Introduction
Osteoarthritis (OA) was histologically thought of as
regular ‘wear and tear’ disease whose immune system was unlikely to
be affected (1). With the
understanding of pathogenesis of OA, it is now generally accepted
to be a low-grade inflammatory disease affecting the whole joint
(2). Although the pathogenesis and
progression of OA seem to be the result of the complex interplay
between mechanical, cellular and inflammatory factors, the
mechanism remains unknown (3).
Knee OA is the most common type of OA, and also the
most common cause of disability of people worldwide. Deterioration
of articular cartilage is clinically regarded as the main cause
leading to knee OA (4), but the
impairment of chondrogenesis from inflammation remains elusive and
deserves to be elucidated. Despite this, macrophage polarization
(5,6)
has been documented to be implicated in the deterioration of
cartilage. Especially, M1 macrophage has been identified as the
major mediator in the anti-chondrogenic process (7). In consideration of the two main
subtypes of macrophages (8), M1
macrophage is believed to be pro-inflammatory whereas M2 macrophage
anti-inflammatory, together with a previous relevant report
(6); we hypothesized that the
balance between M1 and M2 macrophages might be skewed in knee OA.
To test our hypothesis, we measured the ratio of M1 to M2
macrophages from synovial fluids and peripheral blood from patients
with knee OA and matched healthy controls. It was found that
imbalance of M1/M2 macrophages occurs in knee OA and the degree of
imbalance was associated with severe level of knee OA, suggesting
that re-balance of the ratio might be used as a novel therapeutic
alternative for knee OA.
Materials and methods
Clinical samples
This study was approved by the Medical Ethics
Committee of The Affiliated Qingdao Hiser Hospital of Qingdao
University (Qingdao Hospital of Traditional Chinese Medicine;
Qingdao, China). Written informed consent was obtained from each
participant whose synovial fluids as well as peripheral blood were
collected for the investigation. A total of 80 cases of patients
with knee OA were mainly diagnosed using X-ray in combination with
magnetic resonance image (MRI), and were recruited from those who
were hospitalized from May 2014 to May 2017 in the Department of
Rheumatology in the Affiliated Qingdao Hiser Hospital of Qingdao
University. As corresponding healthy control, 80 cases of healthy
patients whose peripheral blood only was obtained from the
Department of Spine Surgery in Qingdao Hiser Hospital. The synovial
fluid from healthy control was unavailable. It should be noted
that, in order to be comparable for the detection results, the
demographic characteristics including age, sex and education level
were strictly matched. In addition, the patients who complained of
pain in the knee had Kellgren-Lawrence (K-L) grade ≥1. In our
enrollment, patients were excluded had they have any other form of
arthritis; body mass index (BMI) >24 kg/m2; any
disorder needing the use of systemic corticosteroids; history of
knee replacement; chronic infectious disease and other chronic
inflammation disease.
K-L and visual analogue scale (VAS)
scoring
In consideration that the K-L grading scheme is the
most widely used and accepted standard for measurement of knee OA
here, scoring criteria using K-L was as follows (9): Grade 0, no radiographic features of OA
are present; grade I, there is doubtful narrowing of joint space
narrowing and possible osteophytes; grade II, there is presence of
osteophytes and possible joint space narrowing on anteroposterior
weight-bearing radiograph; grade III, multiple osteophytes were
present, definite joint space narrowing, sclerosis and possible
bony deformity; and grade IV, large osteophytes were present,
marked joint space narrowing, severe sclerosis and definitely bony
deformity (Fig. 1). The VAS scoring
system used was based on reference (10), the range of VAS was from no pain (0–4
points), mild (5–44 points), moderate (45–74 points) to severe pain
(75–100 points).
 | Figure 1.Roentgenograph of knee OA with
different degree of severity ranging from K-L grade 1 to 4
diagnosed using X-ray. K-L 1, doubtful narrowing of joint space and
possible bone spurs. K-L 2, presence of bone spurs and possible
joint space narrowing on anteroposterior weight-bearing radiograph.
K-L 3, multiple bone spurs are present, definite joint space
narrowing, sclerosis and possible bony deformity. K-L 4, large
spurs are present, marked joint space narrowing, severe sclerosis
and definitely bony deformity. The bone spurs are present and
indicated by the yellow arrow. OA, osteoarthritis; K-L,
Kellgren-Lawrence. |
Reverse transcription-quantitative PCR
(RT-qPCR)
Extraction of total RNA from synovial fluids and
peripheral whole blood was performed with TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc., Carlsbad, CA, USA) method following
its manufacturer's protocol. To avoid possible degradation by
RNase, total RNA was immediately reverse transcribed into cDNA
using the iScript™ cDNA Synthesis kit according to the
manufacturer's protocol. Reverse transcription-quantitative PCR
(RT-qPCR) was performed using FastStart Universal SYBR-Green Master
(ROX) Mix (2X) (Roche Diagnostics, Indianapolis, IN, USA), with 2.5
µl cDNA per reaction in a total volume of 20 µl. RT-qPCR reactions
were run in duplicates on the IQ5™ real-time PCR system (Bio-Rad
Laboratories, Hercules, CA, USA). The comparative Cq (cycle
threshold) method (−ΔΔCq) was applied for the quantification of
gene expression (11). The values
were normalized against β-actin as internal loading control.
Standard curve as algorithm was used to calculate the relative
expression of CD markers to β-actin. The results were expressed as
fold-changes on mRNA level. The thermocycling conditions used were
as follows: 30 sec at 95°C; at 60°C; and at 72°C for 35 cycles. The
following primers were used: CD86 forward,
5′-CTGCTCATCTATACACGGTTACC-3′, and reverse,
5′-GGAAACGTCGTACAGTTCTGTG-3′; CD163 forward,
5′-CAGGAAACCAGTCCCAAACA-3′, and reverse,
5′-AGCGACCTCCTCCATTTACC-3′; β-actin forward,
5′-CGTGACATTAAGGAGAAGCTG-3′; and reverse,
5′-CTAGAAGCATTTGCGGTGGAC-3′.
Flow cytometry
The expression of specific markers was investigated
in monocytes from synovial fluid by fluorescence-activated cell
sorting analysis after surface or intracellular staining with
specific monobodies that were conjugated to different fluorescent
dyes. For the extracellular staining, cells were washed in
phosphate-buffered saline (PBS) containing 1% bovine serum albumin
and 0.02% sodium azide and were incubated with specific antibodies
for 30 min at 4°C. Flow cytometry was performed using a FACS Canto
(CytoFLEX; Beckman Coulter, CA, USA) and analyzed with Flowjo
analysis software (Tree Star, Inc., Ashland, OR, USA). Specific
monoclonal antibodies to human CD11c (Alexa Fluor® 700;
cat. no. 56-0116-42), CD206 (Alexa Fluor® 488; cat. no.
53-2069-42), CD86 (Alexa Fluor® 488; cat. no. A51007)
and CD163 (PerCP-eFluor® 710; cat. no. 46-1639-42) were
all from Thermo Fisher Scientific, Inc. Staining with mouse IgG
isotype control (PerCP-eFluor® 710 labeled, cat. no.
46-4714; Thermo Fisher Scientific, Inc.) was used as control for
analysis of monocytes in the monocyte gate.
Statistical analysis
The data are expressed as mean ± standard error of
means (SEM). The continuous data were subjected to
Kolmogorov-Smirnov test for normal distribution. When the data of
RT-qPCR detection were not normal distribution, Mann-Whitney U test
was employed; the data from flow cytometry analysis had normal
distribution, and independent sample t-test was used. Categorical
data were analyzed using Chi-square or Fisher's exact test. SPSS
17.0 software version (SPSS Inc., Chicago, IL, USA) was used to
analyze the statistical difference and GraphPad Prism 5.0 version
(GraphPad Software, Inc., La Jolla, CA, USA) was employed to
analyze and draw the relevant figures. P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
Although the fine needle aspiration biopsy of
synovial fluids is a routine for patients with knee OA before
therapy in our department, there were still some who were unwilling
to have their synovial fluids biopsied and analyzed. Consequently,
we retrieved the synovial fluids stored in liquid nitrogen device
in the Biobank of The Affiliated Qingdao Hiser Hospital of Qingdao
University in accordance with our requirement. Of the 80 patients
with knee OA we enrolled from outset of the study, only 35 cases
whose synovial fluids were available, yielding the median synovial
fluid volume of 4 ml (interquartitle range 3–8 ml). By contrast,
peripheral blood was obtained from each participant, including
healthy control. There was no significant difference in terms of
age, knee injury, and VAS score between patients who did and those
who did not have their synovial fluids biopsied and analyzed
(Table I). In addition, Chi-square
or Fisher's exact test samples were used to determine which
exclusion of patients (<500 cells/mm3). Eventually,
eligible samples were obtained from 28 subjects. As shown in
Table I, the mean ± SD age of those
28 subjects was 58.0±8.4 years, and 53% were men. The majority of
participants had a K-L grade of 2 or 3. The mean ± SD VAS score at
baseline was 50±20.35.
 | Table I.Baseline characteristics of the
enrolled 80 patients with knee OA. |
Table I.
Baseline characteristics of the
enrolled 80 patients with knee OA.
| Characteristics | Data |
|---|
| Age (mean ± SEM,
years) | 58.0±8.4 |
| Sex (%) |
|
| Men | 53 (66.3) |
|
Women | 27 (33.7) |
| K-L grade (%) |
|
| I | 7 (8.75) |
| II | 28 (35) |
| III | 40 (50) |
| IV | 5 (6.25) |
| Pain evaluation |
|
| Nominated
activity VAS score | 50±20.35 |
| No
pain | 5 |
| Mild
pain | 30 |
| Moderate
pain | 35 |
| Severe
pain | 10 |
| Knee injury gross
assessment |
|
|
Synovitis | 12 |
| Cartilage
lesion | 29 |
|
Osteophytes appeared | 34 |
| Bone
marrow lesion | 5 |
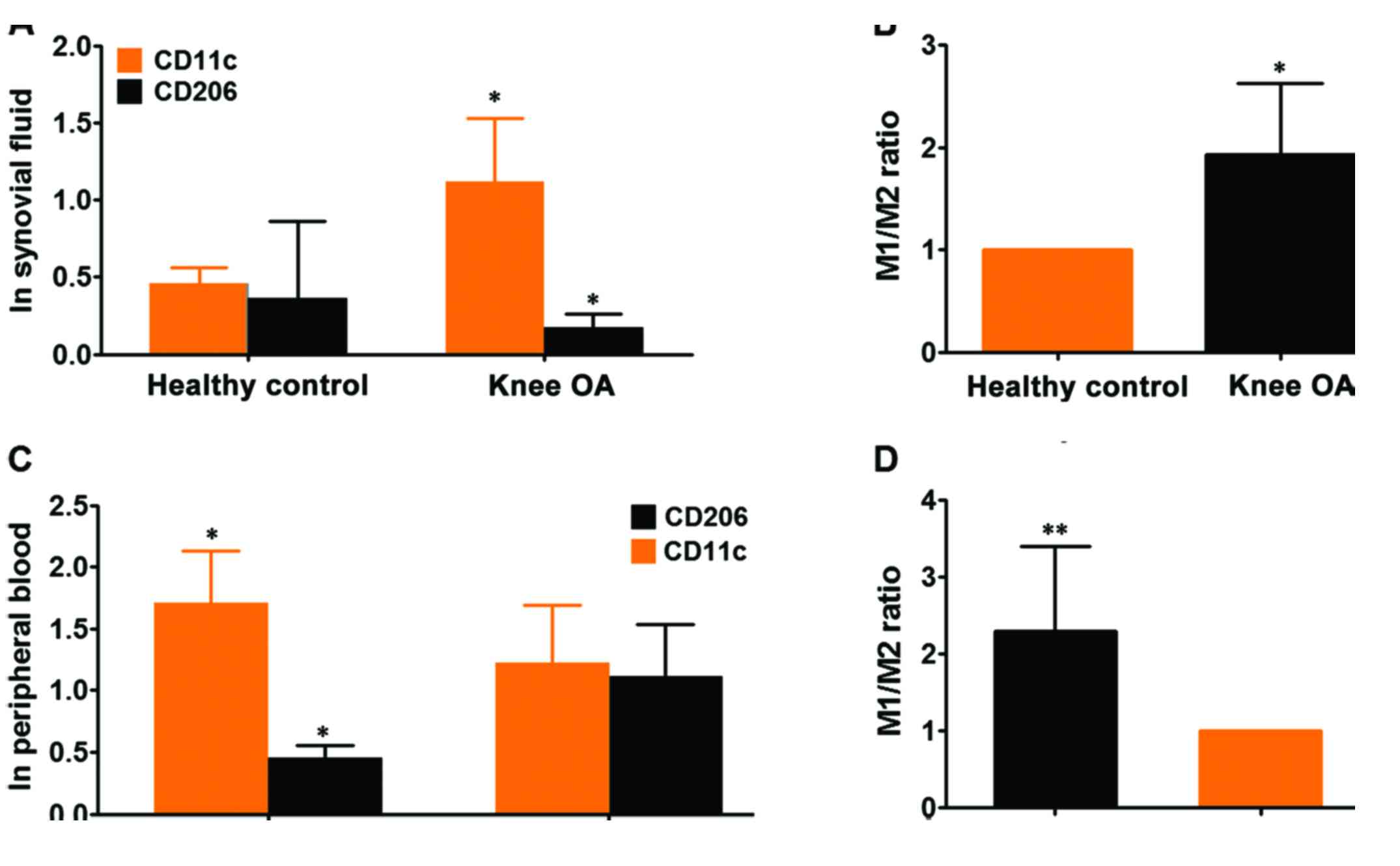
The ratio of M1 to M2 macrophages is
elevated in knee OA
Having made clear the baseline characteristics of
patients with knee OA, subsequently, we determined the state of M1
and M2 macrophages in the synovial fluids and peripheral blood from
participants, by performing flow cytometry analysis of given number
of monocytes (10,000 cells) in these 28 cases of synovial fluids
using specific monoclonal antibody to human CD11c, surface marker
of M1 subtype macrophage and CD206, surface marker of M2
macrophage. Flow cytometry analyses for expression of CD11c as M1
and CD206 as M2 marker exhibited that both M1 and M2 macrophages
were present in the synovial fluids from knee OA. However, the
variation in the case of expression of each marker between patients
was large (data not shown), thus we calculated the total of M1 and
M2 macrophages in each group. It showed that the expression CD11c
was remarkably higher than CD208, suggesting that M1 macrophages
predominantly existed in synovial fluids of knee OA compared with
M2 macrophages (Fig. 2A and B). To
verify the finding from synovial fluids of knee OA, we extended the
flow cytometry analysis from synovial fluid to peripheral blood. It
exhibited that both monocytes with cell surface markers CD11c and
CD208 were also present in whole blood from knee OA and healthy
control. The expression of CD11c was shown to be significantly
higher in knee OA than that of control whereas expression of CD208
was markedly lower in knee OA than that of control (Fig. 2C). Taken as a whole, the ratio of
M1/M2 macrophages was pronouncedly higher in peripheral blood of
knee OA relative to healthy control, indicating that there was an
association between knee OA and ratio of M1/M2 macrophages
(Fig. 2D). While no significant
skewing ratio of M1/M2 macrophages can be observed within the
control group (data not shown). Likewise, in consideration that
there were several well-established surface markers for M1 and M2
macrophages except of CD11c and CD208 we've used, to further
confirm the findings, we've employed RT-qPCR technique to detect
the expression of CD86 (another established surface marker of M1
macrophage) and CD163 (M2 macrophage) in peripheral blood from the
same cohort as CD11c and CD208. It was found that the ratio of
M1/M2 macrophage was remarkably higher in knee OA than that of
control, which was totally congruent with observations made by flow
cytometry (Fig. 3). Taken together,
the results demonstrated that the ratio of M1/M2 macrophages were
significantly higher in knee OA, suggesting a relationship between
disequilibrium of ratio M1 to M2 macrophages versus knee OA
development.
The ratio of M1/M2 macrophages is
associated with severity level of knee OA
Having understood the status of M1 and M2
macrophages in knee OA, next, we sought to analyze the clinical
significance of the ratio of M1/M2 macrophages. Given that all the
patients with knee OA enrolled in our study whose damage was scored
using K-L grading system from the outset, we've tried to explore
whether there was association between ratio of M1/M2 macrophages
and progression of knee OA. In light of the large variation of
expression of each marker between patients, the patients were
stratified further into two groups in accordance with the value of
the ratio of M1/M2 macrophages obtained by RT-qPCR in peripheral
blood. The group whose ratio was >1.5 and group whose ratio was
≤1.5 but >1. As shown in Table
II, it can be seen that there was significant positive
association between K-L scores and value of the ratio M1/M2
macrophages, suggesting that the greater the value of ratio the
more severe the knee OA seems to be. Interestingly, no significant
association was observed between VAS scores and the ratio of M1/M2
macrophages. No significant correlation was observed either between
the ratio of M1/M2 macrophages and knee injury gross assessment,
although there seems to be a trend toward knee injury degree. These
results suggest that the ratio M1/M2 macrophages might be
predictive of the severity of knee OA.
 | Table II.Association between ratio of M1/M2
macrophages and clinical variables at baseline. |
Table II.
Association between ratio of M1/M2
macrophages and clinical variables at baseline.
|
|
| Ratio of M1/M2 |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristics | No. of cases | 1<R≤1.5 | >1.5 | χ2 (or
F) | P-value |
|---|
| Age (years) | 58.0±8.4 | 38 | 42 |
|
|
| Sex (%) |
| Men | 53 (66.3%) | 25 | 28 | 0.053 | 1.000 |
|
Women | 27 (33.7%) | 12 | 15 |
|
|
| K-L grade (%) |
| I | 7 (8.75%) | 4 | 3 | 9.702 | 0.014 |
| II | 28 (35%) | 9 | 29 |
|
|
| III | 40 (50%) | 2 | 28 |
|
|
| IV | 5 (6.25%) | 0 | 5 |
|
|
| Pain evaluation |
| Nominated
activity | 50±20.35 |
|
|
|
|
| VAS
score |
|
No pain | 5 | 2 | 3 | 1.013 | 0.864 |
|
Mild pain | 30 | 16 | 14 |
|
|
|
Moderate pain | 35 | 21 | 14 |
|
|
|
Severe pain | 10 | 6 | 4 |
|
|
| Knee injury gross
assessment |
|
Synovitis | 17 | 6 | 11 | 4.015 | 0.125 |
| Cartilage
lesion | 29 | 7 | 22 |
|
|
|
Osteophyte appear | 34 | 4 | 30 |
|
|
| Bone
marrow lesion | 0 | 0 | 0 |
|
|
Discussion
This is the first report of the ratio of M1/M2
macrophages in synovial fluids of knee OA and clinical significance
of the ratio of M1/M2 macrophages in knee OA. Ratio of M1/M2
macrophages was shown to be remarkably higher in knee OA compared
with healthy control. We further showed that ratio of M1/M2
macrophages was significantly associated with K-L grading of knee
OA, indicating that ratio of M1/M2 macrophages might be used as a
predictor for the damage or severity for the knee with OA. These
observations also suggest that ratio of M1/M2 macrophages in knee
OA might be potentially used as a novel quantitative scoring system
measuring the severity of knee OA. The comparison study therefore
may be warranted.
OA was previously viewed as regular wear and tear of
the body and the immune system was unlikely to be affected, but it
is now generally appreciated to be a low-grade inflammatory disease
affecting the whole joint. Despite extensive investigations in
vitro mechanistic and in vivo animal models implied that
macrophages were implicated (12)
even found to play a certain role in the pathogenesis of knee OA
(12,13), there has been a paucity of direct
evidence regarding the macrophages in the setting of knee OA except
one recent study by Kraus et al (14) who provided direct evidence of
macrophages in the synovial fluid of knee OA. Our present
investigation was also prompted by the study in that although Kraus
et al (14) quantitatively
presented direct evidence for the involvement of macrophages in
patients with symptomatic OA, nevertheless, they did not evaluate
the status of M1 and M2 macrophages in patients with symptomatic OA
in consideration that macrophages used to being subtyped into M1
and M2 types. Furthermore, they determined the localization of
macrophages in joints, including finger joints, thumb bases,
shoulders, toes and ankles, which is to say, the focus was not
merely restricted to the knee. In this sense, our study totally
differs from the previous one. From the outset of our study, we
paid attention on OA limited to the knee, regardless of other types
of OA. Besides, considering the two common subtypes of macrophage
implicated in inflammation, we determined the status of both M1 and
M2 macrophages in the knee OA, the former was believed to be
pro-inflammatory while the later anti-inflammatory.
M1 macrophage was initially mentioned in the murine
model of knee OA in the setting of obesity induced by high-fat diet
(6,13). The study by Wu et al (13) showed that M1 macrophages played a
vital role in modulating the homeostasis of immune cells in the
inflammation associated with the development of OA in obesity,
which was suggestive of the important involvement of M1 macrophages
in OA associated with obesity. However, no direct evidence has
emerged regarding M1 macrophages in OA irrespective of the obesity
factor. We tried to explore the status of M1 and M2 macrophages
both in synovial fluids and whole blood from patients with OA,
tentatively regardless of the confounder factor, obesity in OA. We
showed that the number of monocytes stained with specific surface
marker pertaining to M1 macrophage was significant higher than
monocytes with specific surface marker of M2 macrophage in knee OA,
suggesting that M1 macrophages predominantly existed in knee OA
compared with M2 macrophages. To avoid the confounder of obesity,
the BMI index was set within 24 kg/m2. Any case whose
BMI was above the criteria was excluded. Under such condition, we
presented that the ratio of M1/M2 macrophages was markedly higher
in knee OA compared with healthy control. However, as for the role
M1 and M2 macrophages mediated in knee OA, it remains to be further
investigated.
The pathogenesis of knee OA is believed to be
dependent on the impairment of chondrogenic differentiation.
Previous report has identified that M1 macrophage polarization in
the synovial fluid can exert a significant inhibition on the
differentiation of chondrocytes (7),
indicating that M1 macrophage plays the major role in the mediation
of chondrogenesis. In our present investigation, the ratio of M1/M2
macrophages was shown to be pronouncedly associated with K-L
grading of knee OA, which was fundamentally in line with the study
by Kraus et al (14)
reporting that knee OA severity was associated with activated
macrophage in synovial fluid of systemic OA. Notably, despite the
ratio was found to be unassociated with the knee injury gross
assessment in our analysis, there seems to be a trend of increasing
ratio towards the impairment degree of knee, which was indicative
of the more the ratio the greater the severity degree tends to be.
Nonetheless, caution is needed when interpreting our data because
of the relatively small number of studied patients. To confirm our
observations, we interchangeably used flow cytometry and RT-qPCR
approach, which was complementary to each other in the detection of
synovial and peripheral blood from knee OA and healthy
controls.
There were still some limitations that should have
been acknowledged from the outset of our study. First, the synovial
fluid from knee OA to be analyzed was cryopreserved in liquid
nitrogen for nearly 1 year. In view of relevant studies showing
that storage in liquid nitrogen could have a bearing on the
expression of surface markers of cells (15,16), our
results regarding flow cytometry detection of CD11c and CD206 in
synovial fluids could be compromised. Secondly, our observations
were made on the premise that all the participants whose BMI index
was within 24 kg/m2, that is obesity as a confounder was
excluded at the outset. Consequently, extrapolation of direct ratio
of M1/M2 macrophages in knee OA from this study should be
approached with caution. Thirdly, more mechanistic data should have
been given regarding M1 and M2 macrophages in the pathogenesis of
knee OA, in addition to merely presenting of phenotype of
macrophages.
In conclusion, to our knowledge this was the first
report of the ratio of M1/M2 macrophages in knee OA. The imbalance
of M1/M2 macrophages can feature the severity level of knee OA.
Acknowledgements
Not applicable.
Funding
The present study was supported by Department of
Rheumatology, The Affiliated Qingdao Hiser Hospital of Qingdao
University (Qingdao, China).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL drafted the manuscript and performed PCR. MZ and
JZ was responsible for K-L and VAS scoring. MZ and HY contributed
to flow cytometry. All authors read and approved the final
study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Qingdao Hiser Hospital of Qingdao University
(Qingdao, China). Patients who participated in this research had
complete clinical data. The signed informed consents were obtained
from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elias-Jones CJ, Farrow L, Reilly JH, Kerr
S, Meek RM, Kelly MP, Campton JL and Millar NL: Inflammation and
neovascularization in hip impingement: Not just wear and tear. Am J
Sports Med. 43:1875–1881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scanzello CR: Role of low-grade
inflammation in osteoarthritis. Curr Opin Rheumatol. 29:79–85.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mobasheri A, Rayman MP, Gualillo O, Sellam
J, van der Kraan P and Fearon U: The role of metabolism in the
pathogenesis of osteoarthritis. Nat Rev Rheumatol. 13:302–311.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Makris EA, Gomoll AH, Malizos KN, Hu JC
and Athanasiou KA: Repair and tissue engineering techniques for
articular cartilage. Nat Rev Rheumatol. 11:21–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaffney L, Warren P, Wrona EA, Fisher MB
and Freytes DO: Macrophages' role in tissue disease and
regeneration. Results Probl Cell Differ. 62:245–271. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barboza E, Hudson J, Chang WP, Kovats S,
Towner RA, Silasi-Mansat R, Lupu F, Kent C and Griffin TM:
Profibrotic infrapatellar fat pad remodeling without M1 macrophage
polarization precedes knee osteoarthritis in mice with diet-induced
obesity. Arthritis Rheumatol. 69:1221–1232. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fahy N, de Vries-van Melle ML, Lehmann J,
Wei W, Grotenhuis N, Farrell E, van der Kraan PM, Murphy JM,
Bastiaansen-Jenniskens YM and van Osch GJ: Human osteoarthritic
synovium impacts chondrogenic differentiation of mesenchymal stem
cells via macrophage polarisation state. Osteoarthritis Cartilage.
22:1167–1175. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ivanova EA and Orekhov AN: Monocyte
activation in immunopathology: Cellular test for development of
diagnostics and therapy. J Immunol Res. 2016:47892792016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthrosis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallasch CH and Alexandre NM: The
measurement of musculoskeletal pain intensity: A comparison of four
methods. Rev Gaucha Enferm. 28:260–265. 2007.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daghestani HN, Pieper CF and Kraus VB:
Soluble macrophage biomarkers indicate inflammatory phenotypes in
patients with knee osteoarthritis. Arthritis Rheumatol. 67:956–965.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu CL, McNeill J, Goon K, Little D,
Kimmerling K, Huebner J, Kraus V and Guilak F: Conditional
macrophage depletion increases inflammation and does not inhibit
the development of osteoarthritis in obese macrophage fas-induced
apoptosis-transgenic mice. Arthritis Rheumatol. 69:1772–1783. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kraus VB, McDaniel G, Huebner JL, Stabler
TV, Pieper CF, Shipes SW, Petry NA, Low PS, Shen J, McNearney TA,
et al: Direct in vivo evidence of activated macrophages in human
osteoarthritis. Osteoarthritis Cartilage. 24:1613–1621. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Del Pino A, Ligero G, López MB, Navarro H,
Carrillo JA, Pantoll SC and Díaz de la Guardia R: Morphology, cell
viability, karyotype, expression of surface markers and plasticity
of three human primary cell line cultures before and after the
cryostorage in LN2 and GN2. Cryobiology. 70:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davies OG, Smith AJ, Cooper PR, Shelton RM
and Scheven BA: The effects of cryopreservation on cells isolated
from adipose, bone marrow and dental pulp tissues. Cryobiology.
69:342–347. 2014. View Article : Google Scholar : PubMed/NCBI
|