Introduction
Cancer is a leading cause of mortality worldwide and
includes numerous diseases characterized by the uncontrolled
proliferation of anaplastic cells, which are able to invade
surrounding tissues and metastasize to other organs (1). Many currently available antitumor drugs
have been demonstrated to cause intolerable side effects and
complications; for instance, doxorubicin may induce severe cardiac
toxicity (2). Therefore, it is
important to identify natural, less toxic and effective products to
prevent and treat cancer. For a number of years, humans have
benefited from green plants as a source of pharmacological agents
and herbal remedies (3,4). For example, mushrooms have attracted
attention as a traditional food and medicine for a long time. The
polysaccharides of mushrooms were proven to be an effective
ingredient for the treatment of diseases such as lung cancer and
colorectal carcinoma (5). In
addition to mushroom polysaccharides, other polysaccharides
isolated from natural materials have also been demonstrated to
prevent ailments including gastroenteric dysfunction, diarrhea and
cancer (6,7).
Phellinus igniarius (PI), one of the most
famous traditional Chinese medicines, is classified into
Hymenochaetaceae Basidiomycete, and is widely used in Asia
(8,9). PI polysaccharides (PIP), the aqueous
extraction of PI, have been demonstrated to have an antitumor
bioactivity (10). Previous research
into the antitumor mechanisms have focused on the direct inhibition
of cancer cells (11,12). However, polysaccharides have
attracted more attention due to their immunomodulatory effects
(13,14). It has been demonstrated that
developing cancer is able to avoid detection and escape the immune
response (15). By adjusting or
stimulating immune functions, the tumor cells may be recognized and
targeted by the immune system (16).
As such, immunotherapy has typically been employed in clinical
settings to achieve an improved treatment and outcome of cancer
(17). Therefore, in the present
study, the immunomodulatory effects of PIP were assessed and the
potential for PIP to provide antitumor immunotherapy was
investigated.
The biological activities of polysaccharides
primarily depend on a number of structural features including
monosaccharide composition, molecular weight, type of glycosidic
bond and branch structures (18,19). For
PI, the structure and bioactivity of polysaccharides may be
affected by the origin and species. Therefore, in the present
study, six different origins or species of PI were collected from
China. These PIs were subsequently screened and the one with the
best antitumor effect was preliminary studied to assess the
antitumor mechanisms. PIP was extracted using a microwave
extraction method. The in vivo antitumor efficacy, spleen
index and thymus index was assessed in Kunming (KM) mice bearing
H22 tumors to identify the PI with the highest antitumor efficacy.
The antitumor mechanism was investigated using MTT assay and by
testing the concentration of serum immune cytokines including
interleukin-2 (IL-2), interleukin-12 (IL-12) and interferon-γ
(IFN-γ).
Materials and methods
Materials
A total of six types of PI fruiting bodies
(including Dongbei mulberry Phellinus igniarius and Dongbei
white birch Phellinus igniarius) were obtained from
Shandong, Gansu, Hunan, unknown origin and Dongbei province of
China. All chemicals and kits were obtained commercially.
Cyclophosphamide (CTX) and Trametes versicolor
polysaccharopeptide (PSP) were purchased from the Jinan Central
Hospital (Jinan, China). HepG2 and murine hepatocellular liver
carcinoma (H22) cells were obtained from the Shandong Institute of
Immunopharmacology and Immunotherapy (Jinan, China). MTT and
RPMI-1640 were purchased from Sigma-Aldrich (Merck KGaA; Darmstadt,
Germany). IL-2, IL-12 and IFN-γ kits were purchased from Jinan
Rebecca Trading Co. (Jinan, China).
Animals
A total of 140 4-week-old female KM mice with a
weight of 18–22 g were supplied by Laboratory Animals Center of
Shandong University (Jinan, China). The mice were housed under
normal laboratory conditions (24±2°C, 50±20% humidity, 12/12-h
light-dark cycle) with free access to standard rodent chow and
water. Following acclimatizing to the lighting conditions for 3
days, 132 mice of similar weights were selected for the
investigation. The experiment was performed following the
guidelines of the Ethical Committee for Animal Experiments of
Shandong University. All animal experiments complied with the
requirements of the National Act on the Use of Experimental
Animals. There were 11 groups in total and 12 mice were used in
each group.
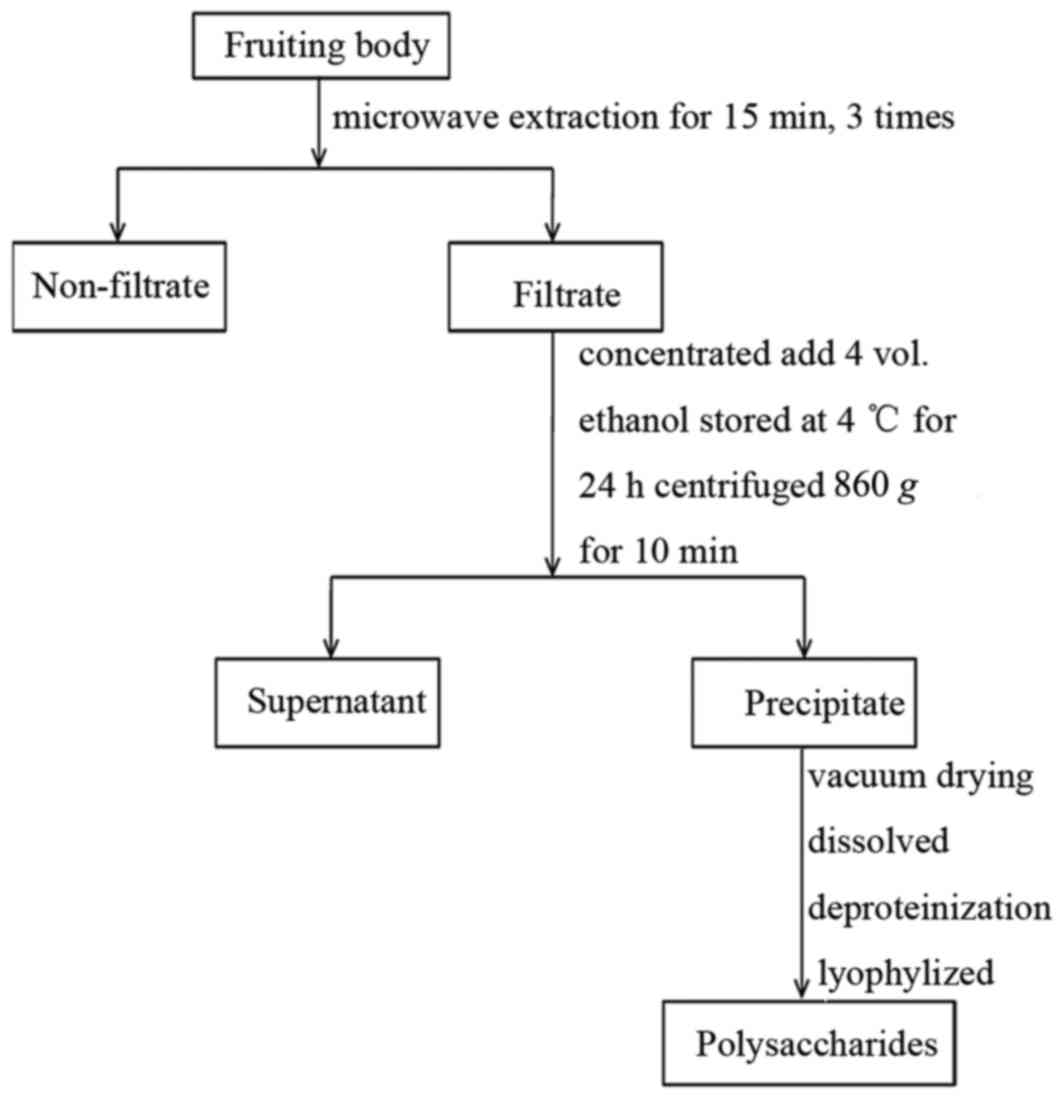
Preparation of PIP
A total of six different types of PI fruiting bodies
were dried in an oven at 60°C for 24 h, ground to powdered-form and
kept in an air-tight plastic bag at room temperature until use. To
prepare crude polysaccharides, 10 g of each PI sample was extracted
with 500 ml distilled water using microwave extraction for 15 min,
three times. The extract was concentrated with RE52-98 rotary
evaporation apparatus (Shanghai Ya Rong Biochemical Instrument
Factory, Shanghai, China), precipitated with 80% ethanol and stored
at 4°C for 24 h. The method of preparation was as previously
described (20) and a clear
description of the process is demonstrated by the flow diagram
presented in Fig. 1. The
concentration of total PIP from Dongbei mulberry, Dongbei birch,
Gansu mulberry, Hunan mulberry, Shandong mulberry and mulberry from
an un-known place were 5.37, 2.21, 3.04, 3.35, 2.77 and 3.58%,
respectively (20). The precipitate
was subsequently centrifuged at 4°C and 860 × g for 15 min, dried
in a vacuum and deproteinized using sevage reagent (chloroform;
Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai,
China).
In vivo tumor growth inhibition
study
KM mice implanted with H22 cells were used to
qualify the relative efficacy of six types of PIP through oral
administration. All animal experiments were performed in full
compliance with guidelines approved by the Animal Care Committee of
Shandong University.
All 132 mice, including the control group, were
subcutaneously injected in the right axillary space with
1×107 H22 cells to establish the hepatoma model mice at
day 0. Treatments were started 24 h following tumor cell
injection.
On Day 0, the mice were randomly divided into 11
groups with 12 mice in each which received the following
treatments: Group i) saline; group ii) CTX (intraperitoneal
injection; CTX concentration of 25 mg/kg; diluted in saline); group
iii): PSP (intragastrically; 200 mg/kg; diluted in distilled
water); groups iv-ix): Dongbei mulberry Phellinus igniarius
polysaccharide (DMPIP), Hunan mulberry Phellinus igniarius
polysaccharide (HPIP), Gansu mulberry Phellinus igniarius
polysaccharide (GPIP), Dongbei white birch Phellinus
igniarius polysaccharide (DWPIP), Shandong mulberry
Phellinus igniarius polysaccharide (SPIP) and unknown origin
Phellinus igniarius polysaccharide (UPIP), respectively
(intragastrically; 200 mg/kg; diluted in distilled water); group
x): GPIP plus CTX at the same time; group xi): PSP plus CTX. The
treatments were administered once daily for two weeks. All mice
were tagged and tumor size was measured daily with calipers during
the period of study. The tumor volume was calculated according to
the following formula: (W2 × L)/2, where W is the tumor
measurement at the widest point and L is the tumor dimension at the
longest point. Each mouse was weighed at the time of treatment, so
that dosages were adjusted to achieve the correct mg/kg amounts.
The body weights of mice were monitored as an index of systemic
toxicity. At the end of the experiment, the animals were sacrificed
by cervical dislocation, and the tumor, spleen and thymus were
harvested by dissection and weighed. The tumor inhibition ratio was
calculated according to the following equation: Inhibition rate (%)
= (mean tumor weight of saline group-mean tumor weight of treated
group)/mean tumor weight of saline group ×100. The thymus and
spleen index were calculated using the following formula: Thymus or
spleen index = the weight of the thymus or spleen/total weight of
mouse.
Cell culture
HepG2 cells were grown in RPMI-1640 medium
containing 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA) for 3
days and then used in the in vitro experiment. The cultures
were maintained at 37°C under a humidified 5% CO2
atmosphere. H22 cells were maintained as ascites in the KM mice by
weekly passage.
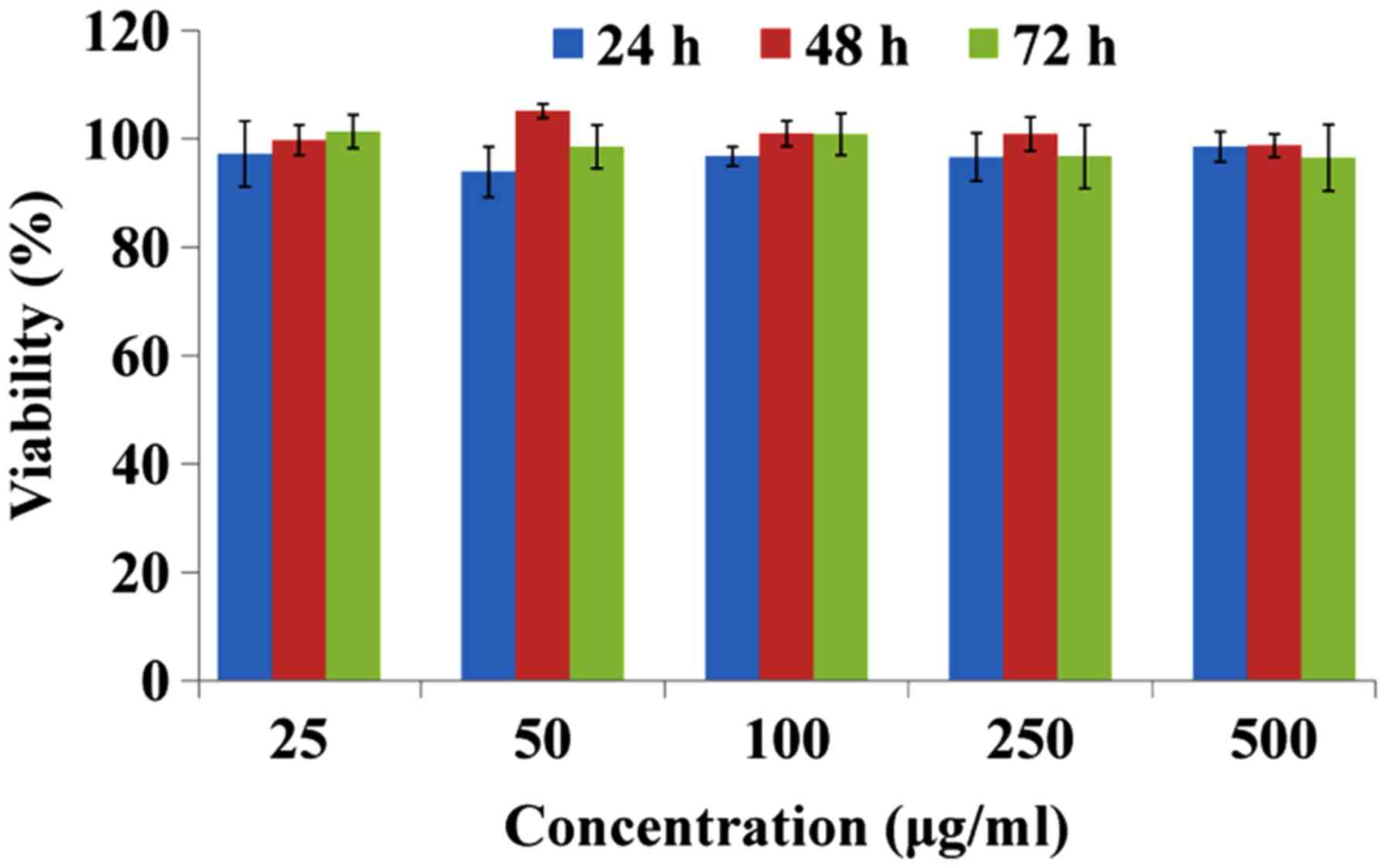
In vitro cytotoxic activity
Direct cytotoxicity of GPIP against HepG2 cells was
evaluated via MTT assay. Cancer cells were inoculated to 96 well
plates at a concentration of 8,000 cells/well and cultured in
RPMI-1640 containing 10% serum for 24 h. Following the addition of
25, 50, 100, 250 and 500 µg/ml GPIP, the cancer cells were further
incubated for 24, 48 and 72 h respectively. Then 20 µl MTT (5
mg/ml) was added into each well and incubated for a further 4 h.
The supernatant was removed carefully and 150 µl dimethyl sulfoxide
was added to each well. The absorbance at 490 nm was measured with
an ELISA reader. Untreated cells were used as a control with 100%
viability and cells without addition of MTT were used as blank to
calibrate the spectrophotometer to zero absorbance. The inhibition
ratio (IR) was calculated according to the following formula: IR
(%) = (1-absorbance of experimental group/absorbance of blank
control group) × 100.
Cytokine measurements
The concentration of serum IL-2, IL-12 and IFN-γ
were measured in the normal (non-tumorous, received saline),
control, CTX, PSP, GPIP, GPIP + CTX and PSP + CTX groups by a
sandwich ELISA method using mouse cytokine ELISA kits (Jinan
Rebecca Trading Co.) according to the manufacturer's protocol.
Assays were performed according to the manufacturer's protocol.
Statistical analysis
Treated and control groups were compared using
Student's t-test SPSS version 21 (IBM Corp., Armonk, NY, USA) was
used for statistical analysis. Results are expressed as means ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results and Discussion
Tumor-inhibitory effect of PIP against
H22
The in vivo antitumor effect of six different
types of PIP was assessed via oral administration using KM mice
bearing H22 tumors as model animals. The tumor weight and
inhibition rate are presented in Table
I. Among the PIP groups, only the average tumor weight in the
GPIP group decreased significantly vs. the saline group (P<0.05)
and the inhibition rate was 33.71%. Furthermore, the tumor weight
in the GPIP group had no significant difference compared with PSP
positive control group. The tumor volumes in all groups were
measured during the experiment. Fig.
2A demonstrates the changes in tumor volumes. It was also
identified that the tumor volumes in the GPIP group were smaller
than those of other PIP groups, together with the tumor weight
changes all indicating that the antitumor effect of GPIP was of the
most promising among the six different PIP. These results revealed
that the antitumor effect was associated with the origin and
species. Therefore it was important to study the association
between the bioactivity and the origin for better development of
PI. Through comparison with other articles, it was identified that
PI from different origins had different antitumor activities and
the mechanisms were not exactly the same (9,21), which
also supported the importance of the present study. Furthermore, in
the study from Li et al (9),
proteoglycans with different purity or structures exhibited
different antitumor activities. Therefore, it was necessary to
initially clarify the association between activity and origin,
screen the PIP with the best antitumor effect and then further
study the structure, because this may save a lot of material
resources, manpower, financial resources and improve the efficiency
of scientific research.
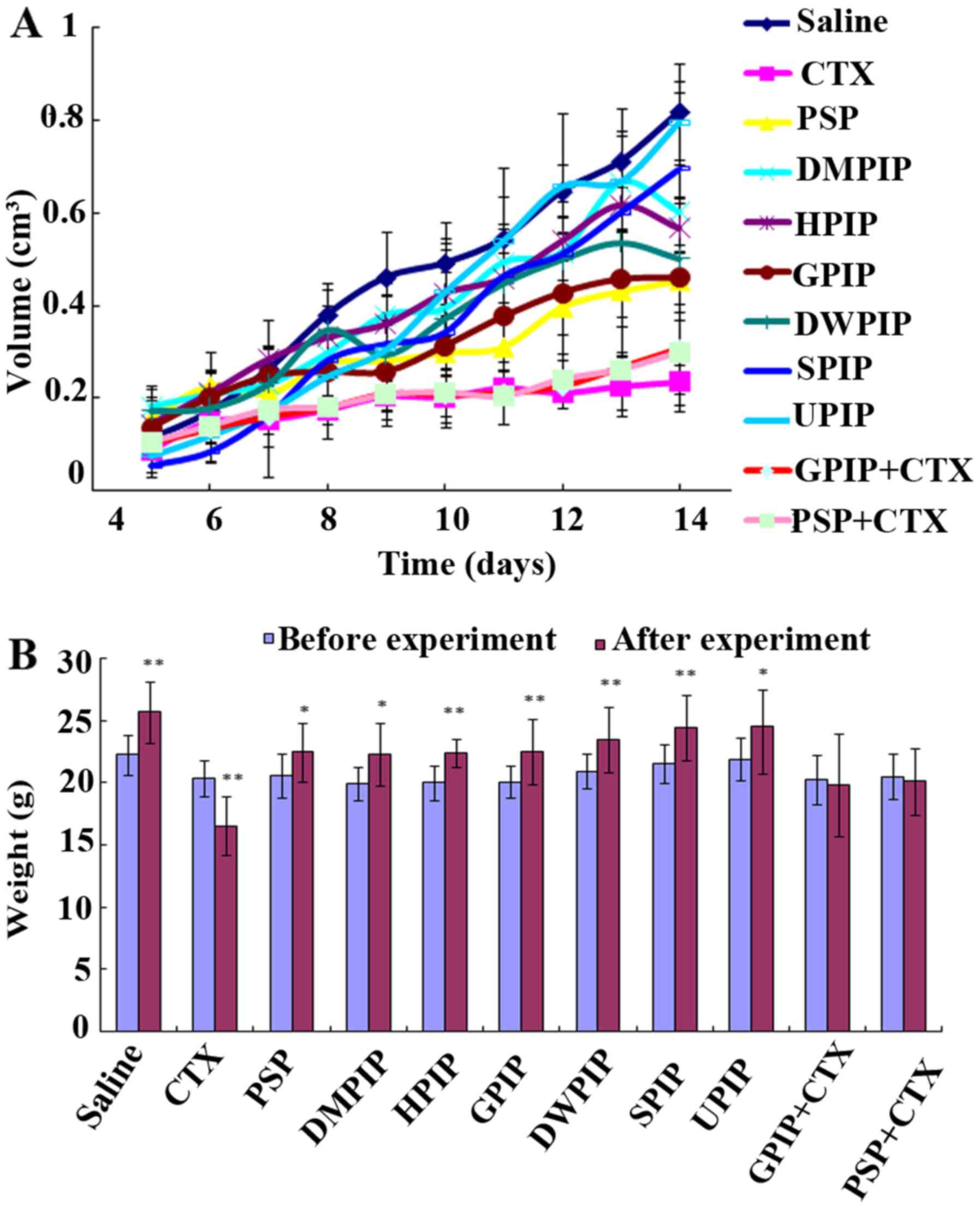 | Figure 2.In vivo antitumor effect of
PIP. (A) Tumor volume changes in different groups; (B) body weight
changes of the mice. *P<0.05, **P<0.01 vs. weight before the
experiment. PIP, Phellinus igniarius polysaccharides; CTX,
cyclophosphamide; PSP, Trametes versicolor
polysaccharopeptide; DMPIP, Dongbei mulberry PIP; HPIP, Hunan
mulberry PIP; GPIP, Gansu mulberry PIP; DWPIP, Dongbei white birch
PIP; SPIP, Shandong mulberry PIP; UPIP, unknown origin PIP. |
 | Table I.Effect of PIP on the number of living
mice, tumor weight and inhibitory rate of H22 cells. |
Table I.
Effect of PIP on the number of living
mice, tumor weight and inhibitory rate of H22 cells.
|
| Total no. of
mice |
|
|
|---|
|
|
|
|
|
|---|
| Groups | Before | After | Tumor weight, g | Inhibition, % |
|---|
| Saline | 12 | 12 | 0.798±0.214 |
|
| CTX | 12 | 10 |
0.234±0.050b | 70.68 |
| PSP | 12 | 12 |
0.493±0.230a | 38.22 |
| DMPIP | 12 | 11 | 0.624±0.300 | 21.80 |
| HPIP | 12 | 11 | 0.602±0.276 | 24.56 |
| GPIP | 12 | 12 |
0.529±0.240a | 33.71 |
| DWPIP | 12 | 12 | 0.601±0.298 | 24.69 |
| SPIP | 12 | 11 | 0.576±0.270 | 27.57 |
| UPIP | 12 | 11 | 0.800±0.370 | 0.00 |
| GPIP+CTX | 12 | 12 |
0.262±0.104b | 67.11 |
| PSP+CTX | 12 | 12 |
0.294±0.069b | 63.11 |
Fig. 2B demonstrates
the body weight changes following the 2-week experimental period.
The results indicated that, with the exception of the CTX group,
the body weight in all PIP groups markedly increased following
treatment. The analysis of body weight variations may be used to
define the systemic toxicity (22).
The primary results suggested that PIP was almost non-toxic. CTX,
as the chemotherapy drug, served an important role in inhibiting
tumor growth, yet its side effects, such as arrest of bone marrow
and liver damage, limited its applications. However, the body
weight in the two combination groups did not change significantly
and thus the results also indicated that GPIP may decrease the
toxicity caused by CTX. The safety of PI was the major advantage
over conventional chemotherapeutics. The importance of
multidisciplinary treatment of cancer in improving patient quality
of life has been discussed and the maintenance of quality of life
greatly affects decision-making in the therapeutic strategies
(23).
Effect of PIP on immunity
The weight of the thymus and spleen reflects the
immune functional strength. In the present study, the relative
spleen and thymus index are presented in Table II. GPIP significantly increased the
spleen index (P<0.01) of H22-bearing mice compared with the
saline and CTX group, whereas CTX significantly decreased thymus
index (P<0.01) compared with the saline group. The spleen index
in other PIP groups didn't demonstrate any significant increase
(P>0.05) compared with the saline group, which was in accordance
with aforementioned antitumor activity. CTX inhibited the growth of
tumor, but damaged the immunity of the mice. However, the spleen
index in GPIP and CTX combination groups significantly increased
compared with CTX group (P<0.05). The relative spleen and thymus
weight were an important index for non-specific immunity.
Therefore, these results indicated that GPIP was a potent
immunomodulating and immunoenhancing agent, which may enhance the
immune function of tumor bearing mice and reduce the immune
suppression caused by CTX.
 | Table II.Immune organ indexes in H22-bearing
mice (n=12). |
Table II.
Immune organ indexes in H22-bearing
mice (n=12).
| Groups | Thymus indexes
mg/10 g | Spleen indexes
mg/10 g |
|---|
| Control | 29.19±6.22 | 44.42±5.63 |
| CTX |
13.69±5.52b | 41.97±9.40 |
| PSP |
31.28±4.68d |
54.71±4.34a,c |
| DMPIP |
19.84±5.33a | 54.66±9.53 |
| HPIP |
23.14±2.12d | 52.52±7.43 |
| GPIP |
30.97±5.45d |
63.40±8.15b,d |
| DWPIP |
25.68±5.69d | 43.12±12.89 |
| SPIP |
28.84±5.90d | 51.26±8.77 |
| UPIP |
26.59±5.24d | 48.03±9.72 |
| GPIP+CTX |
17.63±5.01b |
61.99±9.07b,d |
| PSP+CTX |
20.28±6.79a |
56.30±9.00a,c |
Antitumor mechanism of GPIP
PI has antitumor effects, but the underlying
mechanisms responsible for this phenomenon remained controversial.
Li et al (11) demonstrated
that Phellinus linteus may inhibit the proliferation of
HepG2 cells through inducing S-phase arrest. However, different
origins and structure of polysaccharides may result in different
antitumor mechanism. To further investigate the antitumor mechanism
of GPIP, an MTT assay was performed to demonstrate if the antitumor
activity of GPIP was induced by direct cytotoxicity. The results
are presented in Fig. 3 and indicate
that GPIP exhibited no significant cytotoxicity on HepG2 cells at
any concentration or time point. Therefore, the antitumor mechanism
of GPIP may not be due to inhibition of tumor proliferation
directly.
In view of the aforementioned influence on the
immune organs, GPIP may enhance the immune function. Therefore, it
was speculated that the antitumor mechanisms of GPIP may be
associated with the enhancement of immune function. Macrophages and
lymphocytes belong to two major populations of cells in the host
defense system, which act against invading pathogens. IFN-γ and
IL-2 are secreted by type 1 helper T-cells (Th1 cells) and mediate
cellular immunity. IFN-γ performs a tumoricidal activity and
induces other cells of the innate immune system, including
macrophages and dendritic cells (DCs), to produce IL-12, which
further activates cells mediating the innate response (24). A number of studies have demonstrated
that IFN-γ, IL-12, and IL-2 have a function in the immunomodulation
of polysaccharides (3,4) and the determination of cytokine
concentration was a simple approach to characterize changes in
immune function. Therefore, the serum level of IL-2, IL-12 and
IFN-γ was measured in tumor-bearing mice to further demonstrate the
antitumor mechanisms of GPIP. The results for the concentration of
IL-2, IL-12 and IFN-γ are presented in Figs. 4–6,
respectively. With GPIP stimulation, the serum levels of the three
cytokines significantly increased compared with the saline and CTX
groups (P<0.01). Furthermore, the IL-2 in GPIP group exhibited a
significant increase compared with the PSP group (P<0.05). IL-2
may stimulate the reactivity of numerous types of killer cells,
such as natural killer (NK) cells and cytolytic T lymphocytes
(21,25), thus the high level of IL-2 enhanced
the cytolytic activity of NK cells and cytolytic T lymphocytes. The
trend for the secretion of IFN-γ was the same as that observed for
the secretion of IL-12. IL-12 is one of the crucial cytokines for
transition of the immune response from innate to adaptive as the
antigen-presenting cells, such as monocytes and DCs produce a
substantial amount of IL-12 that primes the Th1 response (26). This in turn induces the production of
IFN-γ and IL-12 by the Th1 cells to further stimulate downstream
cell-mediated immunity (27), which
may explain the consistency of IL-12 and IFN-γ in the present
study. Furthermore, the three cytokines in GPIP and CTX combination
groups also exhibited a significant increase (P<0.01) compared
with CTX, which decreased the level of all these cytokines
significantly (P<0.01). The results indicated that GPIP was not
only an immune enhancing agent but also may decrease the
immunosuppression caused by CTX. According to these results, GPIP
was an effective immunopotentiator and the antitumor mechanism may
be associated with the enhancement of immune function, which was
regulated by GPIP. In conclusion, GPIP was a promising plant
polysaccharide as an immunoregulator that demonstrated great
potential in the treatment of cancer.
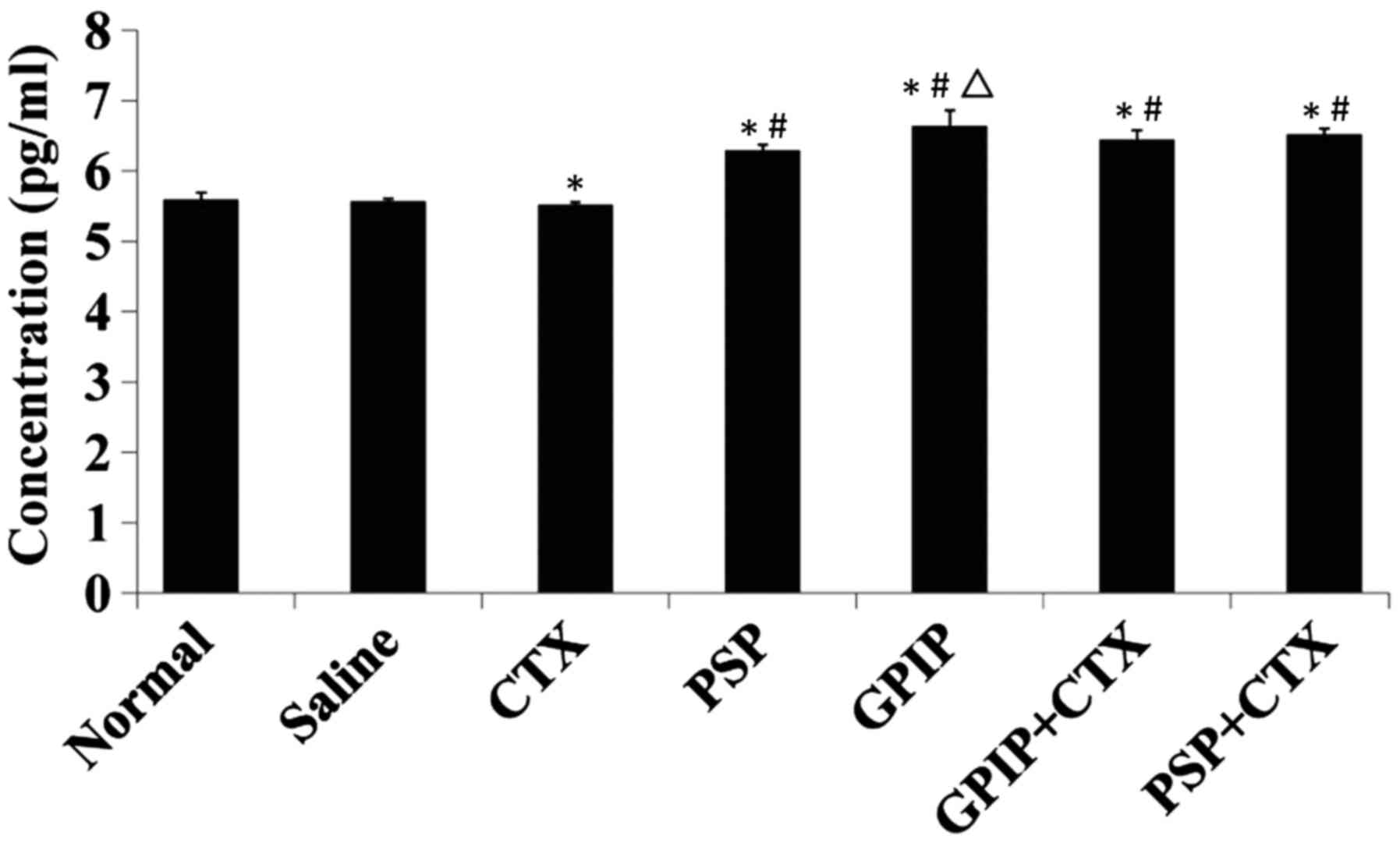 | Figure 4.Serum IL-2 in H22-bearing mice.
H22-bearing mice were administered saline (negative control group),
saline (normal group, non-tumorous), CTX, PSP, GPIP, GPIP + CTX and
PSP + CTX once daily, for 14 days. Sera were collected from the
tumor-bearing mice on day 15. IL-2 concentration was determined
using ELISA method (n=3). *P<0.01 vs. saline group,
#P<0.01 vs. CTX group, and ∆P<0.05 vs.
PSP group. IL-2, interleukin-2; CTX, cyclophosphamide; PSP,
Trametes versicolor polysaccharopeptide; GPIP, Gansu
mulberry Phellinus igniarius polysaccharides; ELISA,
enzyme-linked immunosorbent assay. |
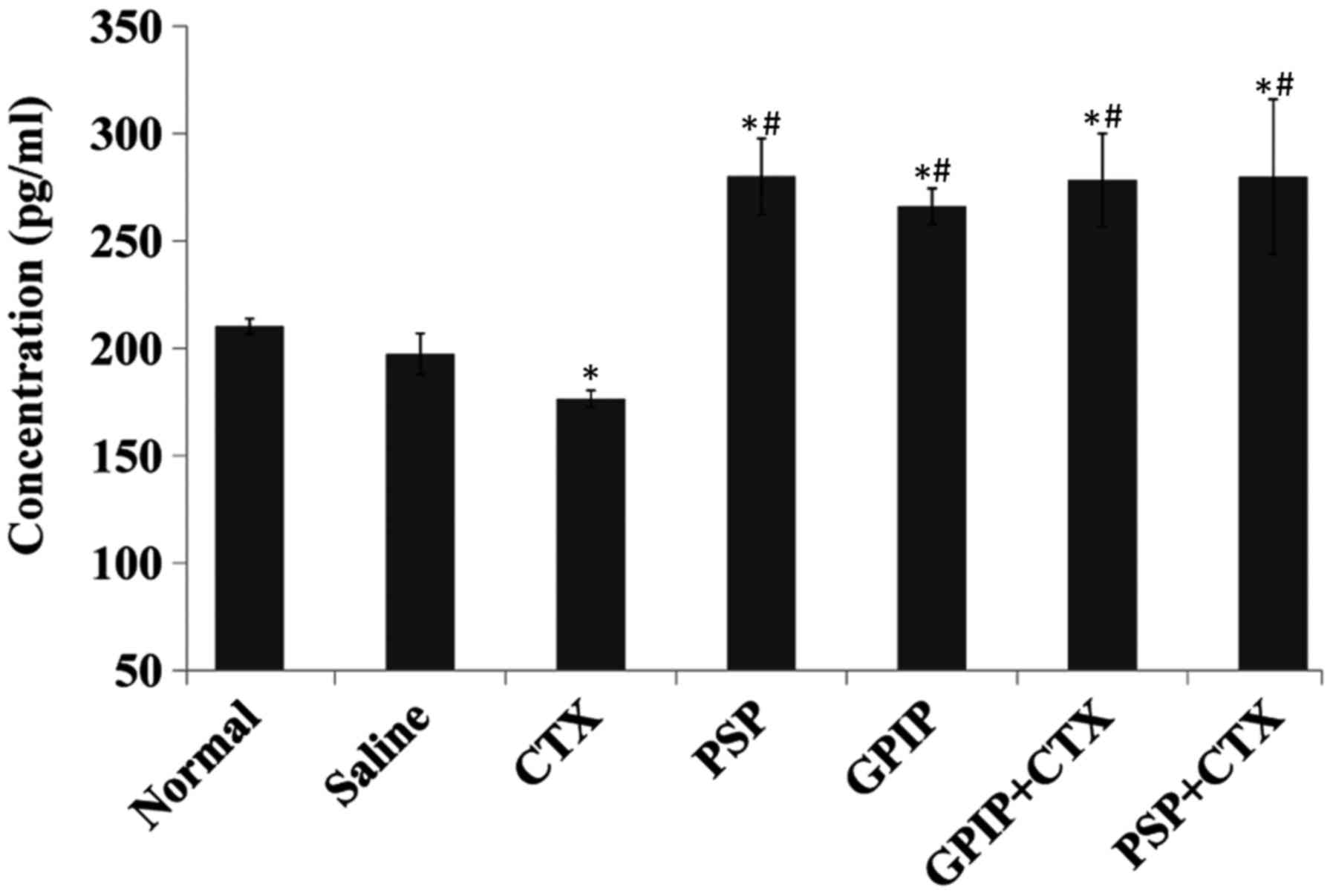 | Figure 6.Serum IFN-γ in H22-bearing mice.
H22-bearing mice were administered saline (negative control group),
saline (normal group, non-tumorous), CTX, PSP, GPIP, GPIP + CTX and
PSP + CTX once daily, for 14 days. Sera were collected from the
tumor bearing mice on day 15. IFN-γ concentration was determined
using ELISA method (n=3). *P<0.01 vs. saline group,
#P<0.01 vs. CTX group. IFN-γ, interferon-γ; CTX,
cyclophosphamide; PSP, Trametes versicolor
polysaccharopeptide; GPIP, Gansu mulberry Phellinus
igniarius polysaccharides; ELISA, enzyme-linked immunosorbent
assay. |
Hepatocellular carcinoma is one of the most
prevalent malignant tumors worldwide, and has an extremely poor
prognosis. The results of the present study suggest that PIP may
have potential therapeutic applications in a clinical setting. Such
immune regulatory effects of PIP should be further investigated to
identify how the components of the PIP interact with immune
cells.
Acknowledgments
The present study was supported by the Development
of Science and Technology Plan Project of Shandong Province (grant
no. 2012GSF1191), Jinan Science and Technology Project (grant no.
201101025).
References
|
1
|
Sliva D, Jedinak A, Kawasaki J, Harvey K
and Slivova V: Phellinus linteus suppresses growth, angiogenesis
and invasive behaviour of breast cancer cells through the
inhibition of AKT signalling. Br J Cancer. 98:1348–1356. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Wan Y, Wang Y, Zhang H and Jiao Z:
Anticancer efficacy enhancement and attenuation of side effects of
doxorubicin with titanium dioxide nanoparticles. Int J
Nanomedicine. 6:2321–2326. 2011.PubMed/NCBI
|
|
3
|
Sun Y, Sun T, Wang F, Zhang J, Li C, Chen
X, Li Q and Sun S: A polysaccharide from the fungi of Huaier
exhibits anti-tumor potential and immunomodulatory effects.
Carbohydr Polym. 92:577–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yi Y, Zhang MW, Liao ST, Zhang RF, Deng
YY, Wei ZC, Tang XJ and Zhang Y: Structural features and
immunomodulatory activities of polysaccharides of longan pulp.
Carbohydr Polym. 87:636–643. 2012. View Article : Google Scholar
|
|
5
|
Fabricant DS and Farnsworth NR: The value
of plants used in traditional medicine for drug discovery. Environ
Health Perspect 109 Suppl. 1:S69–S75. 2001. View Article : Google Scholar
|
|
6
|
Gao C, Zhong L, Jiang L, Geng C, Yao X and
Cao J: Phellinus linteus mushroom protects against tacrine-induced
mitochondrial impairment and oxidative stress in HepG2 cells.
Phytomedicine. 20:705–709. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao R, Gao X, Cai Y, Shao X, Jia G, Huang
Y, Qin X, Wang J and Zheng X: Antitumor activity of Portulaca
oleracea L. polysaccharides against cervical carcinoma in vitro and
in vivo. Carbohydr Polym. 96:376–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zou X, Sun M and Guo X: Quantitative
response of cell growth and polysaccharide biosynthesis by the
medicinal mushroom Phellinus linteus to NaCl in the medium. World J
Microb Biot. 22:1129–1133. 2006. View Article : Google Scholar
|
|
9
|
Li X, Jiao LL, Zhang X, Tian WM, Chen S
and Zhang LP: Anti-tumor and immunomodulating activities of
proteoglycans from mycelium of Phellinus nigricans and culture
medium. Int Immunopharmacol. 8:909–915. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He P, Geng L, Wang J, Wang Z, Mao D and Xu
C: Purification, characterization and bioactivity of an
extracellular polysaccharide produced from Phellinus igniarius. Ann
Microbiol. 62:1697–1707. 2012. View Article : Google Scholar
|
|
11
|
Li YG, Ji DF, Zhong S, Liu PG, Lv ZQ, Zhu
JX, Chen JE and Chen HP: Polysaccharide from Phellinus linteus
induces S-phase arrest in HepG2 cells by decreasing calreticulin
expression and activating the P27kip1-cyclin A/D1/E-CDK2 pathway. J
Ethnopharmacol. 150:187–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong S, Ji DF, Li YG, Lin TB, Lv ZQ and
Chen HP: Activation of P27kip1-cyclin D1/E-CDK2 pathway by
polysaccharide from Phellinus linteus leads to S-phase arrest in
HT-29 cells. Chem Biol Interact. 206:222–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zong A, Cao H and Wang F: Anticancer
polysaccharides from natural resources: A review of recent
research. Carbohydr Polym. 90:1395–1410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu SJ, Liaw CC, Pan SZ, Yang HC and Ng LT:
Phellinus linteus polysaccharides and their immunomodulatory
properties in human monocytic cells. J Funct Foods. 5:679–683.
2013. View Article : Google Scholar
|
|
15
|
Blattman JN and Greenberg PD: Cancer
immunotherapy: A treatment for the masses. Science. 305:200–205.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gajewski TF, Woo SR, Zha Y, Spaapen R,
Zheng Y, Corrales L and Spranger S: Cancer immunotherapy strategies
based on overcoming barriers within the tumor microenvironment.
Curr Opin Immunol. 25:268–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Xiong S, Li A, Huang N, Lu F and Hou D:
Antioxidant and immunoregulatory activity of different
polysaccharide fractions from tuber of Ophiopogon japonicus.
Carbohydr Polym. 86:1273–1280. 2011. View Article : Google Scholar
|
|
19
|
Jeff IB, Yuan X, Sun L, Kassim RM, Foday
AD and Zhou Y: Purification and in vitro anti-proliferative effect
of novel neutral polysaccharides from Lentinus edodes. Int J Biol
Macromol. 52:99–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mou ZZ, Wang MF, Gao WW, Zhang N and Yu
SW: Extraction of Phellinus igniarius polysaccharides and
composition analysis of monosaccharide. Chin J Experimental
Traditional Medical Formulae. 20:13–15. 2014.(In Chinese).
|
|
21
|
Chen L, Pan J, Li X, Zhou Y, Meng Q and
Wang Q: Endo-polysaccharide of Phellinus igniarius exhibited
anti-tumor effect through enhancement of cell mediated immunity.
Int Immunopharmacol. 11:255–259. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang HY, Chieh SY, Tso TK, Chien TY, Lin
HT and Tsai YC: Orally administered mycelial culture of Phellinus
linteus exhibits antitumor effects in hepatoma cell-bearing mice. J
Ethnopharmacol. 133:460–466. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Silva DD, Rapior S, Fons F, Bahkali AH
and Hyde KD: Medicinal mushrooms in supportive cancer therapies: An
approach to anti-cancer effects and putative mechanisms of action.
Fungal Diversity. 55:1–35. 2012. View Article : Google Scholar
|
|
24
|
Wong KH, Lai CK and Cheung PC:
Immunomodulatory activities of mushroom sclerotial polysaccharides.
Food Hydrocolloids. 25:150–158. 2011. View Article : Google Scholar
|
|
25
|
Xie G, Schepetkin IA and Quinn MT:
Immunomodulatory activity of acidic polysaccharides isolated from
Tanacetum vulgare L. Int Immunopharmacol. 7:1639–1650. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cella M, Scheidegger D, Palmer-Lehmann K,
Lane P, Lanzavecchia A and Alber G: Ligation of CD40 on dendritic
cells triggers production of high levels of interleukin-12 and
enhances T cell stimulatory capacity: T-T help via APC activation.
J Exp Med. 184:747–752. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin Z and Blankenstein T: CD4+ T
cell-mediated tumor rejection involves inhibition of angiogenesis
that is dependent on IFN gamma receptor expression by
nonhematopoietic cells. Immunity. 12:677–686. 2000. View Article : Google Scholar : PubMed/NCBI
|