Introduction
The 2008 World Health Organization (WHO)
classification defines hydroa vacciniforme-like lymphoma (HVLL) as
a clinicopathological type of cutaneous lymphoma with Epstein-Barr
virus (EBV) infection (1). HVLL is a
type of EBV+ T-cell lymphoproliferative disorder (LPD) that
generally occurs during childhood (1). Studies have demonstrated that the
prevalence of HVLL is particularly high across Asia, and Central
and South America (2–6). The 2016 revision of the WHO
classification now formally classifies childhood systemic EBV+
T-cell LPD as childhood systemic EBV+ T-cell lymphoma, in order to
emphasize its aggressive clinical course (7). Furthermore, HVLL is no longer
categorized as a subtype of childhood EBV+ T-cell lymphoma and
‘HVLL’ is now referred to hydroa vacciniforme-like
lymphoproliferative disorder (HVLPD) in the 2016 WHO
classification, since this disease has distinctive
clinicopathological features (7).
HVLPD is a primarily cutaneous disorder with a broad spectrum of
clinical aggressiveness and HVLL is considered a syndrome
accompanying this disease. Unlike in classical hydroa vacciniforme
(HV), HVLPD skin lesions are located on sun-exposed and
non-sun-exposed areas of the skin and tend to worsen with age. The
majority of patients with HVLPD present with systemic symptoms,
including fever, lymphadenopathy and hepatosplenomegaly (5,8,9). The majority of patients with HVLPD
exhibit a T-cell phenotype, however some also exhibit the positive
expression of cluster of differentiation (CD)56 in tumor cells,
suggesting that HVLPD may originate from natural killer (NK) cells
(10–14). Differentiating between HVLPD with
CD56+ expression and cutaneous natural killer T-cell lymphoma
(CNKTL) remains challenging. These two diseases share many
histopathological features, particularly as they exhibit similar
immunophenotypic markers (11).
Therefore, in the 2005 WHO classification, the HVLPD-NK cell
phenotype is considered to be a variant of NK/T-cell lymphoma,
nasal type.
The present study investigated the
clinicopathological features of 5 patients with a HVLPD-NK cell
phenotype and compared these to the clinicopathological features of
11 patients with CNKTL, to emphasize that the HVLPD-NK cell
phenotype should be considered as a separate entity to CNKTL and
evaluated with caution.
Patients and methods
Patients
The biopsied tissues of 5 patients (4 females and 1
male) with the HVLPD-NK cell phenotype attending the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China)
between February 2011 and December 2014 were obtained. The median
age of these patients was 7 years old (range, 4–14 years old).
Tissues from patients diagnosed with CNKTL were also obtained and
analyzed as the control group. A total of 11 patients with CNKTL
were included in the current study, of which 7 were male and 4 were
female. The median age of these patients was 53 years old (range,
35–69 years old). All cases were diagnosed according to the 2016
WHO classification criteria (7).
Among the 5 cases of HVLPD, one case was initially considered to be
NK/T cell lymphoma; the final diagnosis of HVLPD was reached
following a review of the patient's tissue specimen. All specimens
were routinely processed, embedded in paraffin, sectioned and
stained with hematoxylin and eosin.
Immunohistochemistry (IHC)
Paraffin-embedded tissue was fixed using 10%
formalin at 37°C for >6 h, which were divided into 4-µm-thick
sections on an automated immunostainer (Ventana Medical Systems,
Inc., Tucson, AZ, USA). Endogenous peroxidase and phosphatase
activity was blocked by 3% hydrogen peroxide for 4 min at 37°C. The
following antibodies were used: CD20 (dilution, ready-to-use; cat.
no. 14357208), CD4 (dilution, ready-to-use; cat. no. 15367406), CD8
(dilution, ready-to-use; cat. no. 15456809), CD56 (dilution,
ready-to-use; cat. no. 14004708), Granzyme-B (dilution,
ready-to-use; cat. no. 16691906), CD30 (dilution, ready-to-use;
cat. no. 15331003), Ki-67 (dilution, ready-to-use; cat. no.
14567378) were purchased from (OriGene Technologies, Inc., Beijing,
China). CD3 (dilution, ready-to-use; cat. no. 130801543E) and TIA-1
(dilution, ready-to-use; cat. no. 160426599E) were purchased from
Fuzhou Maixin Biotech, Co., Ltd. (Fuzhou, China). Endogenous
peroxidase activity blocked by 3% hydrogen peroxide for 4 min at
37°C. Secondary anti-rat antibodies (dilution, ready-to-use; cat.
no. 760-500; Roche Diagnostics, Basel, Switzerland) conjugated to
horseradish peroxidase were applied for 30 min at 37°C. The
sections were counterstained with hematoxylin at 37°C for 2 min and
a coverslip was applied.
In situ hybridization (ISH) for
EBV
EBV RNA was detected using the ISH technique using
the Epstein-Barr Virus Early RNA kit (cat. no. ISH-5021; OriGene
Technologies, Inc.), following the manufacturer's protocol.
Briefly, 4–6 µm sections were cut from paraffin-embedded tissues,
deparaffinized with xylene at 37°C for 10 min, rehydrated,
predigested with proteinase K (OriGene Technologies, Inc.) and
hybridized with DIG-labeled RNA probe. Following washing, the
reaction was accomplished using anti-DIG horseradish peroxidase
conjugate (OriGene Technologies, Inc.), followed by staining with
3,3′-diaminobenzidine substrate at 37°C for 5 min.
Polymerase chain reaction (PCR)
PCR was performed on all 5 cases of HVLPD and only
certain cases of CNKTL (the tissues from a number of cases were not
sufficient for PCR due to necrosis or amounts) to evaluate T-cell
receptor gene rearrangement, following the BIOMED-2 protocol as
previously described (15). The 56
primers for the clonal rearrangement analysis of the TCR gene were
all selected from the BIOMED-2 primer system (cat. no. 200008;
Shanghai Yuanqi Biotechnology Co., Ltd., Shanghai, China), which
was used to detect the TCRβ, TCRγ, and TCRδ chains. The BIOMED-2
primer system also contained five internal control primers. Total
DNA from the tissue samples was amplified using GoldStar Best DNA
Polymerase (CWBIO, Beijing, China) according to the manufacturer's
protocol. The thermocycling conditions were as follows: 7 min at
95°C, followed by and 40 cycles for 45 sec at 95°C and 1 min at
72°C. The primer sequences reference to commercial kit (Shanghai
Yuanqi Biotechnology Co., Ltd., Shanghai, China).
Results
Clinical features
The clinical features of the 5 patients are
summarized in Table I. All patients
presented with skin lesions that were marked by recurring
outbreaks, including papulovesicular eruption, ulceration and
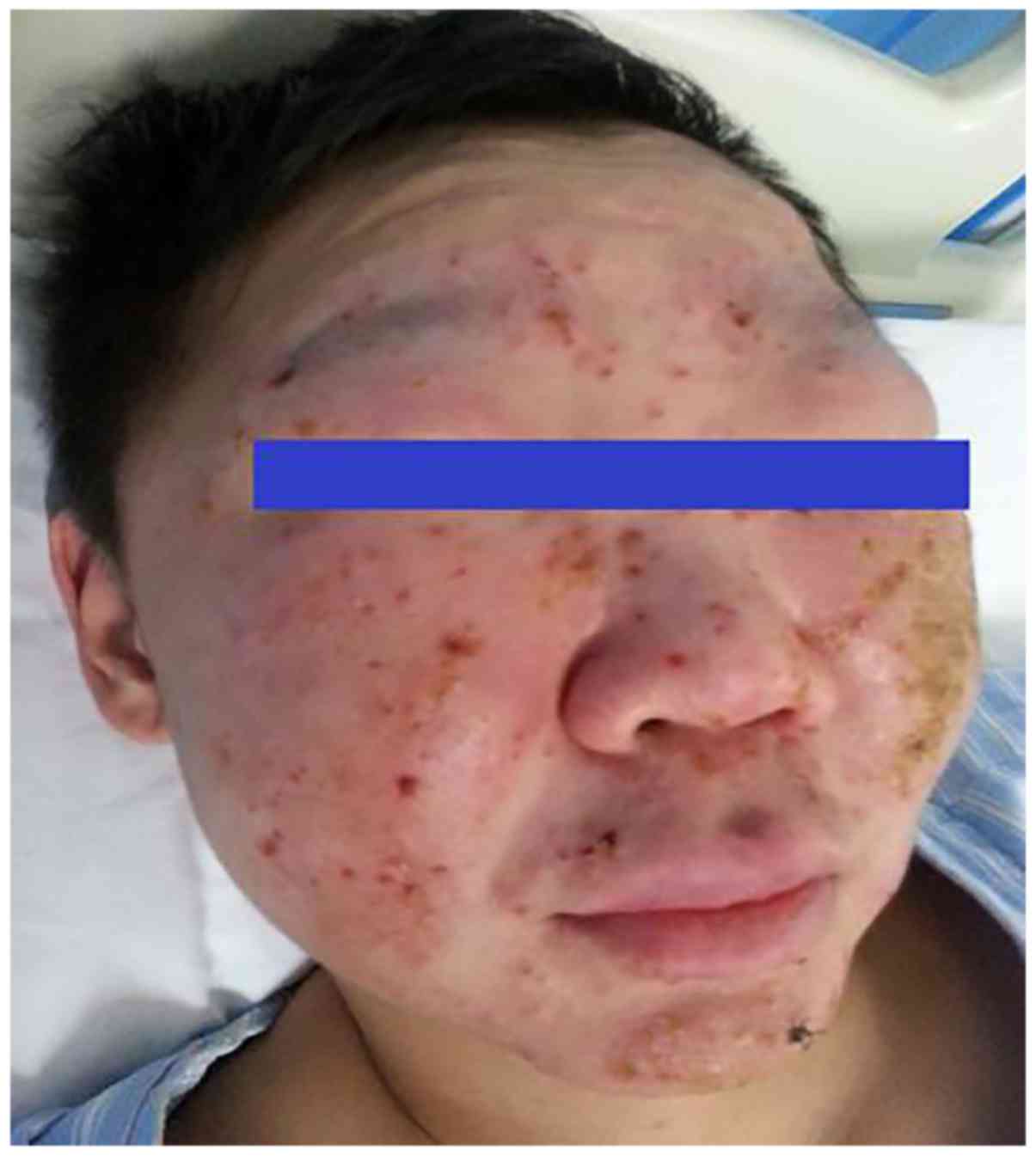
scarring. In 4 of the 5 patients, cutaneous lesions were present on
the face (Fig. 1); another patient
(case 1) had lesions in the trunk and extremities but did not
present with lesions on the face involvement. All patients
presented with systemic symptoms, including fever, lymphadenopathy
and hepatosplenomegaly. The median duration of the disease from the
occurrence of skin lesions to the onset of systemic symptoms was 33
months. None of the patients had bone marrow involvement. Of the 5
cases, 2 (cases 2 and 5) received cyclophosphamide, adriamycin,
vincristine, prednisone (CHOP) chemotherapy; the other two cases
(cases 3 and 4) received glucocorticoids (dexamethasone) as
symptomatic treatment for ~1 week. Case 1 was lost prior to follow
up. At the end of the study, 3 patients were alive; however, case 4
succumbed to systemic disease, including infection and hepatic
failure <13 months after being admitted to the First Affiliated
Hospital of Zhengzhou University.
 | Table I.Clinical features of the HVLPD-NK cell
phenotype. |
Table I.
Clinical features of the HVLPD-NK cell
phenotype.
| Patient | Sex | Age (years) | Distribution of
lesions | Fever | Bone marrow
involvement | Initial
treatment | Status | Duration prior to
progression to systemic symptoms (months) | Survival following
diagnosis (months) |
|---|
| 1 | F | 4 | Haunch and
extremities | Yes | No | − | − | 24 | − |
| 2 | F | 4 | Face | Yes | No | CHOP | Alive | 19 | N/A |
| 3 | F | 9 | Face and
extremities | Yes | No |
Ganciclovir/Steroids | Alive | 33 | N/A |
| 4 | M | 14 | Face and
extremities | Yes | No | Steroids | Succumbed | 54 | 13 |
| 5 | F | 7 | Face and upper
limbs | Yes | No | CHOP | Alive | 36 | N/A |
Histopathological features
The histopathological features of patients are
presented in Table II.
Histopathologically, all cases exhibited polymorphic lymphoid
infiltration throughout the dermis and subcutaneous tissue. In the
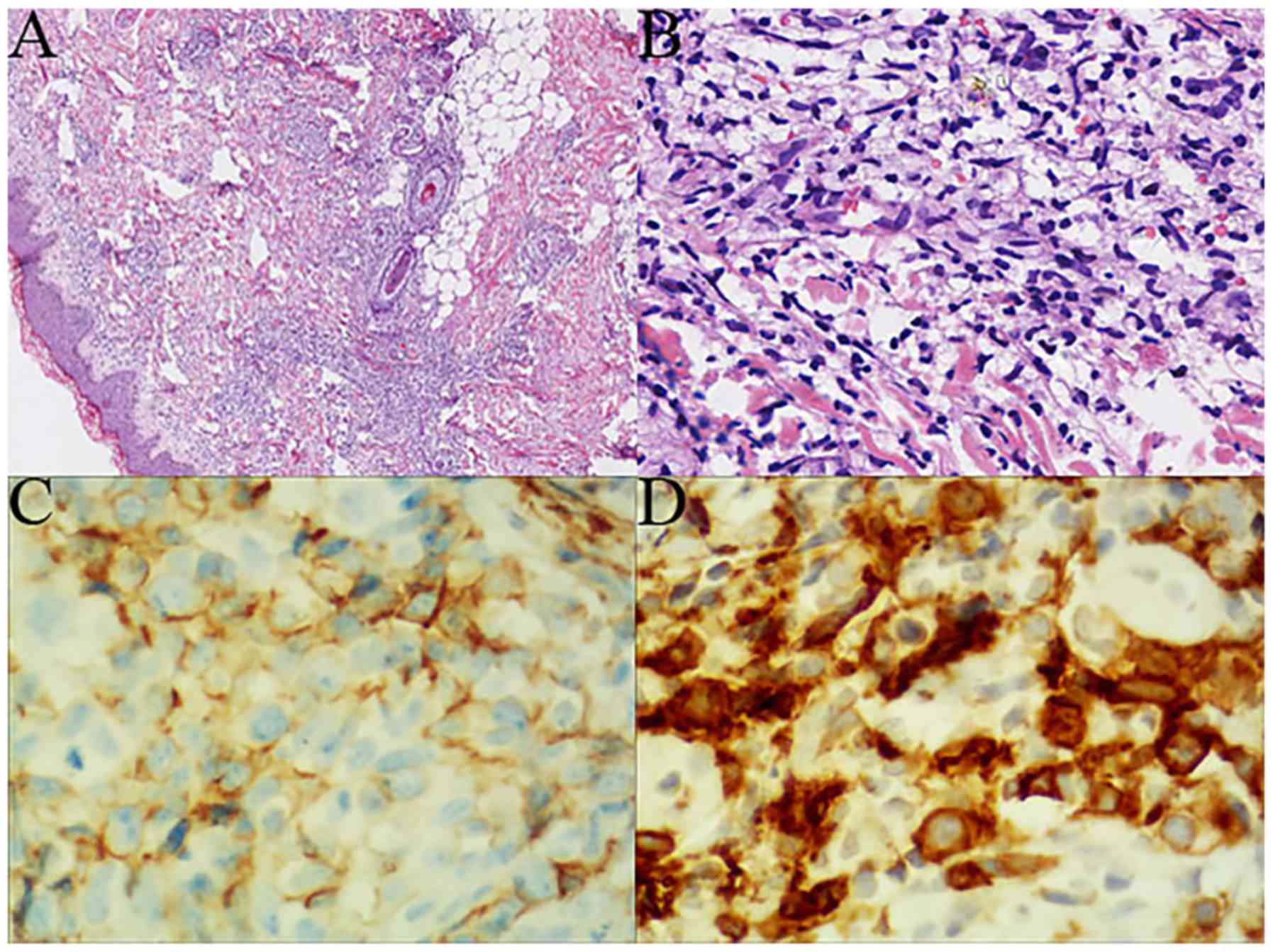
majority of cases (4/5), the squamous epithelium was not
infiltrated or destroyed (Fig. 2A).
There was a patchy or nodular dense lymphoid infiltrate, with
prominent skin adnexa involvement and vascular destruction in the
majority of cases. However, tumor necrosis was only observed in one
case (case 4; Table III). Lymphoid
cells were small-to-medium-sized, exhibiting mild atypia and
irregular nuclear contours (Fig.
2B). In case 2, a large number of neutrophils were found within
the corium layer.
 | Table II.Histopathological features of HVLPD-NK
cell phenotype. |
Table II.
Histopathological features of HVLPD-NK
cell phenotype.
| Patient | Epidermis
involvement | Skin adnexa
involvement | Vascular
destruction | Necrosis | Subcutaneous
infiltrate | Cellular
morphology |
|---|
| 1 | No | Yes | Yes | No | Yes | Small |
| 2 | No | Yes | No | No | Yes | Small-medium |
| 3 | No | Yes | Yes | No | Yes | Small-medium |
| 4 | Yes | Yes | Yes | Yes | Yes | Medium |
| 5 | No | Yes | Yes | No | Yes | Small-medium |
 | Table III.Immunohistochemical analysis and EBER
results of this series. |
Table III.
Immunohistochemical analysis and EBER
results of this series.
| Patient | CD20 | CD3 | CD4 | CD8 | CD56 | Gran-B | TIA-1 | CD30 | Ki-67 (%) | EBV | TCR |
|---|
| 1 | − | + | + | − | + | + | + | − | 30 | + | Polyclonal |
| 2 | − | +/− | − | − | + | + | + | − | 40 | + | Polyclonal |
| 3 | − | +/− | −/+ | − | + | + | + | −/+ | 60 | + | Polyclonal |
| 4 | − | + | − | − | + | + | + | − | 60 | + | Monoclonal |
| 5 | − | + | − | −/+ | + | + | + | + | 40 | + | Polyclonal |
Immunohistochemical analysis and ISH
for EBV
Neoplastic lymphoid cells from all cases expressed
T-cell- and NK-cell-associated antigens, including CD3 and CD56
(Fig. 2C); however they were all
negative for CD20 (Table III).
Weak expression of CD3 was observed in two cases (cases 2 and 3).
One case (case 5) was weakly positive for CD8 and two cases (cases
1 and 3) were positive for CD4. The other two cases (cases 2 and 4)
were negative for CD4 and CD8. The cytotoxic markers TIA-1 and
Granzyme-B were positive in all cases. CD30 was heterogeneously
expressed in cases 3 and 5 (Fig.
2D). Proliferative activity was assessed by Ki-67, which ranged
between 30 and 70% of the tumor cells. All 5 cases were determined
to be EBV positive following ISH detection.
Molecular studies
All 5 cases were analyzed via PCR. Four cases
revealed evidence of polyclonal T cell receptor gene rearrangement,
while 1 case (case 4) was positive for monoclonal T cell receptor γ
chain gene rearrangement (Table
III).
Comparison of clinicopathological
features of patients with HVLPD-NK compared with patients with
CNKTL
Table IV provides an
analysis of the clinicopathological features of patients with CNKTL
(n=11) compared with those that had the HVLPD-NK cell phenotype
(n=5). A total of 8 patients (73%) with CNKTL presented with
nodules or plaques on the extremities and only 1 case (9%) had
lesions on the trunk alone. A further 2 cases (18%) revealed the
involvement of the trunk and extremities. Infiltrations of the
nasal cavity were observed in 2 cases. One case was admitted to the
hospital with a presumptive diagnosis of skin cancer, as it
revealed a large crateriform ulcer on the leg.
 | Table IV.Comparison of the clinicopathological
characteristics of patients with HVLPD-NK cell phenotype and those
with CNKTL. |
Table IV.
Comparison of the clinicopathological
characteristics of patients with HVLPD-NK cell phenotype and those
with CNKTL.
|
Characteristics | HVLPD-NK cell
phenotype (n=5) | CNKTL (n=11) |
|---|
| Median age
(years) | 7 (4–14) | 53 (35–69) |
| Distribution of
lesions (%) |
|
|
|
Face | 80 (4/5) | 18 (2/11) |
|
Trunk | 20 (1/5) | 27 (3/11) |
|
Extremities | 80 (4/5) | 91 (10/11) |
| Initial treatment
(%) |
|
|
| CHOP
(or other chemotherapies) | 50 (2/4) | 45 (5/11) |
|
Radiotherapy | 0 (0/4) | 18 (2/11) |
|
Chemo-radiotherapy | 0 (0/4) | 18 (2/11) |
|
Steroids | 50 (2/4) | 0 (0/11) |
| No
treatment | 0 (0/4) | 18 (2/11) |
| Epidermis
involvement | 20 (1/5) | 64 (7/11) |
| Skin adnexa
involvement | 100 (5/5) | 100 (11/11) |
| Vascular
destruction | 80 (4/5) | 100 (11/11) |
| Necrosis | 20 (1/5) | 91 (10/11) |
| Cellular
morphology |
|
|
|
Small-medium | 100 (5/5) | 0 (0/11) |
|
Medium-large | 0 (0/5) | 82 (9/11) |
|
Large | 0 (0/5) | 18 (2/11) |
A total of 9 patients (82%) received at least one of
the following therapies: Chemotherapy alone (45%; 5 of 11),
radiotherapy alone (18%; 2 of 11) and concurrent chemo-radiotherapy
(18%; 2 of 11). Out of the 11 patients, 5 (45%) had succumbed by
the end of the follow-up period and the median follow-up period was
23 months.
Skin adnexa involvement and vascular destruction
were observed in all patients with CNKTL (Table IV), which was also the case in
HVLPD-NK (Fig. 3A). A total of 7
cases with CNKTL (64%) exhibited epidermis involvement and necrosis
(Fig. 3B) was observed in the
majority of cases with CNKTL (91%). Tumor cells had a monomorphic
appearance and were medium-to-large or large sized with obvious
atypia. All CNKTL cases were EBV positive.
Discussion
There have been few studies regarding the HVLPD-NK
cell phenotype and half of these have been case reports (10,12,13). The
current study assessed 5 patients with HVLPD-NK and indicated that
all 5 cases exhibited clinical and histological features similar to
the typical HVLPD-T phenotype. The difference was that these cases
revealed an unusual immunophenotype of CD56 expression, revealing
that they had a NK cell phenotype of HVLPD. Although all cases
exhibited positive expression of CD3, 2 of the 5 cases exhibited
weak expression of CD3. Two cases exhibited the CD4-/CD8-
immunophenotype, highlighting the NK cell origin. However, another
two cases exhibited CD4 expression and one case had a weak CD8
expression, which revealed it to be a T cell phenotype. Thus,
CD4/CD8 immunophenotype expression could not be used to diagnose
HVLPD. These results differ from the results of studies by Doeden
et al (10) and Magaña et
al (14) and demonstrate that a
variety of CD4/CD8 immunophenotypes may be observed in the HVLPD-NK
cell phenotype.
The HVLPD-NK cell phenotype may be particularly
difficult to distinguish from CNKTL for the reason that these two
diseases share similar immunophenotypic markers for CD3, CD56,
Granzyme-B and TIA-1. These two diseases are also EBV positive and
have a high KI-67 proliferation index (11). Among the 5 cases included in the
current study, case 2 was initially misdiagnosed as NK/T cell
lymphoma and the patient received an inappropriate treatment.
The clinicopathological features of these two
diseases have been summarized in the current study to identify the
differences between them. Firstly, there was a marked difference
regarding the median age of patients with these two diseases. The
HVLPD-NK cell phenotype was more likely occur in children and
adolescents, whereas patients with CNKTL were generally middle-aged
or elderly. Secondly, there was a difference in the distribution of
lesions between these two diseases. The current study demonstrated
that patients with the HVLPD-NK cell phenotype were more likely to
present with cutaneous lesions on the face, whereas patients with
CNKTL generally exhibited lesions on the trunk and extremities.
Consistent with the results of the current study, Mraz-Gernhard
et al (16) reported that 70%
of patients with CNKTL presented with lesions on one or more
extremities and only a few cases (13%) presented with lesions in
the head or neck regions. Furthermore, in the current study, 2
cases with CNKTL exhibited systemic symptoms and revealed nasal
cavity involvement. The nasal skin of these 2 cases exhibited
swelling and ulceration. However, these symptoms differed between
those exhibited by patients with HVLPD presenting with lesions on
the face, which were marked by multiple papulovesicular eruption
and scarring.
Furthermore, the clinical manifestations and courses
of these two diseases differ markedly. HVLPD is marked by slow
progression prior to the development of systemic symptoms;
subsequently patients present with fever and erythema, which recur
repeatedly. The lesions of patients with HVLPD are often
characterized by papulovesicular eruption, ulceration and scarring
(6). All 5 young patients with HVLPD
in the current study experienced acute erythema, which may be
relieved with the use of anti-viral drugs in the early stages of
the disease. The duration of the disease prior to progression to
systemic symptoms, including fever, lymphadenopathy and
hepatosplenomegaly, was ~1.5 years in the current study. By
contrast, NK/T-cell lymphoma presenting in the skin generally
exhibits an aggressive clinical course without remission following
diagnosis and the outcome of the majority of such patients is poor
and may lead to mortality (16–19).
Skin lesions of patients with NK/T cell lymphoma are generally
nodular or present with large plaques and sometimes with ulcers
(16).
The histopathological features of the two diseases
may be differ somewhat, although the immunohistochemical expression
of the HVLPD-NK cell phenotype may mimic that of NK/T cell
lymphoma. The results of the current study indicated that the
involvement of the epidermis in HVLPD, as well as tumor necrosis,
is rare; the opposite was the case regarding skin lesions in
patients with CNKTL. Patients with HVLPD usually exhibit multiple
skin lesions (6). The fact that the
epidermis is not involved in 4 out of 5 cases may be due to the
biopsy site-in the current study, the majority of biopsy sites were
skin rashes, where early pathological changes were observed. Skin
adnexa involvement and vascular destruction occurred in patients
with HVLPD and CNKTL. Lymphoid cells from patients in the HVLPD
group were small-to-medium-sized with mild atypia, whereas in the
majority of cases from the CNKTL group, the tumor was composed of
medium-sized or large cells exhibiting marked pleomorphism. Taken
together, these subtle histological clues may provide an additional
diagnostic basis to distinguish between these two diseases.
In addition to CNKTL, the differential diagnosis for
the HVLPD-NK cell phenotype includes benign inflammatory diseases
and primary cutaneous CD30-positive T-cell LPD. Inflammatory
disorders of the skin may microscopically resemble HVLPD. In the
current study, the lymphocytes of all 5 cases were
small-to-medium-sized with less atypia, which closely mimics
chronic inflammation. The lesions of 1 case were accompanied by a
large number of neutrophils, meaning that it was difficult to
detect the tumor cells. The majority of cases (4/5) with the
HVLPD-NK cell phenotype in the current study exhibited a polyclonal
gene arrangement that closely mimicked benign lesions. Furthermore,
patients with HVLPD tend to exhibit lymphocytic vascular
destruction, which is very rare in inflammatory disorders, such as
cutaneous eczema (20).
Investigating the clinical course and performing ISH for EBV may
help to distinguish between HVLPD and inflammatory disorders
(20). Furthermore, CD30+ T-cell LPD
should also be excluded. Primary cutaneous CD30+ T-cell LPD
includes lymphomatoid papulosis, primary cutaneous anaplastic large
cell lymphoma and borderline cases, which is characterized by CD30+
EBV- T-cells. In the current study, 2 cases with HVLPD exhibited
heterogeneous expression of CD30. The phenotype of these 2 cases
may mimic that of CD30+ T-cell LPD. However, these 2 cases also
exhibited positive EBV expression, meaning that it was determined
to be HVLPD.
The sample size in the current study was very
limited; therefore, further studies involving larger samples are
required to confirm the results. The optimal approach for treatment
remains unknown and previous studies have demonstrated that the
prognosis of patients with HVLPD is variable (11,14,21,22).
Patients in the current study received different treatments; even
so, one case succumbed even following treatment with CHOP.
In conclusion, the current study revealed marked
differences in the clinicopathological features between patients
with HVLPD-NK and those with CNKTL, particularly regarding the
clinical course, even though these two diseases share similar
immunophenotypes and some histopathological features. Therefore,
the results of the current study suggest that the HVLPD-NK cell
phenotype should be classified as a separate entity.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Key research
project of He'nan Educational Committee (Grant number:
17A310035).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
GNW, WCL and MZZ designed and directed the research.
GNW and YCu performed the experiments and drafted the manuscript.
WGZ, LL and XDZ analyzed and interpreted data. YCh and XZG
collected the clinical data. YL collected the experimental data.
All authors read and approved the final for publication.
Ethics approval and consent to
participate
The current study was approved by the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). Signed informed consents were obtained from all
patients prior to the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Thiele Jurgen HS and Vardiman JW: World Health
Organization Classification of Tumors of Hematopoietic and Lymphoid
Tissues. 4th. IARC Press; Lyon: 2008
|
|
2
|
Cho KH, Kim CW, Heo DS, Lee DS, Choi WW,
Rim JH and Han WS: Epstein-Barr virus-associated peripheral T-cell
lymphoma in adults with hydroa vacciniforme-like lesions. Clin Exp
Dermatol. 26:242–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng S, Jin P and Zeng X: Hydroa
vacciniforme-like primary cutaneous CD8-positive T-cell lymphoma.
Eur J Dermatol. 18:364–365. 2008.PubMed/NCBI
|
|
4
|
Magaña M, Sangüeza P, Gil-Beristain J,
Sánchez-Sosa S, Salgado A, Ramón G and Sangüeza OP: Angiocentric
cutaneous T-cell lymphoma of childhood (hydroa-like lymphoma): A
distinctive type of cutaneous T-cell lymphoma. J Am Acad Dermatol.
38:574–579. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barrionuevo C, Anderson VM,
Zevallos-Giampietri E, Zaharia M, Misad O, Bravo F, Cáceres H, Taxa
L, Martínez MT, Wachtel A and Piris MA: Hydroa-like cutaneous
T-cell lymphoma: A clinicopathologic and molecular genetic study of
16 pediatric cases from Peru. Appl Immunohistochem Mol Morphol.
10:7–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu Z and Lian S: Epstein-Barr
virus-associated hydroa vacciniforme-like cutaneous lymphoma in
seven Chinese children. Pediatr Dermatol. 27:463–469. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
(WHO) classification of lymphoid neoplasms. Blood. 127:2375–2390.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwatsuki K, Ohtsuka M, Akiba H and Kaneko
F: Atypical hydroa vacciniforme in childhood: From a smoldering
stage to Epstein-Barr virus-associated lymphoid malignancy. J Am
Acad Dermatol. 40:283–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwatsuki K, Satoh M, Yamamoto T, Oono T,
Morizane S, Ohtsuka M, Xu ZG, Suzuki D and Tsuji K: Pathogenic link
between hydroa vacciniforme and Epstein-Barr virus-associated
hematologic disorders. Arch Dermatol. 142:587–595. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doeden K, Molina-Kirsch H, Perez E, Warnke
R and Sundram U: Hydroa-like lymphoma with CD56 expression. J Cutan
Pathol. 35:488–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rodriguez-pinilla SM, Barrionuevo C,
Garcia J, Martínez MT, Pajares R, Montes-Moreno S, Casavilca S,
Montes J, Bravo F, Zaharia M, et al: EBV-associated cutaneous
NK/T-cell lymphoma: Review of a series of 14 cases from Peru in
children and young adults. Am J Surg Pathol. 34:1773–1782. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quintanilla-Martínez L, Ridaura C, Nagl F,
Sáez-de-Ocariz M, Durán-McKinster C, Ruiz-Maldonado R, Alderete G,
Grube P, Lome-Maldonado C, Bonzheim I and Fend F: Hydroa
vacciniforme like lymphoma: A chronic EBV+ lymphoproliferative
disorder with risk to develop a systemic lymphoma. Blood.
122:3101–3110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santos M, Nogueira L, Talahri C, Massone
C, Cerroni L, Mira MT and Talhari S: Hydroavacciniforme-like
lymphoma in a patient from the Brazilian Amazon. Int J Dermatol.
52:641–643. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Magaña M, Massone C, Magaña P and Cerroni
L: Clinicopathologic features of hydroa vacciniforme-like lymphoma:
A series of 9 patients. Am J Dermato pathol. 38:20–25. 2016.
View Article : Google Scholar
|
|
15
|
Patel KP, Pan Q, Wang Y, Maitta RW, Du J,
Xue X, Lin J and Ratech H: Comparison of BIOMED-2 versus
laboratory-developed polymerase chain reaction assays for detecting
T-cell receptor-gamma gene rearrangements. J Mol Diagn. 12:226–237.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mraz-Gernhard S, Natkunam Y, Hoppe RT,
LeBoit P, Kohler S and Kim YH: Natural killer/natural killer-like
T-cell lymphoma, CD56+, presenting in the skin: An increasingly
recognized entity with an aggressive course. J Clin Oncol.
19:2179–2188. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Child FJ, Mitchell TJ, Whittaker SJ,
Calonje E, Spittle M, Crocker J and Russell-Jones R: Blastic
natural killer cell and extranodal natural killer cell-like T-cell
lymphoma presenting in the skin: Report of six cases from the UK.
Br J Dermatol. 148:507–515. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu JB, Zuo Z, Tang Y, Zhao S, Zhang YC, Bi
CF, Wang WY, Zhang WY, Wang L and Liu WP: Extranodal nasal-type
natural killer/T-cell lymphoma of the skin: A clinicopathologic
study of 16 cases in China. Hum Pathol. 40:807–816. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takata K, Hong ME, Sitthinamsuwan P, Loong
F, Tan SY, Liau JY, Hsieh PP, Ng SB, Yang SF, Pongpruttipan T, et
al: Primary cutaneous NK/T-cell lymphoma, nasal type and
CD56-positive peripheral T-cell lymphoma: A cellular lineage and
clinicopathologic study of 60 patients from Asia. Am J Surg Pathol.
39:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsi AC1 and Rosman IS: Histopathology of
cutaneous inflammatory disorders in children. Pediatr Dev Pathol.
21:115–149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sangueza M and Plaza JA: Hydroa
vacciniforme-like cutaneous T-cell lymphoma: Clinicopathologic and
immunohistochemical study of 12 cases. J Am Acad Dermatol.
69:112–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang YQ, Fan L, Wang L, Xu J, Zhang R, Ge
Z, Li JY and Xu W: Systemic lymphoma arising from hydroa
vacciniforme-like lymphoma: Report of two cases with review of
literature. Int J Clin Exp Pathol. 7:6403–6408. 2014.PubMed/NCBI
|