
through suppression of apoptosis and inflammation. Neurochem Int.
63:522–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SH and Lee WJ: Role of DUOX in gut
inflammation: Lessons from Drosophila model of gut-microbiota
interactions. Front Cell Infect Microbiol. 3:1162014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Y, Antony S, Meitzler JL and Doroshow
JH: Molecular mechanisms underlying chronic inflammation-associated
cancers. Cancer Lett. 345:164–173. 2014. View Article : Google Scholar : PubMed/NCBI
|