Introduction
SOX family proteins are a group of transcriptional
factors that recognize and bind DNA via the high-mobility group
domain (1). Sex determining region
Y-box 2 (SOX2) is located on chromosome 3q26.3 and belongs to the
SOXB1 group (2–4). An increasing number of studies have
implicated SOX2 in the tumorigenesis of gastric, lung and breast
cancer (5,6).
Lung cancer, which includes non-small-cell lung
cancer (NSCLC) and small-cell lung cancer, is one of the most
common causes of cancer-associated mortality worldwide, among which
NSCLC accounts for ~85%. Currently, NSCLC is a non-curable disease
with marked genetic complexity (1).
A fluorescence in situ hybridization analysis performed in
447 resected NSCLC tissue samples indicated that amplification of
SOX2 was positively associated with increased copy number of the
FGFR1 and PI3KCA genes (1). SOX2 has
been demonstrated to promote cellular proliferation via evading
apoptotic signals in NSCLC (7,8).
However, the role of SOX2 in the metastasis and
epithelial-to-mesenchymal transition (EMT) regulation of NSCLC
remains poorly understood.
MicroRNAs (miRNAs) comprise a large family of
regulatory RNAs that may repress expression of target mRNAs in a
sequence-specific manner. miRNAs participate in diverse biological
processes, including cell development, differentiation,
proliferation and apoptosis. In addition, aberrant miRNA expression
patterns have been identified in a number of types of human cancer,
including lung cancer. According to a previous study, the
expression of miR-590-5p significantly suppressed the
proliferation, migration and invasion of NSCLC cells (9), and bioinformatics analysis using
TargetScan also demonstrated that SOX2 is targeted by miRNA-590-5p
(10). The aim of the present study
was to investigate the role of SOX2 in the regulation of NSCLC
metastasis and EMT, and determine whether miR-590-5p is a regulator
of SOX2 in NSCLC.
Materials and methods
Patients and specimens
Between June 2014 and March 2016, 33
paraffin-embedded tumor specimens and adjacent normal specimens
from patients with NSCLC were collected from The Second Affiliated
Hospital of Zhejiang University (Zhejiang, China). The patients
were aged between 32–60 years old with a median age of 48 years;
the cohort contained 25 females and 8 males. The specimens were
definitively diagnosed by experienced pathologists. The
pathological Tumor-Node-Metastasis classification stage was
determined by the 2009 staging system of the Union for
International Cancer Control (11).
Patients were separated into high or low expression groups based on
the median expression value of mRNA for miR-590-5p (cut-off value,
0.036) or SOX2 (cut-off value, 0.0469). Written informed consent
was obtained from all patients regarding the use of their tissues
for research purposes. All the procedures were performed in
accordance with the guidelines of the Ethics Committee of The
Second Affiliated Hospital of Zhejiang University.
Computational target prediction
For the identification of miR-590-5p targets,
TargetScan release 7.2 (http://www.targetscan.org/vert_72/) was used with the
default parameters.
Plasmids
The SOX2 over-expression plasmid (SOX2-OE), SOX2
3untranslated region (UTR) and SOX2 3UTR-mut were purchased from
GenePharma (Shanghai, China). Briefly, the SOX2 cDNA was inserted
into the pcDNA3.1 vector (cat. no. 20011; Addgene, Inc.), which
harbors a FLAG epitope sequence to generate the SOX2-OE plasmid.
The complete SOX2 3UTR was inserted into the pmirGLO
Dual-luciferase plasmid (Promega Corporation) to generate the SOX2
3UTR plasmid. To generate SOX2 3UTR-mut plasmid, the predicted
miR-590-5p binding site in the SOX2 3UTR was mutated (mut) and
inserted into the pmirGLO plasmid to generate SOX2 3UTR-mut
plasmid.
Cell culture and transfection
The lung cancer A549 and H1299 cell lines (American
Type Culture Collection) were cultured in RPMI-1640 medium (Life
Technologies; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Hyclone; GE Healthcare), 100 U/ml penicillin and 100 U/ml
streptomycin. The cells were cultured in an incubator with 5%
CO2 at 37°C. According to the manufacturer's
instructions, cells were seeded in 12-well plates for 24 h and then
transfected with 100 pmol short interfering (si)RNAs and 10 nM miR
mimics with Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h. The SOX2 siRNA sequence was
5′-ACCTCCGGGACATGATCAGCA-3′ (Shanghai GenePharma Co., Ltd.).
Scrambled siRNA (siRNA NC; 5′-TTCTCCGAACGTGTCACGT-3′; Shanghai
GenePharma Co., Ltd.) was used as a negative control. miR-590-5p
mimic (5′-GAGCUUAUUCAUAAAAUGCAG-3′) and miRNA NC
(5′-GUCCAGUGAAUUCCCAG-3′) were obtained from Guangzhou RiboBio Co.,
Ltd.
Luciferase activity assay
A total of 1×105 A549 cells were seeded
in 24-well plates for 24 h. Then the mixture of 1 µg SOX2 3UTR or
SOX2 3UTR-mut, 0.1 µg of Renilla luciferase plasmids
(Promega Corporation) and 25 nM miR-590-5p mimic or miRNA NC were
incubated with Lipofectamine 2000 at room temperature for 15 min
and added to each well. After 48 h, the cells were lysed and the
luciferase activity was detected using the Dual Luciferase Reporter
Assay System (Promega Corporation) on an LD400 luminometer (Beckman
Coulter, Inc.). The firefly luciferase activity was normalized to
the Renilla luciferase activity.
RNA extraction and reverse
transcription quantitative polymerase chain reaction (RT-qPCR)
assay
TRIzol® reagent (Thermo Fisher
Scientific, Inc.) was used to extract total RNA, and the cDNA
library was generated using a cDNA kit (Thermo Fisher Scientific,
Inc.). According to the manufacturer's protocol, SYBR Green Master
mix (Thermo Fisher Scientific) was used to perform RT-qPCR in
triplicate with the following thermocycler conditions: Denaturation
of DNA at 95°C for 5 min, followed by 40 cycles of amplification:
95°C for 60 sec, 55°C for 60 sec and 72°C for 60 sec. To analyze
the relative expression of mRNAs or miRNA, the 2−ΔΔCq
method (12) was used and normalized
by GAPDH or U6 expression. The primer sequences were as follows:
SOX2 forward, 5′-CTCGTGCAGTTCTACTCGTCG-3′; SOX2 reverse,
5′-AGCTCTCGGTCAGGTCCTTT-3′; GAPDH forward,
5′-CTCCTCCTGTTCGACAGTCAGC-3′; GAPDH reverse,
5′-CCCAATACGACCAAATCCGTT-3′.
Cell migration assay
To analyze the cell migration ability, a wound
healing assay was performed. The transfected A549 and H1299 cells
were plated into 12-well plates at a density of 5×104
for 24 h, followed by creation of a wound in the cell monolayer
using a sterile 100 µl micropipette tip, washed with PBS twice and
then cultured in serum-free medium for an additional 24 h. A
phase-contrast inverted microscope (Olympus IX71; Olympus
Corporation) at a magnification of ×100 was used to measure and
capture images of the wound width.
Transwell invasion assay
To analyze the cell invasive ability, a Transwell
assay was performed. The upper chamber was pre-coated with 1 mg/ml
Matrigel (Growth Factor Reduced BD Matrigel™ Matrix; BD
Biosciences) for 1 h in an incubator with 5% CO2 at
37°C, followed by seeding cells (5×104) in the upper
chamber with RPMI-1640 medium without FBS (8 µm pore size membrane;
EMD Millipore). Complete medium (600 µl) was added to the lower
chamber and the cells were incubated for 48 h. The non-invading
cells in the top chamber were removed with a cotton swab, and the
cells on the lower surface of the membrane were fixed with 4%
formaldehyde for 15 min at room temperature and stained with 0.1%
crystal violet solution (both Sigma-Aldrich; Merck KGaA) for 20 min
at room temperature. Images were captured using an IX71 microscope
and cells were counted at a magnification of ×200.
Western blot analysis
Radioimmunoprecipitation assay lysis buffer was used
to obtain the cell lysates, protein was quantified by a
bicinchoninic acid protein assay (Pierce; Thermo Fisher Scientific,
Inc.) and the protein expression was analyzed via standard western
blot analysis. Briefly, equal amounts of protein (50 µg/lane) were
subjected to 10% SDS-PAGE and subsequently transferred to
polyvinylidene fluoride membrane (EMD Millipore). Following
blocking with 5% non-fat milk for 1.5 h at room temperature, the
membrane was incubated with the primary antibodies [anti-SOX2 (cat.
no. ab75485), anti-epithelial cadherin (E-cadherin; cat. no.
ab15148), anti-vimentin (cat. no. ab92547), anti-zinc finger
protein SNAI1 (Snail; cat. no. ab216347), anti-zinc finger protein
SNAI2 (Slug; cat. no. ab106077) and anti-GAPDH (cat. no. ab9484;
all 1:500; Abcam)] at 4°C overnight, and then rinsed twice with
PBS, followed by incubation with the horseradish
peroxidase-conjugated secondary anti-rabbit antibody (cat. no.
ab150077; 1:2,000; Abcam) for 1 h at room temperature. The membrane
was subsequently incubated with an ECL Chemiluminescent Substrate
kit (Thermo Fisher Scientific, Inc.) and exposed to X-ray film.
ImageJ version 1.41 software (National Institutes of Health,
Bethesda, MD, USA) was used for the quantitative analysis of band
densities.
Statistical analysis
All statistical analyses were performed using the
GraphPad software 8 (GraphPad Software, Inc.). A t-test was used to
compare data between two groups. For the comparison of multiple
groups, a one-way analysis of variance followed by Tukey's post-hoc
test was performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
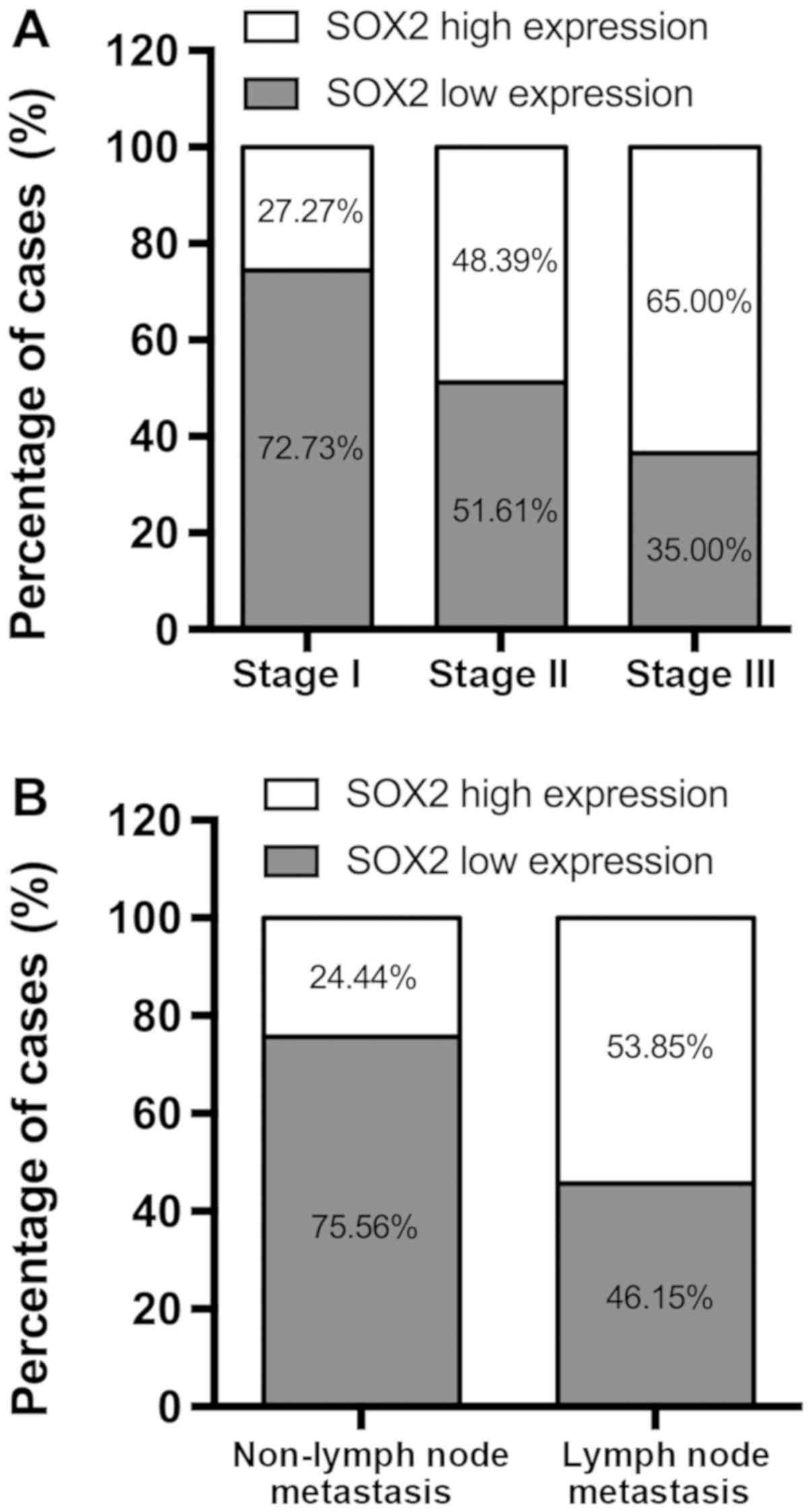
SOX2 is upregulated in NSCLC tissues
and is associated with pathological stage and lymph node
metastasis
Of the 33 NSCLC tissue samples at pathological stage
I, 24 (72.73%) exhibited low expression of SOX2, while 16/31
(51.61%) stage II samples and 7/20 (35.00%) stage III cases
exhibited low SOX2 expression rates (Fig. 1A). Changes in SOX2 expression were
also significantly associated with lymph node metastasis. Of the 39
cases of NSCLC with lymph node metastasis, 21 (53.85%) exhibited
high expression of SOX2. By contrast, of the 45 cases without lymph
node metastasis, only 11 (24.44%) exhibited high SOX2 expression
(Fig. 1B).
Knockdown of SOX2 suppresses NSCLC
cell migration
To examine the role of SOX2 in regulating NSCLC cell
migration, wound healing and Transwell assays were performed. The
SOX2 mRNA expression was measured to evaluate the small interfering
RNA (siRNA) knockdown efficiency in cancer cells, and the results
are presented in Fig. 2A. The wound
healing assay results revealed that, after 48 h, the wound in the
SOX2 downregulated group was markedly narrower compared with that
in the negative control group (Fig.
2B). The Transwell assay demonstrated that, at 48 h after
transfection with SOX2 siRNA, the number of migrated cells was
decreased compared with that in the negative control group
(Fig. 2C). These results
demonstrated that knockdown of SOX2 may suppress NSCLC cell
migration.
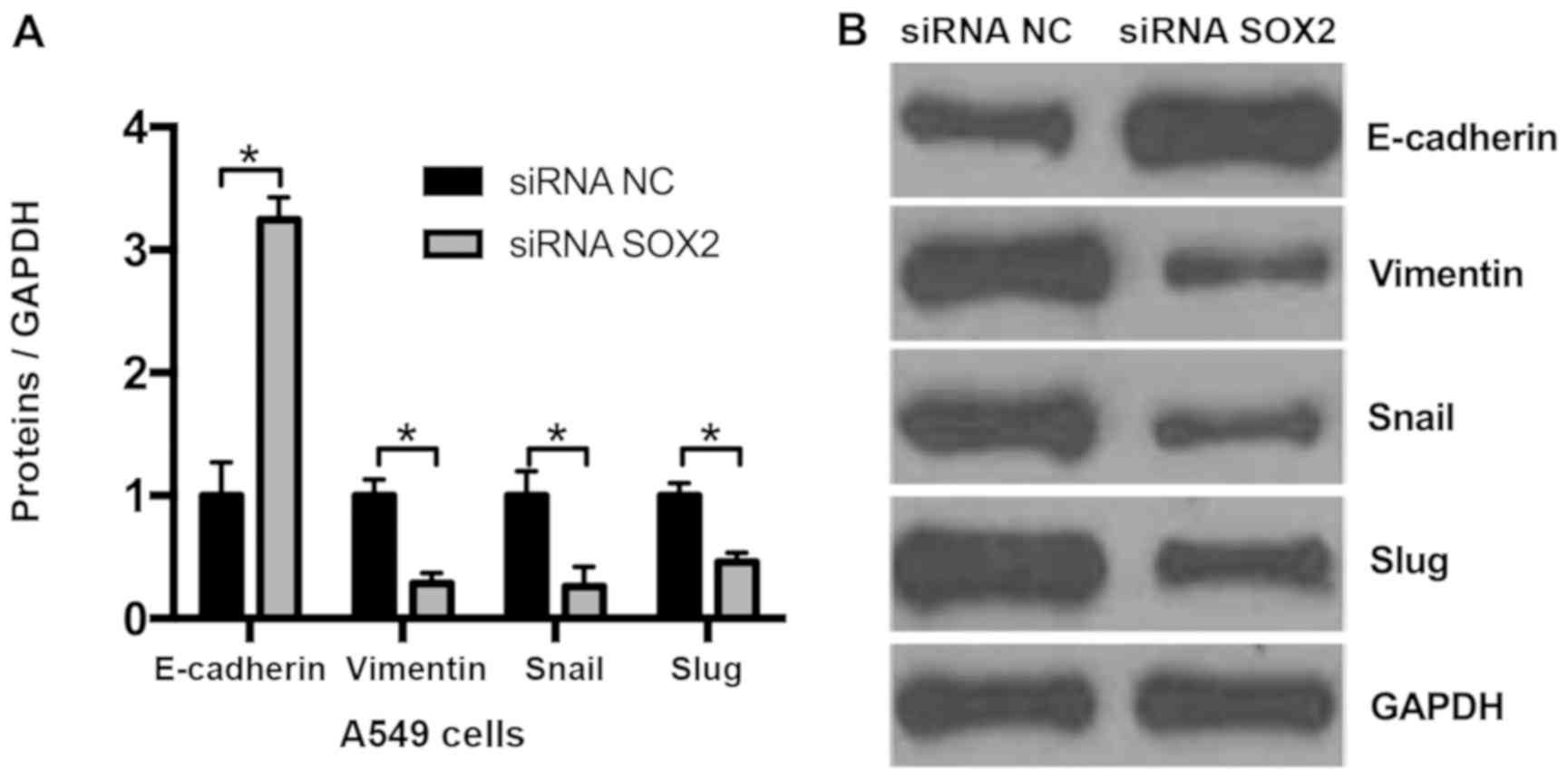
Knockdown of SOX2 suppresses EMT in
NSCLC cells
As knockdown of SOX2 may decrease cell migration, it
was hypothesized that SOX2 may affect EMT. Compared with the
negative control (siRNA NC), it was observed that the expression of
the epithelial marker E-cadherin was markedly upregulated and that
of the mesenchymal marker vimentin was downregulated in A549 cells
transfected with SOX2 siRNA (Fig.
3). The 2 EMT-associated genes, Snail and Slug, were
additionally assessed by western blot analysis, and the results
demonstrated that knockdown of SOX2 decreased Snail and Slug
expression. These results indicate that knockdown of SOX2 may
suppress EMT in NSCLC cells.
miR-590-5p targets SOX2 and suppresses
EMT in NSCLC cells
In order to explore the factors that may regulate
SOX2 expression, TargetScan software was used to identify miRNAs
targeting SOX2. The bioinformatics analysis demonstrated that SOX2
may be targeted by miR-590-5p (Fig.
4A). RT-qPCR data indicated that miR-590-5p mimics markedly
increased its expression by 2.8-fold (Fig. 4B). To confirm whether miR-590-5p
directly targeted SOX2, a 3′-untranslated region (UTR) luciferase
reporter assay was performed. The results revealed that miR-590-5p
mimics repressed the luciferase activity of the wild-type SOX2
3′UTR, but not that of the mutated SOX2 3′UTR (Fig. 4C). To additionally confirm these
results, A549 cells were transfected with the miR-590-5p mimics and
their negative control. The results of RT-qPCR and western blot
analysis demonstrated that miR-590-5p mimics markedly decreased the
mRNA and protein levels of SOX2 (Fig. 4D
and E).
To determine the expression pattern of miR-590-5p in
patients with NSCLC, the expression of miR-590-5p was measured in
33 NSCLC tissue specimens. The results revealed that, among the
cases at pathological stage I, 75.65% exhibited high expression of
miR-590-5p, 61.31% of pathological stage II and 31.63% of
pathological stage III cases exhibited high miR-590-5p expression
(Fig. 5A). Changes in miR-590-5p
expression were also significantly associated with lymph node
metastasis. Among the 39 cases of NSCLC without lymph node
metastasis, 69.75% exhibited high expression of miR-590-5p. By
contrast, of the 45 cases without lymph node metastasis, only
37.63% exhibited high miR-590-5p expression (Fig. 5B).
 | Figure 5.Expression of miR-590-5p in NSCLC
tissues and effect of miR-590-5p on cell migration and
epithelial-to-mesenchymal transition-associated protein expression.
(A) The expression of miR-590-5p was significantly associated with
pathological stage in 33 patients. (B) The expression of miR-590-5p
was associated with lymph node metastasis in NSCLC. (C and D)
Densitometric analysis of western blot analysis data of E-cadherin,
vimentin, Snail and Slug expression. (D) Representative western
blot analysis gel performed in A549 cells transfected with miRNA
NC, miR-590-5p mimics, miR-590-5p mimics + SOX2 overexpression.
*P<0.05. SOX2, sex determining region Y-box 2; NSCLC,
non-small-cell lung cancer; miRNA/miR, microRNA; OE,
overexpression; E-cadherin, epithelial cadherin; Snail, zinc finger
protein SNAI1; Slug, zinc finger protein SNAI2; NC, negative
control. |
The effect of miR-590-5p on cell migration and
EMT-associated protein expression was also investigated in the
present study. The results demonstrated that, compared with the
negative control (miRNA NC), the expression of the epithelial
marker E-cadherin was markedly upregulated and that of the
mesenchymal marker vimentin was downregulated in A549 cells
transfected with miR-590-5p mimics, whereas restoring SOX2
expression reversed these effects (Fig.
5C and D).
Discussion
SOX2 has been demonstrated to be an oncogene
regulating the transcription of downstream target genes. It has
also been revealed that the alteration of SOX2 expression may
affect the progression and prognosis of multiple tumors via
regulating various cell signaling pathways. SOX2 enhances tumor
growth via the RAC-alpha serine/threonine-protein kinase signaling
pathway in esophageal squamous cell carcinoma (13); in hepatocellular cancer, SOX2
enhances cell invasion through activating Slug (14), whereas in lung cancer, cell apoptosis
may be inhibited by SOX2 through the mitogen-activated protein
kinase 4/survivin signaling pathway (15). In addition, SOX2 is involved in the
epidermal growth factor receptor, Bcl-2 like 1 and Wnt signaling
pathways. The emerging role of SOX2 in cell proliferation and
survival and its crosstalk with oncogenic signaling was previously
described in lung cancer (16–18). In
the present study, the expression of SOX2 was evaluated in NSCLC
tissues. The specimens were collected from patients with stage
I–III NSCLC, and the SOX2 mRNA expression in the tissues was
measured using RT-qPCR. The results revealed that an increased
level of SOX2 expression was markedly associated with a more
advanced pathological stage and lymph node metastasis. Due to the
limitation of clinical specimen collection, the sample size of the
present study was not very large. In the future, more specimens
must be collected to validate the results.
EMT enhances tumor cell migration and invasion
ability, eventually promoting the dissemination of tumor cells to
various parts of the body (19). The
process involves downregulation of E-cadherin and upregulation of
vimentin, Snail and Slug expression (20). Functional associations have been
established between the SOX protein family and key effectors of EMT
occurring in the context of carcinogenesis and embryonic
development. In the present study, it was observed that the
knockdown of SOX2 increased the expression of the epithelial marker
E-cadherin, while the mesenchymal markers vimentin, Snail and Slug
and their associated genes were downregulated, indicating that SOX2
may promote EMT in NSCLC.
miR-590-5p appears to exhibit different functions in
various types of cancer. In cervical cancer cells, miR-590-5p was
demonstrated to facilitate cell growth due to its close homology
with the major capsid protein L1 (21). However, miR-590-5p has the opposite
function in glioblastoma multiforme cells (22), suggesting that the role of miR-590-5p
is context-dependent. A recent study demonstrated that ectopic
expression of miR-590-5p significantly suppressed the
proliferation, migration and invasion of NSCLC cells (9). Evidence has demonstrated that miRNAs
may regulate multiple cellular functions, including EMT, through
modulating the expression of their target genes in NSCLC. Using
bioinformatics analysis, SOX2 was targeted by miR-590-5p, and the
3′UTR luciferase assay confirmed that SOX2 expression is regulated
by miR-590-5p. It was also observed that the expression of the
epithelial marker E-cadherin was markedly upregulated and that of
the mesenchymal marker vimentin downregulated in A549 cells
transfected with miR-590-5p mimics, whereas restoring SOX2
expression reversed these effects. The results of the present study
suggested that the miR-590-5p/SOX2 axis may serve as an EMT
regulator in NSCLC, which may be useful as a target for NSCLC
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Ethics approval and consent to
participate
Written informed consent was obtained from all the
patients regarding the use of their tissues for research purposes.
All the procedures were performed in accordance with the guidelines
of the Ethics Committee of Zhejiang Medical University.
Patient consent for publication
Written informed consent was obtained from all the
patients regarding the use of their tissues for research
purposes.
Availability of data and materials
All the datasets generated/analyzed in the present
study are included in the published manuscript.
Authors' contributions
ZBC is the only contributor of this work, who
analyzed and interpreted the patient data, performed the
experiments, and wrote and approved the final manuscript.
Competing interests
The author declares that they have no competing
interests.
References
|
1
|
Sarkar A and Hochedlinger K: The sox
family of transcription factors: Versatile regulators of stem and
progenitor cell fate. Cell Stem Cell. 12:15–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schepers GE, Teasdale RD and Koopman P:
Twenty pairs of sox: Extent, homology, and nomenclature of the
mouse and human sox transcription factor gene families. Dev Cell.
3:167–170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stevanovic M, Zuffardi O, Collignon J,
Lovell-Badge R and Goodfellow P: The cDNA sequence and chromosomal
location of the human SOX2 gene. Mamm Genome. 5:640–642. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collignon J, Sockanathan S, Hacker A,
Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN
and Lovell-Badge R: A comparison of the properties of Sox-3 with
Sry and two related genes, Sox-1 and Sox-2. Development.
122:509–520. 1996.PubMed/NCBI
|
|
5
|
Lengerke C, Fehm T, Kurth R, Neubauer H,
Scheble V, Müller F, Schneider F, Petersen K, Wallwiener D, Kanz L,
et al: Expression of the embryonic stem cell marker SOX2 in
early-stage breast carcinoma. BMC Cancer. 11:422011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Boumahdi S, Driessens G, Lapouge G, Rorive
S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E,
et al: SOX2 controls tumour initiation and cancer stem-cell
functions in squamous-cell carcinoma. Nature. 511:246–250. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie C, Han Y, Liu Y, Han L and Liu J:
miRNA-124 down-regulates SOX8 expression and suppresses cell
proliferation in non-small cell lung cancer. Int J Clin Exp Pathol.
7:6534–6542. 2014.PubMed/NCBI
|
|
8
|
Toschi L, Finocchiaro G, Nguyen TT, Skokan
MC, Giordano L, Gianoncelli L, Perrino M, Siracusano L, Di Tommaso
L, Infante M, et al: Increased SOX2 gene copy number is associated
with FGFR1 and PIK3CA gene gain in non-small cell lung cancer and
predicts improved survival in early stage disease. PLoS One.
9:e953032014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang FF, Wang S, Xue WH and Cheng JL:
microRNA-590 suppresses the tumorigenesis and invasiveness of
non-small cell lung cancer cells by targeting ADAM9. Mol Cell
Biochem. 423:29–37. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou L, Zhao LC, Jiang N, Wang XL, Zhou
XN, Luo XL and Ren J: MicroRNA miR-590-5p inhibits breast cancer
cell stemness and metastasis by targeting SOX2. Eur Rev Med
Pharmacol Sci. 21:87–94. 2017.PubMed/NCBI
|
|
11
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gen Y, Yasui K, Nishikawa T and Yoshikawa
T: SOX2 promotes tumor growth of esophageal squamous cell carcinoma
through the AKT/mammalian target of rapamycin complex 1 signaling
pathway. Cancer Sci. 104:810–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun C, Sun L, Li Y, Kang X, Zhang S and
Liu Y: Sox2 expression predicts poor survival of hepatocellular
carcinoma patients and it promotes liver cancer cell invasion by
activating Slug. Med Oncol. 30:5032013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Li X, Lu D, Xu Y, Mou W, Wang L,
Chen Y, Liu Y, Li X, Li LY, et al: SOX2 regulates apoptosis through
MAP4K4-survivin signaling pathway in human lung cancer cells.
Carcinogenesis. 35:613–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou YT, Lee CC, Hsiao SH, Lin SE, Lin SC,
Chung CH, Chung CH, Kao YR, Wang YH, Chen CT, et al: The emerging
role of SOX2 in cell proliferation and survival and its crosstalk
with oncogenic signaling in lung cancer. Stem Cells. 31:2607–2619.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stewart DJ: Wnt signaling pathway in
non-small cell lung cancer. J Natl Cancer Inst. 106:djt3562014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T,
Liu Y, Li X, Xiang R and Li N: SOX2 promotes tumor metastasis by
stimulating epithelial-to-mesenchymal transition via regulation of
WNT/β-catenin signal network. Cancer Lett. 336:379–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan XW, Wang DM, Hu Y, Tang YN, Shi WW,
Guo XJ and Song JG: Hepatocyte nuclear factor 6 suppresses the
migration and invasive growth of lung cancer cells through p53 and
the inhibition of epithelial-mesenchymal transition. J Biol Chem.
288:31206–31216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kitamura K, Seike M, Okano T, Matsuda K,
Miyanaga A, Mizutani H, Noro R, Minegishi Y, Kubota K and Gemma A:
MiR-134/487b/655 cluster regulates TGF-β-induced
epithelial-mesenchymal transition and drug resistance to gefitinib
by targeting MAGI2 in lung adenocarcinoma cells. Mol Cancer Ther.
13:444–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu Y, Ouyang Y, Wang F, Zheng A, Bai L,
Han L, Chen Y and Wang H: MicroRNA-590 promotes cervical cancer
cell growth and invasion by targeting CHL1. J Cell Biochem.
115:847–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pang H, Zheng Y, Zhao Y, Xiu X and Wang J:
miR-590-3p suppresses cancer cell migration, invasion and
epithelial-mesenchymal transition in glioblastoma multiforme by
targeting ZEB1 and ZEB2. Biochem Biophys Res Commun. 468:739–745.
2015. View Article : Google Scholar : PubMed/NCBI
|