Introduction
Remifentanil (RF) is widely used in general
anesthesia as a potent ultra-short-acting opioid µ receptor
agonist, with a rapid onset and short action time (1,2). RF is
able to cause opioid-induced hyperalgesia (OIH), enhancing pain
sensitivity and making it more difficult to manage postoperative
pain (3,4). It is known that hyperalgesia induced by
a high-dose (0.40–0.2 µg/kg/min) of RF results in increased
morphine consumption after surgery (5,6).
OIH is associated with decreased levels of
endogenous opioid peptides and increased activation of microglia
and N-methyl-D-aspartate receptors (NMDARs) (7). Microglia differentiate from spinal cord
monocytes and are representative immune cells in the central
nervous system that are thought to serve a role in central
sensitization and pain regulation (6–8). Pro-inflammatory cytokines
are associated with the activation of spinal nociceptive neurons
and inflammatory pain maintenance (9). Furthermore, microglia activation is
associated with a significant increase in the production of
pro-inflammatory cytokines, including tumor necrosis factor
(TNF)-α, interleukin (IL)-1β and IL-6 (10,11).
Previous studies have suggested that these cytokines, in
combination with the abnormal NMDAR activation, serve an important
role in central sensitization and hyperalgesia in the spinal dorsal
horn, possibly promoting OIH development and maintenance (12,13).
Clinical studies have revealed that a number of
pharmacological agents, including ketamine, propofol and nitric
oxide, attenuate RF-induced hyperalgesia (14,15);
however, these agents may have adverse effects, including chest
pain, confusion, paresthesia and hypotension, limiting their
clinical application (16).
Electro-acupuncture (EA) stimulation has been used for thousands of
years in traditional Chinese medicine to treat acute and chronic
pain with few complications (17).
The efficiency and safety of EA (18,19) have
made it one of the primary complementary methods for pain treatment
(9). Furthermore, EA has potential
as a treatment for postoperative pain (20) and has previously been applied in
postoperative analgesia (18,21). In
a previous study, our group demonstrated that EA reduces the
analgesic dose required and ameliorates pain in patients
postoperatively (22). The analgesic
mechanism of EA mainly involves the release of endogenous opioid
peptides, adenosine and 5-hydroxytryptamine (23,24).
The effects of EA on RF-induced postoperative
hyperalgesia (RIPH) remain unclear. Therefore, the aim of the
present study was to assess how EA impacts RIPH and explore the
underlying mechanisms. A rat model of RIPH (25,26) was
established and the effects of EA were assessed. The results
indicated that EA prevents RIPH, likely by suppressing spinal
microglia.
Materials and methods
Animals
A total of 96 adult male Sprague-Dawley rats (8–10
weeks; weighing 210–250 g) were provided by the animal center of
Anhui Medical University (Hefei, China) and housed in the animal
facility for 3–4 days prior to experiments. All rats were fed with
a 12-h light/dark cycle at a constant room temperature of 22±2°C
and relative humidity of 60–80%. The animals had access to food and
water ad libitum. The experimental protocols were approved
by the Institutional Animal Experimental Ethics Committee of Anhui
Medical University. All procedures were performed in accordance
with the ethical standards of the Institutional Animal Care and Use
Committee of Anhui Medical University.
Experimental protocol
A total of 96 rats were randomly divided into four
groups (n=24 in each): Normal saline (NS), RF, RF + EA (RF/EA) and
RF + sham acupuncture (RF/EA-sham). Rats were anesthetized with 30
mg/kg pentobarbital intraperitoneally. The Huantiao and
Yanglingquan acupoints or corresponding sham acupoints were
stimulated by EA in the RF/EA and RF/EA-sham groups, respectively,
during incision and medication procedures. Plantar incisional pain
was induced in each group. As appropriate, NS (0.8 ml/h for 60 min)
and RF (Yichang Renfu Pharmaceutical, Yichang, China; batch no.
6130502; 0.08 mg/kg at 0.8 ml/h for 60 min) (25,26) were
injected intravenously with a pump (Fig.
1).
EA procedure
Stainless steel acupuncture needles (0.18×30 mm)
were inserted 5 mm into the right hind leg at the Huantiao (GB30,
posterior upper edge of the hip joint) and Yanglingquan (GB34, 5 mm
below capitulum fibulae) acupoints, as previously described
(27,28) (Fig.
2). Stimulation was performed with a constant current pulse
generator model EL-608 (NKL Electronic Products, Brusque, Brazil)
for ~90 min (prior to incision until the end of RF administration).
The stimuli were set as 0.3 msec wide square waves at a frequency
of 2 Hz. Current intensity was increased in a stepwise fashion
until a muscle twitch was observed (~1 mA at 2 Hz) as described
previously (29,30). Rats in the RF/EA-sham group underwent
the same procedure but needles were inserted 0.5 cm right to the
correct acupoints (31).
 | Figure 2.Equivalence of human acupoints in
rats based on the Academic Department of China Association for
Acupuncture and Moxibustion and the Jiangsu Institute of
Traditional Chinese Medicine (28).
The two acupoints used in the present study are marked with red
stars. Each number represents an acupoint; the Chinese name and
standard international acupuncture nomenclature are also provided.
1, Shui gou (GV26); 2, Bai hui (GV20); 3, Tian men (BL2); 4, Er
jian (EX-HN6); 5, Da zhui (GV14); 6, Fei shu (UB13); 7, Xin shu
(UB15); 8, Ge shu (UB17); 9, Ji zhong (GV6); 10, Pi shu (UB20); 11,
Shen shu (UB23); 12, Hou hui; 13, Huan tiao (GB30); 14, Hou hai;
15, Yang ling quan (GB34); 16, Hou san li; 17, Zhao hai (KID6); 18,
San yin jiao (SP6); 19, Gen duan; 20, Shen mai (UB62); 21, Tai
chong (LIV3); 22, Guan yuan (CV4); 23, Xi qian; 24, Shen jue; 25,
Zhong wan (CV12); 26, Wei jian; 27, Qian san li; 28, Wai guan
(TE5); 29, Nei guan (PC6); 30, Qu chi (LI11); 31, Zhou jie; 32, Tan
zhong (CV17); 33, Cheng jiang (CV24). |
Plantar incision
Plantar incision was performed as previously
described by Brennan (32).
Following sterilization of the right hind paw with 10% iodophor
(Aitefu Co., Ltd., Huai'an, China), a 1 cm longitudinal incision
was made through the skin and fascia of the plantar aspect,
starting 0.5 cm from the proximal edge of the heel and extending
toward the toes. The plantaris muscle was elevated and incised
longitudinally. The muscle origin and insertion remained intact.
Following hemostasis with gentle pressure, the skin was apposed
with mattress sutures. The wound was covered with an ointment
containing polymyxin B, neomycin and bacitracin (Zhejiang Reachall
Pharmaceutical Co., Ltd., Dongyang, China) (32).
Behavioral tests
Paw withdrawal threshold (PWT) and paw withdrawal
latency (PWL) were assessed 24 h prior to RF infusion and at 4, 12,
24 and 48 h following the completion of RF infusion. Rats were
placed in individual wire cages with a mesh bottom and allowed to
adapt for 60 min prior to testing.
Mechanical hyperalgesia was assessed using an
electronic Von Frey filament (Harvard Apparatus, Holliston, MA,
USA) as described by Yuan et al (33). The filament was applied vertically to
the area adjacent to the wound on the right hind paw and pressure
was increased until a positive response occurred. The effective
pressure was then as the PWT. The test was repeated three times at
5-min intervals. A positive response was defined as clear paw
withdrawal, licking or squeaking. A cutoff pressure of 60 g was
used to prevent tissue damage.
Thermal hyperalgesia was measured using YLS-6B
intelligent hotplate equipment (Zhenghua Biologic Apparatus
Facilities Co., Ltd., Huaibei, China) as described by Yuan et
al (33). Rats were placed on a
50°C hotplate until a positive response was observed. The response
time was recorded as the PWL. The test was repeated three times at
10-min intervals. A positive response was defined as a clear paw
withdrawal and a cutoff time of 40 sec was used to prevent tissue
damage.
ELISA
Following RF infusion and behavioral tests, a total
of 6 rats per group were sacrificed at each time point. TNF-α (cat.
no. SC-52746), IL-1β (cat. no. SC-12742) and IL-6 (cat. no.
SC-57315) levels were measured in the spinal cord at lumbar
segments (L4–5) using ELISA kits (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) according to the
manufacturer's protocol.
Immunohistochemistry
At 4, 24 and 48 h following the completion of RF
infusion, following behavior testing, 2 rats were anesthetized with
an intraperitoneal injection of 350 mg/kg chloral hydrate. The
chest was opened and the right atrium was cut, 200 ml saline was
perfused rapidly into the left ventricular at 4°C and then the
right atrium was perfused with 200 ml paraformaldehyde at 4% for 6
h. The spinal arch plate was cut off, the spinal cord was exposed,
and L4–5 lumbar segments were dissected and removed.
Specimens were fixed in 4% paraformaldehyde at room temperature for
24 h and then embedded in paraffin. Each paraffin-cut section was 4
mm in thickness. CD11b expression was measured in the spinal cord
at lumbar segments (L4–5) using immunostaining with the
primary antibody OX-42 (cat. no. ab33827; 1:50; Abcam, Cambridge,
MA, USA) at 4°C for 12 h. Spinal cord sections were washed and
incubated for 30 min at 37°C with horseradish peroxidase-labeled
secondary antibodies (cat. no. PV-6000; 1:50; OriGene Technologies,
Inc., Beijing, China). A total of 6–10 images were captured for
each sample using an inverted microscope with a magnification of
×600. The area of positive staining for CD11b was assessed using
computerized morphometry (Image-Pro Plus software version 6.0;
Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc.,
Chicago, USA). Differences were assessed using one-way analysis of
variance with a post hoc least-significant difference test for
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
EA alleviates RF-induced
hyperalgesia
PWT and PWL values were similar in all groups prior
to infusion and decreased gradually following infusion, with the
lowest values at 24 h (Fig. 3). PWT
and PWL values were significantly decreased in the RF and
RF/EA-sham groups at 4, 12, 24 and 48 h following RF infusion
compared with the NS group (all P<0.05; Fig. 3), indicating RF-induced hyperalgesia.
Higher PWT and PWL values were observed in the RF/EA group compared
with the RF/EA-sham group at 4, 12, 24 and 48 h following RF
infusion (all P<0.05; Fig. 3).
These findings suggest that EA alleviates RF-induced hyperalgesia.
No significant differences in PWT and PWL values were observed
between the NS and RF/EA groups, or between the RF and RF/EA-sham
groups. These results suggest suggested that RF-induced
hyperalgesia was almost completely reversed following after EA
treatment, while sham EA had no effect.
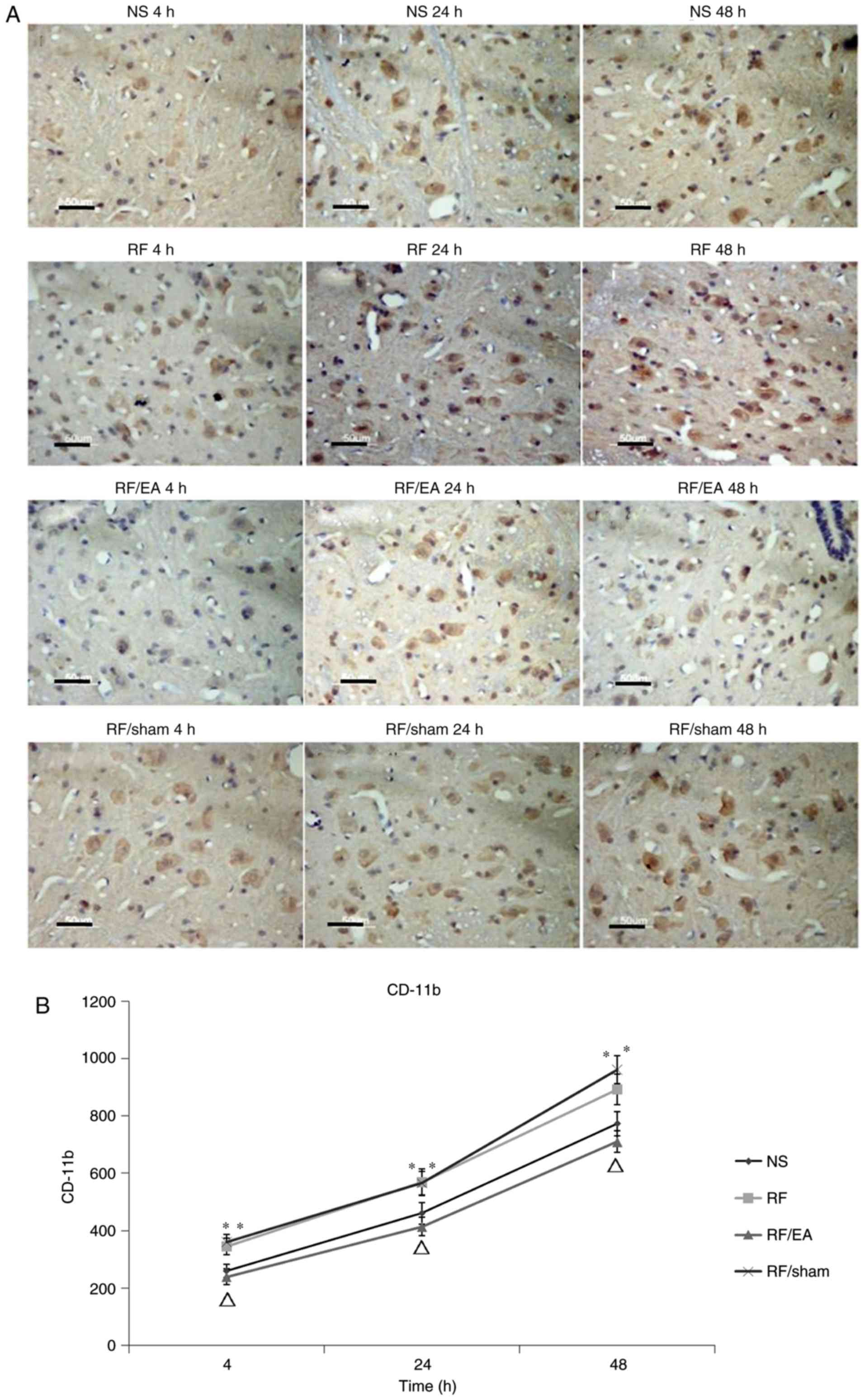
EA decreases CD11b levels
CD11b levels in the RF and RF/EA-sham groups were
significantly increased compared with those of the NS group at 4,
24 and 48 h following RF infusion (all P<0.05; Fig. 4), which indicates that RF treatment
increased the amounts of spinal microglia. Compared with the RF
group, CD11b levels were significantly decreased at 4, 24 and 48 h
following RF infusion in the RF/EA group (all P<0.05; Fig. 4), suggesting that EA decreased the
number of spinal microglia. No significant differences in CD11b
expression were observed between the NS and RF/EA groups or between
the RF and RF/sham groups at any time point. These results
demonstrate that EA treatment is able to completely recover the
number of spinal microglia following RF infusion, while sham EA has
no significant effect.
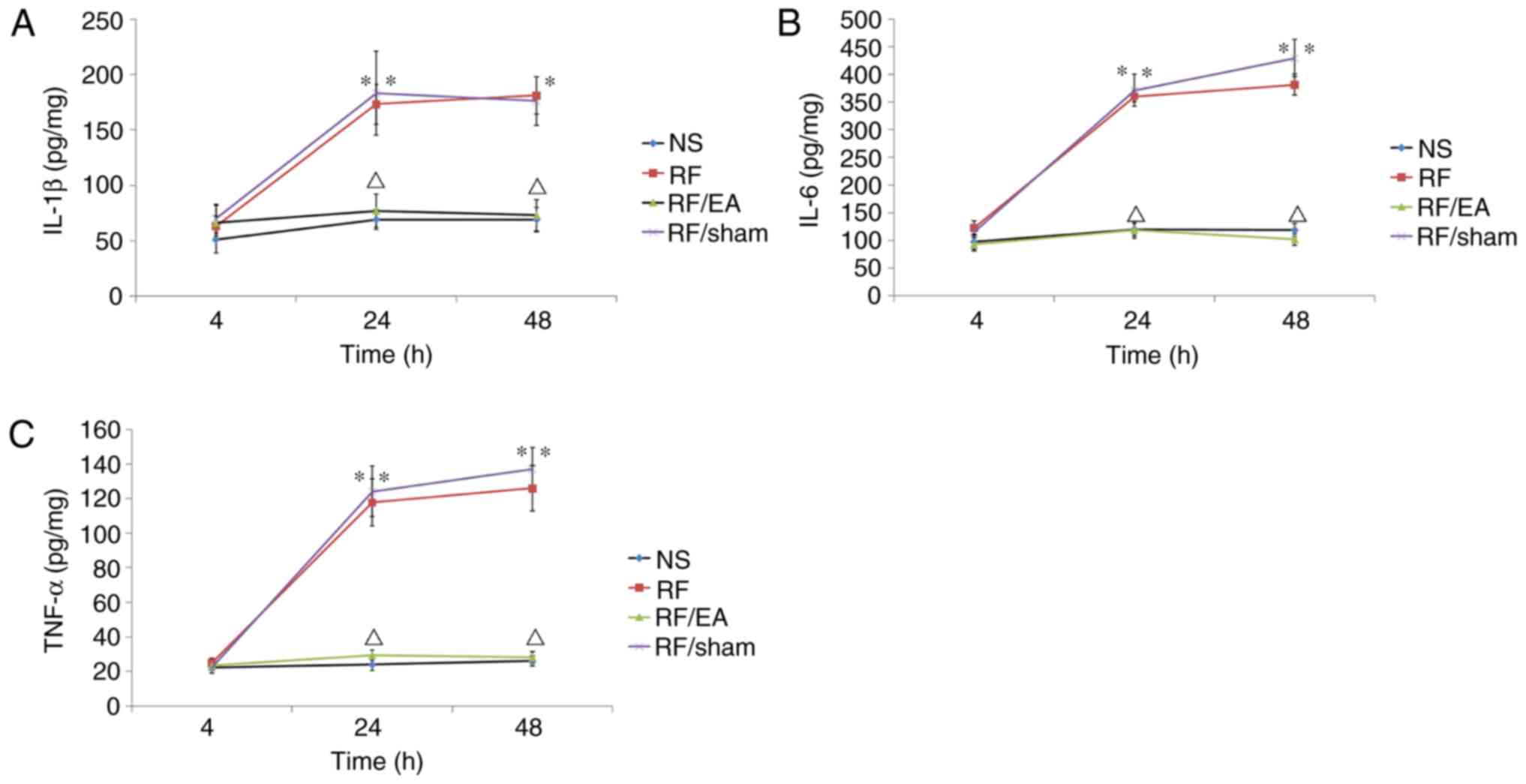
EA decreases TNF-α, IL-1β and IL-6
levels
TNF-α, IL-1β and IL-6 levels in the spinal cord at
lumbar segments (L4–5) were significantly higher in the
RF and RF/EA-sham groups compared with the NS control group at 24
and 48 h following RF infusion (P<0.05; Fig. 5), indicating that RF-induced
hyperalgesia was associated with spinal inflammation. Furthermore,
TNF-α, IL-1β and IL-6 levels in the RF/EA group were significantly
reduced compared with those of the RF/EA-sham group at 24 and 48 h
following infusion (P<0.05; Fig.
5), which suggests that EA suppresses the inflammatory response
in the spine. No significant differences were observed in TNF-α,
IL-1β or and IL-6 levels between the NS and RF/EA groups at any
time point. These results suggest that EA completely suppresses the
inflammatory response in the spine following RF infusion.
Discussion
In the present study, it was demonstrated that RF
administration reduces the PWT and PWL values in rats, while the
number CD11b positive cells is increased and TNF-α, IL-1β and IL-6
are upregulated. These effects were significantly alleviated by
treatment with EA at the Huantiao and Yanglingquan acupoints.
In the present study, RF was injected for 60 min to
construct a rat model of RIPH. Reduced PWT and PWL values confirmed
that the model had been successfully established. Cooper et
al (26) reported that the area
of mechanical hyperalgesia is significantly extended for 30 min
following the end of RF infusion for 90 min. It has also been
reported that RIPH occurs 2 h after anesthesia, peaking at 24–48 h
(34). In addition, Celerier et
al (35) demonstrated that 0.04
mg/kg RF-induced hyperalgesia occurs at 24 h and peaks at 24–48 h
post-surgery. These studies corroborate those of the present
study.
According to Traditional Chinese Medicine,
acupuncture at the Huantiao and Yanglingquan acupoints is effective
for the treatment of sciatica (36).
EA has been used successfully in patients treated with RF for pain
relief, both postoperatively (22,37,38) and
during surgery (39). It has has
been demonstrated that administering EA 30 min before anesthesia
improves cognitive function postoperatively, with reduced
inflammation (40).
In the present study, PWT and PWL values were higher
in the RF/EA group compared with the RF/EA-sham group, with no
significant differences observed between the RF/EA and NS groups,
indicating that electrical stimulation at the Huantiao and
Yanglingquan acupoints significantly alleviated RIPH. These
findings corroborate a previous study in which it was demonstrated
that RIPH decreases mechanical stimuli required and the thermal
pain threshold around the incision (41). The results are consistent with a
previous study by our group in which it was demonstrated that EA
alleviates postoperative pain in patients undergoing thoracic
esophagectomy (22).
The underlying mechanism responsible for the action
of EA in RF-induced hypoanalgesia remains to be elucidated. EA at
acupoints may release endogenous analgesics, including opioid
peptides, adenosine and 5-hydroxytryptamine (23–25,32). EA at the
Huantiao and Yanglingquan acupoints decreased the number of
microglia and suppressed the RF-induced inflammatory response in
the spinal cord. These findings suggest that EA likely alleviates
RIPH by suppressing activated spinal colloid cells that release
large amounts of proinflammatory cytokines. Using this as a basis,
specific targeting of microglia may be an effective method for
reducing postoperative pain and deserves further attention.
The main limitation of the present study is that it
was performed in a rat model, which may not translate exactly to
humans. However, rat acupoints do correspond with human acupoints
to a certain degree in terms of anatomy and physiological functions
(42–44). Previously studies have used pathological rat models to
assess the curative effects of acupuncture (42–44). In the present
study, EA was demonstrated to have curative effects when used to
stimulate specific acupuncture points in a rat model, which
suggests that these acupoints have a similar regulatory effect to
those in humans. Nevertheless, animal experiments are only intended
to provide a tentative exploration of possible mechanisms and these
hypotheses remain to be further explored in humans.
In summary, the results of the present preliminary
study demonstrate that EA inhibits RIPH in an incision pain rat
model, likely by decreasing the number of activated microglia in
the spinal cord and therefore reducing the expression of
proinflammatory cytokines. As such, controlling the activation of
spinal microglia may be a novel method for managing postoperative
pain.
Glossary
Abbreviations
Abbreviations:
|
EA
|
electro-acupuncture
|
|
NMDARs
|
N-methyl-D-aspartate receptors
|
|
NS
|
normal saline
|
|
OIH
|
opioid-induced hyperalgesia
|
|
PWL
|
paw thermal withdrawal latency
|
|
PWT
|
paw withdrawal threshold
|
|
RF
|
remifentanil
|
|
RF/EA
|
remifentanil and
electro-acupuncture
|
|
RF/EA-sham
|
remifentanil and sham acupuncture
|
|
RIPH
|
remifentanil-induced postoperative
hyperalgesia
|
Acknowledgements
The authors would like to thank Professor Liecheng
Wang at the Department of Physiology, Anhui Medical University.
Funding
The present study was supported by the Department of
Health of Anhui Province (grant no. 2012zy45).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX, JM and DI conceived and designed the
experiments. YX and JM performed the experiments. YX and CG
analyzed the data. DI and XC revised the manuscript and approved
the final version.
Ethics approval and consent to
participate
The experimental protocols were approved by the
Institutional Animal Experimental Ethics Committee of Anhui Medical
University (Hefei, China). All procedures were performed in
accordance with the ethical standards of the Institutional Animal
Care and Use Committee of Anhui Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khanykin B, Siddiqi R, Jensen PF, Bigler
DR and Atroshchenko GV: Comparison of remifentanil and low-dose
fentanyl for fast-track cardiac anesthesia: A prospective
randomized study. Heart Surg Forum. 16:E324–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Douma MR, Verwey RA, Kam-Endtz CE, van der
Linden PD and Stienstra R: Obstetric analgesia: A comparison of
patient-controlled meperidine, remifentanil, and fentanyl in
labour. Br J Anaesth. 104:209–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fletcher D and Martinez V: Opioid-induced
hyperalgesia in patients after surgery: A systematic review and a
meta-analysis. Br J Anaesth. 112:991–1004. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rivosecchi RM, Rice MJ, Smithburger PL,
Buckley MS, Coons JC and Kane-Gill SL: An evidence based systematic
review of remifentanil associated opioid-induced hyperalgesia.
Expert Opin Drug Saf. 13:587–603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrenko AB, Ishii H, Kohno T and Baba H:
When similar is not alike: Decreased sensory thresholds after
intravenous infusion of remifentanil may not be
remifentanil-induced hyperalgesia. Anesth Analg. 115:9772012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lü
N, Liu YC and Ji RR: Extracellular caspase-6 drives murine
inflammatory pain via microglial TNF-α secretion. J Clin Invest.
124:1173–1186. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang CT, Chiang RP, Chen CL and Tsai YJ:
Sleep deprivation aggravates median nerve injury-induced
neuropathic pain and enhances microglial activation by suppressing
melatonin secretion. Sleep. 37:1513–1523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mika J, Popiolek-Barczyk K, Rojewska E,
Makuch W, Starowicz K and Przewlocka B: Delta-opioid receptor
analgesia is independent of microglial activation in a rat model of
neuropathic pain. PloS One. 9:e1044202014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vickers AJ, Rusch VW, Malhotra VT, Downey
RJ and Cassileth BR: Acupuncture is a feasible treatment for
post-thoracotomy pain: Results of a prospective pilot trial. BMC
Anesthesiol. 6:52006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milligan ED, Twining C, Chacur M,
Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF and
Watkins LR: Spinal glia and proinflammatory cytokines mediate
mirror-image neuropathic pain in rats. J Neurosci. 23:1026–1040.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Berta T, Xu ZZ, Liu T, Park JY
and Ji RR: TNF-α contributes to spinal cord synaptic plasticity and
inflammatory pain: Distinct role of TNF receptor subtypes 1 and 2.
Pain. 152:419–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maresz K, Pryce G, Ponomarev ED, Marsicano
G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK,
et al: Direct suppression of CNS autoimmune inflammation via the
cannabinoid receptor CB1 on neurons and CB2 on autoreactive T
cells. Nat Med. 13:492–497. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y, Zhang W, Liu Y, Liu X, Ma Z and Gu
X: Intrathecal injection of JWH015 attenuates remifentanil-induced
postoperative hyperalgesia by inhibiting activation of spinal glia
in a rat model. Anesth Analg. 118:841–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song JW, Lee YW, Yoon KB, Park SJ and Shim
YH: Magnesium sulfate prevents remifentanil-induced postoperative
hyperalgesia in patients undergoing thyroidectomy. Anesth Analg.
113:390–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Echevarria G, Elgueta F, Fierro C, Bugedo
D, Faba G, Iñiguez-Cuadra R, Muñoz HR and Cortínez LI: Nitrous
oxide (N(2)O) reduces postoperative opioid-induced hyperalgesia
after remifentanil-propofol anaesthesia in humans. Br J Anaesth.
107:959–965. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elterman KG, Mallampati SR, Kaye AD and
Urman RD: Postoperative alterations in taste and smell. Anesth Pain
Med. 4:e185272014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen T, Wang K, Xu J, Ma W and Zhou J:
Electroacupuncture reduces postoperative pain and analgesic
consumption in patients undergoing thoracic surgery: A randomized
study. Evid Based Complement Alternat Med. 2016:21264162016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Linde K, Vickers A, Hondras M, ter Riet G,
Thormählen J, Berman B and Melchart D: Systematic reviews of
complementary therapies - an annotated bibliography. Part 1:
Acupuncture. BMC Complement Altern Med. 1:32001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park JH, Han JB, Kim SK, Park JH, Go DH,
Sun B and Min BI: Spinal GABA receptors mediate the suppressive
effect of electroacupuncture on cold allodynia in rats. Brain Res.
1322:24–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ma W, Zhu YM, Zhou H, Fu GQ, Pan H and
Shen WD: Protecting action of acupuncture-drug compound anesthesia
with different frequency electroacupuncture on stress reaction in
pneumonectomy. Zhongguo Zhen Jiu. 31:1020–1024. 2011.(In Chinese).
PubMed/NCBI
|
|
21
|
Robinson CR, Zhang H and Dougherty PM:
Astrocytes, but not microglia, are activated in oxaliplatin and
bortezomib-induced peripheral neuropathy in the rat. Neuroscience.
274:308–317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xie YH, Chai XQ, Wang YL, Gao YC and Ma J:
Effect of electro-acupuncture stimulation of Ximen (PC4) and
Neiguan (PC6) on remifentanil-induced breakthrough pain following
thoracal esophagectomy. J Huazhong Univ Sci Technolog Med Sci.
34:569–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su TF, Zhang LH, Peng M, Wu CH, Pan W,
Tian B, Shi J, Pan HL and Li M: Cannabinoid CB2 receptors
contribute to upregulation of β-endorphin in inflamed skin tissues
by electroacupuncture. Mol Pain. 7:982011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fais RS, Reis GM, Silveira JW, Dias QM,
Rossaneis AC and Prado WA: Amitriptyline prolongs the
antihyperalgesic effect of 2- or 100-Hz electro-acupuncture in a
rat model of post-incision pain. Eur J Pain. 16:666–675. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onda A, Jiao Q, Nagano Y, Akimoto T,
Miyamoto T, Minamisawa S and Fukubayashi T: Acupuncture ameliorated
skeletal muscle atrophy induced by hindlimb suspension in mice.
Biochem Biophys Res Commun. 410:434–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cooper ZD, Truong YN, Shi YG and Woods JH:
Morphine deprivation increases self-administration of the fast- and
short-acting mu-opioid receptor agonist remifentanil in the rat. J
Pharmacol Exp Ther. 326:920–929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanisch UK and Kettenmann H: Microglia:
Active sensor and versatile effector cells in the normal and
pathologic brain. Nat Neurosci. 10:1387–1394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hua X, LI C, Zhou H, Song D and Hu Y: The
trituration of the atlas of the rat acupoints. Shiyan Dongwu Yu
Dongwu Shiyan. 3:1–5. 1991.
|
|
29
|
Romita VV, Suk A and Henry JL: Parametric
studies on electroacupuncture-like stimulation in a rat model:
Effects of intensity, frequency, and duration of stimulation on
evoked antinociception. Brain Res Bull. 42:289–296. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lao L, Zhang RX, Zhang G, Wang X, Berman
BM and Ren K: A parametric study of electroacupuncture on
persistent hyperalgesia and Fos protein expression in rats. Brain
Res. 1020:18–29. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fang JL, Krings T, Weidemann J, Meister IG
and Thron A: Functional MRI in healthy subjects during acupuncture:
Different effects of needle rotation in real and false acupoints.
Neuroradiology. 46:359–362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brennan TJ, Vandermeulen EP and Gebhart
GF: Characterization of a rat model of incisional pain. Pain.
64:493–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yuan Y, Wang JY, Yuan F, Xie KL, Yu YH and
Wang GL: Glycogen synthase kinase-3β contributes to
remifentanil-induced postoperative hyperalgesia via regulating
N-methyl-D-aspartate receptor trafficking. Anesth Analg.
116:473–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gu X, Wu X, Liu Y, Cui S and Ma Z:
Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B
subunit in spinal cord contributes to remifentanil-induced
postoperative hyperalgesia: The preventive effect of ketamine. Mol
Pain. 5:762009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Celerier E, Gonzalez JR, Maldonado R,
Cabanero D and Puig MM: Opioid-induced hyperalgesia in a murine
model of postoperative pain: Role of nitric oxide generated from
the inducible nitric oxide synthase. Anesthesiology. 104:546–555.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ji M, Wang X, Chen M, Shen Y, Zhang X and
Yang J: The efficacy of acupuncture for the treatment of sciatica:
A systematic review and meta-analysis. Evid Based Complement
Alternat Med. 2015:1928082015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iacobone M, Citton M, Zanella S, Scarpa M,
Pagura G, Tropea S, Galligioni H, Ceccherelli F, Feltracco P, Viel
G and Nitti D: The effects of acupuncture after thyroid surgery: A
randomized, controlled trial. Surgery. 156:1605–1612. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang H, Xie Y, Zhang Q, Xu N, Zhong H,
Dong H, Liu L, Jiang T, Wang Q and Xiong L: Transcutaneous electric
acupoint stimulation reduces intra-operative remifentanil
consumption and alleviates postoperative side-effects in patients
undergoing sinusotomy: A prospective, randomized,
placebo-controlled trial. Br JAnaesth. 112:1075–1082. 2014.
View Article : Google Scholar
|
|
39
|
Sator-Katzenschlager SM, Wolfler MM,
Kozek-Langenecker SA, Sator K, Sator PG, Li B, Heinze G and Sator
MO: Auricular electro-acupuncture as an additional perioperative
analgesic method during oocyte aspiration in IVF treatment. Hum
Reprod. 21:2114–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Q, Li YN, Guo YY, Yin CP, Gao F, Xin
X, Huo SP, Wang XL and Wang QJ: Effects of preconditioning of
electro-acupuncture on postoperative cognitive dysfunction in
elderly: A prospective, randomized, controlled trial. Medicine
(Baltimore). 96:e73752017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao M and Joo DT: Enhancement of spinal
N-methyl-D-aspartate receptor function by remifentanil action at
delta-opioid receptors as a mechanism for acute opioid-induced
hyperalgesia or tolerance. Anesthesiology. 109:308–317. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang Y, Zhang RX, Zhang M, Shen XY, Li A,
Xin J, Ren K, Berman BM, Tan M and Lao L: Electroacupuncture
inhibition of hyperalgesia in an inflammatory pain rat model:
Involvement of distinct spinal serotonin and norepinephrine
receptor subtypes. Br J Anaesth. 109:245–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu W, Wu J, Huang J, Zhuo P, Lin Y, Wang
L, Lin R, Chen L and Tao J: Electroacupuncture regulates
hippocampal synaptic plasticity via miR-134-Mediated LIMK1 function
in rats with ischemic stroke. Neural Plast. 2017:95456462017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu Y, Deng L, Tang H, Gao X, Wang Y, Guo
K, Kong J and Yang C: Electroacupuncture improves neurobehavioral
function and brain injury in rat model of intracerebral hemorrhage.
Brain Res Bull. 131:123–132. 2017. View Article : Google Scholar : PubMed/NCBI
|