Introduction
Diet may have a pivotal role in oncogenesis and
tumor suppression. In view of the increase in cancer mortality
rates, numerous previous studies have indicated a close association
between diet and cancer prevention (1–4). This is
particularly true of types of cancer of the digestive tract, as
certain diets such as bacon, sausage and hot dogs have been
revealed to be associated with an increased risk of gastric cancer
(5). The P-glycoprotein (multidrug
resistance-related protein) mdr-1 gene is expressed at high levels
in normal human liver, gastrin, colon and kidney and in a number of
tumors derived from these organs (6–8).
Therefore, colon and gastric cancer have often acquired intrinsic
drug resistance, which causes a very weak response to chemotherapy
and thus an ineffective treatment (9). Gastric cancer is characterized by not
only intrinsic drug resistance but also by acquired multidrug
resistance, which may cause resistance to and failure in
chemotherapy (10,11). Therefore, the prevention of gastric
cancer is important and dietary factors are also important
(12,13). Consequently, gastric cancer has
become one of the most common causes of mortality worldwide
(14,15). The nutritionally high-quality seafood
(licenced by the Hazard analysis and critical control points)
referred to as ‘hard clam’ (Meretrix lusoria) is consumed
regularly by Asian populations, and has been reported to possess
antileukemic activity (16,17). Therefore, prevention and treatment of
gastric cancer via the diet is an important and challenging
subject.
The freshwater clam (Corbicula fluminea) and
hard clam (Meretrix spp.) are very popular in Southern Asia.
The latter are more popular from a dietary perspective than
freshwater clams as river pollution may contaminate the waters
where freshwater clams thrive (16,18).
Hard clams (HCs) have now become one of the most pivotal aquatic
products of marine cultivation, serving as a source of nutrition
for people living in countries adjacent to the sea (19). HCs contain essential amino acids,
nitrogenous compounds and other essential components such as
taurine, glycogen, lipids and minerals such as calcium,
phosphorous, iron and zinc (19–21). Due
to its high protein and low cholesterol and salt content, this
easily-digested organism is considered to be a high-quality food
with regard to nutrients vital to humans (19,21).
Additionally, the nutrients within HCs are suggested to be
beneficial for regulating physiological and biochemical functions
of the human body, and for attenuating the progression of disease
(22,23). Specifically, HCs are rich in taurine
(2-aminoethanesulfonic acid). This is an organic compound that is
involved in neuromodulation, cholesterol metabolism, regulation of
growth and development, glucose metabolism and regulation,
maintenance of homeostasis, antioxidant function and protection
from the harmful risks of radiation in humans (24,25).
Therefore, HC consumption may have a beneficial role in the normal
physiological functioning of the brain, lungs, blood, heart, liver,
pancreas, gall bladder, and kidneys (24,26). In
addition to their nutritional benefits, the production of HCs has
increased due to their pharmacological value around the world,
particularly in Asia. Previous rat model studies have revealed that
extracts of freshwater clams reduce cholesterol levels (16,27) and
hepatic lipids, and ameliorate acute liver injuries induced by
hemorrhaging (16).
Hot-water extracts of HCs have been established to
have immune system-modulating properties (28). The muscle lysate of HCs has been
revealed to include angiotensin I-converting enzyme (ACE)
inhibitory peptides, which regulate blood pressure by converting
angiotensin I to angiotensin II in humans. Therefore, HCs have the
potential to prevent hypertension by inhibiting ACE activity
(29,30). Identifying the diets with antitumor
effects against gastric cancer and developing novel
chemotherapeutic agents are critical endeavors for this field of
study. However, previous studies concerning the cancer preventative
and therapeutic effects of HCs have been scarce and basic (17).
In the present study, specific components of HCs
were extracted without the use of hot water or any organic solvent,
methods previously reported in a study by Pan et al
(17). A novel method was required
as hot water and organic solvent extraction methods may cause loss
of active antitumor components (17,31) that
may be used in the development of supplemented food products. In
this novel method, HCs are homogenized and bioactive substances are
extracted with buffers containing protease inhibitors in a series
of subsequently described processes. A nutritional database of the
key free amino acids (FAA) in HCs was established, and the
tumor-suppressive effects of HC extracts in four cancer cell lines
were examined. Finally, the atypical apoptotic mechanisms that may
be induced by HC extracts were investigated in AGS human gastric
cancer cells.
Materials and methods
Chemicals and cell culture medium
L-15 medium was purchased from Gibco (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Fetal bovine serum (FBS) and
antibiotics were obtained from Hyclone (GE Healthcare Life
Sciences, Logan, UT). Other chemicals and supplies were obtained
from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) unless
otherwise indicated.
Cell culture and determination of
growth curves
Cells were maintained in L-15 medium with 10% FBS at
37°C in a humidified 5% CO2 atmosphere. To prevent
serum-containing medium from affecting the anticancer activities of
HC extracts, logarithmic growth phase cells to be used for
experiments were maintained in an FBS-free medium for 72 h. To
determine growth curves, cells were treated with Trypan blue dye,
and the cell numbers were counted using a hemocytometer under a
Zeiss Axiophot light microscope.
Sample extraction
HCs were purchased from the Ni-Shiming aquaculture
artificial breeding farm in Zhanghua, Taiwan. For preparation of
the extracts, the HCs were sectioned and homogenized in 10 mM
Tris-HCl buffer solution with protease inhibitors, including
aprotinin, leupeptin, pepstatin and PMSF, at 4°C using a blender.
The crude extracts were centrifuged at 130,000 × g for 30 min. The
supernatant was then carefully collected and freeze-dried into
powder form using a FD-5N EYELA freeze dryer (EYELA, Tokyo
Rikakikai, Tokyo, Japan). From 500 g of the HCs, ~5 g of powder was
obtained, and this was stored in ice until analysis. For use, 0.5 g
extract powder was dissolved in 10 ml serum-free medium, filtered
through a 0.45-nm membrane filter and analyzed.
Analysis by capillary electrophoresis
(CE)
To confirm the stability and consistency of the
extracts, the HC extracts were evaluated using CE; four HC extracts
that had been isolated at different time points were analyzed. An
Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara,
CA, USA) was used with a LabChip GX (Caliper Life Sciences; Perkin
Elmer, Hopkinton, MA, USA) for the analysis of proteins, according
to the manufacturer's protocol. This involved combining 4 µl HC
extract with 2 µl denaturing solution in a microcentrifuge tube.
Samples were then denatured in boiling water for 3–5 mins and then
loaded into the protein chip. This is a microfabricated chip that
is designed with microchannels to separate sample components
electrophoretically. Samples were treated with a protein 200 Plus
Dye concentrate fluorescent dye (Molecular Probes, Thermo Fisher
Scientific, Inc.) that labels proteins. The components were
detected by fluorescence and converted into gel-like images (bands)
and electropherograms (peaks). Each chip contains an interconnected
set of microchannels that separates proteins by size using
electrophoresis. Use of this lab-on-a-chip approach eliminates
handling sodium dodecyl sulfate polyacrylamide gels, staining or
imaging steps. Two batches of samples were used with two repeats
each for this experiment.
High-performance liquid chromatography
(HPLC) analysis for FAA and stability of HC extracts
To control for consistent quality of the HC extracts
and to determine the FAAs present, the prepared extracts were
evaluated using modifications of the HPLC method as previously
described by Häkkinen et al (32). All HPLC experiments on HC extracts
were performed on an RP-18 column (Merck Millipore, Darmstadt,
Germany) using a fluorescence detector (L-7420 UV detector;
Hitachi, Ltd., Tokyo, Japan) with wavelengths at 210 and 436 nm for
the determination of stability and composition, respectively. For
the stability assay, the extracts were eluted using two buffers: A
(acetonitrile) and B (30 mM citrate phosphate buffer in
double-distilled water; pH 2.5). These were performed using the
following gradient: 100% B, 0% A between 0–5 min; 20% B, 80% A at
5–60 min. The flow rate was 1 ml/min at 40°C. For the FAA estimate,
the extracts were eluted using the buffers: A (4%
N',N-dimethylformamide in 30 mM sodium acetate buffer; pH 6.4) and
B (acetonitrile).
Cell viability assay
Cytotoxicity was assessed using 3-(4,
5-dimethylthiazol)-2, 5-diphenyl-tetra-zoliumbromide (MTT) dye
(Sigma-Aldrich) as previously described (33). Briefly, 2.5 ml cell solution
(2.4×104 cells/ml in L-15 medium) was plated into
96-well plates and incubated overnight at 37°C to enable
attachment. The cells were then incubated at 37°C with a serum-free
medium and different concentrations (0.3125, 0.625, 1.25 and 2.5
mg/ml) of HC extracts. After 0, 12, 24, 48 or 72 h of culture, 20
µl of MTT solution (500 µg/ml) was added to each well and incubated
for 4 h to generate the formazan crystals. Subsequently, the
crystals were solubilized with dimethylsulfoxide. The 570-nm
absorbance of the resulting colored solution was determined with a
microplate reader (Dynatech Laboratories, Chantilly, VA, USA). The
survival of cells was calculated by dividing the absorbance value
with that of the control, and converting this to a percentage.
Hoechst 33258 and immunofluorescence
staining for cytoskeleton and cell nucleus
Hoechst 33258 fluorescence labeling was used to
determine chromatin condensation or nuclear fragmentation and
immunofluorescence was used to analyze actin distribution. Briefly,
the cells were washed twice with phosphate-buffered saline (PBS)
and fixed with a 3:1 solution of methanol and acetate for 15 min.
Subsequent to rinsing in PBS, the cells were permeabilized with
0.5% Triton X-100 in PBS at room temperature for 20 min and
incubated in a blocking buffer (1 g bovine serum albumin BSA in
PBS) for 1 h. Cells were then incubated with a mouse anti-actin
monoclonal antibody (dilution, 1:400; Q-11221MP; Thermo Fisher
Scientific Inc.) at 4°C overnight, washed again with PBS and 0.2%
Tween, then incubated with the secondary antibody
fluorescein-conjugated goat-anti mouse immunoglobulin G (dilution,
1:200) and stained with 10 mg/ml Hoechst 33258 at room temperature
for 1 h. Finally, the cells were rinsed with PBS 3–4 times and
observed using epifluorescence microscopy.
Fluorescence staining with acridine
orange
The cells were grown for 24 h on a chamber slide
(Corning; Sigma-Aldrich) and treated with 0.3125, 0.625, 1.25 and
2.5 mg/ml HC extracts. The cells were then fixed with a 3:1
solution of methanol and acetate for 15–30 min and 1% glacial
acetic acid was added for 30 min. The cells were stained with
0.001% acridine orange (0.05 g acridine orange powder in 50 ml
normal saline, kept away from light) for 30 sec and were treated
with 0.1 M CaCl2 for 2 min. Finally, the excess acridine
orange dye was removed using PBS and fluorescent micrographs were
obtained using fluorescence microscopy.
Cell cycle analysis by flow
cytometry
The cells were grown on a chamber slide (Corning;
Sigma-Aldrich) and treated with HC extracts for 0 and 18 h, and
were then collected using trypsinization. The cells were fixed in
70% ethanol at 4°C overnight, and centrifuged at 500 × g for 5 min.
The cell pellets were washed with ice-cold PBS. The cells were
labeled with 1 ml propidium iodide (PI) solution (comprising 20
µg/ml PI, 0.1 % Triton X-100 and 0.2 mg/ml RNaseA, made up in PBS)
for 30 min at room temperature in the dark. The cells were then
assayed by flow cytometry (FACSCalibur; BD Biosciences, Franklin
Lakes, NJ, USA), and >10,000 cells were counted in each
sample.
Expression analysis of
cell-cycle-related genes by human cell cycle Oligo GEArray
The Oligo GEArray® Human Cell Cycle Microarray (cat.
no. OHS-020) profiles the expression levels of 128 important cell
cycle genes, using a side-by-side hybridization experiment to
determine differential gene expression. Initially, RNA was isolated
and prepared from untreated or HC extract-treated AGS cells using a
phenol-based method (TRIzol; 15596026; Invitrogen, Thermo Fisher
Scientific, Inc.) followed by further purification with an
ArrayGrade RNA isolation kit (GA-013; SA Biosciences; Qiagen
Sciences, Inc., Gaithersburg, MD, USA). Purified mRNA samples were
then added in 5-µg aliquots to TrueLabeling primers, cRNA synthesis
buffer and cDNA synthesis enzyme mix (SA Biosciences; Qiagen
Sciences, Inc., Gaithersburg, MD, USA) for cDNA synthesis followed
by cDNA amplification using biotinylated-dUTP, RNA polymerase
buffer and RNA polymerase. The cRNA synthesis reaction and
purification used an ArrayGrade cRNA cleanup kit (GA-012; SA
Biosciences) to get rid of unincorporated dUTP and other reaction
buffer. The purified cRNA from the experimental samples was mixed
with a hybridization mixture that was prepared using GEAhyb
Hybridization Solution. This reaction entailed incubation of the
cRNA target hybridization mix at 60°C in shaking incubators
overnight. Chemiluminescence detection (cat no. D-01; SA
Biosciences) was performed in pre-warmed (37°C) GEA blocking
solution Q for 40 min to block the array reaction. This was
followed by incubation with 5X alkaline phosphatase-conjugated
streptavidin buffer at room temperature for 10 min with continuous
but gentle agitation. The GEA blocking buffer was discarded and the
array was rinsed with 3 ml buffer G twice. Finally, 1 ml CDP-Star
chemiluminescent substrate was added, incubated for 2–5 min at room
temperature and detected using a UV Photometer (UVP, Inc., Upland,
CA, USA).
Statistical analysis
Data were analyzed with a one-way analysis of
variance using SPSS 16.0 statistical analysis software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to represent a
statistically significant difference. Data are presented as the
mean ± standard deviation.
Results
Composition of FAAs in HCs
The FAA composition of HCs is reported in Table I. The FAA content in the HCs ranged
from 2.8–297.6 mg/100 g. Notably, the total mass of taurine was
297.6 mg/100 g, which accounted for up to 60.7% of the total mass
of FAAs, the greatest proportion of FAAs in HC extracts. Following
taurine, glutamic acid was the second most prevalent FAA.
 | Table I.Free amino acid contents of hard
clams. |
Table I.
Free amino acid contents of hard
clams.
| Amino acid | Content (mg/100
g) |
|---|
| Essential and
semi-essential |
|
Histidine | 2.8±0.1 |
|
Isoleucine | 12.7±3.2 |
|
Leucine | 16.2±3.4 |
|
Lysine | 5.9±0.1 |
|
Methionine | 5.0±3.4 |
|
Phenylalanine | 9.0±2.0 |
|
Threonine | 8.0±0.0 |
|
Valine | 8.9±1.5 |
| Non-essential |
|
Alanine | 4.4±0.0 |
|
Arginine | 3.2±0.1 |
|
Aspartic acid | 20.1±0.0 |
|
Glutamic acid | 36.9±0.0 |
|
Glycine | 27.0±0.0 |
|
Proline | 10.0±2.0 |
|
Serine | 12.5±2.0 |
|
Taurine |
297.6±1.4a |
|
Tyrosine | 10.2±2.2 |
|
Total | 490.4±2.1 |
Analysis of quality control and
stability of the HC extracts by capillary electrophoresis and an
HPLC assay
To confirm the stability and consistency of
extracts, the prepared HC extracts were evaluated by HPLC and
Agilent 2100 protein capillary electrophoresis analyses. As shown
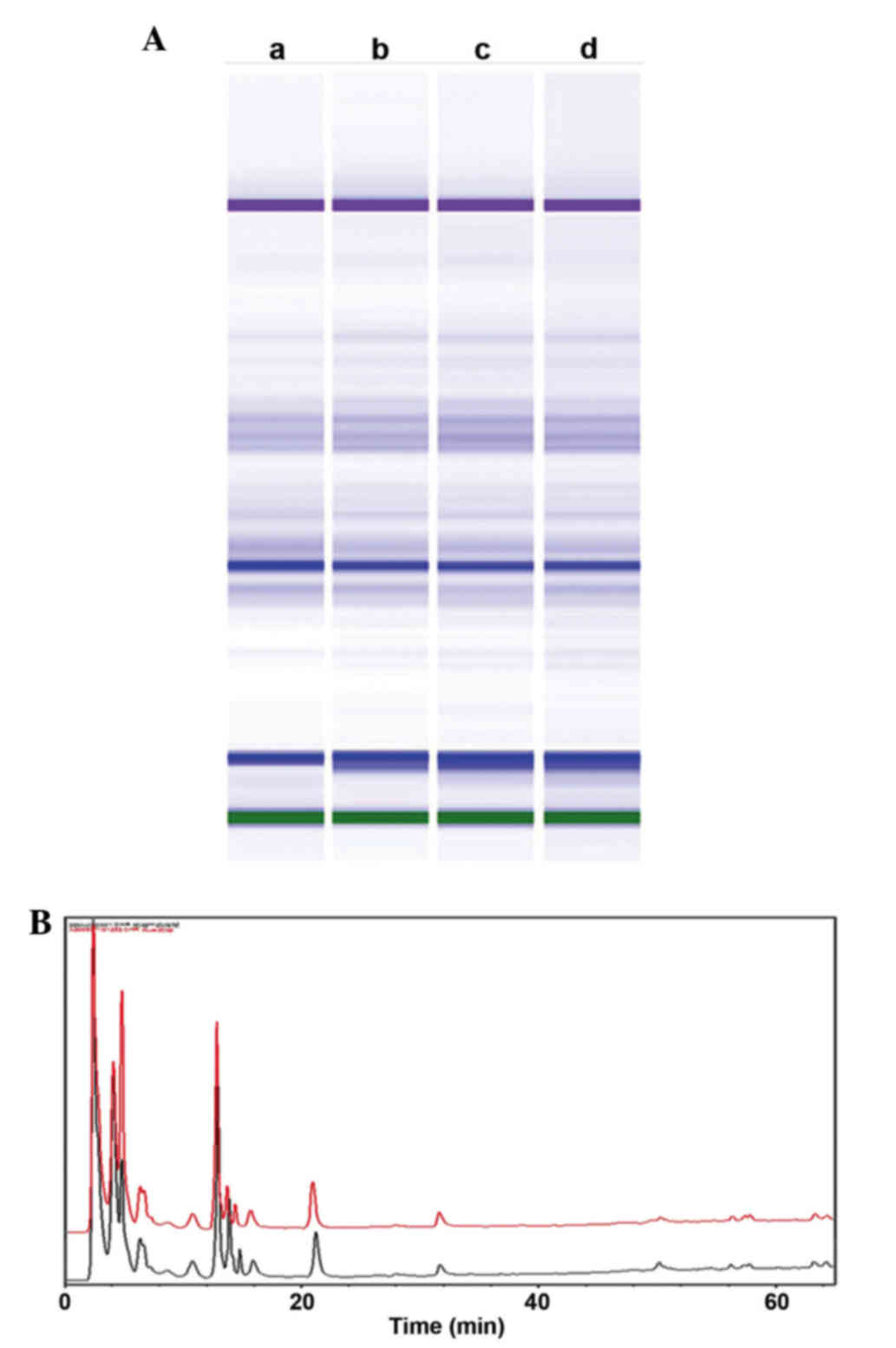
in Fig. 1A, four HC extracts
evaluated by capillary electrophoresis were similar, despite being
isolated at different times. Furthermore, HPLC analyses of the
samples selected from lanes b and c of Fig. 1A also indicated that these samples
were similar (Fig. 1B). These
results indicate that the extraction methods are reliable, and that
the HC extracts are highly stable.
HC extracts induce cytotoxicity in AGS
cells
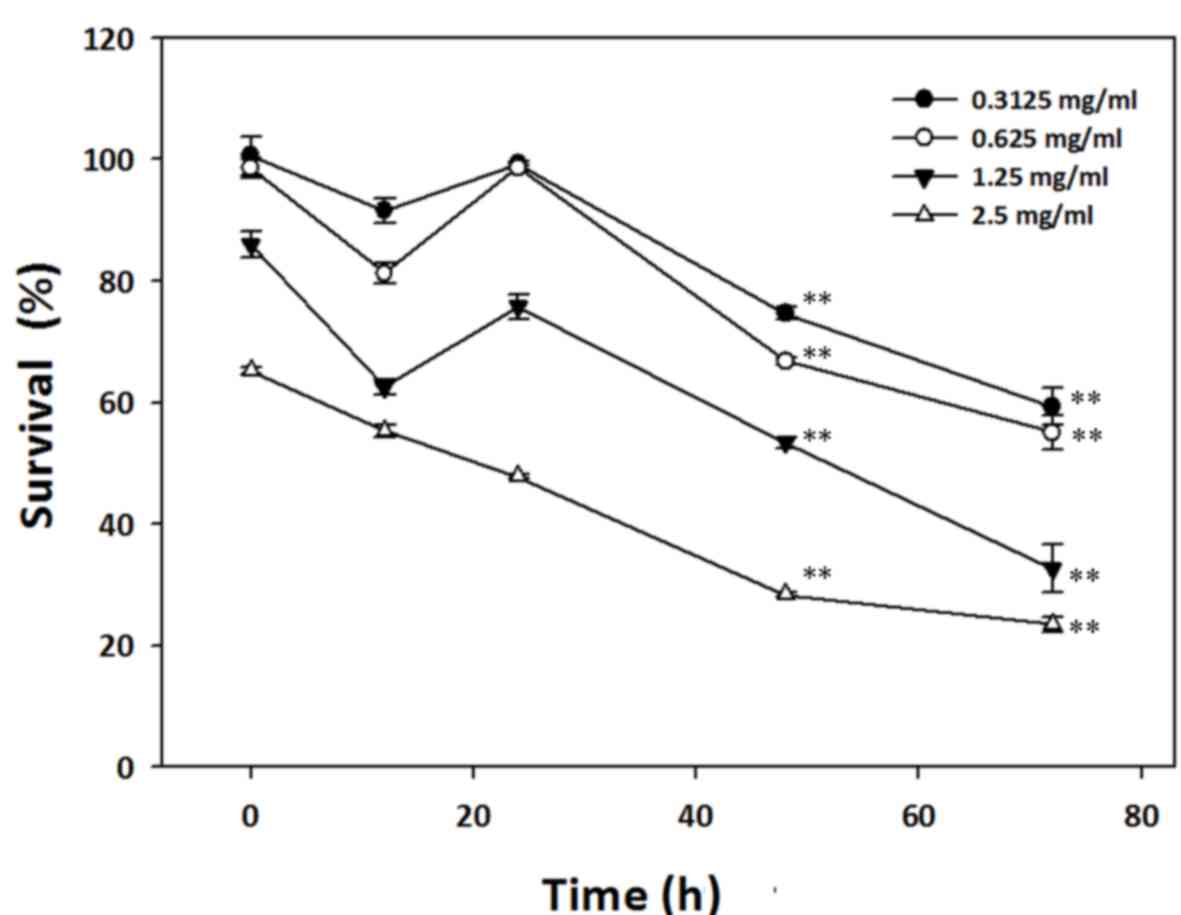
To investigate the effect of 0.3125, 0.625, 1.25 and
2.5 mg/ml HC extracts on the viability of AGS cells over different
time intervals, an MTT assay was performed. The results from
sixteen independent experiments revealed that the HC extracts
caused cytotoxicity in AGS cells in a time- and dose-dependent
manner (Fig. 2). Treatment with HC
extracts immediately decreased the survival of AGS cells up to 65%,
and higher concentrations of HC extracts demonstrated greater
cytotoxicity (Fig. 2). However, over
increasing treatment durations, lower concentrations of HC extracts
were also effective in attenuating the survival of AGS cells.
Subsequent to a 12-h treatment with HC extracts, the relative cell
survival significantly increased suggesting that AGS cells may
perform short-term repair or recovery prior to cell death. This
transient cell repair or recovery is attributed to the increase in
cell survival observed at the 20-h timepoint after treatment with
the HC extracts. These results indicate that HC extracts inhibit
cell growth or induce cell death in AGS cells by one of multiple
possible mechanisms.
Morphological alterations of cells
affected by HC extracts
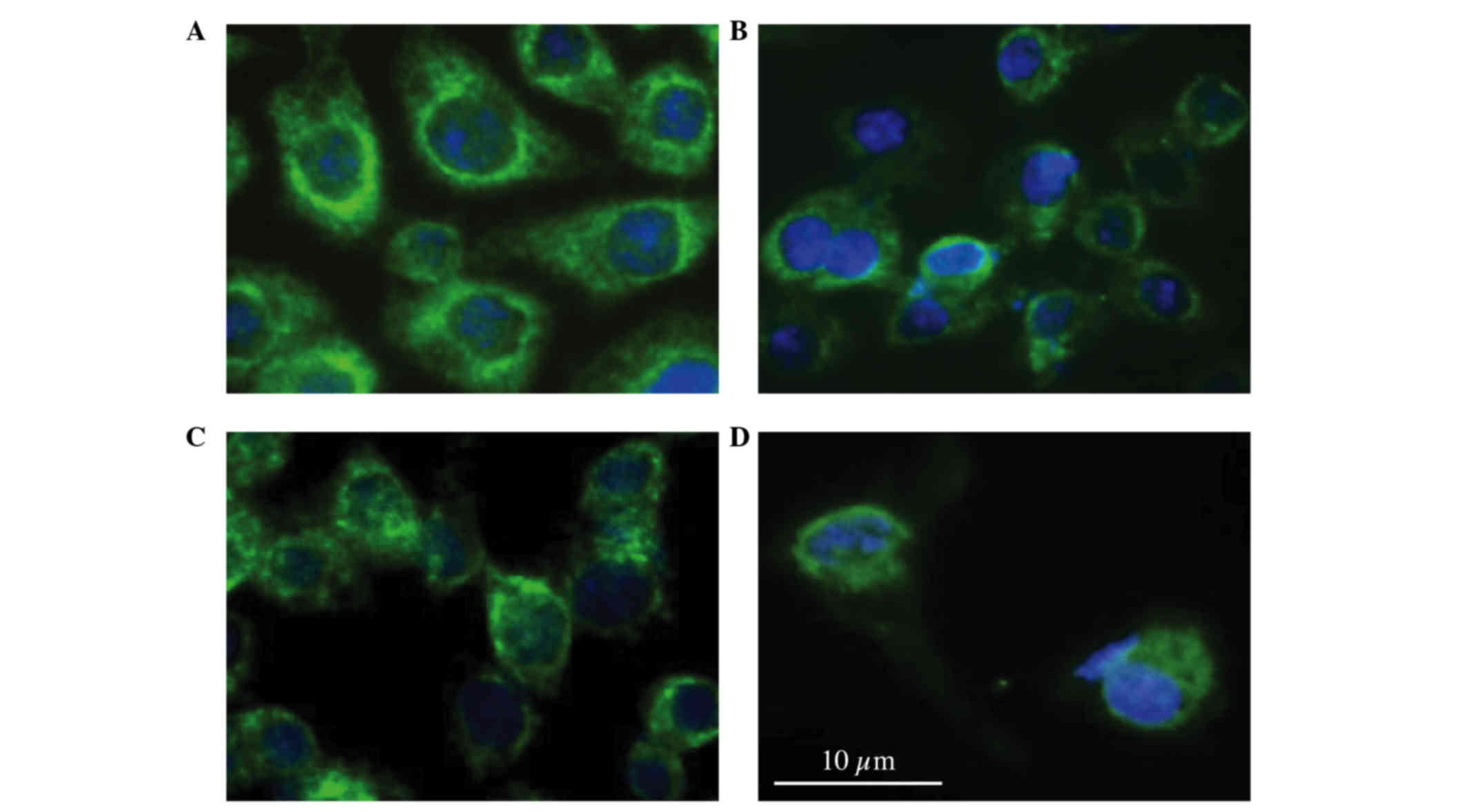
To investigate the morphological changes in AGS
cells induced by HC extracts, AGS cells treated with 0.3125, 0.625,
1.25 and 2.5 mg/ml of HC extracts were examined for changes in
nuclear and cytoskeletal organization using immunofluorescence. As
shown in Fig. 3, HC extract-treated
AGS cells demonstrated marked changes to their morphology,
including disruption of nuclei and the cytoskeleton and cell
morphological changes by actin aggregation (Fig. 3B-D), and these changes were adopted
in a time- and dose-dependent manner. To further characterize the
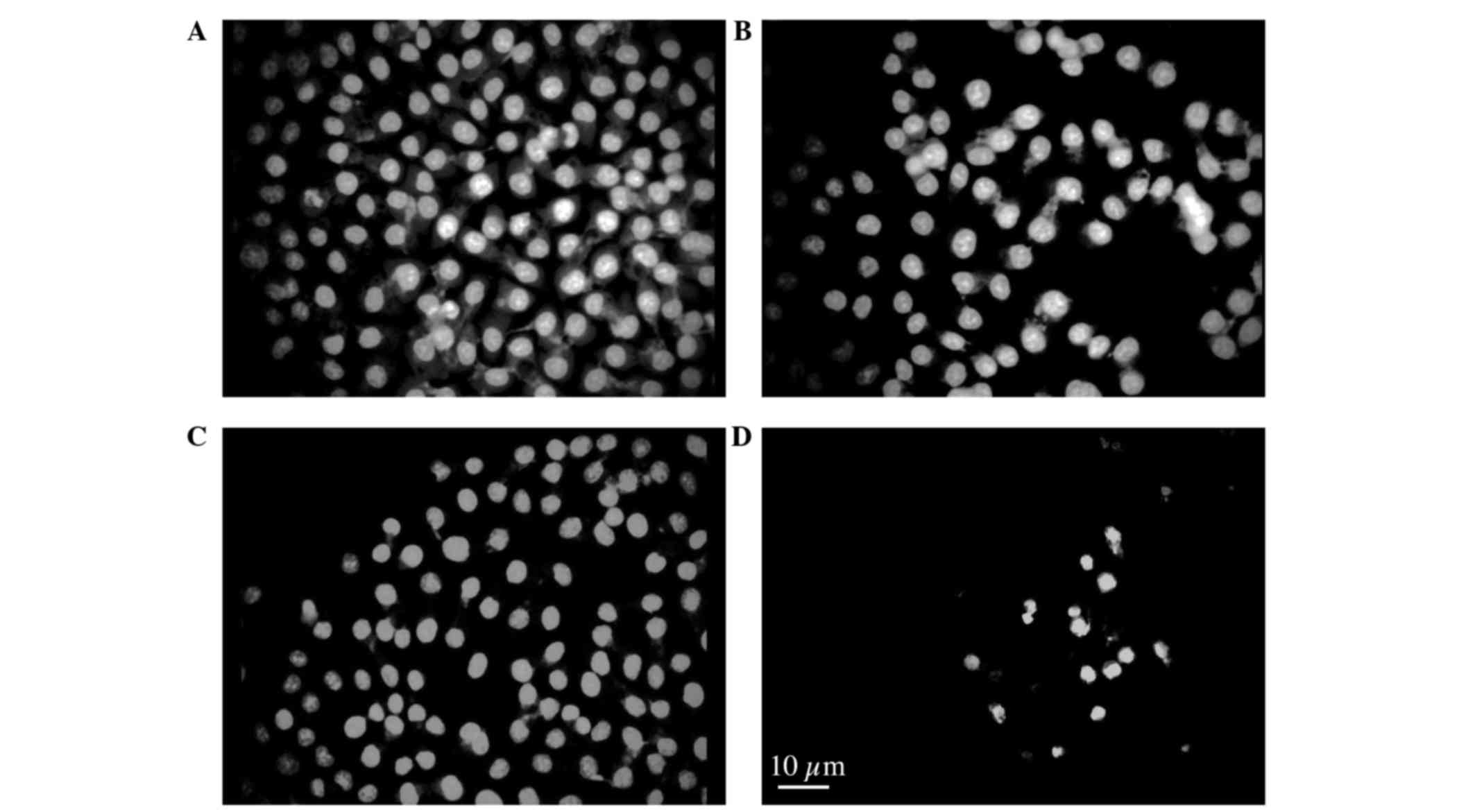
nuclear defects and the apoptotic behavior of these cells, cells
were treated with 2.5 mg/ml HC extracts for 24 h and examined by
acridine orange assay. The control cells had typical cellular
structures (Fig. 4A), including
whole nuclei, clear nucleoli and uniform chromatins. Nuclei
condensation was observed by incubating with different
concentrations of hard clam extract, and according to the dosage
(Fig. 4B-D).
Effect of HC extracts on the cell
cycle in AGS cells
To investigate the mechanisms by which HC extracts
trigger cell death, PI, a DNA chelating dye, was used to determine
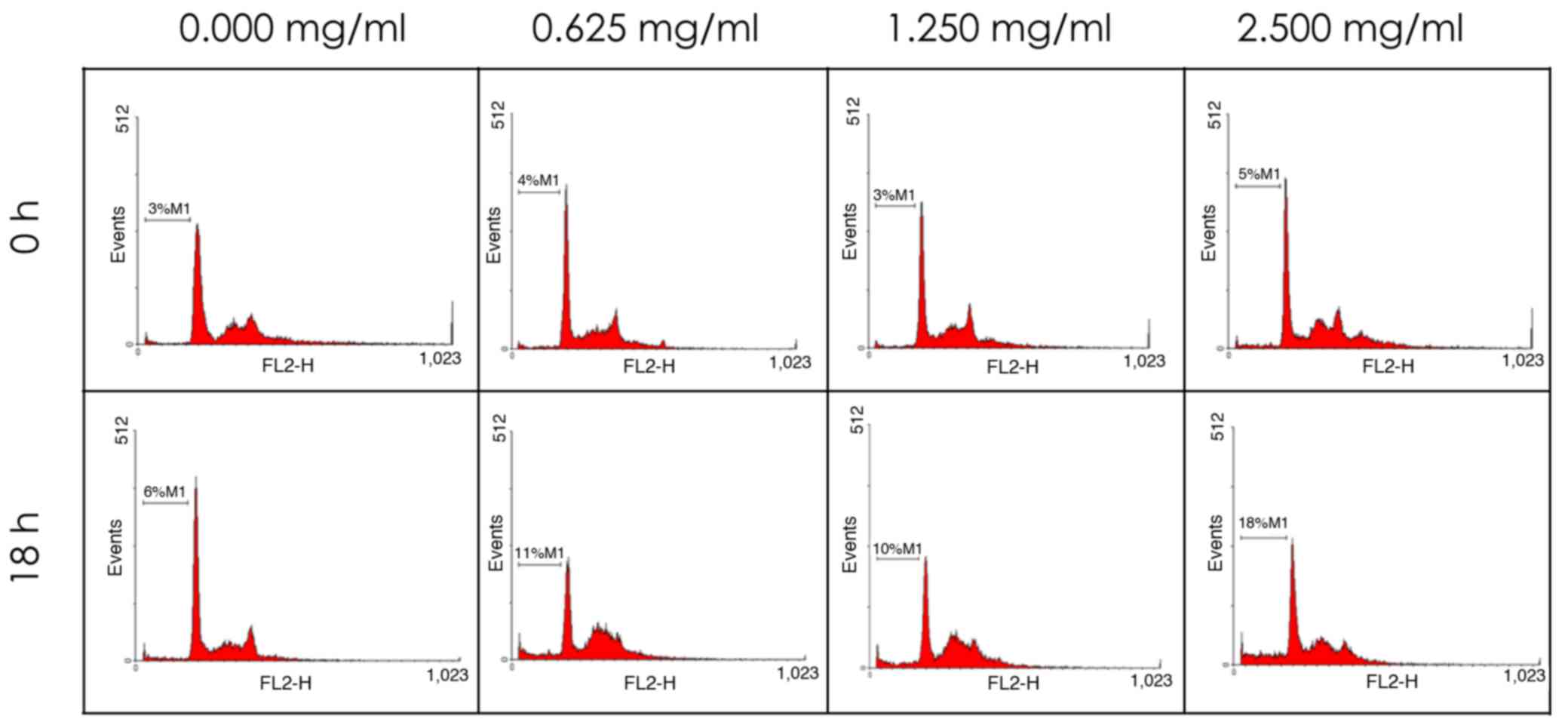
the DNA content and cell cycle distribution by flow cytometry. As
shown in Fig. 5 and Table II, the population of sub-G1 phase
cells increased in a time- and dose-dependent manner.
 | Table II.Dose- and time-dependency effects on
sub-G1 cell number, expressed as % of total cells. |
Table II.
Dose- and time-dependency effects on
sub-G1 cell number, expressed as % of total cells.
|
| Treatment time |
|---|
|
|
|
|---|
| Concentration | 0 h | 18 h |
|---|
| Control | 3.00±0.00 |
6.00±0.00 |
| 0.625 mg/ml | 4.00±0.02 |
11.00±0.00a |
| 1.25 mg/ml | 3.00±0.01 |
10.00±0.01a |
| 2.5 mg/ml |
5.00±0.01b |
18.00±0.00c |
Detection of cell cycle-related gene
expression using micro- arrays
The hallmarks of typical apoptosis, such as DNA
fragmentation, chromatin condensation and apoptotic bodies, were
not observed in the treated AGS cells. However, disruption of the
nuclei and cytoskeleton, and nuclei condensation were observed by
incubation with different concentrations of HC extract. Based on
these results, it is suggested that HC extracts do not cause DNA
fragmentation in AGS cells, but rather obstruct cell cycle
progression. To determine whether genes involved in the cell cycle
arrest were upregulated in AGS cells by HC extracts, a microarray
analysis was performed. AGS cells were treated with HC extracts
(0.625 mg/ml) for 18 h, and were evaluated for differential gene
expression in 128 genes involved in cell cycle regulation,
expression and differentiation (Table
III). Compared with the control groups, this analysis revealed
that the expression of six genes, CDC20, KPNA2, BIRC5, ANAPC2,
CDKN1A and RB1, was affected by HC extracts (Fig. 6). CDC20, KPNA2 and BIRC5 were
revealed to be downregulated while CDKN1A and ANAPC2 were
upregulated. RB1 was not expressed in the control group, but was
expressed in the experimental groups (Fig. 6).
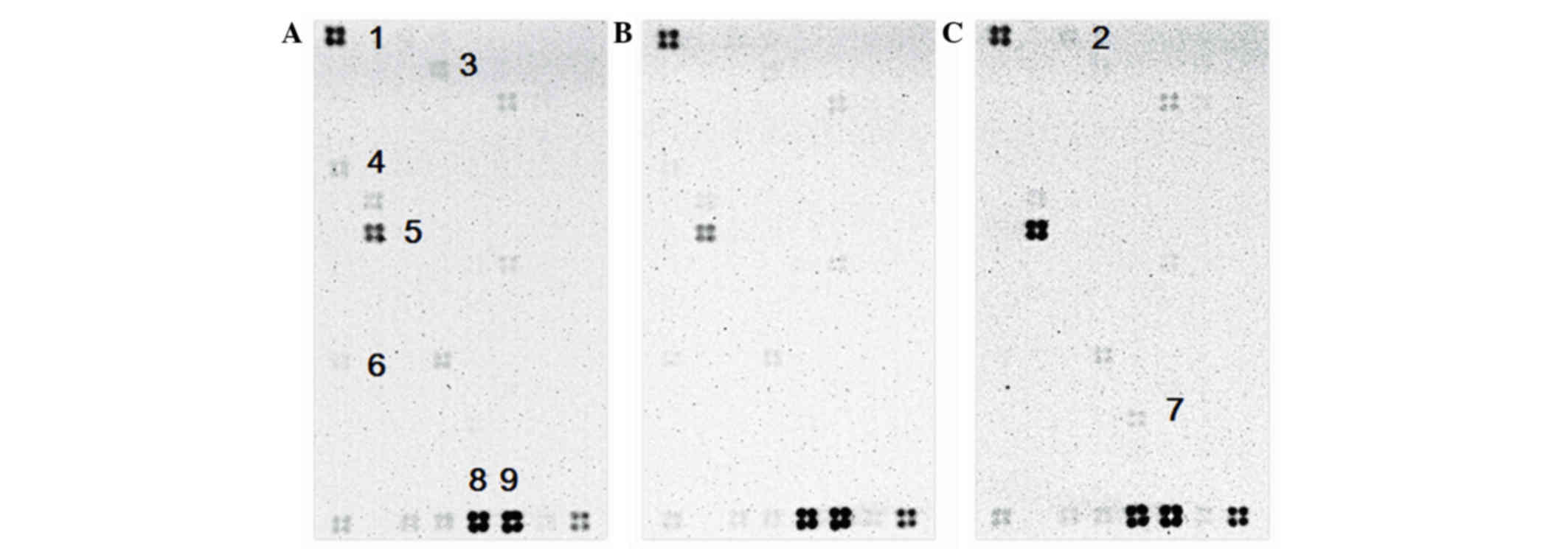 | Figure 6.Microarray profiling the expression
of 128 cell cycle-associated genes in untreated or HC
extract-treated AGS cells. (A) AGS cells only; (B) control membrane
for AGS cells incubated in Tris-HCl lysis buffer with inhibitors,
including to aprotinin, leupeptin, pepstatin and PMSF; (C)
experimental sample, treated with 0.625 mg/ml HC extracts for 18 h.
HC, hard clam. The numbers represent the following genes: 1,
RPS27A; 2, ANAPC2; 3, BIRC5; 4, CDC20; 5, CDKN1A; 6, KPNA2; 7, RB1;
8 and 9, ACTB (used as a control). |
 | Table III.Differentially-expressed genes in the
major GO categories identified in AGS cells following treatment
with HC extracts. |
Table III.
Differentially-expressed genes in the
major GO categories identified in AGS cells following treatment
with HC extracts.
| Gene | Protein name | Accession no. | Location | GO Terms
(biological processesa) |
|---|
| BIRC5 | Baculoviral IAP
repeat-containing 5 (survivin) | NM_001012271 | 17q25 | G2/M transition of
mitotic cell cycle, cytokinesis, establishment of chromosome
localization, mitotic nuclear division, negative regulation of
apoptotic process, positive regulation of cell proliferation,
positive regulation of mitotic cell cycle, protein complex
localization, protein phosphorylation, spindle checkpoint |
| KPNA2 | Karyopherin alpha 2
(RAG cohort 1, importin alpha 1) | NM_002266 | 17q24.2 | DNA metabolic
process, NLS-bearing protein import into the nucleus, regulation of
DNA recombination |
| CDC20 | Cell division cycle
20 homolog (S. cerevisiae) | NM_001255 | 1p34.1 | Anaphase-promoting
complex-dependent catabolic process, cell cycle, positive
regulation of ubiquitin protein ligase activity |
| CDKN1A | Cyclin-dependent
kinase inhibitor 1A (p21, Cip1) | NM_000389 | 6p21.2 | DNA damage response
(signal transduction by p53 class mediator resulting in cell cycle
arrest), G1/S transition of mitotic cell cycle, G2/M transition of
mitotic cell cycle, Ras protein signal transduction, cell cycle
arrest, cellular senescence, intrinsic apoptotic signaling pathway,
negative regulation of G1/S transition of mitotic cell cycle,
negative regulation of cell growth |
| RB1 | Retinoblastoma 1
(including osteosarcoma) | NM_000321 | 13q14.2 | Ras protein signal
transduction, androgen receptor signaling pathway, cell cycle
arrest, chromatin remodeling, maintenance of mitotic sister
chromatid cohesion, mitotic cell cycle checkpoint, negative
regulation of G1/S transition of mitotic cell cycle |
| ANAPC2 | Anaphase promoting
complex subunit 2 | NM_013366 | 9q34.3 | Cell division,
mitotic nuclear division, negative regulation of ubiquitin-protein
ligase activity involved in mitotic cell cycle, regulation of
ubiquitin-protein ligase activity involved in mitotic cell
cycle |
Discussion
The present study developed a method to derive HC
extracts by a method avoiding use of hot water and organic
solvents. The human gastrointestinal cancer cell line AGS
demonstrated a sensitivity to HC extracts, causing reduced
viability of AGS in a dose-dependent manner. First, it was
demonstrated that treatment of AGS cells with HC extracts
immediately abated survival from 98% to ~65% within a short period
of time and continued to occur in a time- and dose-dependent
manner. Following incubation with a high concentration (2.5 mg/ml)
of HC extracts, the survival curve declined consistently with an
increase in time, indicating that lethal cytotoxicity may be
involved in the acute effects observed. By contrast, a delayed
cytotoxicity was observed upon treatment with lower concentrations
of HC extracts. It is possible that initially, HC extracts may
destroy the cytotoxic effects in AGS cells, and may be associated
with apoptosis. In the present study, cells began to proliferate
following 12–24 h of exposure to low doses of HC extracts,
indicating that AGS cells may undergo short-term repair or recovery
prior to death, resulting in a transient increase in the survival
of AGS cells. All survival curves then demonstrated similarly
decreasing patterns of survival after 48 h. Although the exact
mechanism is not yet fully understood, these results may suggest
that at least two major factors, including apoptosis, disruption of
cell cycle progression and proliferation, are involved in the
anticancer effects in AGS cells. Therefore, it may be hypothesized
that HC extracts cause AGS cells to undergo apoptosis and disrupt
cell cycle progression and proliferation; this is supported by
evidence of atypical apoptosis and altered expression of six cell
cycle-associated genes.
Apoptosis is a mechanism by which diet may prevent
tumor formation (31,34), and is a crucial mechanism by which HC
extracts have previously been reported to promote HL-60 cell death
in cancer (17). In the current
study, HC extracts attained by different methods from the
previously described approaches did not cause the hallmarks of
typical apoptosis. Due to the deficiency of the DNA fragmentation
(data not shown), no chromatin condensation and no apoptotic bodies
were observed in treated AGS cells. In the present data, HC
extracts caused an increase in the sub-G1 DNA peaks in AGS cells in
a dose-dependent manner. Following exposure to a high dosage of HC
extracts (2.5 mg/ml) in a continuous culture for >2 days, a
decrease of 70% occurred in the percentage of cells. Furthermore,
nuclear shrinkage, stimulation of detachment of cells during
suspension growth (35,36) and the downregulation of
anti-apoptotic BIRC5 and KPNA2 genes in AGS cells have also been
observed in this and previous studies. These are similar to the
hallmarks of apoptosis (35–38). However, following prolonged treatment
of the cells with 2.5 mg/ml HC extracts for 24 h, karyorrhexis and
plasma membrane bursting of AGS cells were reported. Plasma
membrane bursting, which may have resulted from a loss of plasma
membrane integrity induced by HC extracts, is characteristic of
atypical apoptosis (39). In
summary, the present results suggest that the cytotoxicity of HC
extracts is induced through atypical apoptosis in AGS cells.
Multiple previous studies support a hypothesis of atypical
apoptosis in AGS cancer cells treated with HC extracts, such as the
absence of DNA fragmentation (36),
loss of chromatin condensation (40)
and an absence of detectable apoptotic body formation (41,42).
Other possible evidence of apoptosis induced from HC
extracts could be due to major compositions of taurine. The taurine
content in the HC extracts was higher than that of any other FAAs
(Table I), and this may protect the
gastric mucosa from injury or oncogenesis (43). Taurine has also been reported to
inhibit multiple types of tumor including hepatocellular carcinoma,
head and neck carcinoma, lymphoma and colorectal carcinoma
(44–46). Due to the direct damage caused to the
mitochondria of cancer cells, a high concentration of taurine
accumulating in cancer cells may result in apoptosis (47,48).
However, induction of atypical apoptosis may also occur, as
demonstrated in the current study.
Xu et al (49)
reported that simultaneous occurrence of apoptosis and perturbation
of the cell cycle effectively controls cell proliferation and
induces cancer cell death. Interference to the cell cycle may be
caused by alterations to the distribution of cells across different
stages of the cell cycle (Fig. 6)
and regulation of associated genes, resulting in inhibition of cell
growth and proliferation. In the present study, these cell
cycle-associated genes appeared to indirectly induce death in AGS
cells, mediated by HC extracts. This was demonstrated in 6 genes,
subsequent to a preliminary analysis of the expression of the 128
genes of cell cycle-associated genes. The genes ANAPC2, CDKN1A, and
RB1, which are associated with cell cycle arrest, tumor suppression
and apoptosis, increased in expression in HC extract-treated AGS
cancer cells. However, KPNA2, CDC20 and BIRC5, which are involved
in DNA repair, cell cycle progression, anti-apoptotic activity and
oncogenesis were inhibited. These results indicated the concomitant
effects of interference with cell cycle progression, decreased cell
growth, inhibited DNA repair and increased cell death in AGS cells.
The possible effects on these six genes could also be due to the
up- or downregulation of genes involved in transcription. The RB1
gene, which is a classical tumor suppressor gene and regulates the
cell cycle, has been reported to transactivate the CDKN1A gene
product p21 to induce subsequent transcription and expression
(50). In the current study, it was
determined that the RB1 gene was not expressed in a control group,
but was expressed in the HC extract-treated experimental groups.
Furthermore, CDKN1A and ANAPC2 expression were upregulated in HC
extract-treated AGS cells. These data indicate that RB1, a tumor
suppressor, may threaten the survival of AGS cells, and that the
RB1 gene also activates CDKN1A gene expression. Consequently, these
factors may induce cell cycle exit (51). Subsequent to treatment with HC
extracts for 18 h, an increased expression of the CDKN1A gene,
which is involved in the G1/S cell cycle checkpoint, interrupted
cell cycle progression by inhibiting CDK2 and CDK4, blocked DNA
replication, inhibited repair by binding to proliferating cell
nuclear antigen and is likely to have caused the cell cycle to be
impeded at all stages of G1, S and G2/M phases (52–54).
These results were similar to the downregulation of KPNA2, a DNA
repair gene, observed in HC-extracted-AGS cells (52–54).
Similarly, high levels of CDKN1A gene expression also inhibit the
expression of BIRC5, an anti-apoptotic gene. Consequently, high
levels of CDKN1A gene expression interrupt the progression at
mitosis and anti-apoptotic functioning in cells (55). The products of the CDC20 gene, which
suppresses p21, are activators of the anaphase-promoting
complex/cyclosome (ANAPC/C) required for the metaphase-anaphase
progression during mitosis (56–58).
Reduction in the expression of the CDC20 gene in treated AGS cells
would therefore result in interference with mitotic progression.
The present study indicates that upregulation of ANAPC2, which is a
subunit of the anaphase promoting complex with a function similar
to that of the ANAPC gene, markedly occurs following treatment with
HC extracts. The ANAPC2 gene in treated AGS cells may have a
central role in cell cycle control, tumor suppression and
maintenance of the G0 phase (59–62).
The current study cannot exclude the possibility of
the involvement of other factors such as oxidation (17,34).
However, whether or not other mechanisms are involved, the
mechanism by which HC extracts cause cytotoxicity or growth
inhibition in AGS cells requires additional clarification.
In conclusion, the current in vitro
experimental data demonstrate that HC extracts induce a significant
suppression of cell growth and cytotoxicity in AGS gastric cancer
cells. This was revealed to involve atypical apoptosis, interfere
with cell growth, inhibit DNA repair and block or slow cell cycle
progression. Therefore, it is suggested that HCs may be a healthy
food and may slow down the progress of tumors, serving to promote
anticancer effects. HC extracts may thus have potential as a source
of novel anticancer drugs.
Glossary
Abbreviations
Abbreviations:
|
HC
|
hard clams
|
|
taurine
|
2-aminoethane-sulphonic acid
|
|
CE
|
capillary electrophoresis
|
|
FAA
|
free fatty acid
|
|
ANAPC
|
anaphase-promoting complex
|
References
|
1
|
Ferruzzi MG and Blakeslee J: Digestion,
absorption, and cancer preventative activity of dietary chlorophyll
derivatives. Nutr Res. 27:1–12. 2007. View Article : Google Scholar
|
|
2
|
Kelloff GJ, Boone CW, Crowell JA, Steele
VE, Lubet R and Sigman CC: Chemopreventive drug development:
Perspectives and progress. Cancer Epidemiol Biomarkers Prev.
3:85–98. 1994.PubMed/NCBI
|
|
3
|
Lee HS, Na MH and Kim WK: alpha-Lipoic
acid reduces matrix metalloproteinase activity in MDA-MB-231 human
breast cancer cells. Nutr Res. 30:403–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sutandyo N: Nutritional carcinogenesis.
Acta Med Indones. 42:36–42. 2010.PubMed/NCBI
|
|
5
|
Howson CP, Hiyama T and Wynder EL: The
decline in gastric cancer: Epidemiology of an unplanned triumph.
Epidemiol Rev. 8:1–27. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Suruo T: Mechanisms of multidrug
resistance and implications for therapy. Jpn J Cancer Res.
79:285–296. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thiebaut F, Tsuruo T, Hamada H, Gottesman
MM, Pastan I and Willingham MC: Cellular localization of the
multidrug- resistance gene product P-glycoprotein in normal human
tissues. Proc Natl Acad Sci USA. 84:pp. 7735–7738. 1987; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woolley PV III, Kumar S, Monks TF and
Ortiz JE: Colon cancer as a model for resistance to antineoplastic
drugsMechanisms of Drug Resistance in Neoplastic Cells. Woolley PV
III and Tew KD: Academic Press Inc.; San Diego, CA: pp. 31988,
View Article : Google Scholar
|
|
9
|
Ikeda Y, Mori M, Adachi Y, Matsushima T,
Sugimachi K and Saku M: Carcinoembryonic antigen (CEA) in stage IV
gastric cancer as a risk factor for liver metastasis: A univariate
and multivariate analysis. J Surg Oncol. 53:235–238. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song EJ, Yang VC, Chiang CD and Chao CC:
Potentiation of growth inhibition due to vincristine by ascorbic
acid in a resistant human non-small cell lung cancer cell line. Eur
J Pharmacol. 292:119–125. 1995.PubMed/NCBI
|
|
11
|
Zhang D and Fan D: Multidrug resistance in
gastric cancer: Recent research advances and ongoing therapeutic
challenges. Expert Rev Anticancer Ther. 7:1369–1378. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Graham S, Haughey B, Marshall J, Brasure
J, Zielezny M, Freudenheim J, West D, Nolan J and Wilkinson G: Diet
in the epidemiology of gastric cancer. Nutr Cancer. 13:19–34. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kono S and Hirohata T: Nutrition and
stomach cancer. Cancer Causes Control. 7:41–55. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chijimatsu T, Tatsuguchi I, Oda H and
Mochizuki S: A Freshwater clam (Corbicula fluminea) extract reduces
cholesterol level and hepatic lipids in normal rats and
xenobiotics-induced hypercholesterolemic rats. J Agric Food Chem.
57:3108–3112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan MH, Huang YT, Ho CT, Chang CI, Hsu PC
and Sun Pan B: Induction of apoptosis by Meretrix lusoria through
reactive oxygen species production, glutathione depletion, and
caspase activation in human leukemia cells. Life Sci. 79:1140–1152.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sazimal I and Dangelo GB: The Asian
invasive freshwater clam Corbicula fluminea as prey of two native
waterbirds in South-Eastern Brazil. Folia Malacol. 21:293–295.
2013. View Article : Google Scholar
|
|
19
|
Sugita M, Nakae H, Yamamura T, Takamiya Y,
Itasaka O and Hori T: The occurrence of glycosphingolipids
containing mannose in the sea-water bivalve, Meretrix lusoria
(Hamaguri). J Biochem. 98:27–34. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Karnjanapratum S, Benjakul S, Kishimura H
and Tsai YH: Chemical compositions and nutritional value of Asian
hard clam (Meretrix lusoria) from the coast of Andaman Sea. Food
Chem. 141:4138–4145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kritchevsky D, Tepper SA, Ditullo NW and
Holmes WL: The sterols of seafood. J Food Sci. 32:64–66. 1967.
View Article : Google Scholar
|
|
22
|
Simopoulos AP: Omega-3 fatty acids in
health and disease and in growth and development. Am J Clin Nutr.
54:438–463. 1991.PubMed/NCBI
|
|
23
|
Sinclair AJ, Murphy KJ and Li D: Marine
lipids: Overview ‘news insights and lipid composition of Lyprinol’.
Allerg Immunol (Paris). 32:261–271. 2000.PubMed/NCBI
|
|
24
|
Bevans CG and Harris AL: Regulation of
connexin channels by pH. Direct action of the protonated form of
taurine and other aminosulfonates. J Biol Chem. 274:3711–3719.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu HC and Shiau CY: Proximate composition,
free amino acids and peptides contents in commercial chicken and
other meat essences. J Food Drug Anal. 10:170–177. 2002.
|
|
26
|
Hayes KC: Taurine requirement in primates.
Nutr Rev. 43:65–70. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iritani N, Fukuda E and Inoguchi K: Effect
of feeding the shell fish (Corbicula japonica) on lipid metabolism
in the rat. Atherosclerosis. 34:41–47. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kong ZL, Chiang LC, Fang F, Shinohara K
and Pan P: Immune bioactivity in shellfish toward serum-free
cultured human cell lines. Biosci Biotechnol Biochem. 61:24–28.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsai JS, Chen JL and Pan BS:
ACE-inhibitory peptides identified from the muscle protein
hydrolysate of hard clam (Meretrix lusoria). Process Biochem.
43:743–747. 2008. View Article : Google Scholar
|
|
30
|
Wijesekara I and Kim SK:
Angiotensin-I-converting enzyme (ACE) inhibitors from marine
resources: Prospects in the pharmaceutical industry. Mar Drugs.
8:1080–1093. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin KR: Targeting apoptosis with
dietary bioactive agents. Exp Biol Med (Maywood). 231:117–129.
2006.PubMed/NCBI
|
|
32
|
Häkkinen SH, Kärenlampi SO, Heinonen IM,
Mykkänen HM and Törrönen AR: HPLC method for screening of
flavonoids and phenolic acids in berries. J Sci Food Agric.
77:543–551. 1998. View Article : Google Scholar
|
|
33
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang YT, Huang YH, Hour TC, Pan BS, Liu
YC and Pan MH: Apoptosis-inducing active components from Corbicula
fluminea through activation of caspase-2 and production of reactive
oxygen species in human leukemia HL-60 cells. Food Chem Toxicol.
44:1261–1272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Zhang QH, Wu LJ, Tashiro S,
Onodera S and Ikejima T: Atypical apoptosis in L929 cells induced
by evodiamine isolated from Evodia rutaecarpa. J Asian Nat Prod
Res. 6:19–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zaffaroni N, Pennati M, Colella G, Perego
P, Supino R, Gatti L, Pilotti S, Zunino F and Daidone MG:
Expression of the anti-apoptotic gene survivin correlates with
taxol resistance in human ovarian cancer. Cell Mol Life Sci.
59:1406–1412. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pozarowski P, Halicka DH and Darzynkiewicz
Z: NF-kappaB inhibitor sesquiterpene parthenolide induces
concurrently atypical apoptosis and cell necrosis: Difficulties in
identification of dead cells in such cultures. Cytometry A.
54:118–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hart LS, Ornelles D and Koumenis C: The
adenoviral E4orf6 protein induces atypical apoptosis in response to
DNA damage. J Biol Chem. 282:6061–6067. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hamada M, Nishio K, Doe M, Usuki Y and
Tanaka T: Farnesylpyridinium, an analog of isoprenoid farnesol,
induces apoptosis but suppresses apoptotic body formation in human
promyelocytic leukemia cells. FEBS Lett. 514:250–254. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shiokawa D, Maruta H and Tanuma S:
Inhibitors of poly(ADP-ribose) polymerase suppress nuclear
fragmentation and apoptotic-body formation during apoptosis in
HL-60 cells. FEBS Lett. 413:99–103. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Son M, Kim HK, Kim WB, Yang J and Kim BK:
Protective effect of taurine on indomethacin-induced gastric
mucosal injury. Adv Exp Med Biol. 403:147–155. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Scioscia KA, Snyderman CH and Wagner R:
Altered serum amino acid profiles in head and neck cancer. Nutr
Cancer. 30:144–147. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vecer J, Kvapil M, Sprongl L, Kubátová H,
Hoch J, Jech Z and Charvát J: Tissue amino acids in patients with
colorectal carcinoma. Vnitr Lek. 44:192–194. 1998.(In Czech).
PubMed/NCBI
|
|
46
|
You JS and Chang KJ: Taurine protects the
liver against lipid peroxidation and membrane disintegration during
rat hepatocarcinogenesis. Adv Exp Med Biol. 442:105–112. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Klamt F and Shacter E: Taurine chloramine,
an oxidant derived from neutrophils, induces apoptosis in human B
lymphoma cells through mitochondrial damage. J Biol Chem.
280:21346–21352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Opstad KS, Bell BA, Griffiths JR and Howe
FA: Taurine: A potential marker of apoptosis in gliomas. Br J
Cancer. 100:789–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu XF, Cai BL, Guan SM, Li Y, Wu JZ, Wang
Y and Liu B: Baicalin induces human mucoepidermoid carcinoma Mc3
cells apoptosis in vitro and in vivo. Invest New Drugs. 29:637–645.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Decesse JT, Medjkane S, Datto MB and
Crémisi CE: RB regulates transcription of the p21/WAF1/CIP1 gene.
Oncogene. 20:962–971. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Carreira S, Goodall J, Aksan I, La Rocca
SA, Galibert MD, Denat L, Larue L and Goding CR: Mitf cooperates
with Rb1 and activates p21Cip1 expression to regulate cell cycle
progression. Nature. 433:764–769. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cayrol C, Knibiehler M and Ducommun B: p21
binding to PCNA causes G1 and G2 cell cycle arrest in p53-deficient
cells. Oncogene. 16:311–320. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Harper JW, Adami GR, Wei N, Keyomarsi K
and Elledge SJ: The p21 Cdk-interacting protein Cip1 is a potent
inhibitor of G1 cyclin-dependent kinases. Cell. 75:805–816. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Padua MB and Hansen PJ: Changes in
expression of cell-cycle-related genes in PC-3 prostate cancer
cells caused by ovine uterine serpin. J Cell Biochem.
107:1182–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Löhr K, Möritz C, Contente A and
Dobbelstein M: p21/CDKN1A mediates negative regulation of
transcription by p53. J Biol Chem. 278:32507–32516. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Baker DJ, Dawlaty MM, Galardy P and van
Deursen JM: Mitotic regulation of the anaphase-promoting complex.
Cell Mol Life Sci. 64:589–600. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Harper JW, Burton JL and Solomon MJ: The
anaphase-promoting complex: It's not just for mitosis any more.
Genes Dev. 16:2179–2206. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kidokoro T, Tanikawa C, Furukawa Y,
Katagiri T, Nakamura Y and Matsuda K: CDC20, a potential cancer
therapeutic target, is negatively regulated by p53. Oncogene.
27:1562–1571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Heilman DW, Teodoro JG and Green MR:
Apoptin nucleocytoplasmic shuttling is required for cell
type-specific localization, apoptosis, and recruitment of the
anaphase-promoting complex/cyclosome to PML bodies. J Virol.
80:7535–7545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mo M, Fleming SB and Mercer AA: Cell cycle
deregulation by a poxvirus partial mimic of anaphase-promoting
complex subunit 11. Proc Natl Acad Sci USA. 106:pp. 19527–19532.
2009; View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Morgan D, Eley L, Sayer J, Strachan T,
Yates LM, Craighead AS and Goodship JA: Expression analyses and
interaction with the anaphase promoting complex protein Apc2
suggest a role for inversin in primary cilia and involvement in the
cell cycle. Hum Mol Genet. 11:3345–3350. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wirth KG, Ricci R, Giménez-Abián JF,
Taghybeeglu S, Kudo NR, Jochum W, Vasseur-Cognet M and Nasmyth K:
Loss of the anaphase-promoting complex in quiescent cells causes
unscheduled hepatocyte proliferation. Genes Dev. 18:88–98. 2004.
View Article : Google Scholar : PubMed/NCBI
|