Introduction
Thermal injuries can involve microcirculation
dysfunctions, inflammatory responses and edema in the heated skin,
which can lead to ischemia-induced injury, cellular metabolic
dysfunctions and even, ultimately, cell death (1–3). Jackson
(4) described burn wounds in terms
of three concentric zones based on clinical observations and
histologic investigations. The central zone is the zone of
coagulation that is irreversibly damaged by the heat; this zone is
surrounded by a stasis zone, which is surrounded by a zone of
hyperemia (2). The outermost
hyperemia zone will fully recover. Within the first 48 and 72 h (or
longer) following thermal injury, the stasis zone exhibits
progressive necrosis, which accounts for the varying depth and
extent of skin burns (5,6). Therefore, the optimal intervention at
this stage involves the salvage of the stasis zone. Hence, the
stasis zone has become a focus of burn research and treatment.
Peroxisome proliferator activated receptors (PPARs),
including PPARα, PPARβ/δ and PPARγ, are transcription factors and
members of the steroid nuclear hormone receptor superfamily. PPARs
primarily exist in rodents, amphibians and humans (7). PPARβ/δ is one of three subtypes and
plays a paramount roles in placentation, hepatic stellate cell
proliferation, colon cancer occurrence, cholesterol transfer and
lipid metabolism (8). The activation
of PPARβ inhibits apoptosis, promotes cell proliferation and has
clearly defined roles in burn wound healing (9).
Granulocyte-macrophage colony-stimulating factor
(GM-CSF) is a cytokine that is capable of stimulating proliferation
and differentiation into hematopoietic stem cells and the
subsequent formation of granulocytes, macrophages and
granulocyte-macrophage colonies. Previous studies have demonstrated
that recombinant human granulocyte-macrophage colony-stimulating
factor (rhGM-CSF) has a variety of functions, including the
stimulation of bone marrow hematopoiesis and the induction of the
differentiation of myofibroblasts (10).
Recent evidence suggests that topically applied
rhGM-CSF gel facilitates wound contraction, mediates cell
proliferation and differentiation, induces vascularization and
promotes burn wound healing (11,12). The
entire process of wound healing involves a complex interplay of
different cell populations, growth factors, the extracellular
matrix and cytokines.
PPARβ is also an important factor for promoting
wound healing, and the present study was conducted to investigate
the influence of the topical application of rhGM-CSF gel to
thermally damaged skin on PPARβ gene expression in an animal scald
model.
Materials and methods
Experimental protocol and
reagents
Fifty adult male Sprague-Dawley rats weighing
between 250 and 300 g were provided by the Experimental Animal
Centre of Zhengzhou University for this study. All animals were fed
rat chow and provided water ad libitum. The present study
was approved by the Local Animal Care Committee.
The following reagents and equipment (with the
suppliers indicated in the parentheses) were employed: 100 µg/10 g
rhGM-CSF hydrogel and hydrogel without rhGM-CSF (both provided by
GeneScience Pharmaceuticals Co., Ltd., Changchun, China); BCA
protein quantitative kits (23225; Thermo Fisher Scientific,
Waltham, MA, USA), sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) gel preparation kits (P0012A; Blue Skies
Institute of Biotechnology), polyvinylidene fluoride (PVDF)
membrane (aperture: 0.45 µM; GS0914; Millipore, Billerica, MA,
USA), enhanced HRP-DAB chromogenic substrate kits (PA110; Tiangen
Biotech Co., Ltd., Beijing, China), PPARβ antibodies (1:500;
sc-74517; Santa Cruz Biotechnology, Santa Cruz, CA, USA), two
resistance (7076; Cell Signaling Technology, Danvers, MA, USA),
Bio-Rad protein3 electrophoresis apparatus, Bio-Rad
transblotDianZhuan instrument, Bio-Rad ChemiDoc chemiluminescence
imaging, TRIzol (15596–018; Invitrogen, Carlsbad, CA, USA), reverse
transcription kit (K1622; Fermentas, Waltham, MA, USA), a
quantitative qRT-PCR (SYBR-Green I) kit (FP302-02; Tiangen
Biotech); a rabbit anti-goat ready-to-use two-gait detection kit
and double-amino benzidine chromogenic reagent kit (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China);
optical microscope (Olympus, Tokyo, Japan); and a photographic
microscope system (Leica Microsystems, Wetzlar, Germany).
Thermal injury and treatment
Adult male Sprague Dawley rats weighing 250–300 g
(n=50) were used for the experimental protocol. Fifty rats were
randomly divided into five groups (n=10 per group). The day before
the trial, the back hair of all rats was removed with a sodium
sulfide solution (80 g/l), and symmetric scald models were created
in all five groups of rats on both sides of the dorsal spines. The
scalded skin on the left sides of the five groups served as the
experimental groups, and the right sides served as the control
group. Before the experiment began, rats were anesthetized via an
intraperitoneal injection of 35 mg/kg of chloral hydrate (10%).
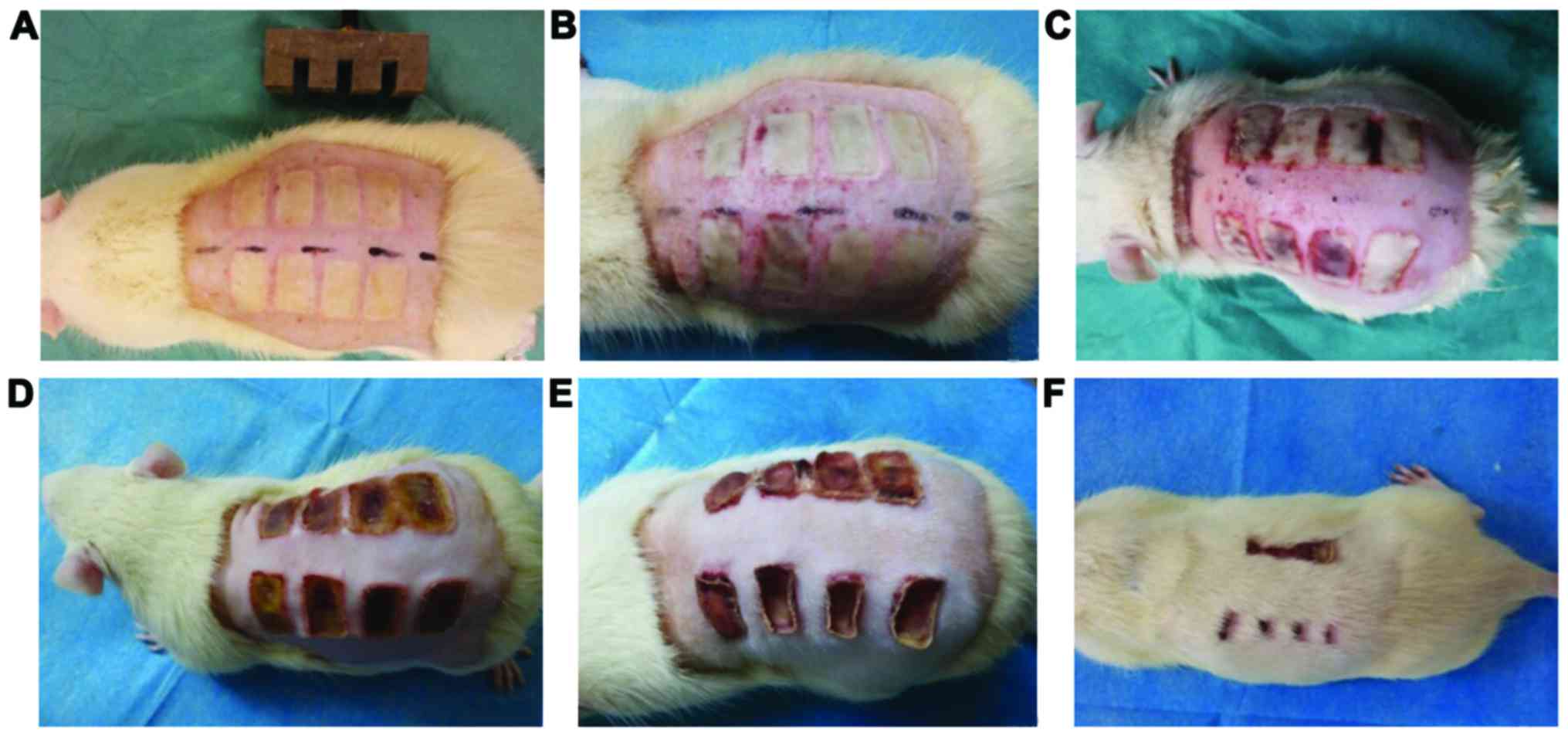
The burn model was established using a brass comb
with four prongs (1 × 2 cm) separated by three 5-mm notches, as
described by Regas and Ehrlich (13). Thus, four patches of burned skin and
three intervening spaces (0.5 × 2 cm) of unburned skin were created
and served as the coagulation zones and the stasis zones,
respectively.
These interspaces in the experimental and control
groups were treated with 100 µg/10 g rhGM-CSF hydrogel and hydrogel
without rhGM-CSF, respectively, once at 30 min after the burn and
once daily thereafter. The gels were applied at a thickness of 1 mm
and subsequently covered with a layer of vaseline and sterile gauze
that was properly fixed with adhesive tape. The scald models on
either side of the dorsal spines were symmetrically dressed
including the stasis zones of 5 × 20 mm. The left side stasis zones
served as the experimental group, and the right side stasis zones
served as the control group.
Macroscopic analysis
In total, there were 300 stasis zones in both groups
or 150 in each of the experimental and control groups. We observed
the macroscopic changes in the organizations of the stasis zones at
the experimental time-point of post-burn days 1, 3, 7, 14 and 21.
The macroscopic assessments involved the assessment of the
survival:necrosis ratios of the stasis zones (interspaces) of the
experimental and control groups.
Tissue specimen collection and
detection
Ten rats from both groups were taken on the 1st,
3rd, 7th,14th and 21st post-burn days. The stasis zones
(interspaces) were exactly excised from the experimental and
control groups following the application of chloral hydrate (10%)
anesthesia. These specimens were stored at −80°C and then subjected
to reverse transcription-polymerase chain reaction (RT-PCR) and
western blot detection. The other stasis zones of the experimental
group were exactly excised on the 3rd day, fixed in 4%
paraformaldehyde for 24 h, embedded in paraffin blocks, and sliced
into 4-micron sections for immunohistochemical detection. After the
specimens were excised, all rats were sacrificed under general
anesthesia.
PPARβ/δ mRNA expression test
RT-PCR analyses were used to examine PPARβ/δ mRNA
expression in both groups according to the following procedure.
Specimens were placed in a mortar and liquid nitrogen was added for
grinding. Total RNA was extracted from the stasis zone tissues with
TRIzol agent. Primer 3 software (Premier Biosoft, Palo Alto, CA,
USA) was used to design the primers, which were as follows: PPRAβ/δ
upstream primer, 5′-CCTTCTCCAAGCACATCTACAA-3′; downstream primer,
5′-TGATGAAGGGTGCGTTATGG-3′. Using a primer amplification procedure
involving a total RNA template and PCR amplification for RT-PCR
amplification, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was
measured relative to an internal control. An ABI Prism 7300
instrument with SDS software (Thermo Fisher Scientific, Waltham,
MA, USA) was used for data analysis. The ΔΔct method was used to
calculate the expression of genes and the efficiency of
silencing.
PPARβ/δ protein expression
detection
The specifications of the BCA working liquid and the
applied liquid protein of the cracking crack cells were collected
to determine the protein levels with the BCA method. Protein
samples were readily placed at −20°C. Twenty grams of protein per
lane were set aside and 60-V runs were performed with enrichment
glue following polypropylene acyl amine gel electrophoresis. The
transfer of the protein from a gel to a PVDF membrane was
performed. PPARβ was closed over a long body of resistance after
the PVDF membrane was treated with skimmed milk powder incubated at
4°C overnight. Horseradish peroxidase (HRP)-labeled sheep
anti-rabbit IgG antibodies (1:2,000; sc-2004; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were then added and the
membrane was incubated for 40 min. A Bio-Rad chemiluminescence
imager was used for development. Analysis of the grey values was
conducted with ImageJ software (National Institutes of Health,
Bethesda, MD, USA) and the grayscale coefficient ratio was then
calculated.
Statistical analysis
The data are expressed as the means and standard
deviations. The data from the control and the experimental groups
were analyzed using independent sample t-tests with the SPSS 17.0
statistical software (SPSS Inc., Chicago, IL, USA). P<0.05 were
regarded as statistically significant.
Results
Macroscopic assessment
On the 21st post-burn day, 87% of the stasis zone
was viable in the rhGM-CSF treatment group, and 14% of the stasis
zone was viable in the control group as shown in Fig. 1.
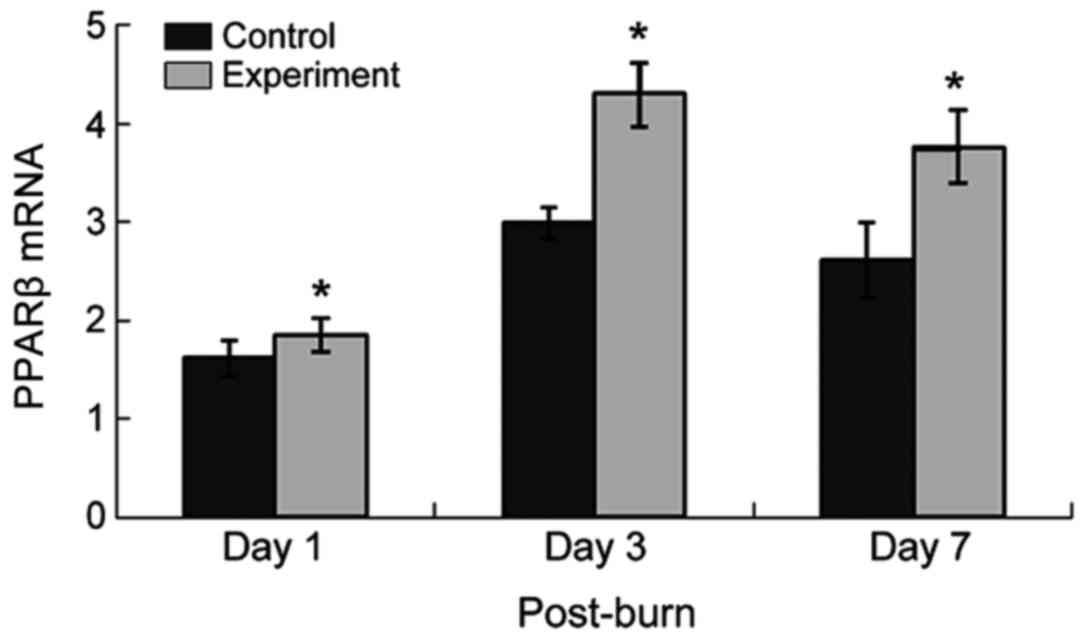
PPARβ mRNA expression
PPARβ expression increased on the 1st postburn day
in the stasis zones of both groups, peaked 3 days after thermal
injury, and subsequently decreased but remained elevated. In the
experimental group, PPARβ expression at every phase was increased
compared with the control group. Dry scabs had formed in some
stasis zones of the control group one week after the injury, as
shown in Fig. 2.
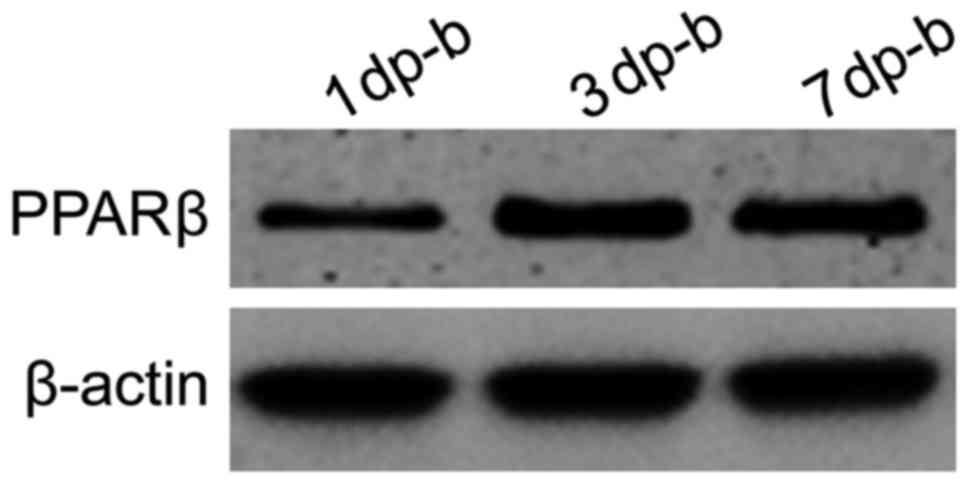
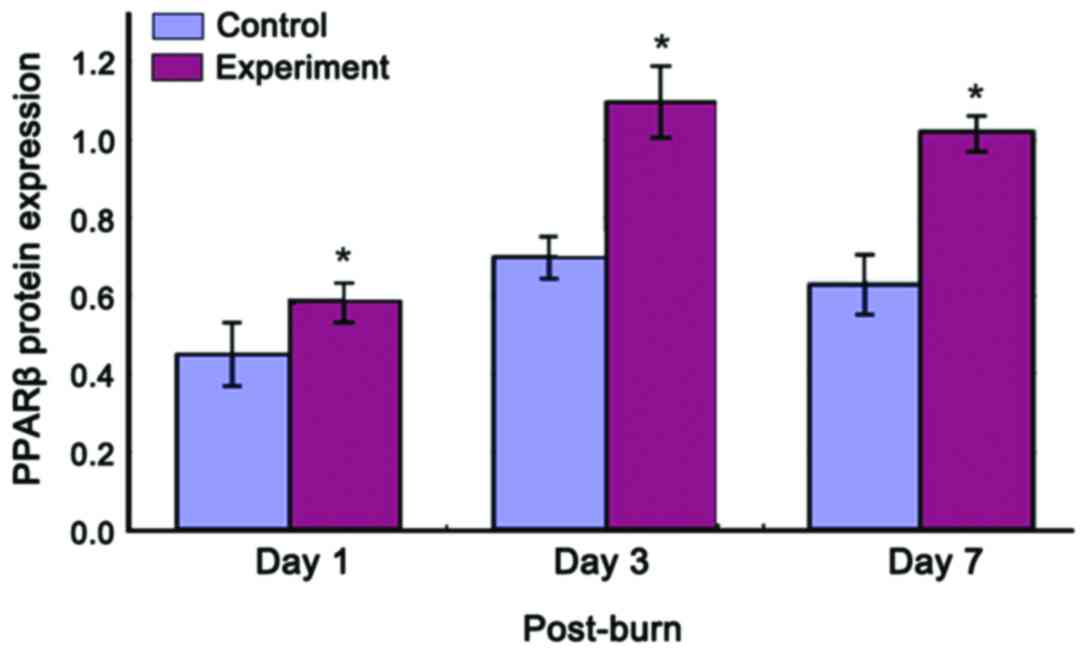
PPARβ protein expression and
localization
PPARβ protein expression increased in both groups
and then decreased following thermal injury. In the experimental
group, the protein expression increased beyond that of the control
group on the 1st post-burn day. The expression level peaked on the
3rd post-burn day and then gradually decreased, but the
experimental group still exhibited a high level of expression on
the 1st post-burn day (Figs. 3 and
4). PPARβ protein expression was
mainly localized to the nuclei of the fibroblasts, keratinocytes
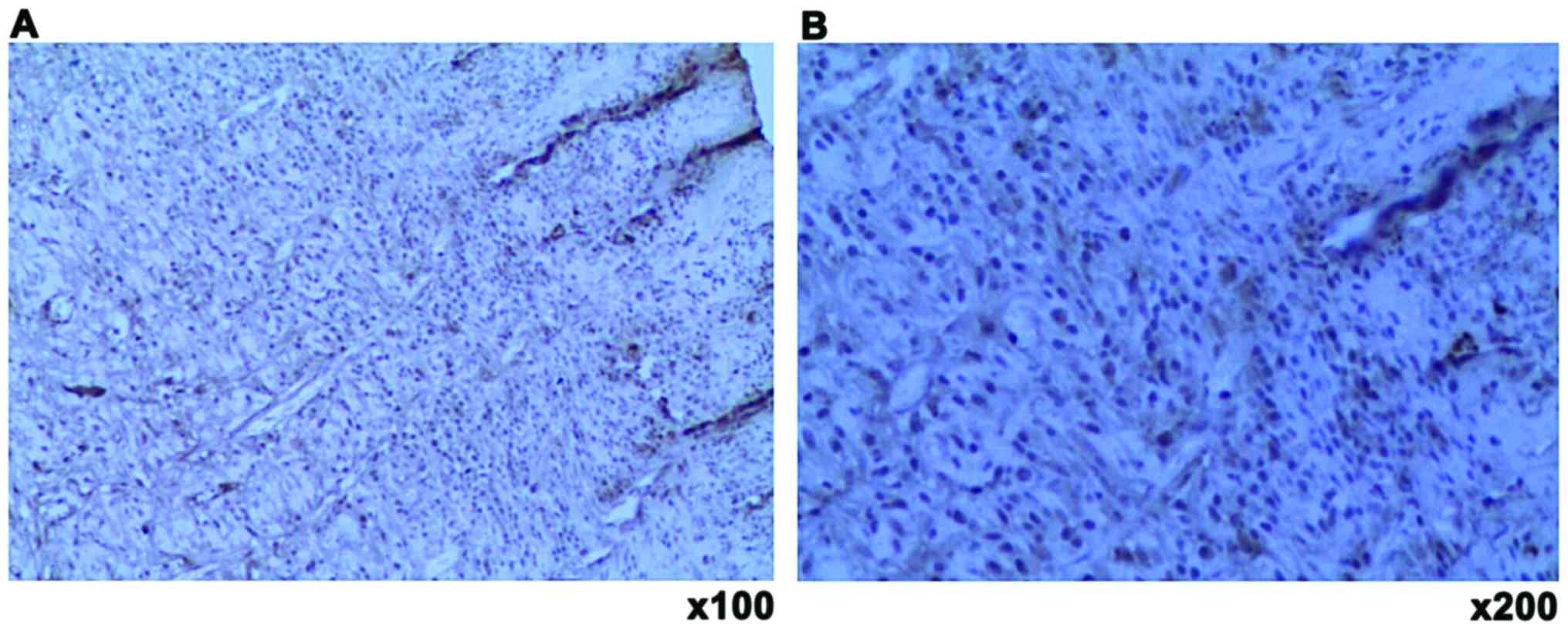
and endothelial cells in the stasis zones of both groups (Fig. 5).
Discussion
After cutaneous thermal injury, the stasis zone may
become necrotic and exhibit hypoperfusion, capillary
vasoconstriction, edema and ischemia, which contribute to the
progression of the overall burn extent or depth (1,14).
During this pathological process, some cytokines and growth
factors, including GM-CSF, other colony-stimulating factors and
interleukins, are synthesized and released by monocytes,
macrophages, keratinocytes, fibroblasts and neutrophils. These
factors can cause further inflammatory injuries but can also
regulate and promote keratinocyte, fibroblast and endothelial cell
proliferation and differentiation in thermally damage tissue, which
provide favorable conditions for preventing the formation of
progressive damage in the interspaces (11,12,14).
Because of these protective factors, the stasis zone has the
characteristic of reversibility. To be precise, there are damage
and protection factors that exist in burn wounds that limit
pathophysiological changes. In experimental studies and clinical
treatment, we attempt to adopt appropriate measures to maximize the
protective factors and minimize the damaging factors to prevent
progressive necrosis in the stasis zone.
As a type of specific glycoprotein, rhGM-CSF has a
variety of functions, such as boosting vaccine immunogenicity and
counteracting the bone marrow suppression caused by radiotherapy,
chemotherapy, stem cell transplantation and severe infections
(15). In recent years, research has
demonstrated that GM-CSF is synthesized and secreted by activated
neutrophils, monocytes/macrophages, keratinocytes, endothelial
cells, and fibroblasts and enhances keratinocyte, endothelial cell
and fibroblast migration and proliferation for wound healing
(16,17). Infection and excessive local
inflammation responses are often present in burn wounds (11). rhGM-CSF effectively improves wound
bacterial clearance rates, increases neutrophil surface adhesion
molecule expression, enhances neutrophil chemotaxis, reduces burn
wound infection rates and prevents necrobiosis in burn wounds to a
certain extent (18–20). Additionally, rhGM-CSF attracts
inflammatory cells, modulates inflammatory responses, and prevents
cascade reactions in burn wounds (11,15,20). In
the process of wound healing, neovascularization may provide oxygen
and nutrients for the inflammatory response and induce
keratinocytes, endothelial cells, and macrophages to secrete
endogenous growth factors that are involved in wound healing
(21). Studies have demonstrated
that rhGM-CSF induces microvascular endothelial cell proliferation,
differentiation, and increased expression of the growth factors
vascular endothelial growth factor (VEGF) and transforming growth
factor (TGF)-1β. rhGM-CSF also promotes the vascularization of the
wound (12). CD31 is activated in
vascular endothelial cells that express the characteristic symbol.
Liu et al (20) proposed that
rhGM-CSF induces CD31 expression in new blood vessel endothelial
cell surfaces, which further demonstrates that rhGM-CSF has a
positive effect on the promotion of angiogenesis. However, the
exact mechanism by which rhGM-CSF influences keratinocytes,
fibroblasts and endothelial cells in burn wound repair are
uncharacterized.
PPARs are important repair genes following thermal
damage and are activated by lipid ligands that bind with PPAR
response elements (PPREs) at loci that are members of the retinoid
X receptor (RXR) family of the steroid hormone receptor superfamily
and act as regulators of transcription. The application of GW0742
(a PPARβ activator) to heat injury-induced fibroblasts
significantly increases PPARβ expression, which subsequently
induces a protective effect by minimizing structural damage and
increasing the cell proliferation response (22). In burn wounds, the activation of
PPARβ inhibits apoptosis, stimulates the proliferation of
keratinocytes, and induces angiogenesis and these processes are
involved in the wound response and healing (23–25). On
the one hand, post-traumatic skin inflammation activates PPARβ
expression. On the other hand, this inflammatory factor also
triggers the production of endogenous PPARβ ligands, ultimately
inhibits cell apoptosis and induces keratinocyte proliferation and
angiogenesis. These processes are involved in the wound response
and the healing of the skin (24–26).
This mechanism may increase PPARβ activity in these cells and
subsequently upregulate the expression of integrin-linked kinase
and 3-phosphoinositide-dependent kinase-1, which phosphorylates
protein kinase B-α (Akt1) (26,27).
Akt1 is a major downstream effector of phosphoinositide 3-kinase
signaling. The resulting increased Akt1 activity suppresses
apoptosis and increases the likelihood of a sufficient number of
viable keratinocytes being present in the wound margin for
re-epithelialization and also increases matrix metalloproteinase-9
production by potentiating nuclear factor-κB activity, which
regulates keratinocyte migration. Additionally, the activation of
PPARβ also reduces the oxidative stress caused by cell apoptosis.
The mechanism of this process involves the induction of alpha
14-3-3 protein, which has anti-apoptotic and anti-inflammatory
effects that promote apoptotic protein degradation and that limit
the promotion of apoptosis due to the caspase cascade (24,28).
Furthermore, activated PPARβ promotes the proliferation of human
and murine vascular endothelial cells in vitro by increasing
VEGFR1 (Flt-1) expression and VEGF production (25,29).
The ‘comb burn’ model has been previously validated
and is widely used in investigations of the stasis zone. Previous
studies have examined the applications of antithrombotic,
anticoagulant, antioxidant and anti-inflammatory agents in
experimental models individually, and these agents exhibit
beneficial effects in terms of salvaging the stasis zone (3,5,6). Although tissues and cells secrete small
amounts of cytokines and growth factors after thermal damage, the
thermal effects of and pathological changes in these factors still
cause anoxic tissue ischemia conditions. Endogenous growth factors
are often not sufficient to continually stimulate and effectively
initiate the thermal damage repair procedure (30). After consulting relevant literature,
we chose the dressing scald model and applied external rhGM-CSF to
the thermally damaged skin. The survival rate in the experimental
group was significantly increased compared with the control group,
and PPARβ was expressed at a higher level in the experimental group
compared with the control group. Moreover, PPARβ expression was
mainly localized to the fibroblasts/endothelial cells and the
cuticular cell nuclei. rhGM-CSF may activate or regulate PPARβ
expression through a specific pathway early after thermal damage
and prevent the stasis zone from exhibiting further irreversible
necrosis. We hypothesize that PPARβ may play an important role in
this process.
Necrobiosis of the stasis zone was associated with
multiple factors. Thermal injury, ischemic reperfusion,
inflammatory reactions and oxidative free radical damage were among
the factors that were closely related to and promoted each other.
In the present study, we used an animal comb thermal model and
found that rhGM-CSF could preliminarily upregulate PPARβ gene and
protein expression via various pathways. In addition, rhGM-CSF had
a certain protective effect on the stasis zone. However, single
intervention factors obviously have certain limitations. We also
observed that not the entire stasis zone could be kept alive.
Therefore, in the process of deep burn treatment, the mechanisms by
which comprehensive interventions focused on the above factors
improve the local environment, prevent secondary injury and promote
benign outcomes in the stasis zone represent difficulties that are
still faced by medical workers dealing with burns.
References
|
1
|
Keck M, Herndon DH, Kamolz LP, Frey M and
Jeschke MG: Pathophysiology of burns. Wien Med Wochenschr.
159:327–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goertz O, Vogelpohl J, Jettkant B,
Daigeler A, Steinau HU, Steinstraesser L and Langer S: Burn model
for in vivo investigations of microcirculatory changes. Eplasty.
9:e132009.PubMed/NCBI
|
|
3
|
Nisanci M, Eski M, Sahin I, Ilgan S and
Isik S: Saving the zone of stasis in burns with activated protein
C: An experimental study in rats. Burns. 36:397–402. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jackson DM: The diagnosis of the depth of
burning. Br J Surg. 40:588–596. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zor F, Ozturk S, Deveci M, Karacalioglu O
and Sengezer M: Saving the zone of stasis: Is glutathione
effective? Burns. 31:972–976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eski M, Ozer F, Firat C, Alhan D, Arslan
N, Senturk T and Işik S: Cerium nitrate treatment prevents
progressive tissue necrosis in the zone of stasis following burn.
Burns. 38:283–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noratto G, Martino HS, Simbo S, Byrne D
and Mertens-Talcott SU: Consumption of polyphenol-rich peach and
plum juice prevents risk factors for obesity-related metabolic
disorders and cardiovascular disease in Zucker rats. J Nutr
Biochem. 26:633–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wagner KD and Wagner N: Peroxisome
proliferator-activated receptor beta/delta (PPARbeta/delta) acts as
regulator of metabolism linked to multiple cellular functions.
Pharmacol Ther. 125:423–435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao PL, Chen L, Dobrzański TP, Zhu B, Kang
BH, Müller R, Gonzalez FJ and Peters JM: Peroxisome
proliferator-activated receptor-β/δ inhibits human neuroblastoma
cell tumorigenesis by inducing p53- and SOX2-mediated cell
differentiation. Mol Carcinog. 56:1472–1483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akay R, Kamisli O, Kahraman A, Oner S and
Tecellioglu M: Evaluation of aqueductal CSF flow dynamics with
phase contrast cine MR imaging in idiopathic intracranial
hypertension patients: Preliminary results. Eur Rev Med Pharmacol
Sci. 19:3475–3479. 2015.PubMed/NCBI
|
|
11
|
Yan H, Chen J and Peng X: Recombinant
human granulocyte-macrophage colony-stimulating factor hydrogel
promotes healing of deep partial thickness burn wounds. Burns.
38:877–881. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rho CR, Park MY and Kang S: Effects of
granulocyte-macrophage colony-stimulating (GM-CSF) factor on
corneal epithelial cells in corneal wound healing model. PLoS One.
10:e01380202015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Regas FC and Ehrlich HP: Elucidating the
vascular response to burns with a new rat model. J Trauma.
32:557–563. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singer AJ, McClain SA, Taira BR, Guerriero
JL and Zong W: Apoptosis and necrosis in the ischemic zone adjacent
to third degree burns. Acad Emerg Med. 15:549–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan L, Minghua C, Feifei D, Runxiu W,
Ziqiang L, Chengyue M and Wenbo J: Study of the use of recombinant
human granulocyte-macrophage colony-stimulating factor hydrogel
externally to treat residual wounds of extensive deep
partial-thickness burn. Burns. 41:1086–1091. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith CH, Allen MH, Groves RW and Barker
JN: Effect of granulocyte macrophage-colony stimulating factor on
Langerhans cells in normal and healthy atopic subjects. Br J
Dermatol. 139:239–246. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Da Costa RM, Jesus Ribeiro FM, Aniceto C
and Mendes M: Randomized, double-blind, placebo-controlled,
dose-ranging study of granulocyte-macrophage colony stimulating
factor in patients with chronic venous leg ulcers. Wound Repair
Regen. 7:17–25. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Chen J and Han C: A multicenter
clinical trial of recombinant human GM-CSF hydrogel for the
treatment of deep second-degree burns. Wound Repair Regen.
17:685–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kobayashi SD, Braughton KR, Whitney AR,
Voyich JM, Schwan TG, Musser JM and DeLeo FR: Bacterial pathogens
modulate an apoptosis differentiation program in human neutrophils.
Proc Natl Acad Sci USA. 100:10948–10953. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JS, Fang Y, Yao M, Yu WR and Du J:
Effects of rhGM-CSF on scalding wound healing and
neovascularization in rats. J Bengbu Med Coll. 37:17–19. 2012.
|
|
21
|
Seegers HC, Hood VC, Kidd BL, Cruwys SC
and Walsh DA: Enhancement of angiogenesis by endogenous substance P
release and neurokinin-1 receptors during neurogenic inflammation.
J Pharmacol Exp Ther. 306:8–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zuo C, Liang P and Huang X: Role of
PPARbeta in fibroblast response to heat injury. Indian J Biochem
Biophys. 49:219–227. 2012.PubMed/NCBI
|
|
23
|
Tyagi S, Gupta P, Saini AS, Kaushal C and
Sharma S: The peroxisome proliferator-activated receptor: A family
of nuclear receptors role in various diseases. J Adv Pharm Technol
Res. 2:236–240. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Di-Poï N, Michalik L, Tan NS, Desvergne B
and Wahli W: The anti-apoptotic role of PPARbeta contributes to
efficient skin wound healing. J Steroid Biochem Mol Biol.
85:257–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Piqueras L, Reynolds AR, Hodivala-Dilke
KM, Alfranca A, Redondo JM, Hatae T, Tanabe T, Warner TD and
Bishop-Bailey D: Activation of PPARbeta/delta induces endothelial
cell proliferation and angiogenesis. Arterioscler Thromb Vasc Biol.
27:63–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Attianese Giordano GM and Desvergne B:
Integrative and systemic approaches for evaluating PPARβ/δ (PPARD)
function. Nucl Recept Signal. 13:e0012015.PubMed/NCBI
|
|
27
|
Di-Poï N, Tan NS, Michalik L, Wahli W and
Desvergne B: Antiapoptotic role of PPARbeta in keratinocytes via
transcriptional control of the Akt1 signaling pathway. Mol Cell.
10:721–733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liou JY, Lee S, Ghelani D,
Matijevic-Aleksic N and Wu KK: Protection of endothelial survival
by peroxisome proliferator-activated receptor-delta mediated 14-3-3
upregulation. Arterioscler Thromb Vasc Biol. 26:1481–1487. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bishop-Bailey D: PPARs and angiogenesis.
Biochem Soc Trans. 39:1601–1605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gravante G, Palmieri MB, Esposito G,
Delogu D, Santeusanio G, Filingeri V and Montone A: Apoptotic cells
are present in ischemic zones of deep partial-thickness burns. J
Burn Care Res. 27:688–693. 2006. View Article : Google Scholar : PubMed/NCBI
|