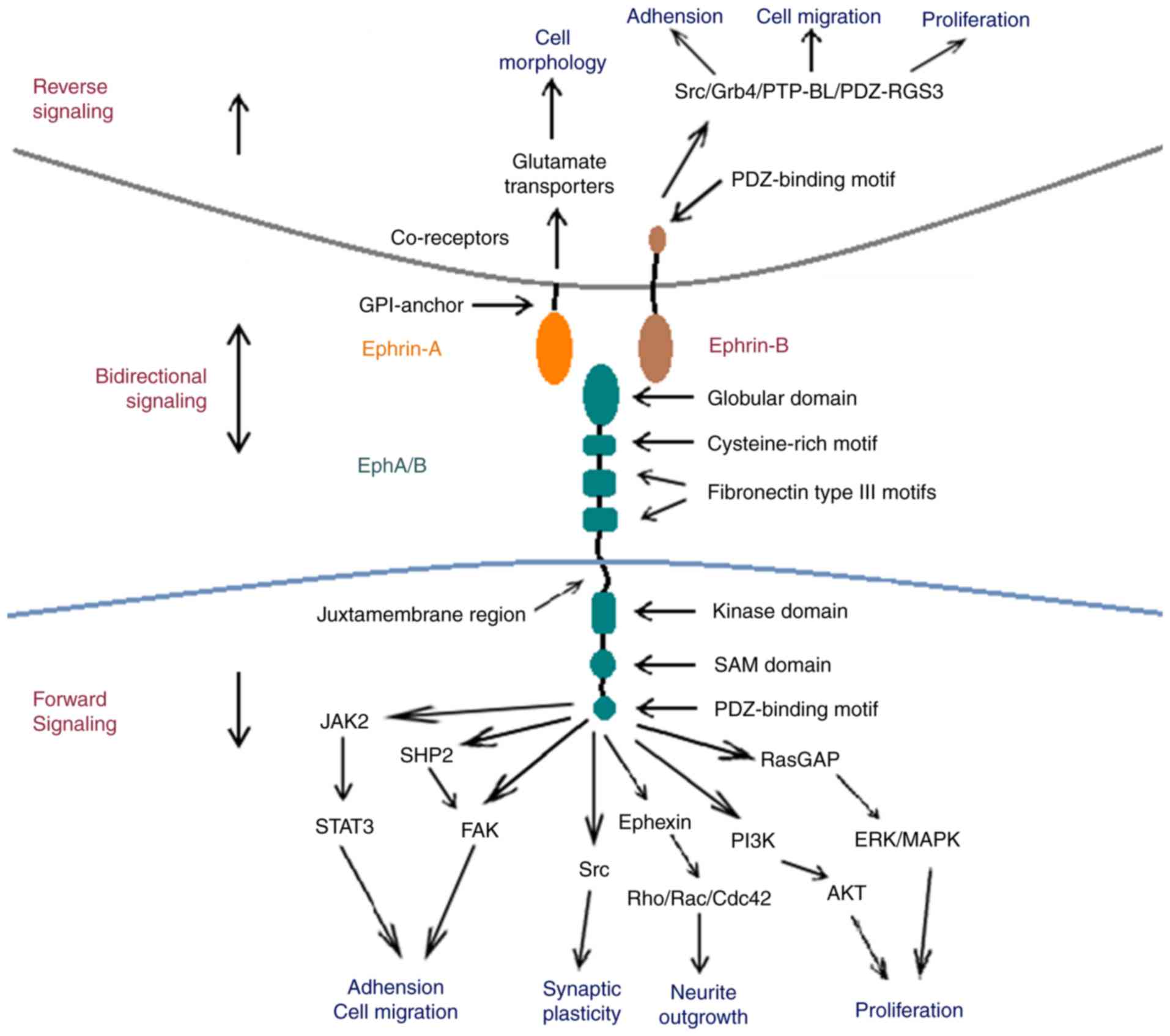
Erythropoietin-producing human hepatocellular (Eph)
proteins constitute the largest known receptor tyrosine kinase
family, and the first identified was the EphA1 receptor in 1987
(1). The Eph receptor family
comprises 14 members in humans and other mammals and is divided
into two subfamilies based on sequence conservation and ligand
binding affinity: EphA (EphA1-EphA8 and EphA10) and EphB
(EphB1-EphB4 and EphB6) (2). Eph
receptors are activated when bound to membrane-combined ephrin
ligands. A total of nine EphA receptors preferentially bind to five
glycosylphosphatidylinositol-anchored ephrin-A ligands
(ephrin-A1-A5). In addition, five EphB receptors possess
high-affinity binding domains to three transmembrane ephrin-B
(ephrin-B1-B3) ligands. EphA4 and EphB2 are exceptions, which can
bind to both A-type and most B-type ligands (2). The formation of the Eph/ephrin complex
initiates bidirectional signaling, which acts upon Eph-expressing
and ephrin-expressing cells. Signaling pathways that are directly
initiated by Eph receptors and ephrin ligand activations are termed
forward signaling and reverse signaling, respectively (3). Previous studies have reported the
diverse roles of Eph/ephrin bidirectional signaling in pathological
and physiological processes (4–7).
Eph receptors exhibit similar structural
characteristics, despite the large number of subtypes. The
extracellular region of Eph receptors is primarily comprised of a
highly-conserved N-terminal globular domain, which is essential for
ephrin identification and binding (8). Following on from the globular domain,
the Eph extracellular region also includes one unique cysteine-rich
and two fibronectin type III motifs, which affect receptor
dimerization (8,9). The intracellular region of Eph contains
four structural and functional units: A juxtamembrane region, a
conserved kinase domain, a sterile-a-motif (SAM) domain and a
PSD95/Dlg/ZO1 (PDZ)-binding motif. The juxtamembrane region is a
highly conserved motif containing two tyrosine residues, which are
the primary autophosphorylation sites for downstream signal
transduction (10,11). The conserved tyrosine kinase domain
is involved in the binding and activating of small guanosine
5′-triphosphate (GTP)ases, which are important for the regulation
of the cytoskeletal structure (12).
The SAM domain is located in the carboxyl-terminal tail of Eph
receptors. It is a conserved region containing 60–70 amino acids,
which regulates receptor dimerization and initiates downstream
signal transduction (13,14). The postsynaptic density of the
protein zona occludens PDZ-binding domain is critical for the
assembly and localization of the Eph/ephrin complex (15).
Ephrin ligands are divided into two subfamilies
based on sequence conservation and their respective affinities for
Eph receptors; ephrin-A and ephrin-B. Ephrin-A ligands
(ephrin-A1-A5) possess a GPI-anchor that attaches ephrin-A to the
membrane at the carboxyl terminal. Ephrin-B ligands (ephrin-B1-B3)
possess a single transmembrane domain and a highly conserved
carboxyl terminal tail, containing five tyrosine phosphorylation
sites and a carboxyl-terminal PDZ-domain binding motif that is the
structural basis for downstream signal transduction (4,16).
A unique feature of the Eph/ephrin complex is that
it initiates bidirectional signaling following its formation; the
Eph receptor may act as a ligand and the ephrin ligand may act as a
receptor (17). Forward and reverse
signaling is involved in numerous physiological processes,
including cell migration, axonal outgrowth, axonal pathfinding,
topographic mapping, axon fasciculation and vascular formation in
the developing nervous system (18,19).
The initiation of Eph/ephrin bidirectional signaling
requires the formation of highly clustered Eph/ephrin complexes.
Previous studies have demonstrated that recombinant soluble ephrin
must be pretreated to form clusters and induce Eph receptor
phosphorylation and downstream signaling (20,21).
Soluble monomeric ephrins act as Eph receptor antagonists instead
of Eph receptor agonists (22,23).
Similarly, reverse signaling through ephrin ligand requires
interactions with clustered Eph receptors (24,25). The
blocking functions of soluble monomeric ephrin or Eph extracellular
domain (ECD) may therefore provide a potential tool for the
manipulation of bidirectional signaling (26).
Eph forward signaling induced by ephrin binding
initiates downstream signal transduction following the
autophosphorylation of two conserved tyrosine residues in the
juxtamembrane region (27).
Downstream pathways of Eph forward signaling have been studied
extensively (15,17,27–36). Eph
receptor-mediated forward signaling modulates the dynamic
rearrangement of the cytoskeleton and is involved in cellular
remodeling, serving a role in certain regenerative processes,
including neurite outgrowth and cell migration (37). Previous studies have demonstrated
that Eph receptors are highly specific to Rac, cell division
control protein 42 (Cdc42), Rho and small GTPases, which are
critical for the regulation of the actin cytoskeleton (37,38). Eph
receptor forward signaling inhibits axonal regeneration in neurons
by stimulating growth cone collapse through Rac and Cdc42 (37,39–41). In
contrast, the blocking of Eph receptors stimulates the activation
of downstream Rac and Cdc42, promoting axonal outgrowth (42). Therefore, EphA receptor signaling may
also provide repulsive guidance for growing axons via the
activation of Rho. It has been demonstrated that Rac and Cdc42
activation promote axonal outgrowth in the absence of Eph forward
signaling (31,42,43).
Notably, EphB1/EphB2/EphB3 triple knockout mice had long, thin and
immature neural spines compared with wild-type mice, suggesting
that ephrin-B/EphB signaling promotes spine formation and
maturation (44). Ephexin is a novel
member of the diffuse B cell lymphoma-like family of guanine
nucleotide exchange factors. It functions to link EphA4 receptors
to Rho GTPases, which serve vital roles in axon guidance (31,43). It
has been demonstrated that ephrin-A3 acts via EphA4 to suppress
Wnt/β-catenin signaling to inhibit the neurogenic potential of
retinal stem cells (45). Eph
forward signaling may also be involved in the mitogen-activated
protein kinase, phosphoinositide 3-kinase (PI3K) and Janus
kinase/signal transducer and activator of transcription (STAT)
pathways (46–48).
Ephrin signal conduction into its host cell is
defined as reverse signaling. Previous studies have revealed that
ephrin reverse signaling is involved in neural progenitor
proliferation (49), axon guidance
(50), neuronal migration (51) and synaptic plasticity (50). However, the intracellular signaling
cascades that are initiated following ephrin activation remain
unknown. Ephrin-As lack a cytoplasmic tail; however, they are
capable of activating downstream Src family kinases (SFKs) and PI3K
with the aid of co-receptors (52,53). It
was demonstrated that associated transmembrane signaling partners,
including topomyosin receptor kinase B and p75 neurotrophin
receptor, may act as co-receptors for ephrin-As (54).
Ephrin-B ligands are composed of a single
transmembrane region and a short, highly conserved cytoplasmic
domain with a carboxy-terminal PDZ domain-binding motif. Together,
these constitute the structural foundation required for reverse
signaling (55). The activation of
ephrin-B ligands leads to the recruitment of SFKs, which
phosphorylate tyrosine residues located in the cytoplasmic domain.
Previous research has revealed that
Src-homology-2-domain-containing adaptor molecules, such as Grb4,
are recruited and phosphorylated by ephrin-B, which further
initiates downstream signaling and regulates cytoskeletal dynamics
(56–60). The basophil-like protein tyrosine
phosphatase is also recruited via its PDZ domain to the
carboxy-terminal tail of ephrin-B, leading to its dephosphorylation
and the inactivation of SFKs. This inactivation acts as a switch
from phosphotyrosine-dependent to PDZ-domain-dependent signaling
(61). PDZ regulation of G-protein
signaling 3 may inhibit C-X-C chemokine receptor type 4-mediated
chemoattraction by inhibiting the Gαβγ-protein complex, which
further regulates the migration of endothelial cells and
angiogenesis (62,63) (Fig.
1).
Previous studies have assessed the role Eph in the
developing CNS. The expression of Eph receptors and ephrin ligands
changes markedly during CNS development (19). Ephs and ephrins continue to be
expressed in the adult CNS and are distributed in most regions and
types of cell (6). Various Eph
receptors and ephrins continue to be highly expressed in adult
brain regions that possess morphological and physiological
plasticity, including the amygdala and hippocampus (64).
Previous studies have elucidated the diverse roles
of Eph receptors and their ephrin ligands in the adult CNS. Eph
receptors and their ligands serve primary roles in the regulation
of synapse formation, function and plasticity (33,65,66),
which is particularly important in the maintenance of hippocampal
plasticity (67) and the processing
of certain types of pain (68).
Previous studies have demonstrated that the activation of EphA4
forward signaling mediates the retraction of dendritic spines and
reduces their number and size by remodeling the actin cytoskeleton
and modifying the properties of adhesion receptors (35,69,70). It
has also been demonstrated that EphA4 blockade leads to
significantly longer and overlapping dendritic spines (71). However, contrasting effects were
observed in triple EphB (EphB1/EphB2/EphB3) knockout mice. A
significant decrease in dendritic spine density and the formation
of headless or small-headed spines were observed, suggesting that
EphB forward signaling is responsible for dendritic spine formation
and synaptic maturation (44,72).
Previous studies have also compared Eph/ephrin knockout with
wild-type mice and demonstrated that pre- and post-synaptic
Eph/ephrins affect memory and learning by controlling synaptic
formation (67,73,74).
Eph/ephrins may recruit cell surface molecules, such as the
N-methyl-D-aspartate receptor (NMDAR), via their PDZ domain
(75,76). EphB2 forward signaling and ephrin-B3
reverse signaling also induces the generation of long-term
potentiation (LTP) via NMDARs (33,50,77–80).
EphA4/ephrin-A3-mediated bidirectional signaling
between neurons and astrocytes was implicated in the alteration of
the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
and spine morphology in the hippocampus (67). In addition, Eph4A forward signaling
and glial ephrin-A3 reverse signaling regulates the astrocyte
glutamate transporter and the plasticity of synapses within the
hippocampus, respectively (81,82).
Furthermore, ephrin-Bs/EphB participate in the processing of spinal
cord pain via the NMDAR, PI3K and downstream signaling pathways
(83–87).
Ephs and their ephrin ligands are also expressed in
the subventricular zone (SVZ) of the lateral ventricle and
subgranular zone (SGZ) of the dentate gyrus, where neural stem
cells maintain neurogenesis throughout the lifetime of mammals
(88). Eph/ephrin bidirectional
signaling influences the proliferation and differentiation of
neural precursor cells (NPCs) (89).
Previous studies have demonstrated that EphB3/ephrin-B3 regulates
the proliferation and differentiation of cells in the SVZ and the
rostral migratory stream (RMS) through altering the expression of
p53 (90–93). In addition, EphA4 knockout mice
exhibit decreased cell proliferation and differentiation disorder
in the SVZ and RMS, resulting in a reduced number of neuroblasts
(94). It has been demonstrated that
EphB1/ephrin-B3 signaling also affects the proliferation and
differentiation of NPCs in the SGZ of the dentate gyrus,
highlighting a potential therapeutic target for neurodegenerative
diseases and brain damage (7). In
addition, the interactions between neural stem cells and blood
vessels in the SVZ function to regulate quiescence and promote
stemness. In particular, ephrin-B2 presented by vascular
endothelial cells suppresses the proliferation and differentiation
of stem cells through the activation of their respective Eph
receptors and downstream notch signaling pathways. Ephrin-B2/Eph
and notch signaling is suspended as stem cells detach from blood
vessels to differentiate and divide (95).
Eph/ephrin signal transduction is involved in CNS
angiogenesis. It has been demonstrated that EphA2 receptor blockade
improves the formation of tight junctions between endothelial cells
and promotes angiogenesis (96). In
addition, an intracerebroventricular injection of ephrin-A1 may
promote angiogenesis by stimulating EphA forward signaling
(26). Small competitive Eph-ephrin
antagonists have also been demonstrated to disrupt the interaction
between EphA2 and ephrin-A1, blocking angiogenesis at low
micromolar concentrations (97). A
previous study also revealed that ephrin-A1 was involved in the
modulation of the actin cytoskeleton, demonstrating its vital role
in re-endothelialization (98).
Eph/ephrin signaling is also involved in
sophisticated pathological processes following CNS injury. Eph
receptors and ephrin ligands are upregulated following CNS injury
and exhibit diverse changes depending on the location or time at
which injuries occur (99–103). Certain types of cell behave
differently following CNS damage: Neurons attempt to regenerate
damaged connections; astrocytes and microglial cells proliferate,
migrate and become activated to maintain homeostasis; and
oligodendrocytes initiate remyelination (104). The alteration of Eph and ephrin
expression under these situations may reveal the function of
Eph/ephrin signaling in the damage response. Ephs and ephrins may
regulate axon guidance in the developing CNS and so may serve a
similar role during CNS regeneration (105). Eph receptors and their ephrin
ligands are also expressed in mature cell types, including neurons
and astrocytes. They may therefore exhibit different effects
compared with those observed in CNS development, including the
mediation of astrocytic gliosis, neural regeneration, vascular
remodeling and neuroinflammation (106).
Eph receptors and their ephrin ligands may influence
the structural and functional reorganization of the CNS during
trauma. Ephs and ephrins may respond to CNS injury by promoting the
formation of glial scars due to their inhibitory effect on axonal
regeneration (105). Previous
results have revealed that the sophisticated processes involving
gliosis include glial reactivation, extracellular matrix alteration
and collagen deposition (107).
Multi-cellular components including astrocytes, microglia,
oligodendrocyte progenitors and fibroblasts participate in the
formation of glial scars (108–110).
Ephs and ephrins are expressed in many types of cells associated
with gliosis and glial scars and affect their response to damage.
Glial cells trigger gliosis in CNS injury as they are highly
sensitive to damage (111). Gliosis
is a process that begins with glial cell activation and
proliferation, and is characterized by morphological and functional
changes in astrocytes and microglia. Astrocytic activation results
in cellular hypertrophy, proliferation and gliosis (112), which are observed in areas distal
to the site of injury (113,114).
However, the astro-glial response has positive and negative effects
on neuronal cell recovery and degeneration. There are various
benefits of glial scar formation, including the separation of the
site of injury from surrounding normal tissues, thus reducing the
spread of damage and filling of the lesion cavity (115–117).
Glial scars help to reconstruct damaged brain areas and re-organize
blood vessels following epithelial cell invasion into the scar
tissue. Previous studies have demonstrated that glial scars also
act as primary barriers to neural regeneration (35,118,119).
There is mounting evidence that Eph/ephrin signaling is involved in
glial scar formation in CNS disorders. It has been demonstrated in
a model of spinal cord injury, that the development of glial scars
and the exclusion of meningeal fibroblasts from the site of damage
are a result of cell contact-mediated bidirectional signaling
cascades, stimulated by the interaction of ephrin-B2 and EphB2 with
reactive astrocytes and meningeal fibroblasts, respectively
(103). Another previous study
demonstrated that ephrin B2 (−/-) mice exhibited a reduction in
astrogliosis and an accelerated regeneration of injured
corticospinal axons, which resulted in the recovery of murine motor
function following spinal cord injury (SCI) (105). It was also demonstrated that
astrocytic gliosis and glial scars were greatly reduced in lesioned
EphA4−/− spinal cords. EphA4−/− astrocytes
also failed to respond to inflammatory cytokines, including
interferon-γ and leukemia inhibitory factor in vitro
(35). In addition, neurons grown in
wild-type astrocytes exhibited shorter neurites compared with
neurons grown in EphA4−/− astrocytes (120). Previous studies have demonstrated
that the use of EphA4 inhibitors moderately reduced astrocytic
gliosis, promoted axonal regeneration and improved functional
outcome following spinal cord hemisection in wild-type mice
(35,121).
Glutamate is the primary excitatory neurotransmitter
in the CNS; however, it is also a potential neurotoxin as excessive
glutamate signaling may lead to excitotoxic cell death (122). The maintenance of extracellular
glutamate homeostasis is a supportive function of astrocytes that
occurs during brain injury, the function of which may be regulated
by Eph/ephrin signaling. The use of clustered EphA4 was
demonstrated to decrease the expression of astrocyte glutamate
transporters and the glutamate uptake capacity of astrocytes via
the activation of ephrin-A3 reverse signaling (123). These results indicated that
EphA4-mediated ephrin-A3 reverse signaling is a vital mechanism for
astrocytes to control glial glutamate transporters and prevent
glutamate excitotoxicity under pathological conditions (123). A novel role of ephrin-B1 was
determined in astrocyte-mediated synapse remodeling following
traumatic brain injury (TBI). The upregulation of astrocytic
ephrin-B1 following injury reduced the vesicular glutamate
transporter 1 positive excitatory presynaptic innervation of CA1
neurons via STAT3-mediated signaling in astrocytes (124). Therefore, the regulation of
ephrin-B1 signaling in astrocytes may provide novel therapeutic
opportunities to aid glutamate homeostasis and functional recovery
following TBI (124).
Endogenous NPCs are present in the SGZ of the
dentate gyrus and in the rostral SVZ of the lateral ventricles in
the mature CNS (125). NPC
proliferation in the SVZ and SGZ is triggered under
pathophysiological conditions. These neuroblasts may migrate to the
lesion area and differentiate into neurons to replace those that
are damaged (126,127).
Eph/ephrin bidirectional signaling influences the
proliferation and differentiation of NPCs, affecting their response
to CNS injury. EphB3/ephrin-B3 regulates the proliferation and
differentiation of cells in the SVZ and the RMS by controlling p53
levels (90–93). Post-ischemic neurogenesis in
ephrin-B3 (−/-) mice was strongly enhanced and associated with the
caspase-3-dependent activation of STAT1 (128). EphB2 has been demonstrated to
control the migration of dentate progenitor cells into the dorsal
half of the developing dentate gyms (129). A previous study revealed that
blockade of EphB2 enhanced neurogenesis in the SVZ and improved
neurological function following cerebral cortical infarction in
hypertensive rats (130). Neurons
adapt their structure and function to microenvironmental changes by
controlling neural plasticity. Previous studies have demonstrated
that Eph/ephrin signaling exhibits an inhibitory effect on neurite
outgrowth in CNS damage (131–133).
For example, ephrin-A5 reverse signaling induces growth cone
collapse and inhibits axonal regeneration by activating RhoA or
dependent protein kinases (131).
Ephrin-A5-mediated EphA4 forward signaling also triggers axonal
growth cone collapse via the downstream Rac GTPase-activating
protein α2-chimera-independent signaling pathway (132). The intervention of ephrin-A5/EphA4
communication may therefore serve a vital role in the suppression
of neuron generation through the phosphorylated (p)-Akt and
p-extracellular signal-related kinase (ERK) pathways (133). EphA4 targeting using miR-93 was
demonstrated to promote neurite outgrowth in spinal cord injury in
rats following a reduction in p-Ephexin and active RhoA levels
(134).
Eph/ephrin bidirectional signaling regulates
oligodendrocyte precursor cells (OPCs) and oligodendrocytes.
Eph-ephrin interactions between axons and OPCs may control the
distribution of OPCs in the optic axonal tracts and the cessation
of their migration (135). It was
revealed that ephrin-B3 is expressed in postnatal myelinating
oligodendrocytes and acts as myelin-based inhibitor through a
combined p75 neurotrophin receptor (136). A previous study demonstrated that
EphB3 functions as a dependence receptor that mediates
oligodendrocyte cell death following SCI, which further supports
the development of ephrin-B3 based therapies to promote recovery
(137).
It is now relatively well accepted that neurogenesis
and angiogenesis are coupled processes. Eph receptors and their
ephrin ligands are also involved in angiogenesis, which is critical
for the remodeling of vasculature following CNS injury (138). EphA2 is an essential regulator of
post-natal angiogenesis. The stimulation of ephrin-A1 induces the
PI3K-dependent activation of Ras-related C3 botulinum toxin 1
(Rac1) in wild-type endothelial cells, and EphA2-deficient cells
fail to activate Rac1 upon stimulation. EphA2-deficient endothelial
cells fail to undergo vascular assembly and migration in response
to ephrin-A1 in vitro (139). The competitive small molecule
UniPR129 acts as an Eph/ephrin antagonist and blocks angiogenesis
at low concentrations in vitro (140). It has been suggested that
increasing ephrin-B2 levels may promote vascular endothelial growth
factor (VEGF)-induced VEGF receptor 2 endocytosis and the
angiogenic function of endothelial cells (141). Previous studies have demonstrated
that the ephrin-A5/EphA4 interaction mediates the ERK and Akt
signaling pathways in pilocarpine-induced epilepsy, and that the
intervention of ephrin/Eph interactions suppresses newborn neuron
generation and microvessel remodeling in a mouse model of temporal
lobe epilepsy (142,143).
Post-injury inflammation is implicated in most types
of CNS injury. Neurodegeneration, trauma and ischemia stimulate an
inflammatory response that causes microglial activation and
circulating immune cell infiltration in the brain (144). Inflammation is generally considered
to be beneficial for the clearance of debris formed by necrotic
cells. However, severe inflammation causes cerebral swelling and
vascular dysfunction, which exaggerates neuronal damage (144). Previous studies have indicated that
Eph/ephrin proteins are involved in the inflammatory process
following CNS injury. EphA2 and ephrin-A1 serve roles in the
maintenance of endothelial junction integrity and cytoskeletal
structure, potentially in response to the upregulation of
inflammatory mediators, resulting in vascular leakage (145,146).
EphA2 inactivation promotes the formation of tight junctions in the
endothelial cells of the brain (96). There is also considerable interest in
ephrin-B2/EphB4 signaling. Ephrin-B2 is a marker of arterial
endothelial cells in the vasculature and EphB4, one of its cognate
receptors, is predominantly expressed in the venous endothelium.
Endothelial ephrin-B2 primarily functions via the VEGF receptor to
mediate vascular responses during inflammation (147). Therefore, therapies that inhibit
the function of ephrin-B2/EphB4 may suppress the inflammatory
response following injury (148).
EphB receptor inhibition using EphB1-fragment crystallizable
reduced formalin-induced inflammation and chronic constrictive
injury-induced neuropathic pain behaviors via the control of PI3K
and PI3K crosstalk to ERK signaling (87). Ephrin-B/EphB signaling also serves a
primary role in the regulation of inflammatory pain via NMDAR
subunit NR2B and PKCγ regulation (76,149).
The underlying mechanisms of Eph/ephrin signaling
remain poorly understood. The key roles of Eph/ephrin signaling in
the progression of a large range of neurological disorders suggest
that Ephs and ephrins may be potential therapeutic targets.
Previous studies have indicated that Eph/ephrin signaling may be a
suitable therapeutic target for the treatment of neurological
diseases.
AD is the most common type of neurodegenerative
disorder that manifests as a progressive decline in cognitive
function. It has been demonstrated that EphB2 and EphA4 are
downregulated in AD (74). Soluble
amyloid-β (Aβ) peptide oligomers are derived from amyloid precursor
proteins (APPs) and are a major causative agent of synaptic
impairment in AD. Previous studies have suggested that Aβ oligomers
indirectly affect the NMDAR NR1 subunit and induce NMDAR depletion
by forming a complex with EphB2 (150,151).
In addition, increased EphB2 expression reverses deficits in
NMDAR-dependent LTP and memory impairments in murine models of AD
(152). EphA4 may also be a
potential therapeutic target of AD. It has been demonstrated that
EphA4 mediates the Aβ-induced impairment of synaptic plasticity;
the depletion or blockade of postsynaptic EphA4 activity reverses
synaptic deficits in murine models of AD. Rhy is a small-molecule
inhibitor of EphA4 that rescues Aβ-induced impairments in
neurotransmission and LTP in murine models of AD (153).
ALS is a neurodegenerative disease that is caused by
the progressive degeneration of the upper and lower motor neurons
in the anterior horn of the spinal cord, brainstem and cerebral
cortex (154). The ALS8 gene leads
to the development of familial ALS and accounts for 10–15% of all
ALS cases (155). The ALS8 protein
vesicle-associated membrane protein-associated B (VAPB) is a ligand
for EphA4. Mutations of VAPB may enhance EphA4/ephrin-A3 signaling
and lead to the dysfunction of glial glutamate transporters, as
observed in ALS (81,156). However, the specific role of EphA4
in the pathology of ALS requires further investigation (81,155).
The observations described in the present review
provide evidence that Ephs and ephrins serve a vital role in
determining the regenerative outcomes of CNS disorders. Signaling
through Eph/ephrin complexes directly regulates neural regeneration
by stimulating growth cone collapse, promoting glial scar
formation, regulating homeostasis, reducing neurogenesis,
inhibiting myelination and exaggerating inflammation together with
injury-induced neuropathic pain. In addition, the interaction
between Ephs and ephrin ligands is essential for angiogenesis.
Therefore, the regulation of these molecules following CNS injury
may serve as therapeutic targets for the treatment of various
neurological diseases.
The present review was supported by the Fujian
Provincial Natural Science Foundation (grant no. 2017J05123), the
Startup Fund for scientific research, Fujian Medical University
(grant no. 2016QH067), the National Key Clinical Specialty
Discipline Construction Program (grant no. 2012-GJLCZD) and the
Fujian Key Clinical Specialty Discipline Construction Program
(grant no. 2012-SLCZD).
|
1
|
Hirai H, Maru Y, Hagiwara K, Nishida J and
Takaku F: A novel putative tyrosine kinase receptor encoded by the
eph gene. Science. 238:1717–1720. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klein R: Bidirectional modulation of
synaptic functions by Eph/ephrin signaling. Nat Neurosci. 12:15–20.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aoto J and Chen L: Bidirectional
ephrin/Eph signaling in synaptic functions. Brain Res. 1184:72–80.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flanagan JG and Vanderhaeghen P: The
ephrins and Eph receptors in neural development. Annu Rev Neurosci.
21:309–345. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilkinson DG: Multiple roles of EPH
receptors and ephrins in neural development. Nat Rev Neurosci.
2:155–164. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Goldshmit Y, McLenachan S and Turnley A:
Roles of Eph receptors and ephrins in the normal and damaged adult
CNS. Brain Res Rev. 52:327–345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chumley MJ, Catchpole T, Silvany RE,
Kernie SG and Henkemeyer M: EphB receptors regulate stem/progenitor
cell proliferation, migration, and polarity during hippocampal
neurogenesis. J Neurosci. 27:13481–13490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Labrador JP, Brambilla R and Klein R: The
N-terminal globular domain of Eph receptors is sufficient for
ligand binding and receptor signaling. EMBO J. 16:3889–3897. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lackmann M, Oates AC, Dottori M, Smith FM,
Do C, Power M, Kravets L and Boyd AW: Distinct subdomains of the
EphA3 receptor mediate ligand binding and receptor dimerization. J
Biol Chem. 273:20228–20237. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bruckner K and Klein R: Signaling by Eph
receptors and their ephrin ligands. Curr Opin Neurobiol. 8:375–382.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holland SJ, Peles E, Pawson T and
Schlessinger J: Cell-contact-dependent signalling in axon growth
and guidance: Eph receptor tyrosine kinases and receptor protein
tyrosine phosphatase beta. Curr Opin Neurobiol. 8:117–127. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nimnual AS, Yatsula BA and Bar-Sagi D:
Coupling of Ras and Rac guanosine triphosphatases through the Ras
exchanger Sos. Science. 279:560–563. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schultz J, Ponting CP, Hofmann K and Bork
P: SAM as a protein interaction domain involved in developmental
regulation. Protein science Protein Sci. 6:249–253. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stapleton D, Balan I, Pawson T and Sicheri
F: The crystal structure of an Eph receptor SAM domain reveals a
mechanism for modular dimerization. Nat Struct Biol. 6:44–49. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kullander K and Klein R: Mechanisms and
functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol.
3:475–486. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin D, Gish GD, Songyang Z and Pawson T:
The carboxyl terminus of B class ephrins constitutes a PDZ domain
binding motif. J Biol Chem. 274:3726–3733. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pasquale EB: Eph receptor signalling casts
a wide net on cell behaviour. Nat Rev Mol Cell Biol. 6:462–475.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mellitzer G, Xu Q and Wilkinson DG: Eph
receptors and ephrins restrict cell intermingling and
communication. Nature. 400:77–81. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klein R: Eph/ephrin signaling in
morphogenesis, neural development and plasticity. Curr Opin Cell
Biol. 16:580–589. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davis S, Gale NW, Aldrich TH, Maisonpierre
PC, Lhotak V, Pawson T, Goldfarb M and Yancopoulos GD: Ligands for
EPH-related receptor tyrosine kinases that require membrane
attachment or clustering for activity. Science. 266:816–819. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stein E, Lane AA, Cerretti DP,
Schoecklmann HO, Schroff AD, Van Etten RL and Daniel TO: Eph
receptors discriminate specific ligand oligomers to determine
alternative signaling complexes, attachment, and assembly
responses. Genes Dev. 12:667–678. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carter N, Nakamoto T, Hirai H and Hunter
T: EphrinA1-induced cytoskeletal re-organization requires FAK and
p130(cas). Nat Cell Biol. 4:565–573. 2002.PubMed/NCBI
|
|
23
|
Lawrenson ID, Wimmer-Kleikamp SH, Lock P,
Schoenwaelder SM, Down M, Boyd AW, Alewood PF and Lackmann M:
Ephrin-A5 induces rounding, blebbing and de-adhesion of
EphA3-expressing 293T and melanoma cells by CrkII and Rho-mediated
signalling. J Cell Sci. 115:1059–1072. 2002.PubMed/NCBI
|
|
24
|
Cowan CA and Henkemeyer M: Ephrins in
reverse, park and drive. Trends Cell Biol. 12:339–346. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davy A and Soriano P: Ephrin signaling in
vivo: Look both ways. Dev Dyn. 232:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jing X, Miwa H, Sawada T, Nakanishi I,
Kondo T, Miyajima M and Sakaguchi K: Ephrin-A1-mediated
dopaminergic neurogenesis and angiogenesis in a rat model of
Parkinson's disease. PLos One. 7:e320192012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Holland SJ, Gale NW, Gish GD, Roth RA,
Songyang Z, Cantley LC, Henkemeyer M, Yancopoulos GD and Pawson T:
Juxtamembrane tyrosine residues couple the Eph family receptor
EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO
J. 16:3877–3888. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Henkemeyer M, Marengere LE, McGlade J,
Olivier JP, Conlon RA, Holmyard DP, Letwin K and Pawson T:
Immunolocalization of the Nuk receptor tyrosine kinase suggests
roles in segmental patterning of the brain and axonogenesis.
Oncogene. 9:1001–1014. 1994.PubMed/NCBI
|
|
29
|
Becker E, Huynh-Do U, Holland S, Pawson T,
Daniel TO and Skolnik EY: Nck-interacting Ste20 kinase couples Eph
receptors to c-Jun N-terminal kinase and integrin activation. Mol
Cell Biol. 20:1537–1545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wahl S, Barth H, Ciossek T, Aktories K and
Mueller BK: Ephrin-A5 induces collapse of growth cones by
activating Rho and Rho kinase. J Cell Biol. 149:263–270. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shamah SM, Lin MZ, Goldberg JL, Estrach S,
Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A and
Greenberg ME: EphA receptors regulate growth cone dynamics through
the novel guanine nucleotide exchange factor ephexin. Cell.
105:233–244. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou X, Suh J, Cerretti DP, Zhou R and
DiCicco-Bloom E: Ephrins stimulate neurite outgrowth during early
cortical neurogenesis. Journal of neuroscience research.
66:1054–1063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takasu MA, Dalva MB, Zigmond RE and
Greenberg ME: Modulation of NMDA receptor-dependent calcium influx
and gene expression through EphB receptors. Science. 295:491–495.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tong J, Elowe S, Nash P and Pawson T:
Manipulation of EphB2 regulatory motifs and SH2 binding sites
switches MAPK signaling and biological activity. J Biol Chem.
278:6111–6119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Goldshmit Y, Galea MP, Wise G, Bartlett PF
and Turnley AM: Axonal regeneration and lack of astrocytic gliosis
in EphA4-deficient mice. J Neurosci. 24:10064–10073. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lisabeth EM, Falivelli G and Pasquale EB:
Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol.
5:2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dickson BJ: Rho GTPases in growth cone
guidance. Curr Opin Neurobiol. 11:103–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giniger E: How do Rho family GTPases
direct axon growth and guidance? A proposal relating signaling
pathways to growth cone mechanics. Differentiation. 70:385–396.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lehmann M, Fournier A, Selles-Navarro I,
Dergham P, Sebok A, Leclerc N, Tigyi G and McKerracher L:
Inactivation of Rho signaling pathway promotes CNS axon
regeneration. J Neurosci. 19:7537–7547. 1999.PubMed/NCBI
|
|
40
|
Dergham P, Ellezam B, Essagian C,
Avedissian H, Lubell WD and McKerracher L: Rho signaling pathway
targeted to promote spinal cord repair. J Neurosci. 22:6570–6577.
2002.PubMed/NCBI
|
|
41
|
Fournier AE, Takizawa BT and Strittmatter
SM: Rho kinase inhibition enhances axonal regeneration in the
injured CNS. J Neurosci. 23:1416–1423. 2003.PubMed/NCBI
|
|
42
|
Nikolic M: The role of Rho GTPases and
associated kinases in regulating neurite outgrowth. Int J Biochem
Cell Biol. 34:731–745. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sahin M, Greer PL, Lin MZ, Poucher H,
Eberhart J, Schmidt S, Wright TM, Shamah SM, O'connell S and Cowan
CW: Eph-dependent tyrosine phosphorylation of ephexin1 modulates
growth cone collapse. Neuron. 46:191–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Henkemeyer M, Itkis OS, Ngo M, Hickmott PW
and Ethell IM: Multiple EphB receptor tyrosine kinases shape
dendritic spines in the hippocampus. J Cell Biol. 163:1313–1326.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fang Y, Cho KS, Tchedre K, Lee SW, Guo C,
Kinouchi H, Fried S, Sun X and Chen DF: Ephrin-A3 suppresses Wnt
signaling to control retinal stem cell potency. Stem Cells.
31:349–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Steinle JJ, Meininger CJ, Forough R, Wu G,
Wu MH and Granger HJ: Eph B4 receptor signaling mediates
endothelial cell migration and proliferation via the
phosphatidylinositol 3-kinase pathway. J Biol Chem.
277:43830–43835. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lai KO, Chen Y, Po HM, Lok KC, Gong K and
Ip NY: Identification of the Jak/Stat proteins as novel downstream
targets of EphA4 signaling in muscle: implications in the
regulation of acetylcholinesterase expression. J Biol Chem.
279:13383–13392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Macrae M, Neve RM, Rodriguez-Viciana P,
Haqq C, Yeh J, Chen C, Gray JW and McCormick F: A conditional
feedback loop regulates Ras activity through EphA2. Cancer Cell.
8:111–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Holmberg J, Armulik A, Senti KA, Edoff K,
Spalding K, Momma S, Cassidy R, Flanagan JG and Frisén J: Ephrin-A2
reverse signaling negatively regulates neural progenitor
proliferation and neurogenesis. Genes Dev. 19:462–471. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Grunwald IC, Korte M, Adelmann G, Plueck
A, Kullander K, Adams RH, Frotscher M, Bonhoeffer T and Klein R:
Hippocampal plasticity requires postsynaptic ephrinBs. Nat
Neurosci. 7:33–40. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
51
|
Davy A, Aubin J and Soriano P: Ephrin-B1
forward and reverse signaling are required during mouse
development. Genes Dev. 18:572–583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Davy A, Gale NW, Murray EW, Klinghoffer
RA, Soriano P, Feuerstein C and Robbins SM: Compartmentalized
signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine
kinase to regulate cellular adhesion. Genes Dev. 13:3125–3135.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Davy A and Robbins SM: Ephrin-A5 modulates
cell adhesion and morphology in an integrin-dependent manner. EMBO
J. 19:5396–5405. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Suetterlin P, Marler KM and Drescher U:
Axonal ephrinA/EphA interactions, and the emergence of order in
topographic projections. Semin Cell Dev Biol. 23:1–6. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Torres R, Firestein BL, Dong H, Staudinger
J, Olson EN, Huganir RL, Bredt DS, Gale NW and Yancopoulos GD: PDZ
proteins bind, cluster, and synaptically colocalize with Eph
receptors and their ephrin ligands. Neuron. 21:1453–1463. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Holland SJ, Gale NW, Mbamalu G,
Yancopoulos GD, Henkemeyer M and Pawson T: Bidirectional signalling
through the EPH-family receptor Nuk and its transmembrane ligands.
Nature. 383:722–725. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bruckner K, Pasquale EB and Klein R:
Tyrosine phosphorylation of transmembrane ligands for Eph
receptors. Science. 275:1640–1643. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kalo MS, Yu HH and Pasquale EB: In vivo
tyrosine phosphorylation sites of activated ephrin-B1 and ephB2
from neural tissue. J Biol Chem. 276:38940–38948. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cowan CA and Henkemeyer M: The SH2/SH3
adaptor Grb4 transduces B-ephrin reverse signals. Nature.
413:174–179. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Palmer A, Zimmer M, Erdmann KS, Eulenburg
V, Porthin A, Heumann R, Deutsch U and Klein R: EphrinB
phosphorylation and reverse signaling: Regulation by Src kinases
and PTP-BL phosphatase. Mol Cell. 9:725–737. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hsueh YP and Sheng M: Eph receptors,
ephrins, and PDZs gather in neuronal synapses. Neuron.
21:1227–1229. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Salcedo R, Wasserman K, Young HA, Grimm
MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ and Oppenheim JJ:
Vascular endothelial growth factor and basic fibroblast growth
factor induce expression of CXCR4 on human endothelial cells: In
vivo neovascularization induced by stromal-derived factor-1alpha.
Am J Pathol. 154:1125–1135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lu Q, Sun EE, Klein RS and Flanagan JG:
Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein
and selectively inhibits G protein-coupled chemoattraction. Cell.
105:69–79. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liebl DJ, Morris CJ, Henkemeyer M and
Parada LF: mRNA expression of ephrins and Eph receptor tyrosine
kinases in the neonatal and adult mouse central nervous system. J
Neurosci Res. 71:7–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Murai KK and Pasquale EB: Can Eph
receptors stimulate the mind? Neuron. 33:159–162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hruska M and Dalva MB: Ephrin regulation
of synapse formation, function and plasticity. Mol Cell Neurosci.
50:35–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Murai KK, Nguyen LN, Irie F, Yamaguchi Y
and Pasquale EB: Control of hippocampal dendritic spine morphology
through ephrin-A3/EphA4 signaling. Nat Neurosci. 6:153–160. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Vasileiou I, Adamakis I, Patsouris E and
Theocharis S: Ephrins and pain. Expert Opin Ther Targets.
17:879–887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bouvier D, Corera AT, Tremblay ME, Riad M,
Chagnon M, Murai KK, Pasquale EB, Fon EA and Doucet G: Pre-synaptic
and post-synaptic localization of EphA4 and EphB2 in adult mouse
forebrain. J Neurochem. 106:682–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
McKinnell IW, Makarenkova H, de Curtis I,
Turmaine M and Patel K: EphA4, RhoB and the molecular development
of feather buds are maintained by the integrity of the actin
cytoskeleton. Dev Biol. 270:94–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Heintz TG, Eva R and Fawcett JW: Regional
regulation of purkinje cell dendritic spines by integrins and
Eph/Ephrins. PLoS One. 11:e01585582016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zhu XN, Liu XD, Zhuang H, Henkemeyer M,
Yang JY and Xu NJ: Amygdala EphB2 signaling regulates glutamatergic
neuron maturation and innate fear. J Neurosci. 36:10151–10162.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Rodenas-Ruano A, Perez-Pinzon MA, Green
EJ, Henkemeyer M and Liebl DJ: Distinct roles for ephrinB3 in the
formation and function of hippocampal synapses. Dev Biol.
292:34–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Cisse M and Checler F: Eph receptors: New
players in Alzheimer's disease pathogenesis. Neurobiol Dis.
73:137–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kalo MS and Pasquale EB: Signal transfer
by eph receptors. Cell Tissue Res. 298:1–9. 1999. View Article : Google Scholar
|
|
76
|
Zhou XL, Zhang CJ, Wang Y, Wang M, Sun LH,
Yu LN, Cao JL and Yan M: EphrinB-EphB signaling regulates spinal
pain processing via PKCgamma. Neuroscience. 307:64–72. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu
L, Gale NW and Greenberg ME: EphB receptors interact with NMDA
receptors and regulate excitatory synapse formation. Cell.
103:945–956. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Grunwald IC, Korte M, Wolfer D, Wilkinson
GA, Unsicker K, Lipp HP, Bonhoeffer T and Klein R:
Kinase-independent requirement of EphB2 receptors in hippocampal
synaptic plasticity. Neuron. 32:1027–1040. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Armstrong JN, Saganich MJ, Xu NJ,
Henkemeyer M, Heinemann SF and Contractor A: B-ephrin reverse
signaling is required for NMDA-independent long-term potentiation
of mossy fibers in the hippocampus. J Neurosci. 26:3474–3481. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lim BK, Matsuda N and Poo MM: Ephrin-B
reverse signaling promotes structural and functional synaptic
maturation in vivo. Nat Neurosci. 11:160–169. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Filosa A, Paixão S, Honsek SD, Carmona MA,
Becker L, Feddersen B, Gaitanos L, Rudhard Y, Schoepfer R and
Klopstock T: Neuron-glia communication via EphA4/ephrin-A3
modulates LTP through glial glutamate transport. Nat Neurosci.
12:1285–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Carmona MA, Murai KK, Wang L, Roberts AJ
and Pasquale EB: Glial ephrin-A3 regulates hippocampal dendritic
spine morphology and glutamate transport. Proc Natl Acad Sci USA.
106:pp. 12524–12529. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Battaglia AA, Sehayek K, Grist J, McMahon
SB and Gavazzi I: EphB receptors and ephrin-B ligands regulate
spinal sensory connectivity and modulate pain processing. Nat
Neurosci. 6:339–340. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
84
|
Song XJ, Cao JL, Li HC, Zheng JH, Song XS
and Xiong LZ: Upregulation and redistribution of ephrinB and EphB
receptor in dorsal root ganglion and spinal dorsal horn neurons
after peripheral nerve injury and dorsal rhizotomy. Eur J Pain.
12:1031–1039. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Slack S, Battaglia A, Cibert-Goton V and
Gavazzi I: EphrinB2 induces tyrosine phosphorylation of NR2B via
Src-family kinases during inflammatory hyperalgesia. Neuroscience.
156:175–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ruan JP, Zhang HX, Lu XF, Liu YP and Cao
JL: EphrinBs/EphBs signaling is involved in modulation of spinal
nociceptive processing through a mitogen-activated protein
kinases-dependent mechanism. Anesthesiology. 112:1234–1249. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yu LN, Zhou XL, Yu J, Huang H, Jiang LS,
Zhang FJ, Cao JL and Yan M: PI3K contributed to modulation of
spinal nociceptive information related to ephrinBs/EphBs. PLoS One.
7:e409302012. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Laussu J, Khuong A, Gautrais J and Davy A:
Beyond boundaries-Eph:ephrin signaling in neurogenesis. Cell Adh
Migr. 8:349–359. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Aoki M, Yamashita T and Tohyama M: EphA
receptors direct the differentiation of mammalian neural precursor
cells through a mitogen-activated protein kinase-dependent pathway.
J Biol Chem. 279:32643–32650. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ricard J, Salinas J, Garcia L and Liebl
DJ: EphrinB3 regulates cell proliferation and survival in adult
neurogenesis. Mol Cell Neurosci. 31:713–722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Theus MH, Ricard J, Bethea JR and Liebl
DJ: EphB3 limits the expansion of neural progenitor cells in the
subventricular zone by regulating p53 during homeostasis and
following traumatic brain injury. Stem Cells. 28:1231–1242.
2010.PubMed/NCBI
|
|
92
|
del Valle K, Theus MH, Bethea JR, Liebl DJ
and Ricard J: Neural progenitors proliferation is inhibited by
EphB3 in the developing subventricular zone. Int J Dev Neurosci.
29:9–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Baumann G, Travieso L, Liebl DJ and Theus
MH: Pronounced hypoxia in the subventricular zone following
traumatic brain injury and the neural stem/progenitor cell
response. Exp Biol Med (Maywood). 238:830–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Khodosevich K, Watanabe Y and Monyer H:
EphA4 preserves postnatal and adult neural stem cells in an
undifferentiated state in vivo. J Cell Sci. 124:1268–1279. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ottone C, Krusche B, Whitby A, Clements M,
Quadrato G, Pitulescu ME, Adams RH and Parrinello S: Direct
cell-cell contact with the vascular niche maintains quiescent
neural stem cells. Nat Cell Biol. 16:1045–1056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhou N, Zhao WD, Liu DX, Liang Y, Fang WG,
Li B and Chen YH: Inactivation of EphA2 promotes tight junction
formation and impairs angiogenesis in brain endothelial cells.
Microvasc Res. 82:113–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hassan-Mohamed I, Giorgio C, Incerti M,
Russo S, Pala D, Pasquale EB, Zanotti I, Vicini P, Barocelli E,
Rivara S, et al: UniPR129 is a competitive small molecule
Eph-ephrin antagonist blocking in vitro angiogenesis at low
micromolar concentrations. Br J Pharmacol. 171:5195–5208. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Wiedemann E, Jellinghaus S, Ende G,
Augstein A, Sczech R, Wielockx B, Weinert S, Strasser RH and Poitz
DM: Regulation of endothelial migration and proliferation by
ephrin-A1. Cell Signal. 29:84–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Miranda JD, White LA, Marcillo AE, Willson
CA, Jagid J and Whittemore SR: Induction of Eph B3 after spinal
cord injury. Exp Neurol. 156:218–222. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Moreno-Flores MT and Wandosell F:
Up-regulation of Eph tyrosine kinase receptors after excitotoxic
injury in adult hippocampus. Neuroscience. 91:193–201. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Rodger J, Lindsey KA, Leaver SG, King CE,
Dunlop SA and Beazley LD: Expression of ephrin-A2 in the superior
colliculus and EphA5 in the retina following optic nerve section in
adult rat. Eur J Neurosci. 14:1929–1936. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Willson CA, Irizarry-Ramírez M, Gaskins
HE, Cruz-Orengo L, Figueroa JD, Whittemore SR and Miranda JD:
Upregulation of EphA receptor expression in the injured adult rat
spinal cord. Cell Transplant. 11:229–239. 2002.PubMed/NCBI
|
|
103
|
Bundesen LQ, Scheel TA, Bregman BS and
Kromer LF: Ephrin-B2 and EphB2 regulation of astrocyte-meningeal
fibroblast interactions in response to spinal cord lesions in adult
rats. J Neurosci. 23:7789–7800. 2003.PubMed/NCBI
|
|
104
|
del Zoppo GJ: Stroke and neurovascular
protection. N Engl J Med. 354:553–555. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ren Z, Chen X, Yang J, Kress BT, Tong J,
Liu H, Takano T, Zhao Y and Nedergaard M: Improved axonal
regeneration after spinal cord injury in mice with conditional
deletion of ephrin B2 under the GFAP promoter. Neuroscience.
241:89–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Pasquale EB: Eph-ephrin bidirectional
signaling in physiology and disease. Cell. 133:38–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lukes A, Mun-Bryce S, Lukes M and
Rosenberg GA: Extracellular matrix degradation by
metalloproteinases and central nervous system diseases. Mol
Neurobiol. 19:267–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Bunge RP, Puckett WR and Hiester ED:
Observations on the pathology of several types of human spinal cord
injury, with emphasis on the astrocyte response to penetrating
injuries. Adv Neurol. 72:305–315. 1997.PubMed/NCBI
|
|
109
|
Fawcett JW and Asher RA: The glial scar
and central nervous system repair. Brain Res Bull. 49:377–391.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Dawson MR, Levine JM and Reynolds R:
NG2-expressing cells in the central nervous system: are they
oligodendroglial progenitors? J Neurosci Res. 61:471–479. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Song I and Dityatev A: Crosstalk between
glia, extracellular matrix and neurons. Brain Res Bull. S0361–9230.
2017.
|
|
112
|
Schnell L, Fearn S, Klassen H, Schwab ME
and Perry VH: Acute inflammatory responses to mechanical lesions in
the CNS: differences between brain and spinal cord. Eur J Neurosci.
11:3648–3658. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
McGraw J, Hiebert GW and Steeves JD:
Modulating astrogliosis after neurotrauma. J Neurosci Res.
63:109–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Xie M, Yi C, Luo X, Xu S, Yu Z, Tang Y,
Zhu W, Du Y, Jia L and Zhang Q: Glial gap junctional communication
involvement in hippocampal damage after middle cerebral artery
occlusion. Ann Neurol. 70:121–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Stichel CC and Muller HW: The CNS lesion
scar: New vistas on an old regeneration barrier. Cell Tissue Res.
294:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Bush TG, Puvanachandra N, Horner CH,
Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH and
Sofroniew MV: Leukocyte infiltration, neuronal degeneration, and
neurite outgrowth after ablation of scar-forming, reactive
astrocytes in adult transgenic mice. Neuron. 23:297–308. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Faulkner JR, Herrmann JE, Woo MJ, Tansey
KE, Doan NB and Sofroniew MV: Reactive astrocytes protect tissue
and preserve function after spinal cord injury. J Neurosci.
24:2143–2155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Jakeman LB and Reier PJ: Axonal
projections between fetal spinal cord transplants and the adult rat
spinal cord: A neuroanatomical tracing study of local interactions.
J Comp Neurol. 307:311–334. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Fernandez-Klett F and Priller J: The
fibrotic scar in neurological disorders. Brain Pathol. 24:404–413.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Joly S, Jordi N, Schwab ME and Pernet V:
The Ephrin receptor EphA4 restricts axonal sprouting and enhances
branching in the injured mouse optic nerve. Eur J Neurosci.
40:3021–3031. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Goldshmit Y, Spanevello MD, Tajouri S, Li
L, Rogers F, Pearse M, Galea M, Bartlett PF, Boyd AW and Turnley
AM: EphA4 blockers promote axonal regeneration and functional
recovery following spinal cord injury in mice. PLos One.
6:e246362011. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Choi DW: Excitotoxic cell death. J
Neurobiol. 23:1261–1276. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yang J, Luo X, Huang X, Ning Q, Xie M and
Wang W: Ephrin-A3 reverse signaling regulates hippocampal neuronal
damage and astrocytic glutamate transport after transient global
ischemia. J Neurochem. 131:383–394. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Nikolakopoulou AM, Koeppen J, Garcia M,
Leish J, Obenaus A and Ethell IM: Astrocytic Ephrin-B1 regulates
synapse remodeling following traumatic brain injury. ASN Neuro.
8:1–18. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Zhao C, Deng W and Gage FH: Mechanisms and
functional implications of adult neurogenesis. Cell. 132:645–660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Butti E, Cusimano M, Bacigaluppi M and
Martino G: Neurogenic and non-neurogenic functions of endogenous
neural stem cells. Front Neurosci. 8:922014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Das A, Gupta T, Davla S, Prieto-Godino LL,
Diegelmann S, Reddy OV, Raghavan KV, Reichert H, Lovick J and
Hartenstein V: Neuroblast lineage-specific origin of the neurons of
the Drosophila larval olfactory system. Dev Biol. 373:322–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Doeppner TR, Bretschneider E, Doehring M,
Segura I, Sentürk A, Acker-Palmer A, Hasan MR, ElAli A, Hermann DM
and Bähr M: Enhancement of endogenous neurogenesis in ephrin-B3
deficient mice after transient focal cerebral ischemia. Acta
Neuropathol. 122:429–442. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Catchpole T and Henkemeyer M: EphB2
tyrosine kinase-dependent forward signaling in migration of
neuronal progenitors that populate and form a distinct region of
the dentate niche. J Neurosci. 31:11472–11483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Xing S, He Y, Ling L, Hou Q, Yu J, Zeng J
and Pei Z: Blockade of EphB2 enhances neurogenesis in the
subventricular zone and improves neurological function after
cerebral cortical infarction in hypertensive rats. Brain Res.
1230:237–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Yue X, Dreyfus C, Kong TA and Zhou R: A
subset of signal transduction pathways is required for hippocampal
growth cone collapse induced by ephrin-A5. Dev Neurobiol.
68:1269–1286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wegmeyer H, Egea J, Rabe N, Gezelius H,
Filosa A, Enjin A, Varoqueaux F, Deininger K, Schnütgen F, Brose N,
et al: EphA4-dependent axon guidance is mediated by the RacGAP
alpha2-chimaerin. Neuron. 55:756–767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shu Y, Xiao B, Wu Q, Liu T, Du Y, Tang H,
Chen S, Feng L, Long L and Li Y: The Ephrin-A5/EphA4 interaction
modulates neurogenesis and angiogenesis by the p-Akt and p-ERK
pathways in a mouse model of TLE. Mol Neurobiol. 53:561–576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chen X, Yang H, Zhou X, Zhang L and Lu X:
MiR-93 Targeting EphA4 promotes neurite outgrowth from spinal cord
neurons. J Mol Neurosci. 58:517–524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Prestoz L, Chatzopoulou E, Lemkine G,
Spassky N, Lebras B, Kagawa T, Ikenaka K, Zalc B and Thomas JL:
Control of axonophilic migration of oligodendrocyte precursor cells
by Eph-ephrin interaction. Neuron Glia Biol. 1:73–83. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Benson MD, Romero MI, Lush ME, Lu QR,
Henkemeyer M and Parada LF: Ephrin-B3 is a myelin-based inhibitor
of neurite outgrowth. Proc Natl Acad Sci USA. 102:pp. 10694–10699.
2005; View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Tsenkina Y, Ricard J, Runko E,
Quiala-Acosta MM, Mier J and Liebl DJ: EphB3 receptors function as
dependence receptors to mediate oligodendrocyte cell death
following contusive spinal cord injury. Cell Death Dis.
6:e19222015. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lodola A, Giorgio C, Incerti M, Zanotti I
and Tognolini M: Targeting Eph/ephrin system in cancer therapy. Eur
J Med Chem. 142:152–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Brantley-Sieders DM, Caughron J, Hicks D,
Pozzi A, Ruiz JC and Chen J: EphA2 receptor tyrosine kinase
regulates endothelial cell migration and vascular assembly through
phosphoinositide 3-kinase-mediated Rac1 GTPase activation. J Cell
Sci. 117:2037–2049. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Hassan-Mohamed I, Giorgio C, Incerti M,
Russo S, Pala D, Pasquale EB, Zanotti I, Vicini P, Barocelli E,
Rivara S, et al: UniPR129 is a competitive small molecule
Eph-ephrin antagonist blocking in vitro angiogenesis at low
micromolar concentrations. Br J Pharmacol,. 171:5195–5208. 2014.
View Article : Google Scholar
|
|
141
|
Tae N, Lee S, Kim O, Park J, Na S and Lee
JH: Syntenin promotes VEGF-induced VEGFR2 endocytosis and
angiogenesis by increasing ephrin-B2 function in endothelial cells.
Oncotarget. 8:38886–38901. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Feng L, Shu Y, Wu Q, Liu T, Long H, Yang
H, Li Y and Xiao B: EphA4 may contribute to microvessel remodeling
in the hippocampal CA1 and CA3 areas in a mouse model of temporal
lobe epilepsy. Mol Med Rep. 15:37–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Shu Y, Xiao B, Wu Q, Liu T, Du Y, Tang H,
Chen S, Feng L, Long L and Li Y: The Ephrin-A5/EphA4 interaction
modulates neurogenesis and angiogenesis by the p-Akt and p-ERK
pathways in a mouse model of TLE. Mol Neurobiol. 53:561–576. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Cherry JD, Olschowka JA and O'Banion MK:
Neuroinflammation and M2 microglia: The good, the bad, and the
inflamed. J Neuroinflammation. 11:982014. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Chan B and Sukhatme VP: Receptor tyrosine
kinase EphA2 mediates thrombin-induced upregulation of ICAM-1 in
endothelial cells in vitro. Thromb Res. 123:745–752. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Fang WB, Ireton RC, Zhuang G, Takahashi T,
Reynolds A and Chen J: Overexpression of EPHA2 receptor
destabilizes adherens junctions via a RhoA-dependent mechanism. J
Cell Sci. 121:358–368. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Yuan K, Hong TM, Chen JJ, Tsai WH and Lin
MT: Syndecan-1 up-regulated by ephrinB2/EphB4 plays dual roles in
inflammatory angiogenesis. Blood. 104:1025–1033. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Shen LL, Zhang LX, Wang LM, Zhou RJ, Yang
CZ, Zhang J and Yang PS: Disturbed Expression of EphB4, but Not
EphrinB2, inhibited bone regeneration in an in vivo inflammatory
microenvironment. Mediators Inflamm. 2016:64304072016. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhao J, Yuan G, Cendan CM, Nassar MA,
Lagerström MC, Kullander K, Gavazzi I and Wood JN:
Nociceptor-expressed ephrin-B2 regulates inflammatory and
neuropathic pain. Mol Pain. 6:772010. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Geng D, Kang L, Su Y, Jia J, Ma J, Li S,
Du J and Cui H: Protective effects of EphB2 on Abeta1-42
oligomer-induced neurotoxicity and synaptic NMDA receptor signaling
in hippocampal neurons. Neurochem Int. 63:283–290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Cissé M, Halabisky B, Harris J, Devidze N,
Dubal DB, Sun B, Orr A, Lotz G, Kim DH, Hamto P, et al: Reversing
EphB2 depletion rescues cognitive functions in Alzheimer model.
Nature. 469:47–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Henderson JT, Georgiou J, Jia Z, Robertson
J, Elowe S, Roder JC and Pawson T: The receptor tyrosine kinase
EphB2 regulates NMDA-dependent synaptic function. Neuron.
32:1041–1056. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Fu AK, Hung KW, Huang H, Gu S, Shen Y,
Cheng EY, Ip FC, Huang X, Fu WY and Ip NY: Blockade of EphA4
signaling ameliorates hippocampal synaptic dysfunctions in mouse
models of Alzheimer's disease. Proc Natl Acad Sci USA. 111:pp.
9959–9964. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Hardiman O, Al-Chalabi A, Chio A, Corr EM,
Logroscino G, Robberecht W, Shaw PJ, Simmons Z and van den Berg LH:
Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 3:170712017.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Tsuda H, Han SM, Yang Y, Tong C, Lin YQ,
Mohan K, Haueter C, Zoghbi A, Harati Y, Kwan J, et al: The
amyotrophic lateral sclerosis 8 protein VAPB is cleaved, secreted,
and acts as a ligand for Eph receptors. Cell. 133:963–977. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Van Hoecke A, Schoonaert L, Lemmens R,
Timmers M, Staats KA, Laird AS, Peeters E, Philips T, Goris A,
Dubois B, et al: EPHA4 is a disease modifier of amyotrophic lateral
sclerosis in animal models and in humans. Nat Med. 18:1418–1422.
2012. View Article : Google Scholar : PubMed/NCBI
|