Introduction
Keratoconus is an idiopathic degenerative eye
disease characterized by a progressive non-inflammatory thinning
and conical protrusion of the cornea, which results in corneal
protrusion, irregular astigmatism, loss of visual acuity and even
the possibility of corneal perforation (1). Keratoconus is the most prevalent form
of corneal ectasia and affects all ethnicities. However, higher
incidence has been reported in the Asian population when compared
with Caucasian individuals (2,3).
Although the etiology and pathology of the disease remain not fully
understood, certain studies have identified that in the process of
keratoconus, the intrafibrillar and interfibrillar collagen fiber
cross-links are diminished and lost due to the apoptosis of
keratocytes or release of proteolytic enzymes (4,5).
Corneal collagen cross-linking (CXL) with the
photo-sensitizer riboflavin and ultraviolet A (UVA) light
represents a landmark in the management of keratoconus since it
directly targets the underlying pathology rather than only
addressing the refractive consequences of the disorder (6,7), due to
its capacity in increasing biomechanical corneal resistance
(8,9)
and intrinsic anti-collagenase activity (10). Conventional CXL (CXL) with a
continuous irradiation of 3 mW/cm2 for 30 min is
considered safe and effective in the prevention of keratoconus
progression according to different clinical trials (11–13).
However, the procedure is time-consuming, lasting ~1 h, which may
result in patient discomfort and reduced physician working
efficiency (14). According to the
Bunsen-Roscoe law of reciprocity, it is theoretically possible to
deliver the same energy dose ensuring a proportional biological
effect by setting a higher UVA power in a shorter exposure time in
the accelerated CXL modality (15–17).
However, a lower experimental and clinical efficacy of accelerated
CXL has been reported, which was attributed to the higher
consumption and shortage of oxygen in the stroma (18,19).
Delivering ultraviolet light with an on-off pattern is expected to
allow more oxygen to diffuse into the corneal stroma, lead to an
enhanced release of singlet oxygen and allow a more effective
cross-linking of the collagen molecules. Considering these
aforementioned factors, pulsed-light accelerated CXL (pl-ACXL) has
been recognized by physicians and is currently gaining popularity.
For instance, Peyman et al (20) demonstrated that the pl-ACXL protocol
induced a significantly deeper stromal demarcation line when
compared with the continuous accelerated CXL protocol.
In the present study, the clinical outcomes of CXL
(3 mW/cm2 for 30 min) and pl-ACXL (30 mW/cm2
for 8 min with 1 sec on/1 sec off) were evaluated in a series of 72
eyes with progressive keratoconus in 58 patients over a 12-month
follow-up period. The treatment penetration was estimated by means
of in vivo confocal microscopy.
Materials and methods
Subjects
This prospective study included 58 patients with 72
eyes with keratoconus who were treated between January 2015 and
August 2016 in the Department of Ophthalmology, Shandong Provincial
Hospital affiliated to Shandong University (Jinan, Shandong). Of
these, 36 eyes of 31 patients were treated with CXL (CXL group) and
36 eyes of 27 patients were treated with pl-ACXL (pl-ACXL group).
The treatment protocols of CXL were randomly selected, and all
patients were followed for at least 12 months. Diagnosis of
keratoconus was established by the Amsler-Krumeich classification,
based on the astigmatism, corneal power, corneal transparency and
corneal thickness, obtained with a rotating Scheimpflug imaging
instrument (Pentacam; Oculus Optikgeräte GmbH, Wetzlar, Germany),
slit-lamp biomicroscopy (21) and
interaocular pressure (IOP) using Goldmann applanation tonometry
(Haag-Streit, Koniz, Switzerland). As keratoconus is characterized
by binocular asymmetry, when both eyes of keratoconus patients were
treated with CXL, the same CXL protocol was selected.
The inclusion criteria were as follows: Progressive
keratoconus of stages 1–3 according to the Amsler-Krumeich
classification (21,22); the thinnest corneal thickness (TCT)
was >400 µm; and patients did not wear contact lenses for one
month prior to the initial evaluation and treatment. Progression of
the disease was considered as confirmed if the loss of corrected
distance visual acuity (CDVA) was more than one line in 1 year, or
when the topographic keratometry increased by >1.0 D in 6 months
or >2.0 D in 12 months. Patients with ocular, corneal or immune
system disorders, as well as those who were pregnant or
breastfeeding, were excluded from the present study. All
participants signed an informed consent form in accordance with the
tenets of the Declaration of Helsinki. The present study also
received Institutional Review Board approval by the Shandong
Provincial Hospital affiliated to Shandong University.
CXL treatment
The CXL procedure was performed under sterile
conditions. Subsequent to topical anesthesia using 0.5%
proparacaine hydrochloride (Alcaine; Alcon Laboratories, Inc., Fort
Worth, TX, USA) eye drops, a lid speculum was inserted. To loosen
the epithelium from the stroma, the central cornea was contacted
with a filter paper (diameter, 9 mm) soaked with 20% alcohol for 60
sec, and then the central 9 mm of the cornea epithelium was removed
with a blunt spatula (AE-2766; Asico LLC, Westmont, IL, USA).
Deepithelialization was followed by measuring of the corneal
thickness with ultrasound pachymetry (Pachy Meter SP3000; Tomey
Corp., Nagoya, Japan) to validate that the TCT was >400 µm.
Following epithelial debridement, 0.1% riboflavin solution in 20%
dextran (Shandong Fangming Pharmaceutical Group Co., Ltd., Heze,
China) was applied to the cornea every 3 min for 30 min. A digital
slit-lamp photograph was performed to ensure the appearance of
riboflavin in the anterior chamber. Next, the eye was irradiated
with UVA light with a 370-nm wavelength (UVX 1000 system; IROC
Innocross AG, Zurich, Switzerland) at a working distance of 5 cm.
An area with 9-mm diameter in the center of the cornea was
irradiated with 3 mW/cm2 for 30 min. During the
irradiation, 0.1% riboflavin solution was applied every 3 min to
maintain the riboflavin saturation in the cornea stroma. The total
exposure dose was 5.4 J/cm2.
At the end of the procedure, a bandage-type corneal
contact lens was applied until complete closure of the corneal
epithelium was observed. Postoperative medication included a
combination of 0.1% dexamethasone and 0.3% tobramycin (TobraDex;
Alcon Laboratories, Inc.) four times a day, and the dose was
tapered over 2 weeks.
pl-ACXL treatment
Patients were prepared using the same process as for
the CXL procedure. Following epithelial debridement, 0.1%
dextran-free riboflavin with hydroxyl, propyl, methyl and cellulose
(VibeX Rapid; Avedro Inc., Waltham, MA, USA) were instilled every 2
for 10 min. After riboflavin had been observed in the anterior
chamber, the KXL system (Avedro Inc.) was applied to irradiate the
cornea with UVA light at a 365-nm wavelength, delivered using 30
mW/cm2 irradiance. The ‘pulsed-light’ irradiation mode
was used to alternate 1 sec of UVA irradiation with a 1 sec pause,
for a total duration of 8 min. The cumulative dose was 7.2
J/cm2. During UVA irradiation, balanced salt solution
was distilled onto the subject's eyes to prevent dry spots on the
surface of the cornea. Postoperatively, a bandage-type corneal
contact lens was applied until complete closure of the corneal
epithelium, followed by application of the same postoperative
medications as those administered subsequent to the CXL
procedure.
Preoperative and postoperative
examination items
All patients received systematic ophthalmologic
examinations preoperatively and at each follow-up visit.
Examination included measurement of the uncorrected distance visual
acuity (UDVA), corrected DVA (CDVA) and manifest refraction
spherical equivalent (MRSE). Tomography data were recorded using
Pentacam, including the maximum keratometry (Kmax) and TCT. Corneal
tomographic images were obtained with optical coherence tomography
(OCT) with the RTVue OCT system (Optovue, Inc., Fremont, CA, USA).
Various microstructural features of the cornea were observed with a
Heidelberg Retina Tomograph confocal microscope with the Rostock
Corneal Module (HRT III; Heidelberg Engineering, Inc., Heidelberg,
Germany). Patients were evaluated preoperatively and at 1, 3, 6 and
12 months postoperatively. The parameters were recorded at the
follow-up visits by the same experienced technician as prior to
surgery.
Statistical analysis
Statistical analyses were performed using SPSS
version 20 software (IBM Corp., Armonk, NY, USA). The
Kolmogorov-Smirnov test was used to check for a normal distribution
of quantitative data, which are provided as the mean ± standard
deviation. Postoperative changes were evaluated using a paired
t-test. If the data were not distributed normally, the Wilcoxon
rank-sum test was performed. An independent sample t-test was
performed to analyze the difference in outcomes between the two
groups, while the Mann-Whitney test was performed when data were
not distributed normally. A P-value of <0.05 was considered to
indicate differences that were statistically significant.
Results
Patient characteristics and baseline
values
In the present study, CXL or pl-ACXL was performed
on 72 eyes of 58 patients with progressive keratoconus. At
baseline, there were no significant differences between the CXL and
pl-ACXL groups in terms of their age, UDVA, CDVA, astigmatism,
MRSE, Kmax, TCT, ECD or IOP values. The baseline parameters are
summarized in Table I.
 | Table I.Baseline demographic and clinical
characteristics of patients in the CXL and pl-ACXL groups. |
Table I.
Baseline demographic and clinical
characteristics of patients in the CXL and pl-ACXL groups.
| Parameter | CXL | pl-ACXL | P-value |
|---|
| No. of patients
(n) | 31 | 27 | – |
| No. of eyes treated
(n) | 36 | 36 | – |
| Male gender
(%) | 18 (58.06) | 13 (48.15) | 0.45 |
| Age (years) | 26.86±5.28 | 25.03±5.2 | 0.142 |
| UDVA (logMAR) | 0.9±0.34 | 0.82±0.37 | 0.356 |
| CDVA (logMAR) | 0.36±0.25 | 0.28±0.23 | 0.159 |
| Astigmatism
(D) | −3.32±1.69 | −2.89±1.43 | 0.251 |
| MRSE (D) | −6.12±3.96 | −5.54±3.21 | 0.496 |
| Kmax (D) | 54.38±5.65 | 53.05±4.8 | 0.284 |
| TCT (µm) | 456.53±27.57 | 444.22±31.81 | 0.084 |
| ECD
(cell/mm2) | 2658.17±265.84 | 2563.92±238.9 | 0.118 |
| IOP (mmHg) | 14.07±2.21 | 13.54±2.12 | 0.302 |
Postoperative complications
Following surgery, stromal haze was observed in 17
eyes (47.22%) in the CXL group and 8 eyes (22.22%) in the pl-ACXL
group at the 1-month postoperative visit. The haze disappeared in
all eyes by 12 months after the procedure. In the two groups, all
patients selected presented no delayed corneal epithelium healing,
corneal melting, permanent scars, endothelial damage, sterile
infiltrates, corneal infection or other complications during the
12-month follow-up period.
Visual acuity and refractive
outcomes
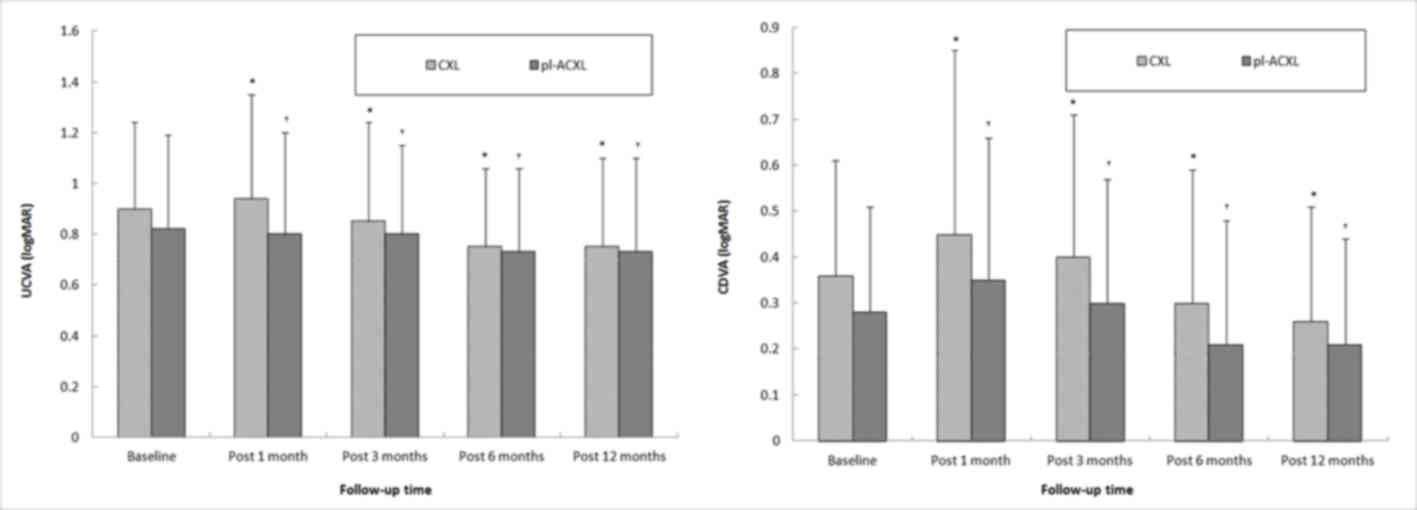
At the 12-month follow-up, UDVA demonstrated a
statistically significant improvement of 0.14±0.05 and 0.12±0.04
logMAR chart scores in the CXL and pl-ACXL groups, respectively
(both P<0.001; Fig. 1A). In
addition, the CDVA exhibited a statistically significant
improvement of 0.12±0.03 and 0.09±0.02 logMAR in the CXL and
pl-ACXL groups, respectively (both P<0.001; Table II and Fig. 1B). However, there were no
statistically significant differences in postoperative astigmatism
or MRSE between the CXL and pl-ACXL groups (all P>0.05).
Furthermore, no statistically significant differences were detected
in the astigmatism or MRSE between the postoperative and baseline
values in the two groups (all P>0.05).
 | Table II.Changes in clinical characteristics
of eyes in the CXL and pl-ACXL groups at 12 months postoperatively
compared with the baseline measurements. |
Table II.
Changes in clinical characteristics
of eyes in the CXL and pl-ACXL groups at 12 months postoperatively
compared with the baseline measurements.
| Parameter | CXL | pl-ACXL |
P-valuea |
|---|
| ΔUDVA (logMAR) |
0.14±0.05 |
0.12±0.04 | 0.769 |
| ΔCDVA (logMAR) |
0.12±0.03 |
0.09±0.02 | 0.323 |
| ΔAstigmatism
(D) |
0.35±1.55 |
0.45±1.34 | 0.198 |
| ΔMRSE (D) |
0.5±1.58 |
0.6±1.78 | 0.189 |
| ΔKmax (D) |
1.80±2.78 |
1.31±2.34 | 0.537 |
| ΔECD
(cell/mm2) |
109.56±327.54 |
246.87±775.59 | 0.317 |
| ΔTCT (µm) |
445.56±26.06 |
440.31±32.04 | 0.448 |
| ΔIOP (mmHg) |
13.39±2.52 |
13.36±1.79 | 0.954 |
Topographic results
Fig. 2 demonstrates
the Kmax readings from the Pentacam system preoperatively and at
the 12-month follow-up visit. Following CXL treatment, the Kmax
values in the pl-ACXL group initially increased at 1 and 3 months,
and later decreased at 6 and 12 months. There was a notable
improvement in the treated eyes, with the Kmax decreasing by
1.80±2.78 D in the CXL group and 1.31±2.34 D in the pl-ACXL group
at 12 months post treatment compared with the baseline. In the CXL
group, 94.44% of the eyes (34/36) presented flattened or stable
Kmax values, as compared with 88.89% of the eyes (32/36) in the
pl-ACXL group.
Demarcation line
A demarcation line can be observed between the
anterior hyper-reflective stroma and posterior stroma with normal
reflectivity. The mean stromal demarcation line depth was
284.94±33.29 µm (range, 236–372 µm) in the CXL group and
201.64±27.72 µm (range, 163–279 µm) in the pl-ACXL group. Thus, the
demarcation line depth of eyes in the CXL group was deeper in
comparison with that in the pl-ACXL group, with a statistically
significant difference observed (P<0.001; Fig. 3).
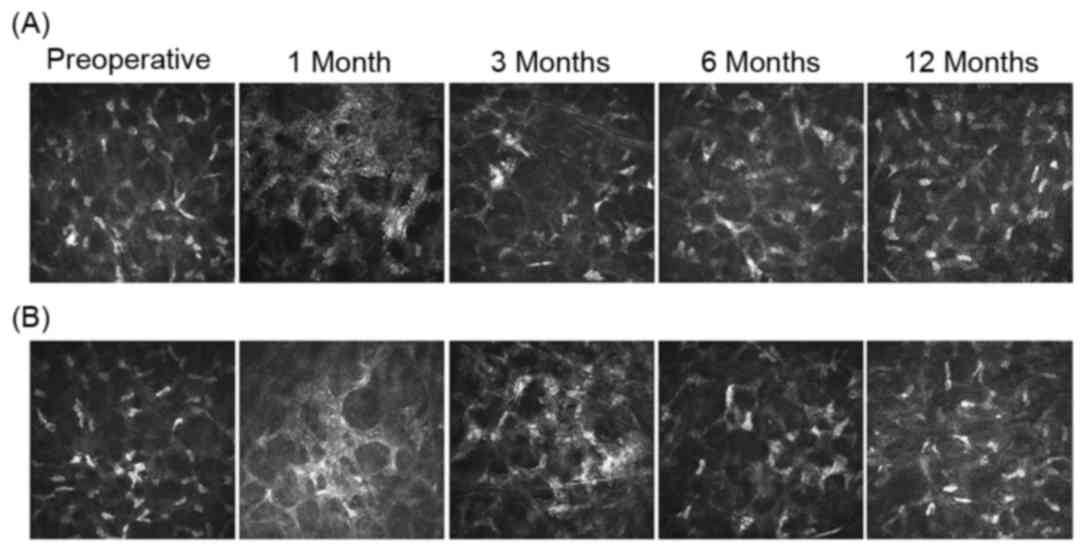
In vivo confocal microscopy and
ECD
Following CXL and pl-ACXL, in vivo confocal
microscopy images demonstrated that the sub-basal nerve plexus was
obliterated, and the density of the nerve plexus decreased at 1, 3
and 6 months postoperatively. However, this density returned to the
preoperative status at 12 months in the two groups. In addition, in
the 1–3-month postoperative period following CXL, anterior stromal
edema with honeycomb-like structures appeared and the keratocyte
density decreased. These changes were similar in the two treatment
groups, however, they were more pronounced following the CXL
procedure. At 3 months, repopulation of the anterior stroma with
keratocyte nuclei was noted in CXL and pl-ACXL-treated eyes, while
the honeycomb-like structures were still apparent, but less
pronounced. At 12 months, the anterior stroma structure was almost
restored to the preoperative status in both groups. The posterior
stromal layers did not appear to have been affected in the CXL or
pl-ACXL groups. Confocal images of the changes following CXL and
pl-ACXL at an anterior corneal depth of ~150 µm are presented in
Fig. 4. Furthermore, a significant
difference in ECD was not observed at any of the follow-up time
points when compared with the baseline value in either group.
Discussion
The efficacy of CXL, using 3 mW/cm2
ultraviolet A (UVA) light for 30 min, has been supported by various
randomized controlled studies (6,23).
However, this protocol is time-consuming, thus research efforts are
focusing on reducing the treatment duration and discomfort, as well
as improving the safety of the procedure. pl-ACXL refers to a
faster procedure with higher radiation and reduced exposure time to
maintain a nearly constant total irradiance and efficacy, according
to the photochemical law of reciprocity (16). Previous studies have demonstrated the
efficacy and safety of accelerated CXL as a corneal stabilizing
method for treating keratoconus (17,24). In
the present study a comparative analysis demonstrated the efficacy
of CXL and pl-ACXL in stabilizing keratoconus progression after 12
months of follow-up, although a small case series was used. To the
best of our knowledge, no previous studies exist in the literature
comparing the results of CXL and pl-ACXL procedures.
At the early stages following CXL and pl-ACXL, all
patients included in the current study presented no delayed corneal
epithelium healing, corneal melting, permanent scars, endothelial
damage, sterile infiltrates, corneal infection or other
complications. However, 17 eyes (47.22%) in the CXL group and 8
eyes (22.22%) in the pl-ACXL group exhibited different degrees of
haze, which reached peak value at 1 month after the surgery and
gradually faded away within 3–12 months after the surgery. Post-CXL
corneal haze is usually a temporary and common complication, which
may occur in 10–90% of eyes treated with CXL (25). Haze may be caused by the complex
structural and physiological wound-healing alterations, such as the
hyperplasia of fibroblasts, in the cornea stroma following CXL.
Thus, it is a distinct clinical component of the basic CXL healing
process (26,27). It has been demonstrated that the
possibility of haze occurrence is not associated with the type of
CXL surgery (14). However, in the
present study, the incidence of haze in the CXL group was
significantly higher in comparison with that in the pl-ACXL group
(P=0.026). This may be due to the longer exposure time of the
corneal stroma during the CXL process. The current study observed
that the occurrence rate of haze following pl-ACXL was ~22.22%,
which is in agreement with the study by Waszczykowska et al
(28) reporting a 25% occurrence
rate following accelerated CXL (6 mW/cm2; 15 min) with a
2-years follow-up. The correlation between CXL and postoperative
haze has to be further analyzed through the analysis of a larger
sample size.
The effects of CXL mainly present as variations of
keratometry over time, as observed on corneal topography. A
randomized control trial of CXL in progressive keratoconus reported
that, after 36 months of follow-up, 41 CXL treated eyes experienced
a mean reduction of Kmax by 1.03 D (6). In a study by Caporossi et al
(29), with a minimum of 4-year
follow-up, the mean value of Kmax decreased by 1.96 D at 1 year
postoperatively. The present study findings were similar to these
aforementioned results. In the current study, a decrease of the
Kmax values was observed at 12 months after the two treatments (CXL
reduced by −1.80±2.78 D; pl-ACXL reduced by −1.31±2.34 D), which
indicated that the corneas became flattened. Therefore, pl-ACXL
treatment appears to be as effective as CXL. In addition, the
difference in treatment protocols resulted in the pl-ACXL group
receiving higher total UVA irradiation (7.2 J/cm2 at a
30 mW/cm2 irradiance for 8 min, with 1 sec on/1 sec off)
as compared with that in the CXL group (5.4 J/cm2 with
an irradiance of 3 mW/cm2 over 30 min). In the study by
Mazzotta et al (30), which
used the same pl-ACXL protocol as the present study, the apical
curvature of the treated cornea demonstrated a decrease by a mean
value of 1.39±0.38 D at 12 months of follow-up. The efficacy of
accelerated CXL is also supported by experimental data on human
donor corneas with scanning acoustic microscopy. The results
demonstrated an increase in stiffness of the corneal tissue by the
same factor of 1.051 following treatment with the convention and
accelerated CXL protocols (31).
The demarcation line represents the effective depth
following CXL (32). In the present
study, the interaction depth of CXL at 1 month after surgery was
observed by performing anterior segment OCT. The CXL treatment
presented a deeper effect, at a stromal depth of 284.94±33.29 µm,
while pl-ACXL treatment presented a penetration of 201.64±27.72 µm.
These findings were consistent with the findings of Mazzotta et
al (30), in which the
demarcation line was 200 µm in depth subsequent to pl-ACXL
treatment. Kohlhaas et al (33) suggested that only the anterior 200 µm
of the cornea is affected in keratoconus. The demarcation line
depth recorded by OCT in the present study suggests that the depth
of cross-linking was sufficient to reach the majority of the
affected cornea.
CXL induces cell apoptosis of stromal keratocytes,
and one potential risk of CXL may be the damage to the corneal
endothelial cells (14). Vinciguerra
et al (34) reported that,
following CXL surgery, stromal cell apoptosis was detected on the
corneal stroma at a depth of ~320 µm. In the CXL procedure, the UVA
irradiation energy was 5.4 J/cm2, markedly lower than
the threshold that may cause injuries to the corneal endothelium,
lens and retina in a cornea of sufficient thickness (34). In the current study, the radiation
energy delivered during pl-ACXL was 7.2 J/cm2. There
were no statistical differences in the ECD between the baseline and
at 12 months postoperatively.
Richoz et al (18) highlighted the slow rate of
replenishment of oxygen in the cornea as a potential limitation of
high-radiation accelerated CXL in an in vitro porcine cornea
experiment. A 1-year follow-up clinical study (30) also confirmed that oxygen represents
the main driver of collagen cross-linking reaction. Pulsed-light
treatment optimized intraoperative oxygen availability, thus
improving the postoperative functional outcomes compared with the
continuous-light treatment of accelerated CXL (30). Although in the current study the
functional results at 1 year after CXL and pl-ACXL demonstrated
keratoconus stability in the two groups, the functional outcomes
were improved in the CXL treatment group, which also presented a
deeper stromal penetration.
In vivo confocal microscopy was used to
observe the microstructural changes over time following CXL
treatment in the present study. Severe loss of sub-basal nerves was
observed in the early postoperative period in both the CXL and
pl-ACXL-treated eyes. Mechanical removal of the epithelium may be
mainly responsible for the loss of corneal nerves. Previous in
vivo confocal microscopy has demonstrated that CXL and pl-ACXL
may cause keratocyte apoptosis in the anterior and middle stroma in
the early postoperative period, while by 6 months, the keratocytes
had gradually repopulated the cornea (35,36),
which is consistent with the results of the present study. The
posterior stromal keratocyte density and the endothelial cell
density were unaffected by the two types of CXL. These observations
are in agreement with previous studies that generally reported that
the posterior stroma and endothelium qualitatively are unaffected
subsequent to CXL (37,38).
The limitations of the current study include the
small number of patients in each group and the short follow-up
period. The long-term effects of the two cross-linking methods
require further investigation. Nevertheless, in the present study,
it was observed that CXL and pl-ACXL were able to control and delay
the development of keratoconus to a certain extent at an early
stage following the surgery. The efficacy of these techniques needs
to be investigated with mid to long-term follow-up and in a large
cohort of patients.
In conclusion, CXL and pl-ACXL were safe and
effective procedures for stabilizing the progression of
keratoconus. The CXL technique offers more effective visual and
topographic outcomes than pl-ACXL; however, pl-ACXL induces less
microstructural damage. The long-term effects of both cross-linking
methods require further study.
Acknowledgements
The authors would like to thank Avedro Inc.
(Waltham, MA, USA) for the loan of the KXL system.
References
|
1
|
Edrington TB, Zadnik K and Barr JT:
Keratoconus. Optom Clin. 4:65–73. 2004.
|
|
2
|
Leccisotti A, Aslanides IM, Moore JE and
Shah S: Keratoconus and Keratoectasia: Advancements in diagnosis
and treatment. J Ophthalmol. 2012:5260582012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Georgiou T, Funnell CL, Cassels-Brown A
and O'Conor R: Influence of ethnic origin on the incidence of
keratoconus and associated atopic disease in Asians and white
patients. Eye. 18:379–383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meek KM, Tuft SJ, Huang Y, Gill PS, Hayes
S, Newton RH and Bron AJ: Changes in collagen orientation and
distribution in keratoconus corneas. Invest Ophthalmol Vis Sci.
46:1948–1956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts CJ and Dupps WJ Jr: Biomechanics
of corneal ectasia and biomechanical treatments. J Cataract Refract
Surg. 40:991–998. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wittig-Silva C, Chan E, Islam FM, Wu T,
Whiting M and Snibson GR: A randomized, controlled trial of corneal
collagen cross-linking in progressive keratoconus: Three-year
results. Ophthalmology. 121:812–821. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meiri Z, Keren S, Rosenblatt A, Sarig T,
Shenhav L and Varssano D: Efficacy of corneal collagen
cross-linking for the treatment of keratoconus: A systematic review
and meta-analysis. Cornea. 35:2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kling S, Remon L, Pérez-Escudero A,
Merayo-Lloves J and Marcos S: Corneal biomechanical changes after
collagen cross-linking from porcine eye inflation experiments.
Invest Ophthalmol Vis Sci. 51:3961–3968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wollensak G, Spoerl E and Seiler T:
Stress-strain measurements of human and porcine corneas after
riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract
Surg. 29:1780–1785. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spoerl E, Wollensak G and Seiler T:
Increased resistance of crosslinked cornea against enzymatic
digestion. Curr Eye Res. 29:35–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raiskup-Wolf F, Hoyer A, Spoerl E and
Pillunat LE: Collagen crosslinking with riboflavin and
ultraviolet-A light in keratoconus: Long-term results. J Cataract
Refract Surg. 34:796–801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hersh PS, Greenstein SA and Fry KL:
Corneal collagen crosslinking for keratoconus and corneal ectasia:
One-year results. J Cataract Refract Surg. 37:149–160. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hashemi H, Seyedian MA, Miraftab M,
Fotouhi A and Asgari S: Corneal collagen cross-linking with
riboflavin and ultraviolet a irradiation for keratoconus: Long-term
results. Ophthalmology. 120:1515–1520. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spoerl E, Mrochen M, Sliney D, Trokel S
and Seiler T: Safety of UVA-riboflavin cross-linking of the cornea.
Cornea. 26:385–389. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wernli J, Schumacher S, Spoerl E and
Mrochen M: The efficacy of corneal cross-linking shows a sudden
decrease with very high intensity UV light and short treatment
time. Invest Ophthalmol Vis Sci. 54:1176–1180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mrochen M: Current status of accelerated
corneal cross-linking. Indian J Ophthalmol. 61:428–429. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurt T, Ozgurhan EB, Yildirim Y, Akcay BI,
Cosar MG, Bozkurt E and Taskapili M: Accelerated (18 mW/cm(2))
corneal cross-linking for progressive keratoconus: 18-Month
results. J Ocul Pharmacol Ther. 32:186–191. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Richoz O, Hammer A, Tabibian D, Gatzioufas
Z and Hafezi F: The Biomechanical effect of corneal collagen
cross-linking (CXL) with riboflavin and UV-A is oxygen dependent.
Transl Vis Sci Technol. 2:62013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hammer A, Richoz O, Mosquera Arba S,
Tabibian D, Hoogewoud F and Hafezi F: Corneal biomechanical
properties at different corneal cross-linking (CXL) irradiances.
Invest Ophthalmol Vis Sci. 55:2881–2884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peyman A, Nouralishahi A, Hafezi F, Kling
S and Peyman M: Stromal demarcation line in pulsed versus
continuous light accelerated corneal cross-linking for keratoconus.
J Refract Surg. 32:206–208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamiya K, Ishii R, Shimizu K and Igarashi
A: Evaluation of corneal elevation, pachymetry and keratometry in
keratoconic eyes with respect to the stage of Amsler-Krumeich
classification. Br J Ophthalmol. 98:459–463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krumeich JH and Kezirian GM: Circular
keratotomy to reduce astigmatism and improve vision in stage I and
II keratoconus. J Refract Surg. 25:357–365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Brart DP, Chan E, Samaras K, Patel P and
Shah SP: A randomised, prospective study to investigate the
efficacy of riboflavin/ultraviolet A (370 nm) corneal collagen
cross-linkage to halt the progression of keratoconus. Br J
Ophthalmol. 95:1519–1524. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Konstantopoulos A and Mehta JS:
Conventional versus accelerated collagen cross-linking for
keratoconus. Eye Contact Lens. 41:65–71. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Razmjoo H, Rahimi B, Kharraji M, Koosha N
and Peyman A: Corneal haze and visual outcome after collagen
crosslinking for keratoconus: A comparison between total epithelium
off and partial epithelial removal methods. Adv Biomed Res.
3:2212014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salomão MQ, Chaurasia SS, Sinha-Roy A,
Ambrósio R Jr, Esposito A, Sepulveda R, Agrawal V and Wilson SE:
Corneal wound healing after ultraviolet-A/riboflavin collagen
cross-linking: a rabbit study. J Refract Surg. 27:401–407. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kymionis GD, Portaliou DM, Diakonis VF,
Kontadakis GA, Krasia MS, Papadiamantis AG, Coskunseven E and
Pallikaris AI: Posterior linear stromal haze formation after
simultaneous photorefractive keratectomy followed by corneal
collagen cross-linking. Invest Ophthalmol Vis Sci. 51:5030–5033.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Waszczykowska A and Jurowski P: Two-year
accelerated corneal cross-linking outcome in patients with
progressive keratoconus. Biomed Res Int. 2015:3251572015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caporossi A, Mazzotta C, Baiocchi S and
Caporossi T: Long-term results of riboflavin ultraviolet a corneal
collagen cross-linking for keratoconus in Italy: The Siena eye
cross study. Am J Ophthalmol. 149:585–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mazzotta C, Traversi C, Paradiso AL,
Latronico ME and Rechichi M: Pulsed light accelerated crosslinking
versus continuous light accelerated crosslinking: One-year results.
J Ophthalmol. 2014:6047312014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beshtawi IM, Akhtar R, Hillarby MC,
O'Donnell C, Zhao X, Brahma A, Carley F, Derby B and Radhakrishnan
H: Biomechanical properties of human corneas following low- and
high-intensity collagen cross-linking determined with scanning
acoustic microscopy. Invest Ophthalmol Vis Sci. 54:5273–5280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seiler T and Hafezi F: Corneal
cross-linking-induced stromal demarcation line. Cornea.
25:1057–1059. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kohlhaas M, Spoerl E, Schilde T, Unger G,
Wittig C and Pillunat LE: Biomechanical evidence of the
distribution of cross-links in corneas treated with riboflavin and
ultraviolet A light. J Cataract Refract Surg. 32:279–283. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vinciguerra P, Camesasca FI, Albè E and
Trazza S: Corneal collagen cross-linking for ectasia after excimer
laser refractive surgery: 1-year results. J Refract Surg.
26:486–497. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mazzotta C, Balestrazzi A, Traversi C,
Baiocchi S, Caporossi T, Tommasi C and Caporossi A: Treatment of
progressive keratoconus by riboflavin-UVA-induced cross-linking of
corneal collagen: Ultrastructural analysis by Heidelberg Retinal
Tomograph II in vivo confocal microscopy in humans. Cornea.
26:390–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jordan C, Patel DV, Abeysekera N and
Mcghee CN: In vivo confocal microscopy analyses of corneal
microstructural changes in a prospective study of collagen
cross-linking in keratoconus. Ophthalmology. 121:469–474. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ku JY, Niederer RL, Patel DV, Sherwin T
and Mcghee CN: Laser scanning in vivo confocal analysis of
keratocyte density in keratoconus. Ophthalmology. 115:845–850.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Messmer EM, Meyer P, Herwig MC, Loeffler
KU, Schirra F, Seitz B, Thiel M, Reinhard T, Kampik A and
Auw-Haedrich C: Morphological and immunohistochemical changes after
corneal cross-linking. Cornea. 32:111–117. 2013. View Article : Google Scholar : PubMed/NCBI
|