Introduction
In the last few years, optical coherence tomography
(OCT) has served as an important method for the diagnosis and
follow-up of retinal diseases (1).
It has also been increasingly used in demonstrating retinal and
optic nerve damage in patients with neurological disorders,
including multiple sclerosis (2–4) and
neuromyelitis optica (5). The
application of Fourier transform techniques in spectral domain OCT
allows for simultaneous measurements of light reflex from different
layers, thus improving image acquisition speed (6). However, certain spectral domain OCT
models are still facing certain challenges (7), one of which is the balancing of scan
time reduction and image quality. Higher pixel density requires a
longer image acquisition time. Elderly patients with Parkinson's
disease (8) or severe eye diseases
may not be able to cooperate well during the measurements and are
more prone to generating motion artifacts. Low data processing
speed may be another issue faced in busy clinics in China treating
a high volume of patients.
With the advancement of imaging technology, a series
of OCT devices have been released and the above-mentioned issues
have been improved to a certain extent. Introduced after the
initial model Topcon 3D OCT-1000, Topcon 3D OCT-2000 is one of the
updated, commercially available OCT instruments from Topcon.
According to the manual (9),
compared to the 18,000 A scans/sec by Topcon 3D OCT-1000, the
enhanced 27,000–50,000 A scans/sec by the OCT-2000 series allow for
faster tomography acquisition and minimize artifacts generated by
eye movement. The computation time of OCT-2000 for data processing
is ~30 sec, while that of OCT-1000 is ~90 sec. With the development
of additional noise reduction and enhanced depth (2.3 vs. 1.68 mm)
by built-in imaging software, OCT-2000 provides better
visualization of the retina and choroid than OCT-1000. The
combination of OCT scan and retinal angiography in OCT-2000 allows
for accurate positioning and facilitates the exploration of disease
pathogenesis. OCT-2000 is a better choice in clinical settings
where time and space is limited. As the replacement of OCT-1000 by
OCT-2000 has become increasingly widespread, it is important to
deal with patient data acquired using OCT-1000. Although Topcon 3D
OCT-2000 allows import and re-analysis of raw data from 3D
OCT-1000, the performance remains elusive and requires to be
evaluated.
The purpose of the present study was to investigate
the reliability of the re-analysis of 3D OCT-1000 data by built-in
algorithm of 3D OCT-2000. The segmentation error (SE) rate and
severity were compared by evaluating each cross-sectional image for
each case and assessing the agreement of retinal thickness
measurement between the two algorithms. Particular attention was
paid to the central subfield, as it is the most important location
associated with visual acuity (10).
Patients and methods
Study subjects
The present study was approved by the Institutional
Review Board of the Joint Shantou International Eye Center (JSIEC)
of Shantou University and the Chinese University of Hong Kong
(Shantou, China), and followed the tenets of the Declaration of
Helsinki. Informed consent was not required from subjects due to
the retrospective nature of the study.
Patients who had complete electronic records in the
JSIEC, including information regarding visual acuity, non-contact
tonometry, slit-lamp biomicroscopy and fundus examination, and who
were examined using Topcon 3D OCT-1000 with an image quality of
>50 were included in the present study. All medical records,
including the diagnosis of these patients, were retrospectively
reviewed. Normal subjects and a variety of patients with retinal
disorders were included. The criteria of ‘normal’ included: i) No
history of retinal disease or glaucoma; and ii) the retinal
structure was normal on fundus examination and OCT scan. The
exclusion criteria of OCT images included: i) Distorted image
caused by motion artifacts; and ii) incomplete image caused by long
axial length or severe opacity changes along the visual axis. In
subjects who had received an OCT scan in the bilateral eyes, only
one eye was randomly selected for assessment. All images were
obtained using the 3D macular 512×128 scan mode (128 B-scans in
total, each B-scan consisting of 512 A-scans, covering an area of
6×6 mm2) on a Topcon 3D OCT-1000 machine (software
version 2.20). The raw data was exported from the OCT-1000 in
‘.fds’ format and imported to an OCT-2000 machine (software version
8.11.003.04) for re-analysis. The built-in algorithms of OCT-1000
and OCT-2000 instruments analyzed the raw data automatically.
SE and thickness measurement
SE assessment in the two algorithms was performed by
a blinded investigator (BC). SE was defined as the disagreement
between automatic and manual identification of the inner and outer
retinal boundaries. The inner and outer boundaries refer to the
anterior border of the internal limiting membrane and the anterior
border of retinal pigment epithelium (RPE), respectively (11). Full-thickness macular hole (MH) was
defined at a center thickness of 0 µm, in which the inner border
overlapped with the outer border (12).
The severity of SE in the anterior and posterior
borders of each B scan was evaluated by a graded scale described by
Sadda et al (13), which has
also been validated in other studies (14–17). The
criteria were the following: i) Any deviation that may be
recognized from the actual boundary was given 1 point; ii) to
emphasize the importance of foveal measurements in clinical
diagnosis and study, 1 additional point was given if the deviation
was located within the central subfield, which was defined as the
central 1-mm area of the macula; iii) 1 point was added when an SE
or sum of multiple discontinuous errors appeared to be longer than
1 mm. If the error/sum of errors was longer than 3 mm, another
point was given; iv) in axial dimension, 1 point was added for a
deviation larger than one third of the actual retinal thickness and
2 points were given when the deviation increased to more than two
thirds of the retina. The total SE score was calculated by adding
all SE points from the inner and outer boundaries of each cross
section (128 B scans in total). The SE score of the central
subfield was further analyzed.
The built-in software of Topcon OCT assumes foveal
fixation and generates an Early Treatment of Diabetic Retinopathy
Study (ETDRS) thickness map centered on this location. The ETDRS
plot includes three circles with diameters of 1, 3 and 6 mm,
dividing the macula into two rings. It is further divided into four
quadrants: Superior, inferior, nasal and temporal. Retinal
thickness given by the two algorithms was compared without
correction in each subfield of the ETDRS map, without adjustment of
center location and retinal boundaries.
Statistical analysis
The difference in image quality score as assessed by
the two algorithms was evaluated using a Student's paired t-test
and Pearson's correlation. The presence of SEs was compared between
normal and abnormal eyes using a Chi-square test, and between
OCT-1000 and OCT-2000 using McNemar's test. The independent-samples
t-test was used to compare the image quality score between groups
with and without SEs, and compare the SE score between normal and
abnormal eyes. Intraclass Correlation Coefficient (ICC) analysis
and a Student's paired t-test were used to analyze the correlation
and difference in thickness measurement between the two algorithms,
respectively. Bland-Altman plots were used to evaluate the
agreement between the two algorithms. SPSS statistical software
(version 18.0.0; SPSS, Inc.) was used to perform the statistical
analysis and draw a scatter plot. MedCalc software (version 15.2.2;
MedCalc Software) was used to draw Bland-Altman plots.
Results
Patient characteristics
A total of 87 eyes from 87 patients, including 41
(47.13%) male and 46 (52.87%) female subjects, were included in the
present study. The mean age of the patients was 44.08±15.12 years.
Among the eyes included, 43 (49.43%) were normal and 44 (50.57%)
were abnormal. The diagnoses of abnormal eyes included central
serous chorioretinopathy (36.36%), retinal vascular diseases
(18.18%), age-associated macular degeneration (AMD) (15.91%), MH
(6.82%) and other retinal diseases (22.73%). The image quality
score re-assessed by the 3D OCT-2000 algorithm was significantly
lower than the original score determined by the 3D OCT-1000
algorithm, with a mean difference of 19.18±1.87 (P<0.001; paired
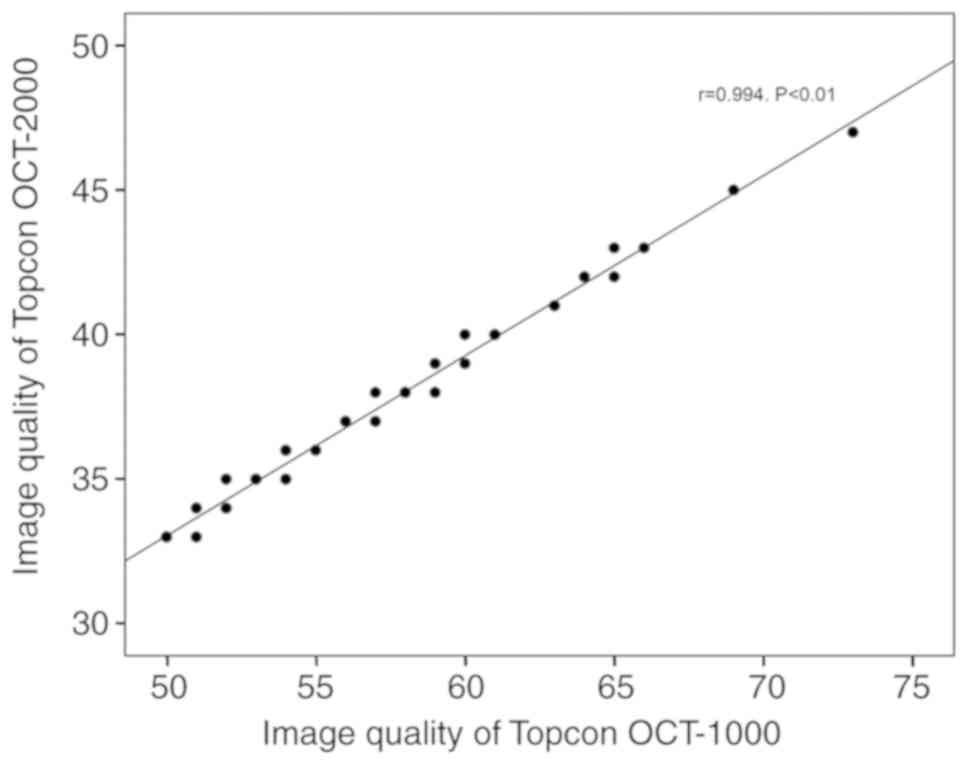
t-test). A highly linear correlation between the image quality
scores of the OCT-1000 and OCT-2000 algorithms was identified
(r=0.994, P<0.001; Fig. 1).
Comparison of SE rate and score
SEs were present in normal as well as abnormal
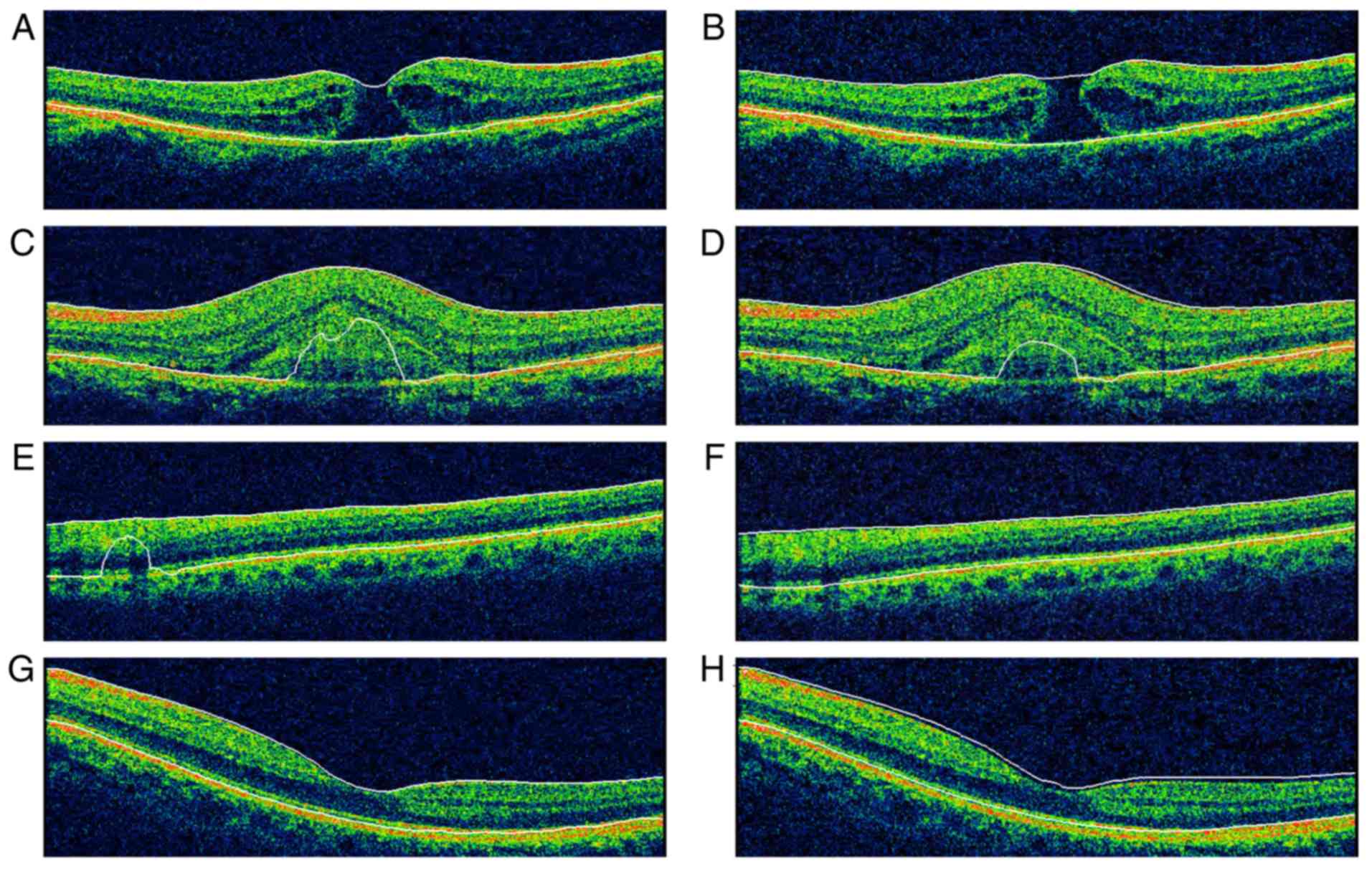
cases, but were more frequent in abnormal ones (Table I). All cases of MH and AMD with
choroidal neovascularization (CNV) exhibited SEs. In eyes with MH,
the misidentification always happened at the inner boundary, while
for CNV eyes, outer boundary SE occurred more frequently. Images of
representative cases with retinal boundary identification errors
are illustrated in Fig. 2.
 | Table I.Comparison of segmentation error rate
between Topcon 3D OCT-1000 and Topcon 3D OCT-2000 algorithms in
analyzing OCT raw data captured with Topcon OCT-1000. |
Table I.
Comparison of segmentation error rate
between Topcon 3D OCT-1000 and Topcon 3D OCT-2000 algorithms in
analyzing OCT raw data captured with Topcon OCT-1000.
|
|
| Number of eyes with
segmentation error |
|
|---|
|
|
|
|
|
|---|
| Region | N | 3D OCT-1000 | 3D OCT-2000 | P-valuea |
|---|
| Entire scan
region |
| Normal
eyes | 43 | 31 (72.09) | 31 (72.09) | 0.999 |
| Abnormal
eyes | 44 | 36 (81.82) | 34 (77.27) | 0.791 |
|
Total | 87 | 67 (77.01) | 65 (74.71) | 0.864 |
| P-valueb |
| 0.281 | 0.578 |
|
| Central subfield |
| Normal
eyes | 43 | 2 (4.65) | 5 (11.63) | 0.453 |
| Abnormal
eyes | 44 | 15 (34.09) | 17 (38.64) | 0.625 |
|
Total | 87 | 17 (19.54) | 22 (25.29) | 0.227 |
| P-valuec |
| 0.001 | 0.004 |
|
No statistically significant differences were
identified in the SE rate between these two algorithms in any
subgroups or total subjects (all P>0.05, McNemar's test;
Table I). Retinal boundary detection
errors in the central subfield were less frequent with OCT-1000
than with OCT-2000, although the difference did not reach
statistical significance (19.54 vs. 25.29%; P=0.227, McNemar's
test; Table I). The SE rate was
higher in the abnormal group compared with that in the normal group
with OCT-1000 (34.09 vs. 4.65%; P=0.001) and OCT-2000 (38.64 vs.
11.63%; P=0.004) in the central subfield, but not in the entire
scan region with OCT-1000 (81.82 vs. 72.09%; P=0.281) or OCT-2000
(77.27 vs. 72.09%; P=0.578).
In OCT-1000, the image quality score did not differ
between groups with and without SEs in either the entire scan
region (56.18 vs. 54.90; P=0.389) or the central subfield (54.94
vs. 56.11; P=0.282). Similar results were found in the OCT-2000
algorithm in entire region (36.65 vs. 36.86; P=0.226) and central
region (36.00 vs. 36.94; P=0.058, independent-samples t-test).
Table II presents the SE score as
assessed by the two algorithms. The SE score was higher when
assessed by the OCT-2000 algorithm, as compared with that obtained
with the OCT 1000 algorithm in the central subfield (mean
difference, 0.93±3.80; P=0.025; paired t-test), but not in the
entire scan region (mean difference, 0.33±10.26; P=0.763; paired
t-test). The SE score of either the entire scan region or the
central subfield was identified to be higher in the abnormal group
than that in the normal group with either algorithm (all P<0.01;
independent-samples t-test). No correlation was identified between
the SE score and image quality in the entire scan region and the
central subfield, as assessed by the two algorithms (all P>0.05;
Pearson's correlation; data not shown).
 | Table II.Comparison of SE scores between Topcon
3D OCT-1000 and Topcon 3D OCT-2000 algorithms. |
Table II.
Comparison of SE scores between Topcon
3D OCT-1000 and Topcon 3D OCT-2000 algorithms.
| Region | 3D OCT-1000 | 3D OCT-2000 | Difference |
P-valuea |
|---|
| Entire scan
region |
|
|
|
|
|
Normal | 3.14±4.54
(0–25) | 5.35±6.37
(0–27) | −2.21±8.43 | 0.093 |
|
Abnormal | 28.50±37.32
(0–136) | 27.00±37.02
(0–140) | 1.50±11.58 | 0.395 |
|
Total | 15.97±29.48
(0–136) | 16.30±28.70
(0–140) | −0.33±10.26 | 0.763 |
|
P-valueb | <0.01 | <0.01 |
|
|
| Central
subfield |
|
|
|
|
|
Normal | 0.09±0.43
(0–2) | 0.98±3.19
(0–16) | −0.88±3.25 | 0.081 |
|
Abnormal | 13.59±24.58
(0–95) | 14.57±24.36
(0–95) | −0.98±4.31 | 0.139 |
|
Total | 6.92±18.66
(0–95) | 7.85±18.67
(0–95) | −0.93±3.80 | 0.025 |
|
P-valueb | <0.01 | <0.01 |
|
|
Comparison of retinal thickness
measurements
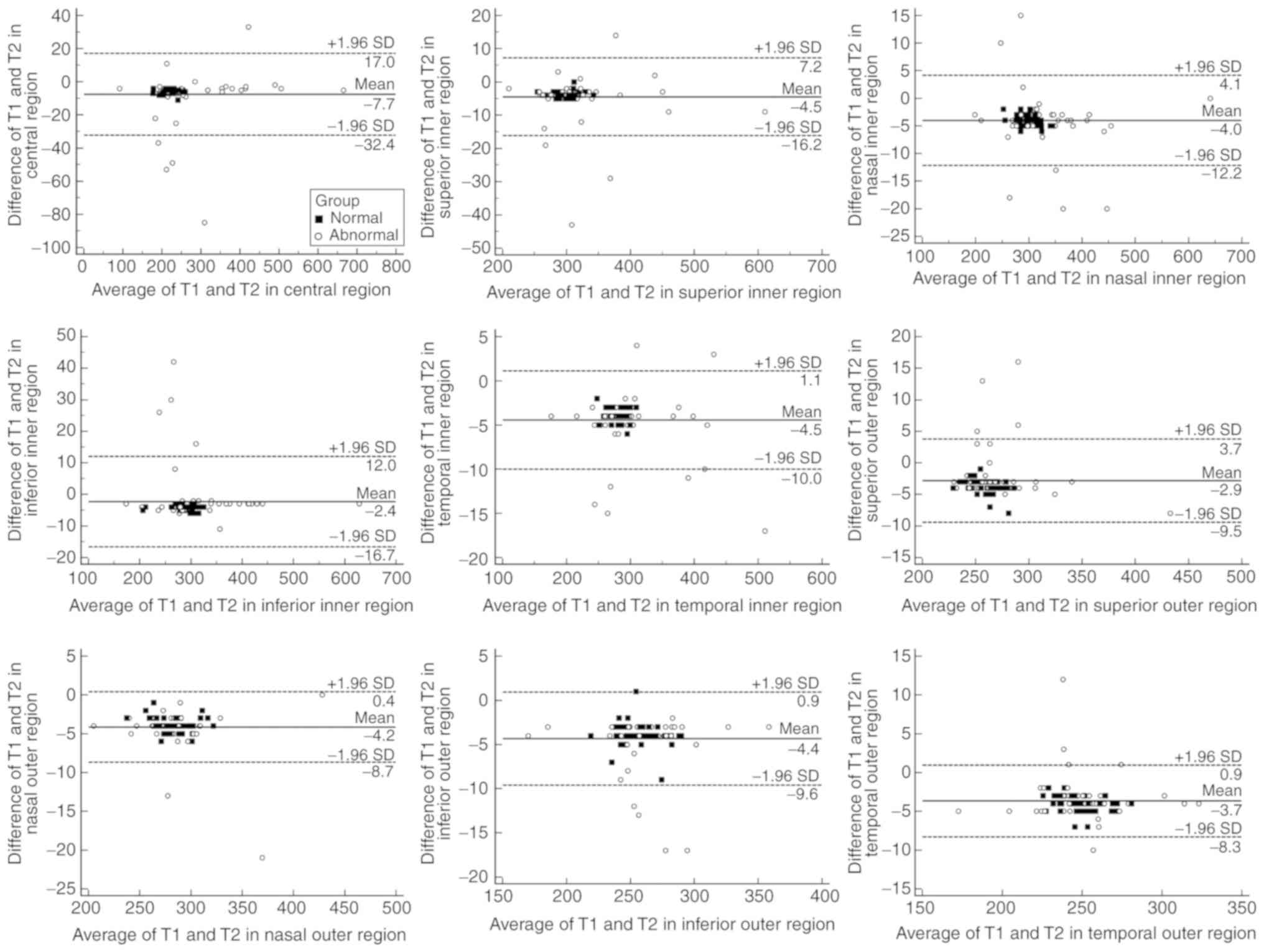
Retinal thickness measured by the two algorithms was
highly correlated [r, 0.987–0.999 (all P<0.001); ICC,
0.951–0.995] in all ETDRS regions for the normal and abnormal eyes
(Table III). The thickness was
greater when assessed by OCT-2000 than by OCT-1000 in all ETDRS
regions for normal and abnormal subjects, except in the inferior
inner region of abnormal subjects. The difference in thickness
between the two algorithms, was 0–11 µm in normal and 0–85 µm (case
with MH) in abnormal group, respectively. The mean difference was
3.72–5.77 µm in the normal and 0.61–9.52 µm in the abnormal group,
respectively (Table III; Fig. 3). It was also indicated that the 95%
confidence interval for the difference was relatively smaller in
the normal group (Fig. 3).
 | Table III.Comparison of macular thickness
analyzed with 3D-OCT-1000 and −2000 algorithms in normal and
abnormal eyes. |
Table III.
Comparison of macular thickness
analyzed with 3D-OCT-1000 and −2000 algorithms in normal and
abnormal eyes.
|
| Retinal thickness
(µm) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Region | 3D OCT-1000 | 3D OCT-2000 | r | ICC value | Difference
(µm) |
|---|
| Normal eyes |
|
|
|
|
|
|
Central | 219.67±19.54 | 225.44±19.53 | 0.998a | 0.956a |
−5.77±1.31a |
|
Superior inner | 299.09±17.12 | 302.81±17.00 | 0.999a | 0.975a |
−3.72±0.93a |
| Nasal
inner | 299.72±19.11 | 303.60±19.46 | 0.999a | 0.979a |
−3.88±0.93a |
|
Inferior inner | 284.95±22.59 | 289.14±22.66 | 0.999a | 0.983a |
−4.19±0.73a |
|
Temporal inner | 281.35±13.78 | 285.30±13.89 | 0.998a | 0.959a |
−3.95±0.82a |
|
Superior outer | 257.86±13.08 | 261.63±13.49 | 0.997a | 0.958a |
−3.77±1.17a |
| Nasal
outer | 278.44±18.44 | 282.26±18.66 | 0.999a | 0.978a |
−3.81±0.98a |
|
Inferior outer | 256.35±16.03 | 260.19±16.23 | 0.996a | 0.969a |
−3.84±1.38a |
|
Temporal outer | 247.02±13.01 | 251.12±13.31 | 0.997a | 0.951a |
−4.09±1.09a |
| Abnormal eyes |
|
|
|
|
|
|
Central | 270.30±110.20 | 279.82±107.02 | 0.987a | 0.983a |
−9.52±17.58a |
|
Superior inner | 315.61±67.63 | 320.89±67.78 | 0.992a | 0.990a |
−5.27±8.32a |
| Nasal
inner | 325.93±74.72 | 330.11±75.37 | 0.997a | 0.995a |
−4.18±5.82a |
|
Inferior inner | 314.91±75.37 | 315.52±77.28 | 0.992a | 0.992a | −0.61±10.01 |
|
Temporal inner | 298.34±61.94 | 303.30±62.56 | 0.998a | 0.995a |
−4.96±3.86a |
|
Superior outer | 267.84±34.58 | 269.82±35.10 | 0.992a | 0.990a |
−1.98±4.44a |
| Nasal
outer | 284.86±32.98 | 289.36±33.55 | 0.996a | 0.987a |
−4.50±3.09a |
|
Inferior outer | 258.48±30.26 | 263.34±30.47 | 0.993a | 0.981a |
−4.86±3.48a |
|
Temporal outer | 248.07±25.42 | 251.36±25.60 | 0.993a | 0.985a |
−3.30±3.11a |
Discussion
In the present study, the raw data acquired with an
OCT-1000 instrument were analyzed using OCT-1000 and OCT-2000
algorithms and their performance and results were compared. It was
revealed that the SE rate and SE score were similar between the two
algorithms in the entire scan region. However, in the central
subfield, the OCT-2000 algorithm performed worse than the OCT-1000
algorithm, with a higher SE score. The retinal thickness
measurements were highly correlated between the two algorithms. The
thickness measurements obtained with OCT-2000 were larger than
those obtained with OCT-1000 by several microns.
Previous studies have demonstrated that the
incidence of SE and discrepancy between different OCTs was
associated with imaging technology, as well as the segmentation
algorithm (18,19). Kim et al (15) investigated the accuracy of the Topcon
3D OCT-1000 algorithm in analyzing Stratus OCT data and revealed
that the OCT-1000 algorithm performed better than Stratus OCT in
segmentation. In the present study, OCT-2000 failed to perform
better than OCT-1000 in analyzing data acquired with OCT-1000. Even
in the central subfield, the OCT-2000 algorithm performed worse
than the OCT-1000 algorithm. One possible reason may be the low
image quality score for OCT-2000. Following re-analysis, the image
quality clearly decreased to 33–47. According to Falavarjani et
al (20), lower image quality
led to a higher SE rate in normal eyes and eyes with clinically
significant macular edema. Since a higher image quality may
increase the sharpness of the retinal boundary (21) and thus decrease the SE rate, the
OCT-2000 system may become less sensitive to the retinal boundary
with poor image quality. However, with the narrow range in the
level of image quality (only 33–47), it was not possible to
demonstrate that a higher image score leads to a better
segmentation performance. Further research is required to elucidate
the effect of image quality on SE.
The differences in thickness were statistically
significant, with OCT-2000 always overestimating retinal thickness
as compared to OCT-1000. The highest mean difference of the 9 ETDRS
areas located in the central subfield was 5.77 µm in the normal and
9.52 µm in the abnormal group. The systemic difference may be due
to differences in built-in software algorithms in the two OCT
devices (22), various degree of
misinterpretation of the double contoured RPE choriocapillaris band
(23) in Topcon OCT-1000 and
misidentification of ILM in Topcon OCT-2000. Another reason may be
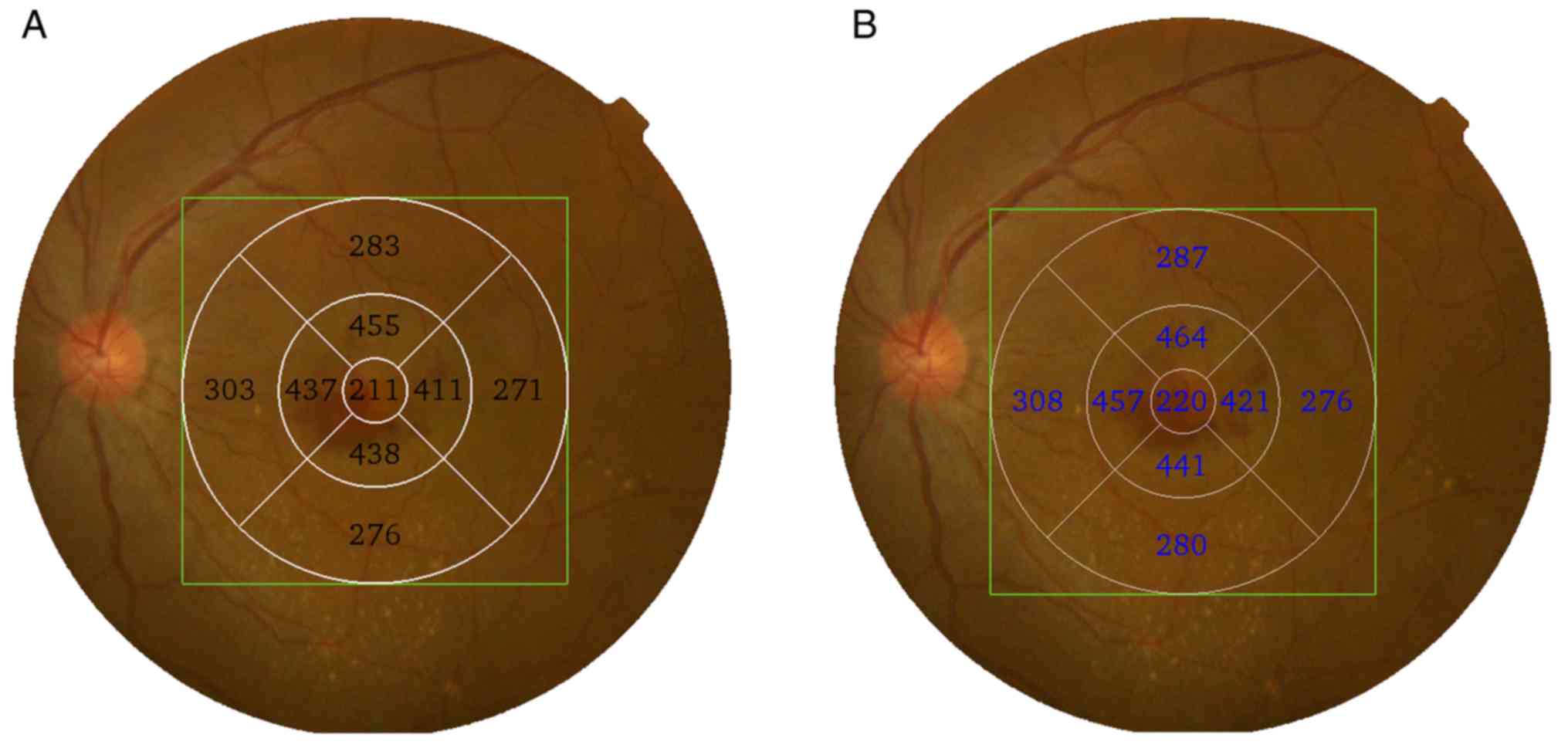
the alteration of the ETDRS map location. Odell et al
(24) compared the ETDRS plot
centered at the location detected by Cirrus HD-OCT with the plot
centered at the fovea and determined that the two plots differed by
14.4 µm on average, reaching 100 µm (sum of difference in all ETDRS
subfields) in normal eyes and even higher in diseased eyes.
Although internal fixation was used with the Topcon OCT-1000 when
scanning eyes, certain aged and abnormal cases may not be able to
sufficiently cooperate, leading to errant fixation (25). The auto fovea function in the
OCT-2000 algorithm may help in the search for the foveal center,
while the OCT-1000 does not have this function. Therefore, the
ETDRS map location may be different between the two algorithms,
causing a difference in the thickness determined (Fig. 4). However, correlation analysis and
ICC values indicated a considerable agreement between the retinal
thickness measurements of the two OCT algorithms. According to the
Bland-Altman plots, the amount of dissimilarity was just a few
microns in the normal group. The difference was insignificant and
indicated that retinal thickness measurements analyzed by the two
OCT built-in algorithms may be used interchangeably in normal
cases. In the abnormal group, the discrepancy ranged from 0 to 85
with various SEs. Therefore, these two measurements should not be
used interchangeably prior to excluding severe SEs in disease
cases, particularly in subjects with MH and CNV.
In the present study, errors in retinal boundary
detection were observed in 77.01% of eyes using the OCT-1000
algorithm and 74.71% using the OCT-2000, a relatively high
percentage when compared to previous studies of SE in association
with OCT (14,15,26,27). Ray
et al (27) reported that
retinal thickness measurement errors occurred in 62.2% of the total
scan area with a Stratus OCT. In a study that analyzed Stratus data
with Topcon 3D OCT viewer, the SE rate was 30.9% (15). Song et al (14) assessed 116 eyes, including normal and
abnormal cases, and determined that the SE rate with the Topcon
OCT-1000 was 63.8% in the 12 radial scan mode. Since various study
designs were used, different retinal boundary error criteria and
different subjects included may explain for the variance in the SE
rate. The higher SE rates in the present study may be due to the
complete analysis of all 128 cross-section images, since a minor
error in any of the 128 images may lead to an SE. In the fast
macular protocol of the stratus OCT, only 6 cross-section images
were evaluated (15), thus lowering
the possibility of SE, even with a ‘relatively low-level
algorithm’. Although Song et al (14) scanned eyes using spectral-domain OCT,
the presence of SEs in the total scan area was assessed in 12
images, in which the sample density was much lower. The SE rate of
the central subfield may be more comparable, since the same scan
mode and sample density were used for the evaluation. In the
present study, a relatively low central subfield SE rate in the
normal and abnormal groups was obtained for the two OCT algorithms,
as compared with those in the study by Song et al (14) (22.5% for normal subjects, 68.9% for
the retinal pathology group and 83.9% for the subretinal pathology
group). Possible explanations include the lower percentage and
severity of abnormal eyes included, as well as a more recent
software version and different criteria used to assess SEs in the
present study.
It is not a surprise that the abnormal group had a
higher SE rate than the normal group, since OCT segmentation
algorithms were designed based on normal data. Diseases may affect
the characteristics of retinal interface and cause
misidentification (28,29). In the present study, the SE score was
considerably lower in the normal group, in the central as well as
the total scan areas, for the assessment by either of the two OCT
algorithms. According to the study by Song et al (14), SEs were more frequent and severe in
the subretinal and retinal pathology groups than in the normal
group. Ho et al (30)
identified a poor agreement between manual and automated
segmentation in eyes with retinal pigment epithelial detachment, as
assessed by the Cirrus HD-OCT. The high percentage of
misidentification of the inner border in MH and outer border in AMD
in the present study also illustrated that disease is an important
factor affecting SEs.
Of note, the present study had certain limitations.
The image quality of the scan data clearly decreased following
re-analysis with OCT-2000. It was not possible to identify the
reason for this, since the standards of image quality grading in
the two OCT devices were not known to us. However, this may be
explained by the higher grading criteria of the OCT-2000, since the
same raw data were analyzed. Images with a poor signal in the
OCT-1000 may be assigned an extremely low-quality score in the
OCT-2000, which may cause severe misidentification of the retinal
boundary, illustrating that OCT data obtained with poor scan
quality are difficult to accurately re-analyze with an advanced OCT
algorithm in the Topcon series. However, this cannot be avoided in
a clinical setting, since patients are not always able to
cooperate, particularly those with poor vision or media opacity.
Another limitation of the present study is that it only focused on
the condition of retinal boundary and thickness deviation,
regardless of SE determinants. Poor scan acquisition (13) and image artifacts (27) were previously reported to also cause
SEs. Due to the retrospective nature of the present study, it was
not possible to gather this information as previous studies did.
Instead, only the effect of disease and image quality on SE was
analyzed. Another limitation was that the present study did not
analyze the SE rate and score in different disease groups. Since
the abnormal cases included exhibited a wide variation and were
difficult to classify, it was not possible to compare the incidence
and severity of SE caused by different diseases.
In conclusion, the SEs obtained with the two OCT
algorithms were relatively high. The OCT-2000 algorithm did not
exhibit a better segmentation performance in the analysis of data
acquired with the OCT-1000. It is, however, noteworthy that not all
SEs have an equally important effect on clinical diagnosis. In
spite of a systemic difference in the measurement of retinal
thickness between the two models, with high ICC values and a small
amount of dissimilarity, it was still possible to use the
measurements interchangeably in normal cases and abnormal cases
without SEs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation
Training Program for the University Students of Guangdong Province
of China (grant no. 1056013105), the Research Grant of Joint
Shantou International Eye Center (grant no. 2012-18) and the
Shantou Medical Science and Technology Planning Project (grant no.
180725224011311).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MZ and HC conceived and designed the experiments. BC
performed the experiments. BC, HC and CZ analyzed the data. BC and
HC wrote the paper. BC, HC, CZ and MZ revised the manuscript.
Ethics approval and informed consent
The present study was approved by the Institutional
Review Board of the JSIEC of Shantou University and the Chinese
University of Hong Kong, and followed the tenets of the Declaration
of Helsinki. Informed consent from the subjects was not required
due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
None of the authors had any competing interests or
financial interests associated with the work presented in this
manuscript.
References
|
1
|
Bhende M, Shetty S, Parthasarathy MK and
Ramya S: Optical coherence tomography: A guide to interpretation of
common macular diseases. Indian J Ophthalmol. 66:20–35. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oberwahrenbrock T, Traber GL, Lukas S,
Gabilondo I, Nolan R, Songster C, Balk L, Petzold A, Paul F,
Villoslada P, et al: Multicenter reliability of semiautomatic
retinal layer segmentation using OCT. Neurol Neuroimmunol
Neuroinflamm. 5:e4492018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waldman AT, Liu GT, Lavery AM, Liu G,
Gaetz W, Aleman TS and Banwell BL: Optical coherence tomography and
visual evoked potentials in pediatric MS. Neurol Neuroimmunol
Neuroinflamm. 4:e3562017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
You Y, Graham EC, Shen T, Yiannikas C,
Parratt J, Gupta V, Barton J, Dwyer M, Barnett MH, Fraser CL, et
al: Progressive inner nuclear layer dysfunction in non-optic
neuritis eyes in MS. Neurol Neuroimmunol Neuroinflamm. 5:e4272018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bennett J, de Seze J, Lana-Peixoto M,
Palace J, Waldman A, Schippling S, Tenembaum S, Banwell B,
Greenberg B, Levy M, et al: Neuromyelitis optica and multiple
sclerosis: Seeing differences through optical coherence tomography.
Mult Scler. 21:678–688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wojtkowski M, Srinivasan V, Fujimoto JG,
Ko T, Schuman JS, Kowalczyk A and Duker JS: Three-dimensional
retinal imaging with high-speed ultrahigh-resolution optical
coherence tomography. Ophthalmology. 112:1734–1746. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reichel E, Ho J and Duker JS: OCT Units:
Which One Is Right for Me? Review of ophthalmology, Boston.
16:622009.
|
|
8
|
Roth NM, Saidha S, Zimmermann H, Brandt
AU, Isensee J, Benkhellouf-Rutkowska A, Dornauer M, Kühn AA, Müller
T, Calabresi PA and Paul F: Photoreceptor layer thinning in
idiopathic Parkinson's disease. Mov Disord. 29:1163–1170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topcon, . Optical Coherence Tomography 3D
OCT-2000 Series. http://pdf.medicalexpo.com/pdf/topcon-europe-medical/brochure-topcon-3d-oct-2000-series/77876-75588-_12.html
|
|
10
|
Larsson J, Zhu M, Sutter F and Gillies MC:
Relation between reduction of foveal thickness and visual acuity in
diabetic macular edema treated with intravitreal triamcinolone. Am
J Ophthalmol. 139:802–806. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ooto S, Hangai M, Sakamoto A, Tomidokoro
A, Araie M, Otani T, Kishi S, Matsushita K, Maeda N, Shirakashi M,
et al: Three-dimensional profile of macular retinal thickness in
normal Japanese eyes. Invest Ophthalmol Vis Sci. 51:465–473. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haouchine B, Massin P, Tadayoni R, Erginay
A and Gaudric A: Diagnosis of macular pseudoholes and lamellar
macular holes by optical coherence tomography. Am J Ophthalmol.
138:732–739. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sadda SR, Wu Z, Walsh AC, Richine L,
Dougall J, Cortez R and LaBree LD: Errors in retinal thickness
measurements obtained by optical coherence tomography.
Ophthalmology. 113:285–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song Y, Lee BR, Shin YW and Lee YJ:
Overcoming segmentation errors in measurements of macular thickness
made by spectral-domain optical coherence tomography. Retina.
32:569–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SW, Oh J, Yang KS, Kim YH, Park JW,
Rhim JW and Huh K: Stratus OCT image analysis with spectral-domain
OCT (Topcon 3D OCT Viewer). Br J Ophthalmol. 96:93–98. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim M, Lee SJ, Han J, Yu SY and Kwak HW:
Segmentation error and macular thickness measurements obtained with
spectral-domain optical coherence tomography devices in neovascular
age-related macular degeneration. Indian J Ophthalmol. 61:213–217.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varga BE, Tátrai E, Cabrera DeBuc D and
Somfai GM: The effect of incorrect scanning distance on boundary
detection errors and macular thickness measurements by spectral
domain optical coherence tomography: A cross sectional study. BMC
Ophthalmol. 14:1482014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ho J, Sull AC, Vuong LN, Chen Y, Liu J,
Fujimoto JG, Schuman JS and Duker JS: Assessment of artifacts and
reproducibility across spectral- and time-domain optical coherence
tomography devices. Ophthalmology. 116:1960–1970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tan CS, Cheong KX, Lim LW and Sadda SR:
Comparison of macular choroidal thicknesses from swept source and
spectral domain optical coherence tomography. Br J Ophthalmol.
100:995–999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Falavarjani KG, Mehrpuya A and
Amirkourjani F: Effect of spectral domain optical coherence
tomography image quality on macular thickness measurements and
error rate. Curr Eye Res. 42:282–286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Boer JF, Cense B, Park BH, Pierce MC,
Tearney GJ and Bouma BE: Improved signal-to-noise ratio in
spectral-domain compared with time-domain optical coherence
tomography. Opt Lett. 28:2067–2069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sander B, Al-Abiji HA, Kofod M and
Jørgensen TM: Do different spectral domain OCT hardwares measure
the same? Comparison of retinal thickness using third-party
software. Graefes Arch Clin Exp Ophthalmol. 253:1915–1921. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Krebs I, Falkner-Radler C, Hagen S, Haas
P, Brannath W, Lie S, Ansari-Shahrezaei S and Binder S: Quality of
the threshold algorithm in age-related macular degeneration:
Stratus versus Cirrus OCT. Invest Ophthalmol Vis Sci. 50:995–1000.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Odell D, Dubis AM, Lever JF, Stepien KE
and Carroll J: Assessing errors inherent in OCT-derived macular
thickness maps. J Ophthalmol. 2011:6925742011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bedell HE: A functional test of foveal
fixation based upon differential cone directional sensitivity.
Vision Res. 20:557–560. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alshareef RA, Dumpala S, Rapole S,
Januwada M, Goud A, Peguda HK and Chhablani J: Prevalence and
distribution of segmentation errors in macular ganglion cell
analysis of healthy eyes using cirrus HD-OCT. PLoS One.
11:e01553192016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ray R, Stinnett SS and Jaffe GJ:
Evaluation of image artifact produced by optical coherence
tomography of retinal pathology. Am J Ophthalmol. 139:18–29. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bahrami B, Ewe SYP, Hong T, Zhu M, Ong G,
Luo K and Chang A: Influence of retinal pathology on the
reliability of macular thickness measurement: A comparison between
optical coherence tomography devices. Ophthalmic Surg Lasers
Imaging Retina. 48:319–325. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Waldstein SM, Gerendas BS, Montuoro A,
Simader C and Schmidt-Erfurth U: Quantitative comparison of macular
segmentation performance using identical retinal regions across
multiple spectral-domain optical coherence tomography instruments.
Br J Ophthalmol. 99:794–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ho J, Adhi M, Baumal C, Liu J, Fujimoto
JG, Duker JS and Waheed NK: Agreement and reproducibility of
retinal pigment epithelial detachment volumetric measurements
through optical coherence tomography. Retina. 35:467–472. 2015.
View Article : Google Scholar : PubMed/NCBI
|