Introduction
Cyclosporine A (CsA) is a commonly used
immunosuppressive agent that specifically targets T cells and
inhibits the secretion of interleukin-2, and dramatically increases
the success rate of liver, kidney and cornea transplantations, as
well as the survival rate of recipients (1–3).
However, immunosuppression induced by long-term CsA use
significantly increases the risk of cardiovascular diseases,
infection and malignant tumors (4).
Eid et al (5) reported that
the concentration and motility of sperm were negatively correlated
with the concentration of serum CsA in male kidney transplant
recipients, suggesting that CsA may induce dysfunction of the
reproductive system in male recipients. Furthermore, it has been
documented that CsA may be responsible for impaired spermatogenesis
and damage to the male reproductive system (6).
Previous studies have demonstrated that CsA
decreased the levels of serum testosterone by influencing
testosterone biosynthesis and secretion in the testes. He et
al (7) observed that rats
treated with 40 mg/kg/day CsA exhibited significantly higher levels
of luteinizing hormone (LH) and significantly lower levels of
testosterone in the serum, leading to impaired testicular
development. Ali et al (8)
reported that treatment with CsA decreased the expression of LH
receptor (LHR) on the membrane of Leydig cells, which subsequently
decreased LH-mediated testosterone synthesis and secretion from
Leydig cells. Another study by Seethalakshmi et al (9) reported that CsA decreased testosterone
levels by inhibiting the activity of 17 alpha-hydroxylase
uncompetitively and 17β-hydroxydteroid dehydrogenase activity
competitively during testosterone biosynthesis, and the primary
site for CsA inhibition was interrupted cyclic adenosine
monophosphate stimulation. Furthermore, this report indicated that
decreased testosterone secretion induced by CsA in Leydig cells
directly influenced development of the male reproductive system and
maintenance of reproductive ability. Seethalakshmi et al
(10) injected exogenous
testosterone into CsA-treated rats to increase endogenous
testosterone levels. It was observed that the elevated doses of
exogenous testosterone significantly restored reproductive organ
weight, serum levels of follicle-stimulating hormone (FSH) and
increased the germ cell count, thus indicating that CsA-induced
impairment of spermatogenesis may be partially prevented by the
exogenous administration of testosterone (10). However, exogenous testosterone was
found to be insufficient in repairing damaged testicular tissue, as
testosterone secretion and spermatogenesis were still influenced by
CsA, and high rates of apoptosis were observed in sperm and
spermatogenic cells (10).
Wenshen Shengjing Decoction (WSSJD) consists of 15
herbal medicines, primarily including Cornu Cervi Nippon
Parvum, Panax ginseng, Cynomorium songaricum,
Cistanche deserticola, Radix Astragali, Epimedium
brevicornum and Angelica sinensis (11). WSSJD has been demonstrated to
alleviate cyclophosphamide-induced spermatogenic apoptosis
(11). To further improve the
therapeutic effect of WSSJD, the present study developed a new
WSSJD based on the mechanism of CsA-induced testicular tissue
damage by adjusting the dosage of the WSSJD components. Modern
pharmacological studies have demonstrated that antler velvet in
WSSJD may function as a sex hormone (12); Radix Astragali may improve the
proliferation and nutrient supply of testicular sertoli cells
(13); and Panax ginseng may
decrease testicular hyperoxide levels and inhibit spermatogenic
apoptosis (14). The above-mentioned
medicines may also increase kidney function and boost the
development of spermatogenic cells (12–14).
Clomifene citrate (CC) has been demonstrated to exert therapeutics
effect on male infertility in previous clinical studies (15,16).
Furthermore, CC has been associated with improved testosterone and
semen parameters, and the protective mechanisms of CC may be
similar to those of WSSJD (15,16). In
addition, in a study by Wang et al (17), CC was used as a treatment control to
investigate the effect of the Chinese medicine Shengjing on
spermatogenesis disturbances in mice. Based on this, CC was used as
a positive control in the present study.
In the present study, the mechanism underlying the
protective effect of new WSSJD in testicular tissues was
investigated to determine the therapeutic efficacy of the modified
medicine. Elucidating the protective mechanism may provide a basis
for the use of new WSSJD in ameliorating CsA-induced spermatogenic
damage.
Materials and methods
Animals
A total of 90 male Kunming mice (8-weeks-old; 35–40
g) were provided by the Changchun Institute of Biological Products
Co., Ltd. (Changchun, China). The present study was conducted with
approval from the Ethics Committee of Jilin Medical University
(Changchun, China). Mice were housed at 21±3°C and 55–65% relative
humidity under a 12 h dark-light cycle. Mice were given free access
to a standard laboratory mouse diet and sterile water.
Preparation of WSSJD
The 15 Chinese medicine components of WSSJD,
including Cornu Cervi Nippon Parvum, Panax ginseng,
Cynomorium songaricum, Cistanche deserticola,
Radix Astragali, Epimedium brevicornum and
Angelica sinensis (11), were
purchased from Tongrentang (Beijing, China). These materials were
decocted according to the traditional method of Chinese medicinal
decoction (18). In brief, the
ingredients were weighed according to the recipe, and a total of
250 ml distilled water was added to submerge the herbs. Herbs were
soaked for 30 min and subsequently heated. The herbs were first
boiled for 10 min and then the heat was reduced to a simmer for 20
min. Following 30 min of heating, the liquid decoction was
separated from the herbs, which were further decocted with 200 ml
distilled water at 80°C for another 30 min. This process was
repeated and the final volume of decoction liquid was filtered
through 4–5 layers of gauze. Liquid was also squeezed from the
herbs into the filtered decoction. The decoction was subsequently
heated at 80°C in a water bath for 6–7 h until the concentration
reached 2 g crude drug/ml, and the decoction was stored at 4°C
prior to use. new WSSJD was prepared based on the formulation of
WSSJD with minor adjustments to the dosages of Panax
ginseng, Cynomorium songaricum, Radix Astragali
and Epimedium brevicornum. The decocting method was the same
as that of WSSJD. new WSSJD comprised of the following: Panax
ginseng, 6 g; Cynomorium songaricum, 9 g; Radix
Astragali, 12 g; Epimedium brevicornum, 6 g; Cornu
Cervi Nippon Parvum, 1 g; Cistanche deserticola, 9 g;
Angelica sinensis, 6 g; Flatstem Milkvetch Seed, 9 g;
Rhizoma Dioscoreae, 15 g; Largehead Atractylodes
Rhizome, 6 g; Ligusticum wallichii, 3 g; Radix
Paeoniae Alba, 6 g; Cinnamomum cassia, 1 g;
Costustoot, 1.5 g and Fructus Foeniculi, 3 g.
The concentrations of crude drug extracts were
determined as follows: Weight of the crude material/final volume.
This calculation method is widely used in the study of traditional
Chinese medicine (19–21).
Drug administration
Mice were randomly divided into 6 groups (15 mice
per group) and were administrated with medicine intragastrically.
The groups were as follows: Control (normal saline);
dimethylsulfoxide (DMSO); CsA; CC; WSSJD; and new WSSJD. Mice in
the CsA, CC, WSSJD and new WSSJD groups were intraperitoneally
(i.p.) injected with 15 mg/kg/day CsA (Shanxi Powerdone
Pharmaceutics Co., Ltd., Datong, China) for 30 days, as described
previously (8). Mice in the control
and DMSO groups underwent a daily i.p. injection with an equal
volume of normal saline or DMSO solvent, respectively, throughout
the 30-day experimental period. The concentration of DMSO diluted
in normal saline was 3.25% (v/v). The CC group was administered
with 21.6 mg/kg/day CC (GKH Pharmaceutical, Ltd., Guangzhou, China)
as described previously (13), which
was also diluted in 3.25% (v/v) DMSO (pH=7.2). Mice in the WSSJD
and new WSSJD groups were administered with 12 g crude drug/kg/day
of WSSJD and new WSSJD, respectively.
Mouse euthanasia
At the end of the experimental period (at day 31),
mice were placed in sealed cages, which were subsequently infused
with 10–30% CO2. When mice ceased to move for 5 min, it
was confirmed that mice had succumbed to CO2
exposure.
Hematoxylin and eosin (H&E)
staining
Mice testes were harvested and immediately fixed in
4% paraformaldehyde (pH=7.2) at room temperature for 24 h, followed
by ethanol dehydration, xylene treatment to remove the ethanol, wax
embedding and sectioning. Histological sections (5 µm) were
obtained using a rotary microtome. These were affixed to glass
slides for H&E staining. Testicular sections were observed
using highlight histopathological microscopy to evaluate the
development of seminiferous tubules and obtain histometric data.
The development of seminiferous tubules was assessed using the
Johnsen scoring system (22).
ELISA
On day 30 of treatment, the mice were anesthetized
with 10% chloral hydrate (Shanghai Guoyao Chemical Reagent Co.,
Ltd) at 0.004 ml/g of body weight i.p. prior to euthanasia with
CO2. Trunk blood was harvested and the serum was
separated and stored at −20°C. Briefly, blood was collected into
1.5-ml tubes and stored at 4°C overnight. The tube was then
centrifuged at 1,300 × g at 4°C for 6 min and the serum was
collected. ELISA kits (Shanghai Elisa Biotech Co., Ltd., Shanghai,
China) were used to determine the serum contents of testosterone
(cat. no. EIA-2380) and LH (cat. no. EIA-2385) according to the
manufacturer's protocol. The optical density (OD) was determined
using a Model 680 microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), and the levels of testosterone and LH were
determined based on the standard curve.
Immunohistochemistry
Testicular tissues were fixed in 4% formaldehyde at
room temperature for 24 h and embedded in paraffin. Paraffin
sections were cut into 5 µm sections and were subsequently dewaxed.
Antigen retrieval was achieved by incubating sections in 0.01 M
citrate buffer (pH=6.0) at 95–98°C for 5 min. The sections were
subsequently blocked with 5% bovine serum albumin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany; A3675) at room temperature for 1 h.
Following blocking, sections were incubated with rabbit polyclonal
antibodies against LHR (cat. no. L6792; Sigma-Aldrich; Merck KGaA;
1:200) or P450 side chain cleavage (P450scc; cat. no. ab75497;
Abcam, Cambridge, UK; 1:200) at 4°C overnight in the dark. The
streptavidin-biotin complex (SABC) method was used to detect the
expression of LHR and P450scc using an SABC kit (SA2010; Boster
Biological Technology, Pleasanton, CA, USA), according to the
manufacturer's protocol. For the tissues isolated from negative
control mice, the primary antibody was replaced with PBS. Leydig
cells with yellow or brown staining on the membrane or plasma were
considered to be LHR-positive cells. Five slides were obtained from
each sample and five fields in the Leydig tissue areas were
selected at random and assessed under a fluorescence microscope
(magnification, ×400). The mean OD of positive cells in the Leydig
tissue areas was obtained using Image Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA). The expressions of LHR and
P450scc were proportional to the OD values, with higher OD values
representing higher protein expressions.
Terminal dexynucleotidyl transferase
(TdT)-mediated dUTP nick-end labeling (TUNEL) assay
Mice testes were dissected, embedded in wax and
sectioned. The sections (5-µm thick) were then stained using the
TUNEL assay kit (MK1024; Boster Biological Technology), according
to the manufacturer's protocol. In brief, testicular tissues form
control (normal saline), DMSO, CsA, CC, WSSJD and new WSSJD groups
were stained using a 1:100 dilution of biotin labeled digoxin
antibody for 30 min at 37°C. Stained rat interstitial epithelial
tissue (provided in the kit) was used as a positive control, and
samples incubated with PBS instead of biotin labeled digoxin
antibody were used as a negative control. The frequency of
TUNEL-positive cells exhibiting green nuclear staining was
evaluated using a laser scanning confocal microscope. A total of 10
random fields were assessed under high-magnification (×400) and
positive cells were counted. The mean number of positive cells per
field was calculated.
Propidium iodide (PI) or Annexin
V-fluorescein isothiocyanate (FITC) staining and flow cytometry
analysis
The cauda epididymidis was harvested following
sacrifice and the epidermis was cut with a blade to release the
sperm from the epididymis into 2 ml PBS at 37°C to obtain
epididymis suspensions. The suspensions were subsequently incubated
at 37°C for 10 min to allow sperm to swim out. Aggregations of
sperm were discarded and the remaining sperm samples were isolated
and resuspended in PBS to give a concentration of 106
cells/ml. Sperm apoptosis was analyzed using a Annexin V-FITC
Apoptosis Detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China), according to the manufacturer's protocol. In brief, 1 ml of
sperm suspension was stained with 10 µl PI or with 500 µl binding
buffer and 5 µl Annexin V-FITC in the dark for 10 min at room
temperature. Cells were immediately analyzed by Epies XL flow
cytometry (Beckman Coulter, Inc.) and using a TetraONE™
System (6915050; Beckman Coulter, Inc.). Cells in the early stages
of apoptosis were identified by Annexin V-positive staining and
necrotic cells were identified by PI-positive staining.
Statistical analysis
Data analysis was performed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA) and expressed as the mean + or ±
standard deviation as indicated. Differences between groups were
evaluated for significance using one-way analysis of variance
followed by a Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of new WSSJD on the
development of testicular seminiferous tubules
To investigate the potential protective effect of
new WSSJD on the development of mouse testicular seminiferous
tubules, the morphology of H&E-stained mouse testes were
compared under light microscopy. The seminiferous tubules of mice
from the CsA and CC groups exhibited shrinkage of the tubule
fringe, decreased tubule diameters and reduced layers of testicular
seminiferous epithelium compared with the control. In addition,
spermatogenic cells exhibited a disordered arrangement, the number
of spermatogenic cells was reduced and few mature sperm were
visible in the lumen (Fig. 1). In
mice from the DMSO, WSSJD and new WSSJD groups, more layers of
testicular seminiferous epithelia were visible and the seminiferous
tubule fringe was integrated without shrinkage or collapse
(Fig. 1). The Johnsen scores did not
differ significantly between the DMSO group and control groups, or
between the CC and DMSO groups. However, the Johnsen score was
significantly decreased in the CsA group and WSSJD group compared
with the control group, and the scores of the WSSJD and new WSSJD
groups were significantly increased compared with the CC or CsA
group (P<0.05; Table I). These
results indicated that new WSSJD and WSSJD promoted the development
of seminiferous epithelium following CsA treatment.
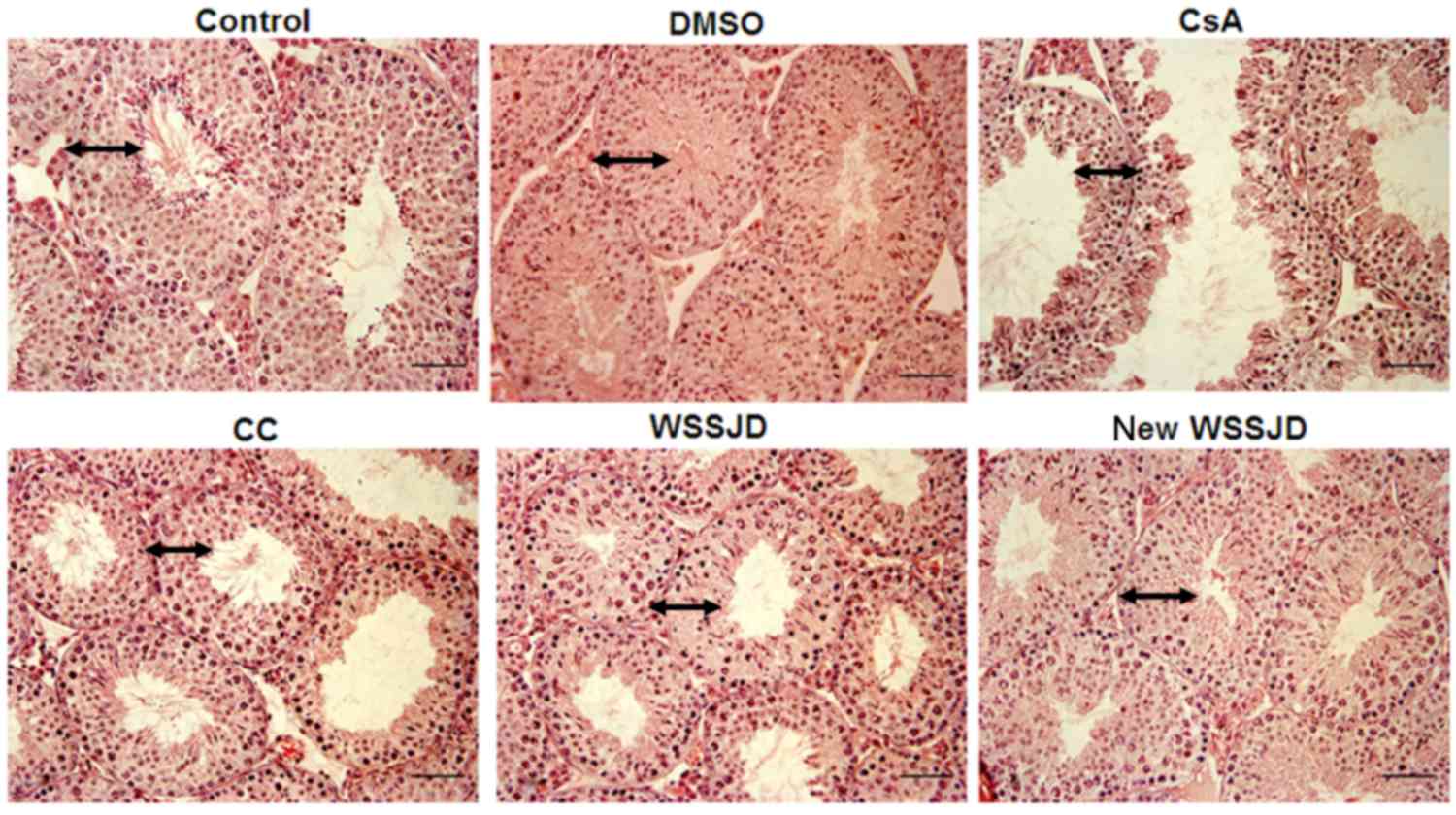 | Figure 1.Hematoxylin and eosin staining of
mouse testicular tissue. Mice were administered with CsA, followed
by CC, WSSJD, new WSSJD or DMSO. Untreated mice were used as a
negative control. The testes were harvested 30 days later and
stained with hematoxylin and eosin. CsA, cyclosporine A; CC,
clomifene citrate; WSSJD, Wenshen Shengjing Decoction; DMSO,
dimethylsulfoxide. The length of arrows represented the thickness
of spermatogenic tubule, and the ratio of their thickness was
1.4:1.4:1.2:1.22:1.3:1.4 in control, DMSO, CsA, WSSJD and new WSSJD
group, respectively. Magnification, ×200; Scale bar, 100 µm. |
 | Table I.Effect of new WSSJD on Johnsen
scoring of the testes. |
Table I.
Effect of new WSSJD on Johnsen
scoring of the testes.
| Groups | Johnsen score
(≤10) |
|---|
| Control |
9.75±0.25 |
| DMSO |
9.50±0.25 |
| CsA |
7.25±0.43a |
| CC |
7.33±0.29a |
| WSSJD |
9.00±0.25a–c |
| New WSSJD |
9.25±0.25b,c |
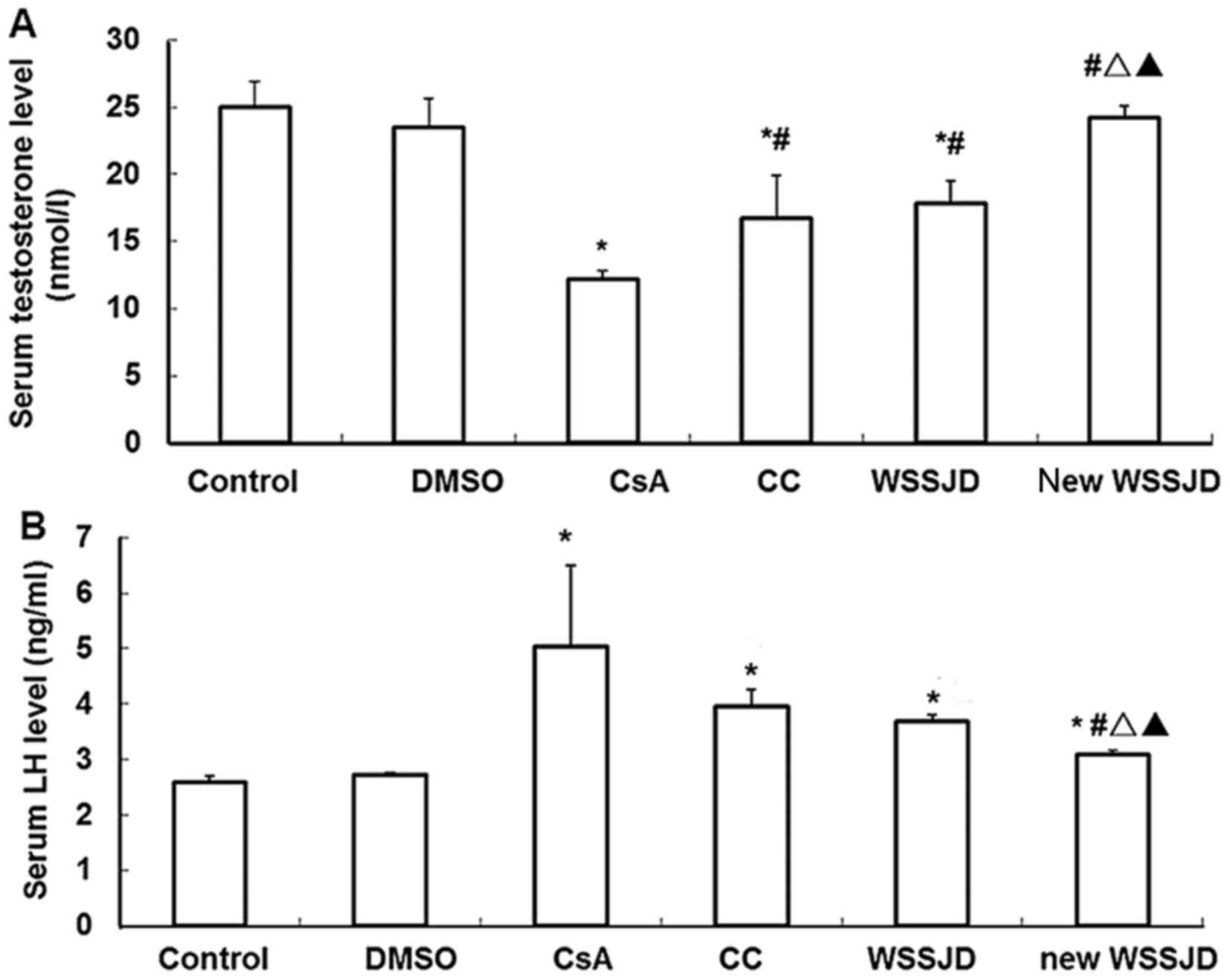
Effects of new WSSJD on serum
testosterone and LH
The levels of testosterone and LH in the serum were
subsequently measured. Serum levels of testosterone and LH were
unaffected following DMSO administration. By contrast, serum
testosterone was significantly downregulated and LH was
significantly upregulated in CsA-treated mice, relative to controls
(P<0.05; Fig. 2), and new WSSJD
administration significantly restored testosterone to near control
levels (P<0.05; Fig. 2). For
serum LH, the protective effects of WSSJD was similar to that of
CC; however, new WSSJD decreased serum testosterone to a
significantly lower level than that observed with CC and WSSJD
(P<0.05; Fig. 2), suggesting a
superior protective effect of new WSSJD over CC or WSSJD.
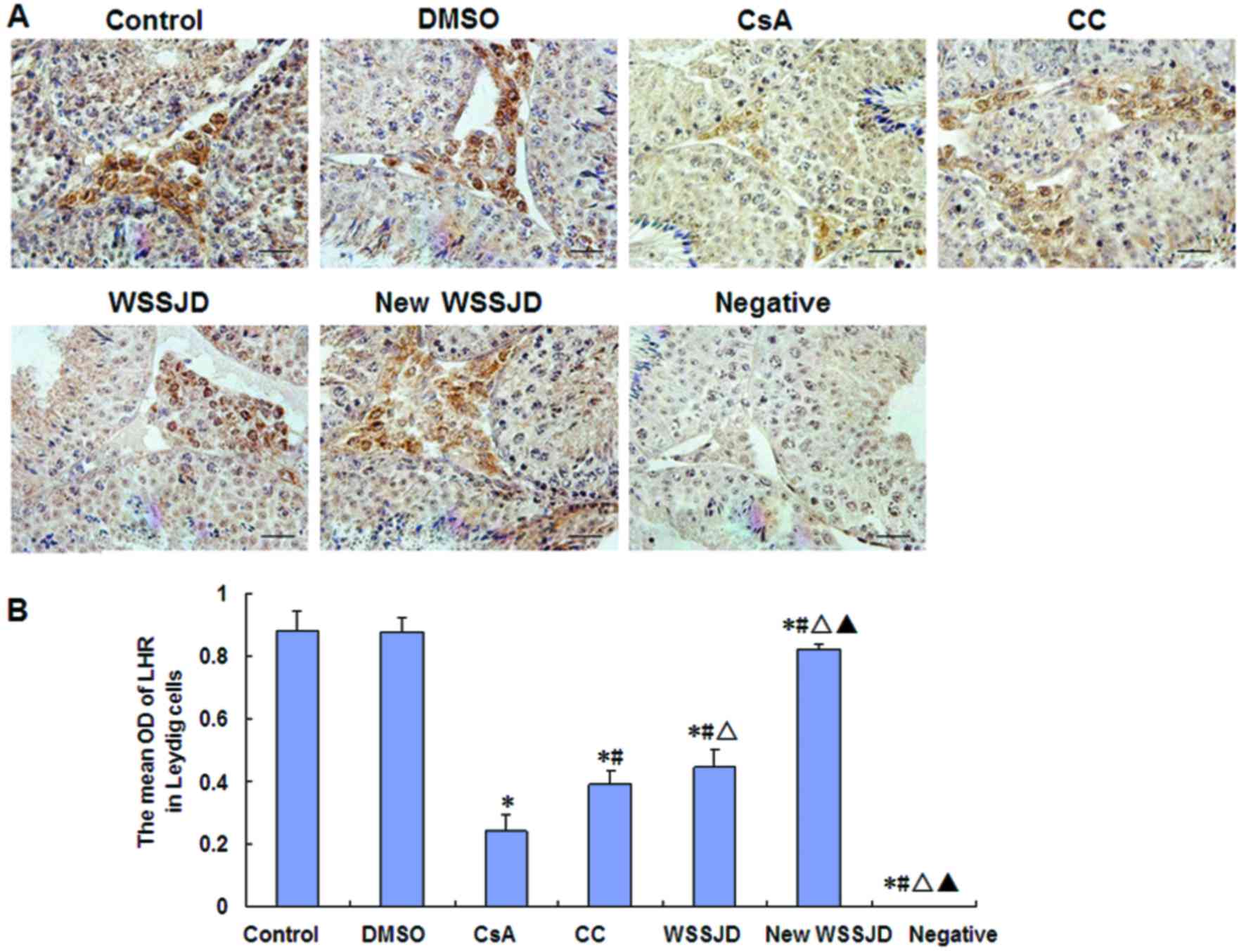
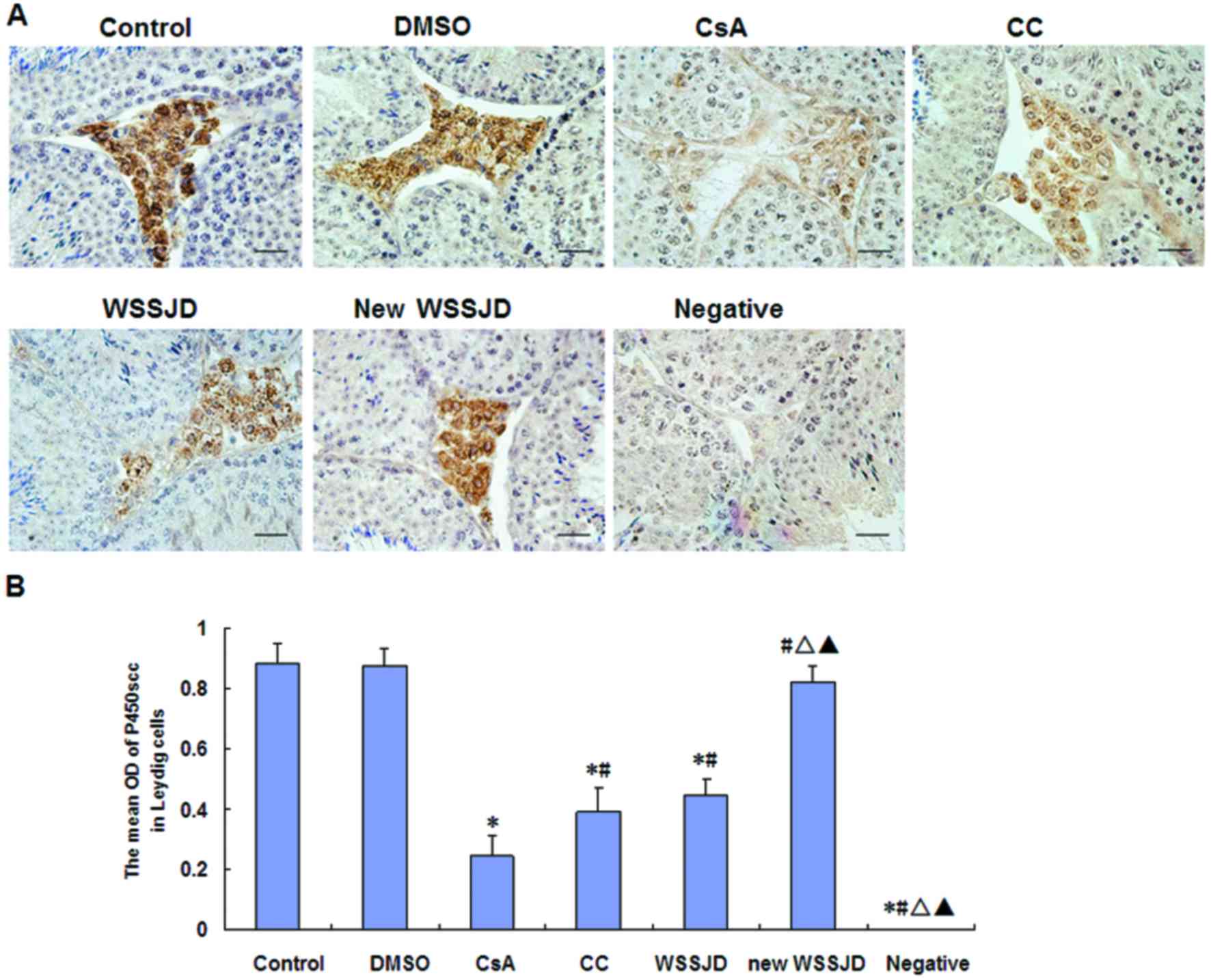
Effects of new WSSJD on the
expressions of LHR and P450scc
The expressions of P450scc and LHR were assessed in
testicular Leydig cells by immunohistochemistry (Figs. 3 and 4). In the control group, LHR was expressed
on the outer membranes of Leydig cells between the seminiferous
tubules (Fig. 3A), and P450cc was
expressed in the cytoplasm of testicular Leydig cells (Fig. 4A). DMSO treatment had no significant
effect on the expressions of LHR and P450scc. By contrast, CsA
treatment significantly decreased the expressions of LHR and
P450scc compared with control mice (P<0.05; Figs. 3B and 4B). In turn, the expressions of LHR and
P450scc were significantly increased by treatment with CC, WSSJD or
new WSSJD compared with the CsA group (P<0.05; Figs. 3B and 4B). In addition, levels of LHR and P450scc
in testicular Leydig cells were significantly higher in WSSJD and
new WSSJD mice compared with CC mice (P<0.05; Figs. 3B and 4B), and new WSSJD induced a significantly
greater upregulation than WSSJD (P<0.05; Figs. 3B and 4B).
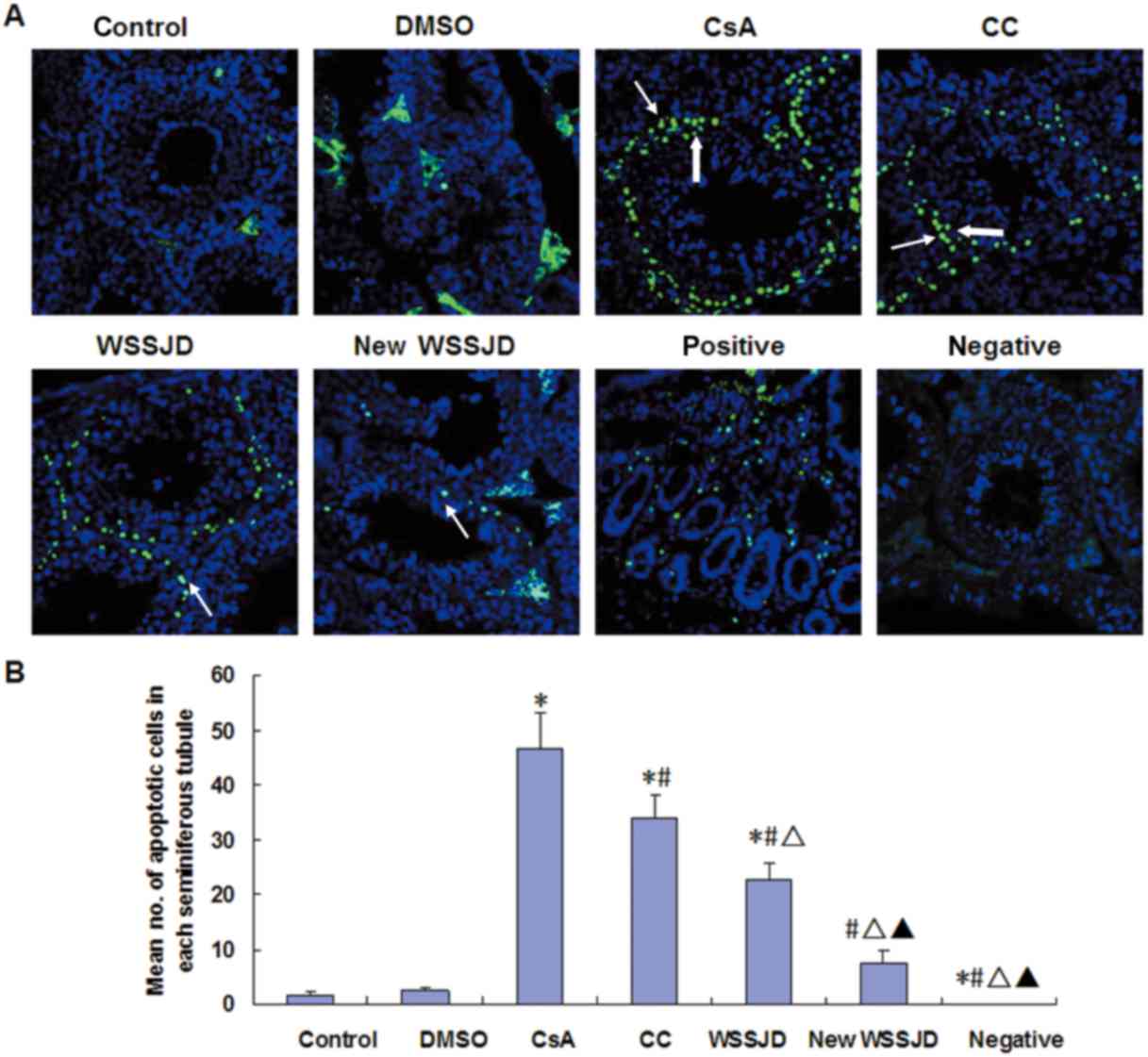
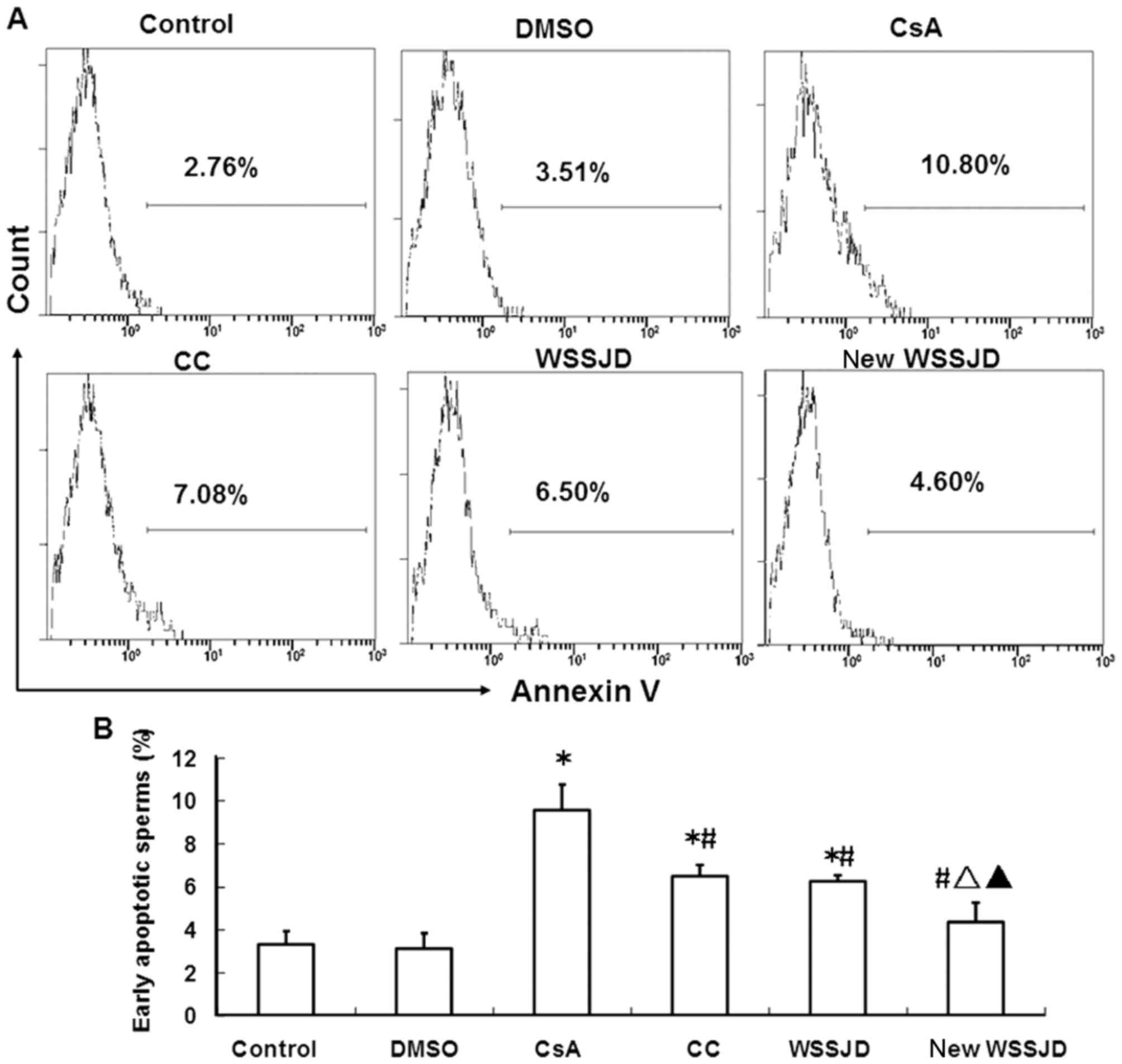
Effect of new WSSJD on spermatogenic
cell apoptosis
The apoptosis of spermatogenic cells in the mouse
testes was analyzed by a TUNEL assay. The nuclei of apoptotic cells
were principally observed in the spermatogonia and primary
spermatocytes (Fig. 5A).
Spermatogonia are larger than spermatocytes and are located closer
to the basement membrane (23). DMSO
treatment had no significant effect on the number of apoptotic
spermatogenic cells in the testes. By contrast, treatment with CsA
significantly increased the number of apoptotic spermatogenic cells
compared with the control and DMSO groups (P<0.05; Fig. 5B). In turn, administration of CC,
WSSJD or new WSSJD significantly reduced CsA-induced apoptosis
(P<0.05; Fig. 5B). Furthermore,
the number of apoptotic testicular spermatogenic cells in the WSSJD
and new WSSJD groups was significantly reduced compared with the CC
group (P<0.05), and new WSSJD was significantly more effective
than WSSJD (P<0.05; Fig. 5B).
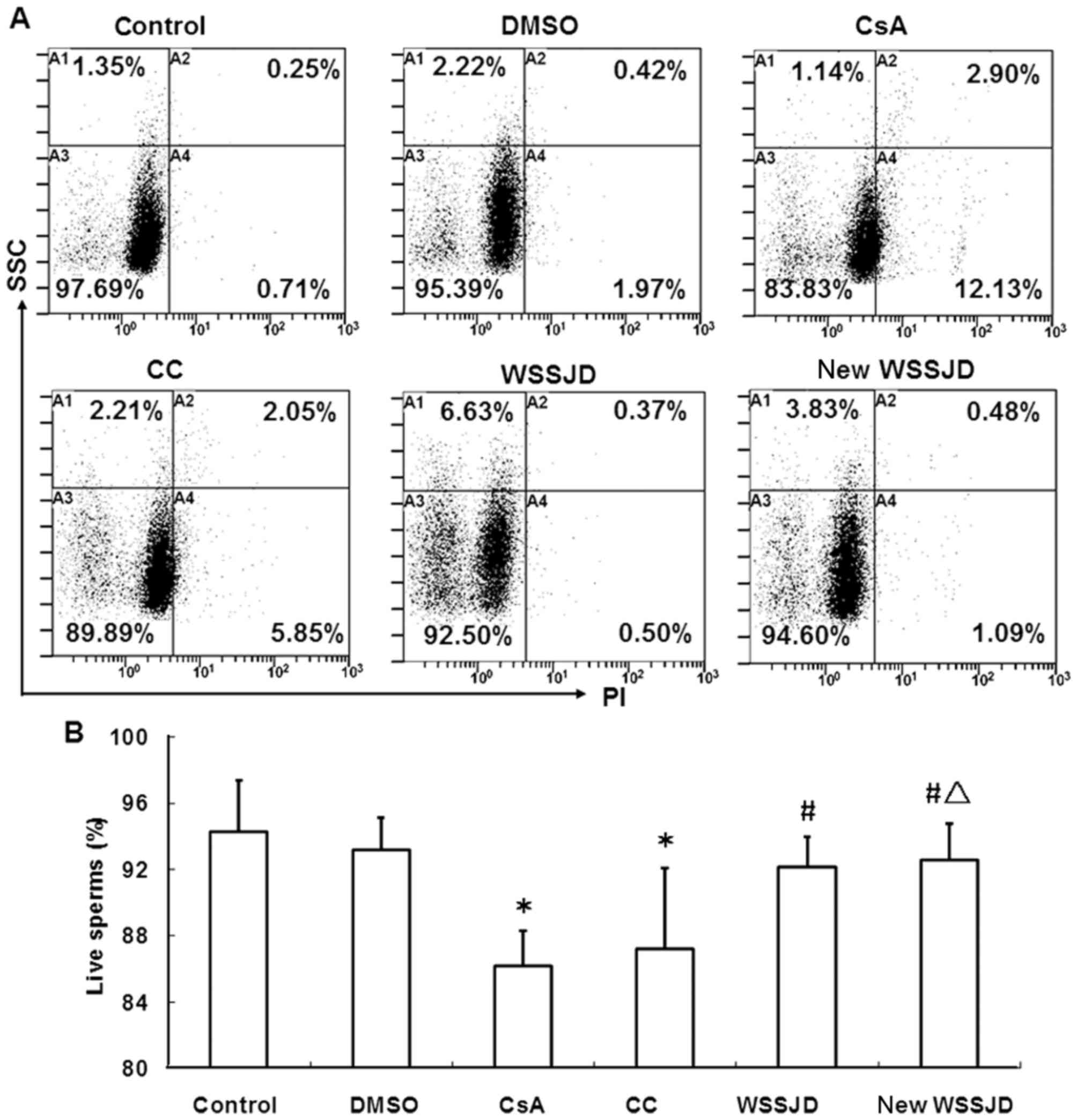
Effects of new WSSJD on the survival
and early apoptosis of sperm
To verify the protective effects of new WSSJD
against CsA-induced sperm apoptosis, the survival and early
apoptosis of sperm in the epididymis were determined. Epididymal
sperm were stained with PI or Annexin V and analyzed by flow
cytometry (Figs. 6 and 7). In accordance with the aforementioned
results, CsA treatment significantly reduced the percentage of live
sperm and significantly increased the percentage of early apoptotic
sperm (P<0.05; Figs. 6B and
7B), while DMSO treatment had no
effect. WSSJD and new WSSJD significantly increased the percentage
of live sperm (P<0.05; Fig. 6B)
and reduced the percentage of early apoptosis sperm (P<0.05;
Fig. 7B) compared with CsA
treatment. The percentages of live and early apoptotic sperm in
WSSJD and CC mice did not differ significantly, while new WSSJD
induced a significantly greater increase in live sperm percentage
compared with the CC group (P<0.05; Fig. 6B) and significantly decreased the
percentage of early apoptotic sperm compared with the CC and WSSJD
groups (P<0.05; Fig. 7B).
Discussion
It has previously been documented that long-term use
of CsA as an immunosuppressive agent affects reproductive capacity
(6). Xu (24) reported that the morphology and
vitality of sperm in patients treated with CsA were significantly
lower than that in an untreated group, as observed in the semen
samples of 26 renal transplant recipients treated with various
doses of CsA and 12 healthy volunteers. It was also observed that
head deformity rates of sperm were significantly higher in
CsA-treated recipients (24). These
results suggested that CsA had a dose-dependent effect on semen
parameters. Therefore, studies are warranted to identify novel
pharmacological agents capable of alleviating CsA-induced
testicular damage and improving male reproductive ability following
organ transplantation.
The specific microenvironment of the testis promotes
spermatogenesis, and thus impairment of testicular structure and
function may lead to spermatogenic arrest (25). Monteiro et al (26) treated Wistar rats with CsA at a dose
of 15 mg/kg per day for 56 days, and observed an increased
volumetric proportion of connective tissue and decreased volumetric
proportion of Leydig cells in CsA-treated rats. It was also
observed that CsA caused seminiferous epithelium degeneration,
resulting in Sertoli cell vacuolization, abnormal round and
elongated spermatids and the accumulation of residual cytoplasm at
the epithelium border adjacent to the lumen (26). In the present study, Kunming mice
were treated with 15 mg/kg CsA daily for 30 days, which lead to
shrinkage and decreased diameters of the seminiferous tubules,
reduction of the seminiferous epithelium layers, disordered
arrangement of the seminiferous cells, a decrease in mature sperm
in the lumen and a significantly decreased Johnsen score. In
addition, treatment with CsA treatment severely damaged the
testicular structure. The present study also evaluated the
protective effects of new WSSJD, as a novel Chinese medicine, on
the testes of CsA-treated mice. It was observed that the testicular
seminiferous epithelia layers and arrangement of the seminiferous
cells were restored following treatment with new WSSJD. In
addition, mice in the new WSSJD group exhibited integrated
seminiferous tubules without shrinkage or collapse, and had a
significantly higher Johnsen score compared with CsA-treated mice.
These results suggested that WSSJD significantly repaired
CsA-induced testicular damage, indicating that this herbal medicine
compound may be an effective treatment in the prevention of
CsA-induced testicular damage. Whether or not this cell repair is
dependent on the niche within the epithelium is also an important
question, which should be investigated in future studies.
The hypothalamic-pituitary-testicular axis serves an
important role in the regulation of genital activity (27). The development and functionality of
the male genital organs are regulated by hormones from the
hypothalamus and pituitary glands (28). Krueger et al (29) treated Sprague Dawley rats with 25
mg/kg/day CsA or 40 mg/kg/day CsA for 6 days and observed that
serum levels of LH and FSH increased by 2–4 fold, while P450scc
expression decreased to 30% of that in the control group. In
addition, serum testosterone levels were significantly decreased in
CsA-treated mice, resulting in impaired spermatogenesis (29). In the present study, it was
demonstrated that CsA treatment significantly decreased the
expression of LHR in Leydig cells. Although serum LH was increased,
decreased expression of LHR may have impaired LH-mediated recovery
of testosterone biosynthesis and decreased the expression of
P450scc, as the rate-limiting enzyme of testosterone biosynthesis
(30), thus affecting testosterone
biosynthesis by Leydig cells. The novel ingredients in new WSSJD,
such as pilose antler, exhibit effects similar to sex hormones
(12), which may improve serum
testosterone levels and decrease serum LH levels, thus increasing
LHR and p450scc expression in Leydig cells. Notably, the present
results indicated that new WSSJD stimulated testosterone
biosynthesis and secretion in Leydig cells to promote
spermatogenesis.
CsA-induced oxidative stress and testis damage
induce the dysplasia of sperm and spermatogenic cells (31). It has been reported that long-term
CsA treatment damages the antioxidant system in animal testicular
tissues, leading to decreased levels of glutathione, glutathione
peroxidase and hydrogen peroxide levels and increased levels of
malonic dialdehyde in the testes (31). Therefore, excessive levels of
reactive oxygen species in the testicular tissues cannot be
eliminated, leading to the peroxidation of sperm membrane lipids,
DNA damage and decreased sperm vitality. In the present study, it
was observed that CsA treatment significantly increased DNA
breakage and the apoptotic rate of spermatogenic cells in
seminiferous tubules. The survival rate of sperm in the epididymis
also decreased, while the percentage of early apoptotic sperm was
increased, indicating that the spermatogenic activity of the testes
was significantly damaged. Türk et al (32) documented that the protective effect
of ellagic acid on CsA-induced testicular damage was associated
with oxidative stress in male rats. The new WSSJD used in the
present study contains various antioxidant components, including
the herbal medicine Ginseng, which has previously been demonstrated
to significantly decrease levels of hyperoxide in the testes
(14).
In the present study, the effects of new WSSJD on
CsA-induced impairment of testosterone synthesis and spermatogenic
apoptosis were investigated, and it was observed that new WSSJD
significantly decreased the apoptotic rates of spermatogenic cells
and sperm, and thus repaired damage to the testicular seminiferous
epithelium. The morphology of testicular seminiferous tubules and
the apoptosis of spermatogenic cells and sperm were investigated
using histochemistry and flow cytometry. Although cell cycle
distribution and daily sperm production were not investigated, they
will be the focus of future studies by our group.
In conclusion, compared with traditional WSSJD, new
WSSJD significantly increased testosterone levels in the testes and
decreased the apoptosis of spermatogenic cells and sperm, which
effectively repaired CsA-induced testicular damage. These results
indicate that new WSSJD may be a useful pharmacological agent in
the treatment and prevention of CsA-induced testicular damage.
Acknowledgements
The present study was supported by the Key Research
Project of the Scientific and Technological Development Program of
Jilin Province (grant no. 20140204033YY), the College Science and
Technology Program of Shandong Province (grant no. J13LL04) and the
Undergraduate Training Programs for Innovation and Entrepreneurship
of Jilin Province (grant no. 2014024).
References
|
1
|
Pedersen M and Seetharam A: Infections
after orthotopic liver transplantation. J Clin Exp Hepatol.
4:347–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vora GK and Ciolino JB: Corneal allograft
reaction associated with nonocular inflammation. Digit J
Ophthalmol. 20:29–31. 2014.PubMed/NCBI
|
|
3
|
De Pasquale C, Veroux M, Indelicato L,
Sinagra N, Giaquinta A, Fornaro M, Veroux P and Pistorio ML:
Psychopathological aspects of kidney transplantation: Efficacy of a
multidisciplinary team. World J Transplant. 4:267–275. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Gowelli HM and El-Mas MM: Central
modulation of cyclosporine-induced hypertension. Naunyn
Schmiedebergs Arch Pharmacol. 388:351–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eid MM, Abdel-Hamid IA, Sobh MA and
el-Saied MA: Assessment of sperm motion characteristics in
infertile renal transplant recipients using computerized analysis.
Int J Androl. 19:338–344. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zahra A, Gholamreza N, Farokhi F and
Shalizar Jalali A: Attenuation of cyclosporine-induced sperm
impairment and embryotoxicity by crataegus monogyna fruit aqueous
extract. Cell J. 15:198–205. 2013.PubMed/NCBI
|
|
7
|
He Z, Qiu J, Li J, Zhao D, Chen G and Chen
L: Long-term effects of conversion from cyclosporine to rapamycin
on testicular function and morphology in a rat transplantation
model. Transplant Proc. 45:pp. 763–769. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ali RB, Klouz A, Boubaker S, Lakhal M and
Belkahia C: An animal model of testicular toxicity by cyclosporine:
Evaluation and protection. Fundam Clin Pharmacol. 23:241–246. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seethalakshmi L, Flores C, Malhotra RK,
Pallias JD, Tharakan D, Khauli RB and Menon M: The mechanism of
cyclosporine's action in the inhibition of testosterone
biosynthesis by rat Leydig cells in vitro. Transplantation.
53:190–195. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seethalakshmi L, Flores C, Carboni AA and
Menon M: Quantitative maintenance of spermatogenesis in
cyclosporine-treated rats by exogenous administration of
testosterone propionate. J Androl. 11:491–497. 1990.PubMed/NCBI
|
|
11
|
Pan XY, Wang XY, Wang XN, Sun ZX, Wang D,
Wang X, Yang YY and Li ZX: Effects of ‘Wenshen Shengjing Decoction’
on cyclophosphamide induced spermatogenic cell apoptosis and
histone H3K9 dimethylation. Shanghai Zhong Yi Yao Za Zhi. 48:82–86.
2014.(In Chinese).
|
|
12
|
Xu ZH, Li SF, Wang JY, Zhou R and Tian SJ:
Extraction of sex hormone from antler velvet with supercritical
CO2. Zhongguo Zhong Yao Za Zhi. 32:2000–2003. 2007.(In Chinese).
PubMed/NCBI
|
|
13
|
Zhao LP, Xu Z, Zhang M, Sun HC and Tang F:
Effects of Fructus Lycii and Radix astragali on the function of
sertoli cells in rat testes. Zhonghua Nan Ke Xue. 13:82–86.
2007.(In Chinese). PubMed/NCBI
|
|
14
|
Xu L, Liu XH and Zhang L: Effects of total
ginsenosides on the NO NOS and total antioxygen capacity in the
testis of mouse. Acta Academiae Medicinae CPAPF. 5:507–508.
2006.(In Chinese).
|
|
15
|
Patel DP, Brant WO, Myers JB, Presson AP,
Johnstone EB, Dorais JA, Aston KI, Carrell DT and Hotaling JM: The
safety and efficacy of clomiphene citrate in hypoandrogenic and
subfertile men. Int J Impot Res. 27:221–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
ElSheikh MG, Hosny MB, Elshenoufy A,
Elghamrawi H, Fayad A and Abdelrahman S: Combination of vitamin E
and clomiphene citrate in treating patients with idiopathic
oligoasthenozoospermia: A prospective, randomized trial. Andrology.
3:864–867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang R, Xu L, Zhang WX, Wu YF, Shi JH and
Zhang YX: Shengjing Granule: An Effective Chinese Medicine for
Spermatogenic Disturbance in Mice. Zhonghua Nan Ke Xue.
14:1046–1049. 2008.(In Chinese). PubMed/NCBI
|
|
18
|
Fan BT: Chinese Medicine Pharmacy.
Shanghai Science and Technology Press; 12. pp. 62–68. 1997, (In
Chinese).
|
|
19
|
Zhang J, Li H, Lu L, Yan L, Yang X, Shi Z
and Li D: The Yiqi and Yangyin Formula ameliorates injury to the
hematopoietic system induced by total body irradiation. J Radiat
Res. 58:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minami M, Konishi T, Jiang Z, Arai T and
Makino T: Effect of Shin'iseihaito on murine allergic reaction
induced by nasal sensitization. J Tradit Complement Med. 6:252–256.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rong R, Li RR, Hou YB, Li J, Ding JX,
Zhang CB and Yang Y: Mahuang-xixin-fuzi decoction reduces the
infection of influenza a virus in kidney-yang deficiency syndrome
mice. J Ethnopharmacol. 192:217–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Johnsen SG: Testicular biopsy score
count-a method for registration of spermatogenesis in human testes:
Normal values and results in 335 hypogonadal males. Hormones.
1:2–25. 1970.PubMed/NCBI
|
|
23
|
Mays-Hoopes LL, Bolen J, Riggs AD and
Singer-Sam J: Preparation of spermatogonia, spermatocytes, and
round spermatids for analysis of gene expression using
fluorescence-activated cell sorting. Biol Reprod. 53:1003–1011.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu LG: Influence of cyclosporin A on the
semen parameters of the patients after renal transplantation. J
Clin Urol. 20:603–605. 2005.
|
|
25
|
Pintus E, Ros-Santaella JL and Garde JJ:
Beyond testis size: Links between spermatogenesis and sperm traits
in a seasonal breeding mammal. PLoS One. 10:e01392402015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Monteiro JC, Predes FS, Matta SL and
Dolder H: Heteropterys aphrodisiaca infusion reduces the collateral
effects of cyclosporine A on the testis. Anat Rec (Hoboken).
291:809–817. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oseko F, Note S, Morikawa K, Endo J,
Taniguchi A and Imura H: Influence of chronic hyperprolactinemia
induced by sulpiride on the hypothalamo-pituitary-testicular axis
in normal men. Fertil Steril. 44:106–111. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wisniewski P, Romano RM, Kizys MM,
Oliveira KC, Kasamatsu T, Giannocco G, Chiamolera MI, Dias-da-Silva
MR and Romano MA: Adult exposure to bisphenol A (BPA) in Wistar
rats reduces sperm quality with disruption of the
hypothalamic-pituitary-testicular axis. Toxicology. 329:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krueger BA, Trakshel GM, Sluss PM and
Maines MD: Cyclosporin-mediated depression of luteinizing hormone
receptors and heme biosynthesis in rat testes: A possible mechanism
for decrease in serum testosterone. Endocrinology. 129:2647–2654.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nolan CJ and Payne AH: Genotype at the
P450scc locus determines differences in the amount of P450scc
protein and maximal testosterone production in mouse Leydig cells.
Mol Endocrinol. 4:1459–1464. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Türk G, Ateşşahin A, Sönmez M, Yüce A and
Ceribaşi AO: Lycopene protects against cyclosporine A-induced
testicular toxicity in rats. Theriogenology. 67:778–785. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Türk G, Sönmez M, Ceribaşi AO, Yüce A and
Ateşşahin A: Attenuation of cyclosporine A-induced testicular and
spermatozoal damages associated with oxidative stress by ellagic
acid. Int Immunopharmacol. 10:177–182. 2010. View Article : Google Scholar : PubMed/NCBI
|