Introduction
Degeneration of the cortex is commonly found with
brain injury (1), stroke (2), limb amputation (3) and aging (4). The long-term pathological state further
hinders future treatments.
Hemiplegia is generally a symptom of stroke (when a
bleed or blood clot damages part of brain) and brain injury, and it
is generally treated with drugs (5,6) such as
acetyl glutamine and amantadine, physical therapy (7), general nursing methods (8), transcutaneous electrical nerve
stimulation (9–12), and implanted nerve electrical
stimulators (13). Many studies have
shown that it is possible for patients with hemiplegia to regain
motor ability in the lower limbs using certain treatments (8,9,14,15).
However, prolonged use of drug treatments may affect other regions
of the brain, and physical care is time intensive. Neuromuscular
electrical stimulation is effective in the short term in improving
upper limb impairment in individuals with chronic stroke (16). Wilson et al (17) studied patients with hemiplegic
shoulder pain who received a fully-implanted electrical stimulator
and were followed up for 24 months; the study demonstrated the
safety and efficacy of a fully-implantable axillary peripheral
nerve stimulation system for chronic hemiplegic shoulder pain. In
general, electrical stimulation of nerves is an efficient approach
(16,18,19) with
minimal side effects (17,20).
By implanting an electrical stimulator, studies have
shown that patients with spinal cord injury can achieve standing
and walking (21–23). Furthermore, research has indicated
that electrical stimulation can improve cognitive deficits
associated with traumatic brain injury (24), and that low-frequency
electroencephalogram (EEG) signals appear when the sub-paresthesia
spinal cord of a rat is stimulated (25). To date, implantable electrical
stimulator microsystems have been rapidly developed and used in
many fields of medicine (13,17,26).
However, to the best of our knowledge, there have been no studies
on the effect of electrical stimulation of the sciatic nerve on the
motor cortex. The present study discusses another method for the
activation of motor brain regions using a peripheral nerve
electrical stimulator.
The present study is based on a set of
self-developed high-density electrodes for rats (27), designed to monitor EEG activity
commonly used in neural interfaces (28,29). A
self-developed fully-implantable electrical stimulator was
implanted subcutaneously in rats, for which the waveform amplitude,
frequency and stimulation time could be set externally.
Materials and methods
Animal selection
A total of 10 healthy 8-week-old male SD rats
(weight range, 250–350 g), 3 healthy 1-year-old male SD rats
(weight range, 600–650 g) were selected and all rats were subjected
to electrical stimulation and non-electrical stimulation. The rats
were housed at 30°C, with 55.3% humidity and a 12-h light/dark
cycle, with access to food and water ad libitum. All animal
experiments were performed in accordance with the Guide for the
Care and Use of Laboratory Animals (30). The procedures in the study were
designed to minimize the pain or discomfort of the animals, in
accordance with the current protocols approved by the Laboratory
Animal Ethics Committee of Beijing Institute of Technology
(Beijing, China). A systematic diagram of the experiment is shown
in Fig. 1.
Implantable electrical stimulator
design
The present study is based on a self-developed
implanted voltage stimulator and a self-developed electrode. The
location of fixing of the electrodes is shown in Fig. 2A. The longitudinal spacing of the
electrode points was 2 mm, the horizontal electrode point spacing
was 1.25 mm, and the total number of electrodes was 34. A schematic
representation of the implantable electrical stimulator and wave of
stimulation is shown in Fig. 2C.
According to function, the stimulator was divided into a power
amplification module, signal processing module, low-power Bluetooth
module, voltage conversion module and power supply module. The
circuit hardware is shown in Fig.
2B.
Characteristics of the electrical
stimulator
The amplitude, period and pulse width of the
electrical stimulation waveform of the stimulator can be adjusted;
there were two independently-programmable channels, so that an
effective stimulation model could be set up according to different
stimulation parameters. Low-power Bluetooth data transmission was
based on Bluetooth 4.0 protocol data transmission, and the stimulus
waveform was outputted by receiving the stimulation parameters
(stimulation waveform, stimulation period and stimulation pulse
width) from the host computer.
Based on the small size and low power consumption of
the silicone package (when not working, the quiescent current loss
was <1 mA, and the power consumption was 0.3 W when working). An
LED on the chip indicated the working status. Due to its wireless
charging function, there is no need to frequently remove the
stimulator from the subject for charging. The stimulator supports
external control terminals, including Bluetooth-enabled devices
such as mobile phones and computers. In this experiment, the
stimulator was controlled using an Android™ application developed
based on the HMBLEComAssistant tool (http://www.huamaosoft.com/download.asp).
Surgical procedure
Firstly, SD rats were anesthetized with isoflurane
and then placed on a rat stereotaxic apparatus. The pressure of the
respirator was maintained at 50 kPa and the air flow rate was 600
ml/min. The anesthetic gas concentration was 2% and a heating
blanket was used to maintain normal body temperature.
The hair on the left hind leg and head of the SD rat
were removed. Then, a 4-cm-long incision in the longitudinal
direction was made with a scalpel on the thigh and expanded to
expose the sciatic nerve. The skin and muscle of the SD rat were
separated carefully using medical scissors to facilitate the
implantation of the stimulator. The stimulator was implanted and
the nerve of the SD rat was wrapped with the flexible electrode
(shown in Fig. 2B). The incision was
sutured after implantation. Next, a 5-cm incision to the head was
cut longitudinally with a scalpel. The subcutaneous tissues were
removed from the skull, and the periosteum was cleaned using cotton
swabs. The aim of this procedure was to make the bregma and lambda
landmarks of the skull more clearly visible. The electrodes were
positioned in alignment with the two markers and fixed to the skull
with micro-screws (Fig. 3). After
fixation of the electrode, the outlet of the electrode was covered
with dental cement to separate the electrode from the fur and
prevent injury caused by the movement of the rats or the insertion
and removal process.
After the surgery, the rats were allowed to recover
for 2–3 days and were then subjected to an electrical stimulation
test. The stimulus parameters were set by amplitude and frequency.
In a previous study, the square wave showed a relatively good
effect on nerve stimulation (31).
Therefore, the square wave waveform was selected as the output
waveform of the stimulator, the carrier frequency was 1 kHz, the
number of carriers was set to 2 and the stimulus voltage was set
between +5 and −5 V. Each set of experiments took 5 min, including
3 min of non-electrical stimulation and a 2 min of electrical
stimulation. Four sets of experiment were performed every two days
and the time interval between each set of experiments was 10 min.
During the process of stimulation lasting 1 month, EEG signals were
acquired using a wired recording system (Cerebus™; Blackrock
Microsystems LLC). After each set of experiments, a 10-min rest
period was given, in order to avoid muscle fatigue, however in the
ending of 1 month, one rat was paralyzed.
Statistical analysis
Statistical analyses were carried out using EEGLAB
(Swartz Center for Computational Neuroscience, University of
California, San Diego, San Diego, CA, USA; http://sccn.ucsd.edu/eeglab/index.php) (32) with MATLAB 2018b (Mathworks Inc). The
Wavelet Analysis v.1.0.3 (https://pypi.org/project/PyWavelets/) and SciPy
v.1.3.0 (https://www.scipy.org/) expansion
packages of Python v.3.7.3 (https://www.python.org/) were also used. The power map
was obtained using EEGLAB by importing stored data, and Wavelet
Analysis was used to analyze the relationship between voltage and
frequency.
Results
Data acquisition and filtering
EEG data of the rats were acquired using a system
from a wired recording system, which includes 32 signal channels
with a sampling frequency of up to 1 kHz. After band-pass
filtering, the signals averaged between 0.1 and 500 Hz using a
sixth-order Chebyshev filter, and signals were notched at 50
Hz.
Visual observation
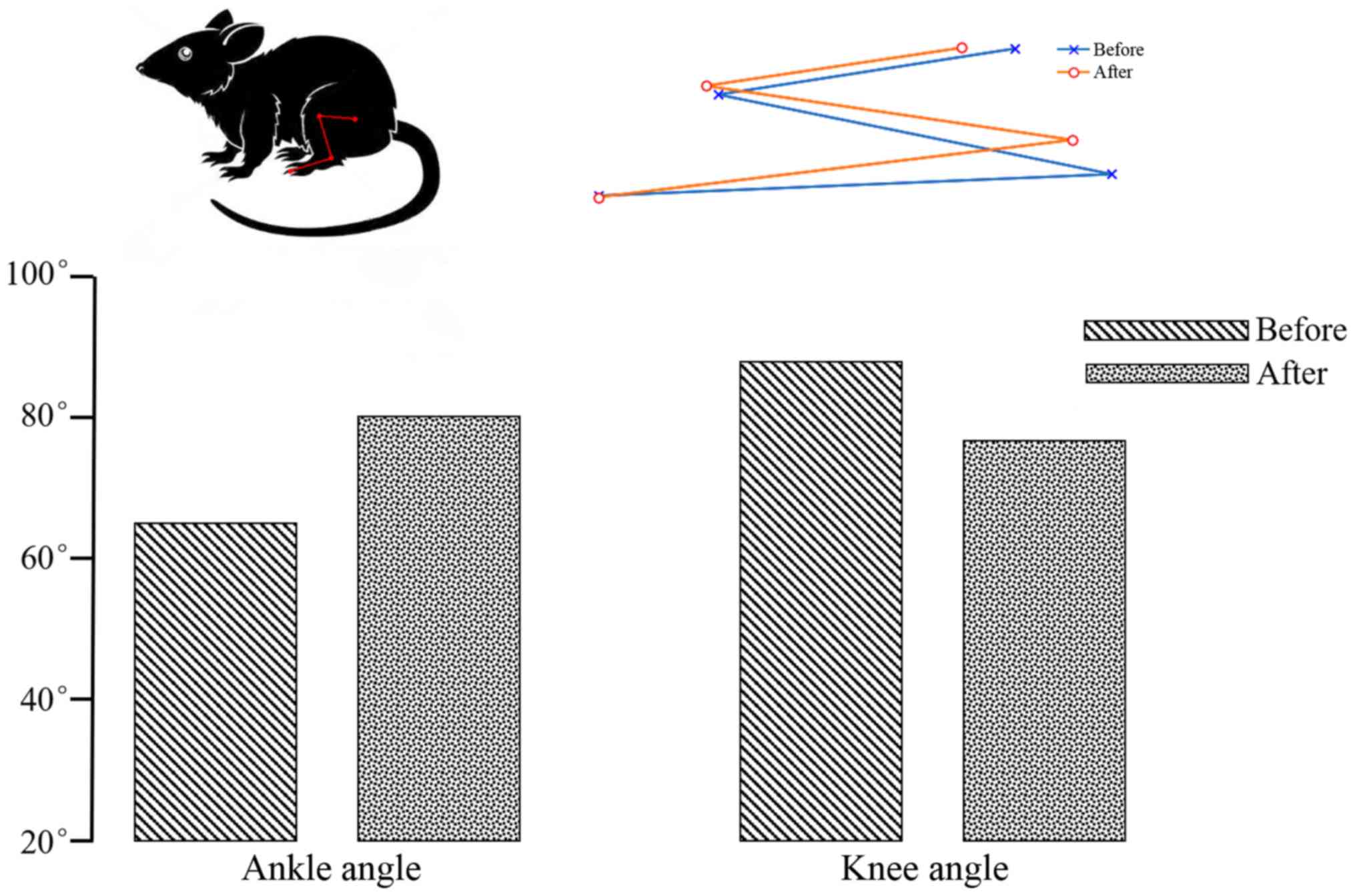
When the sciatic nerve was stimulated, the rat leg
contracted at the knee joint and extended at the ankle. In Fig. 4, the blue line represents the initial
state and the red line represents the end state. Change in ankle
and knee angles are shown in Fig. 4.
The initial ankle and knee angles were ~65° and 90°, and ~80° and
78° after electrical stimulation, therefore, the change in ankle
and knee angles were ~15° and 12°, respectively. The angle of ankle
and knee angles have changed markedly before and after electrical
stimulation.
Power map
As shown in Fig. 5A and
B, the EEG power of the younger rats in the non-stimulation
group was lower than that in the stimulation group, and this was
the same scenario for the older rats shown in Fig. 5C and D. As shown in Fig. 5B and D, all brain regions were
affected; channel 8 was more active than the others when the left
sciatic nerve was stimulated. Based on these phenomena, the
location of channel 8 was considered to be related to the
electrical stimulation of the sciatic nerve. As presented in
Fig. 6, the signal was distributed
relatively evenly throughout the brain at the low frequency (<30
Hz). At 10 Hz, the power density of the brain region at channel 8
was relatively higher than that of other brain regions (according
to the electrode distribution shown in Fig. 2A).
Data processing
The SciPy package in Python was used to generate the
EEG power maps, which are shown in Fig.
7. The EEGs of non-electrical stimulation and electrical
stimulation in younger rats are shown in Fig. 7A and B, respectively. The EEGs of
non-electrical stimulation and electrical stimulation in older rats
are shown in Fig. 7C and D,
respectively. It was demonstrated that the voltage of channel 8 was
higher than that for other channels. When comparing older rats with
younger rats, the same phenomenon was observed (when the left
sciatic was stimulated, all the brain regions were influenced, but
the right hemisphere was more strongly influenced than the left).
As shown in Fig. 8A and B, wavelet
analysis showed a string of low-frequency data in the period
between 220 and 225 sec. At 215 and 232 sec, the rat was stimulated
by the implanted electrical stimulator, and at these two points in
time the wavelet analysis showed more low-frequency components than
high-frequency components, indicating that electrical stimulation
of the sciatic nerve can simultaneously activate the motor cortex.
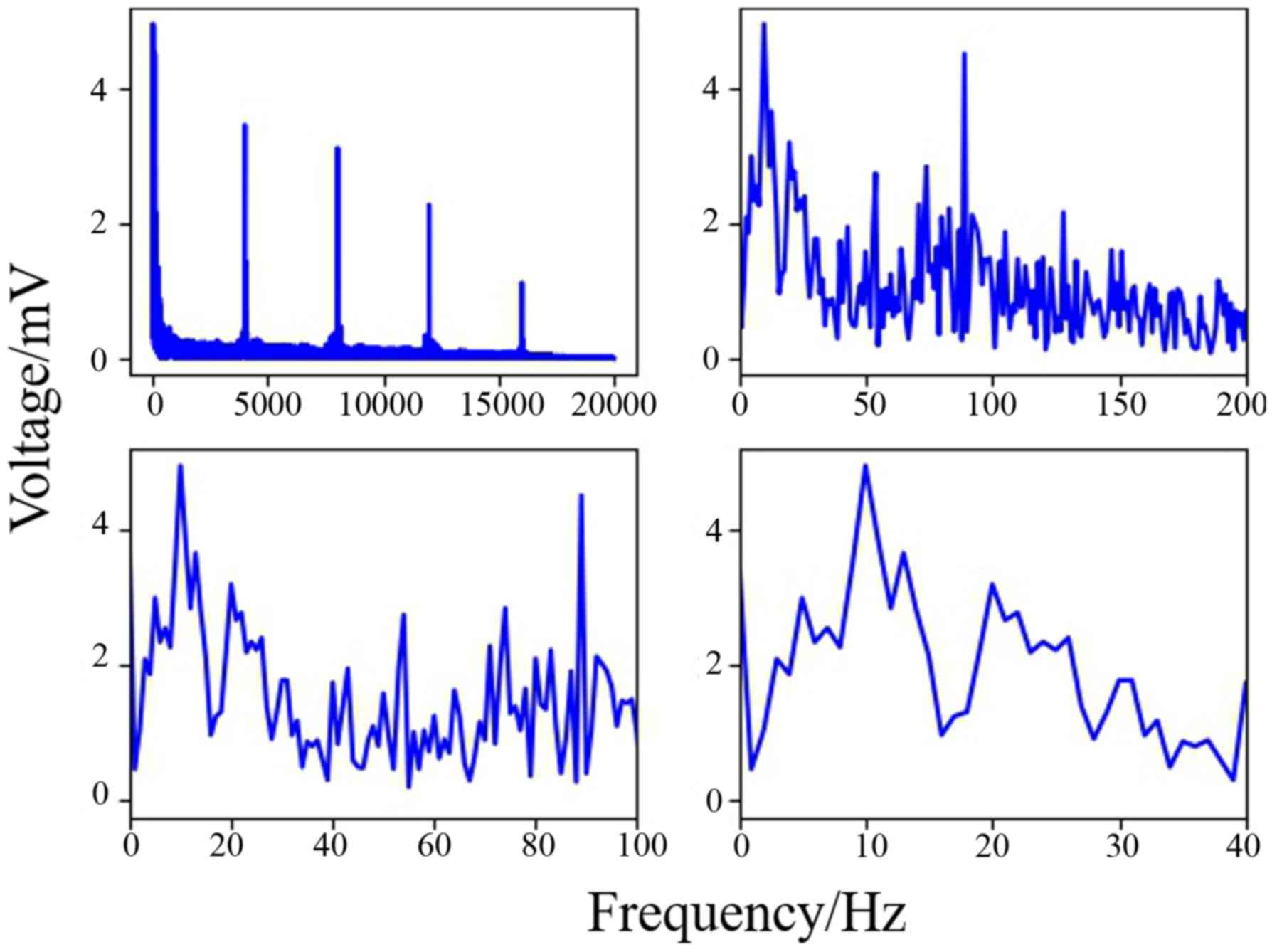
As shown in Fig. 9, the data of
channel 8 was analyzed by computing Fast Fourier Transform. It has
been established that the frequency of motion and perception is
8–16 Hz (33–35), and this frequency at channel 8 was
higher than others in the present experiment. The results indicated
that stimulation of the sciatic nerve excited the brain region
under channel 8.
Discussion
Stroke caused by cerebral hemorrhage is likely to
become increasingly common as the population ages, thus leading to
more cases of degradation of the cortex. The current treatments for
improving this condition include certain medications, physical
exercise and transcutaneous electrical nerve stimulation (10,11),
which are only capable of improving symptoms and are not curative.
The usage of neuro-electrical stimulation to relieve local pain
within a short period was first proposed by Wall and Sweet
(36) in 1967, and it has been
demonstrated that electrical stimulation affects sensory nerves
(37,38). In 1952, Malis et al (39) found that action potentials were
generated in the motor cortex after stimulating a peripheral nerve.
Based on the aforementioned studies, a new idea was proposed in the
present study to treat damage to and degradation of the motor
cortex by stimulating the sciatic nerve. In the present study, a
self-developed fully-implanted neuro-electrical stimulator was
implanted into rats, and the experimental results showed that an
EEG frequency band of 8–16 Hz (α) could be evoked by electrical
nerve stimulation, accompanied by leg movement, and that it had the
highest amplitude of all frequency bands.
Electrodes need to be biocompatible, non-toxic and
not provoke an immune response (40). Gold is utilized in EEG electrode
manufacture, a material that is commonly used in surface
electromyography and invasive extracellular electrodes (41). The stimulus wave in the present study
was square wave, which is more efficient than other wave types
(31), and the frequency of the
carrier wave was 1 kHz (avoiding damage to the nerves by continuous
current). The stimulus voltage was set between +5 V and −5 V
(42,43) (the adjustable voltage range of the
self-developed neurostimulator is between +15 V and −15 V). The
stimulus voltage duty cycle was 50%. The leg movements of the rats
evoked by different stimulus voltages differed, which may be caused
by slight differences in the fixation position of the electrodes.
When the sciatic nerve was stimulated by electrical stimulator, the
most active channel was channel 8. Therefore, nerve electrical
stimulation of the sciatic nerve can activate the motor cortex and
evoke α wave which is related to movement (34,35).
During the experiment, especially for long-term nerve electrical
stimulation, the use of excessive voltages was carefully avoided.
In the present study, electrical stimulation experiments were
performed on a daily basis for 1 month, and paralysis occurred on
the surgical side of the bodies of one rat; the same response was
observed in a patient after spinal nerve electrical stimulation
(44), suggesting that tissue damage
may occur under electrical stimulation (45,46). The
mode and intensity of nerve electrical stimulation require further
study. The intensities of the younger rats' EEG power maps were
higher than those for older rats; this may be because the number of
experiments was small, or because the cortex of a younger rat is
more active than that of an older rat, but this needs to be
clarified in further experiments. However, the location of the
brain area activated by electrical stimulation of the sciatic nerve
was the same in younger and older rats. Therefore, it could be
concluded that electrical stimulation of the sciatic nerve
activated the corresponding motor cortex region.
In the current study, the fully-implanted nerve
electrical stimulator that had been developed was able to more
flexibly set a waveform cycle for nerve electrical stimulation. The
wireless charging strategy avoids the disadvantages of a disposable
device, the control terminal is designed to be diversified, and the
size of the stimulator is small. The experiments indicated that
electrical stimulation of the sciatic nerve could effectively
activate specific brain regions in the rats, suggesting that, to an
extent, sciatic nerve stimulation can stimulate the corresponding
brain region, and also indicating, to an extent, that sciatic nerve
electrical stimulation may contribute to the activation and
recovery of motor cortex injury; this may provide a new method for
treating stroke-induced brain damage.
Acknowledgements
The authors would like to thank Dr Luyao Chen from
the School of Optical and Electronic Information, Huazhong
University of Science and Technology for constructive discussions
and contribution to the experimental design, the design of the
stimulator and data processing for the current study.
Funding
This study was supported by Beijing Municipal
Science and Technology Program (grant no. Z181100003118007), the
Nation Key R&D Program of China (grant nos. 2017YFA0701102 and
2018YFB1307300), National Natural Science Foundation of China
(grant nos. 91648207 and 61673068) and the Research Fund of PLA of
China (grant no. BWS17J024).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, RT, YL and JH contributed to the conception and
design of the study. DH and GL were responsible for the collection
and processing of data. ZG and XL completed data analysis and
interpretation. XL, YL and JH contributed to manuscript writing.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Laboratory
Animal Ethics Committee of Beijing Institute of Technology
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gao X and Chen J: Mild traumatic brain
injury results in extensive neuronal degeneration in the cerebral
cortex. J Neuropathol Exp Neurol. 70:183–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iizuka H, Sakatani K and Young W: Neural
damage in the rat thalamus after cortical infarcts. Stroke.
21:790–794. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie H, Kane JT, Dennis MJ, Mooney RD,
Bauer WR, Wang X and Wall JT: Case series evidence for changed
interhemispheric relationships in cortical structure in some
amputees. J Clin Neurosci. 20:523–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salat DH, Kaye JA and Janowsky JS:
Selective regional degeneration and preservation within the
prefrontal cortex in healthy aging and Alzheimer's disease. Arch
Neurol. 58:1403–1408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kline AE, Chen MJ, Tso-Olivas DY and
Feeney DM: Methylphenidate treatment following ablation-induced
hemiplegia in rat: Experience during drug action alters effects on
recovery of function. Pharmacol Biochem Behav. 48:773–779. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khan MA, Tariq M, Naime M and Akhtar J:
Falij-E-Nisfi (Hemiplegia) cause and treatment in Unani medicine: A
review. J Pharm Pharm Sci. 7:293–300. 2018.
|
|
7
|
Perry JC and Rosen J: Upper-limb powered
exoskeleton design. IEEE/ASME Trans Mechatronics. 12:408–417. 2007.
View Article : Google Scholar
|
|
8
|
Twitchell TE: The restoration of motor
function following hemiplegia in man. Brain. 74:443–480. 1951.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gallas S, Marie JP, Leroi AM and Verin E:
Sensory transcutaneous electrical stimulation improves post-stroke
dysphagic patients. Dysphagia. 25:291–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwong PWH, Ng GYF, Chung RCK and Ng SSM:
Bilateral transcutaneous electrical nerve stimulation improves
lower-limb motor function in subjects with chronic stroke: A
randomized controlled trial. J Am Heart Assoc. 7(pii):
e0073412018.PubMed/NCBI
|
|
11
|
Laddha D, Ganesh GS, Pattnaik M, Mohanty P
and Mishra C: Effect of transcutaneous electrical nerve stimulation
on plantar flexor muscle spasticity and walking speed in stroke
patients. Physiother Res Int. 21:247–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ghoname ESA, White PF, Ahmed HE, Hamza MA,
Craig WF and Noe CE: Percutaneous electrical nerve stimulation: An
alternative to TENS in the management of sciatica. Pain.
83:193–199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldner JL, Nashold BS Jr and Hendrix PC:
Peripheral nerve electrical stimulation. Clin Orthop Relat Res.
33–41. 1982.PubMed/NCBI
|
|
14
|
Davies PM: The comprehensive treatment of
patients with hemiplegia. 2000.
|
|
15
|
Cramer SC: Repairing the human brain after
stroke: I. Mechanisms of spontaneous recovery. Ann Neurol.
63:272–287. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Monte-Silva K, Piscitelli D,
Norouzi-Gheidari N, Batalla MAP, Archambault P and Levin MF:
Electromyogram-related neuromuscular electrical stimulation for
restoring wrist and hand movement in Poststroke hemiplegia: A
systematic review and meta-analysis. Neurorehabil Neural Repair.
33:96–111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wilson RD, Bennett ME, Nguyen VQC, Bock
WC, O'Dell MW, Watanabe TK, Amundson RH, Hoyen HA and Chae J: Fully
implantable peripheral nerve stimulation for hemiplegic shoulder
pain: A multi-site case series with two-year follow-up.
Neuromodulation. 21:290–295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JH, Baker LL, Johnson RE and Tilson
JK: Effectiveness of neuromuscular electrical stimulation for
management of shoulder subluxation post-stroke: A systematic review
with meta-analysis. Clin Rehabil. 31:1431–1444. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paik YR, Park HS, Oh DH, Lee JH and Lee
DH: Effect of mirror therapy and electrical stimulation on upper
extremity function in stroke with hemiplegic patient: A pilot
study. J Phys Ther Sci. 29:2085–2086. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Russo C, Souza Carneiro MI, Bolognini N
and Fregni F: Safety review of transcranial direct current
stimulation in stroke. Neuromodulation. 20:215–222. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guiraud D, Azevedo Coste C, Benoussaad M
and Fattal C: Implanted functional electrical stimulation: Case
report of a paraplegic patient with complete SCI after 9 years. J
Neuroeng Rehabil. 11:152014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Herman R, He J, D'Luzansky S, Willis W and
Dilli S: Spinal cord stimulation facilitates functional walking in
a chronic, incomplete spinal cord injured. Spinal Cord. 40:65–68.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harkema S, Gerasimenko Y, Hodes J, Burdick
J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG
and Edgerton VR: Effect of Epidural stimulation of the lumbosacral
spinal cord on voluntary movement, standing and assisted stepping
after motor complete paraplegia: A case study susan. Lancet.
377:1938–1947. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng ZT, Dong XL, Li YD, Gao WW, Zhou Y,
Jiang RC, Yue SY, Zhou ZW and Zhang JN: Electrical stimulation
improved cognitive deficits associated with traumatic brain injury
in rats. Brain Behav. 7:e006672017. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Koyama S, Xia J, Leblanc BW, Gu JW and
Saab CY: Sub-paresthesia spinal cord stimulation reverses thermal
hyperalgesia and modulates low frequency EEG in a rat model of
neuropathic pain. Sci Rep. 8:71812018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yip M, Jin R, Nakajima HH, Stankovic KM
and Chandrakasan AP: A fully-implantable cochlear implant SoC with
piezoelectric middle-ear sensor and arbitrary waveform neural
stimulation. IEEE J Solid-State Circuits. 50:214–229. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim D, Yeon C, Chung E and Kim K: A
non-invasive flexible multi-channel electrode for in vivo mouse EEG
recordingProceedings of IEEE Sensors Conference. Valencia, Spain:
2-5. Novemb; pp. 1111–1114. 2014
|
|
28
|
Zhang P, Ma X, Chen L, Zhou J, Wang C, Li
W and He J: Decoder calibration with ultra small current sample set
for intracortical brain-machine interface. J Neural Eng.
15:0260192018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang P, Huang J, Li W, Ma X, Yang P, Dai
J and He J: Using high frequency local field potentials from
multi-cortex to decode reaching and grasping movements in monkey.
IEEE Trans Cogn Dev Syst. Sep;2018.DOI: 10.1109/TCDS.2018.2869587.
View Article : Google Scholar
|
|
30
|
National Institute of Health (NIH), .
Guide for the Care and Use of Laboratory AnimalsThe National
Academies Press; Washington, D.C.: 1996
|
|
31
|
Wongsarnpigoon A and Grill WM:
Energy-efficient waveform shapes for neural stimulation revealed
with a genetic algorithm. J Neural Eng. 7:0460092010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Delorme A and Makeig S: EEGLAB: An open
source toolbox for analysis of single-trial EEG dynamics including
independent component analysis. J Neurosci Methods. 134:9–21. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klimesch W: EEG alpha and theta
oscillations reflect cognitive and memory performance: A review and
analysis. Brain Res Rev. 29:169–195. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chatrian GE, Petersen MC and Lazarte JA:
The blocking of the rolandic wicket rhythm and some central changes
related to movement. Electroencephalogr Clin Neurophysiol.
11:497–510. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Babiloni C, Carducci F, Cincotti F,
Rossini PM, Neuper C, Pfurtscheller G and Babiloni F: Human
movement-related potentials vs desynchronization of EEG alpha
rhythm: A high-resolution EEG study related to movement.
Neuroimage. 10:658–665. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wall PD and Sweet WH: Temporary abolition
of pain in man. Science. 155:108–109. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Slavin KV: Peripheral nerve stimulation
for neuropathic pain. US Neurol. 7:1442015. View Article : Google Scholar
|
|
38
|
Levine AB, Steven DA, Parrent AG and
MacDougall KW: Successful long-term nerve root stimulation for
chronic neuropathic pain: A real world, single center Canadian
experience. Pain Physician. 20:95–106. 2017.PubMed/NCBI
|
|
39
|
Malis LI, Pribram KH and Kruger L: Action
potentials in ‘Motor’ cortex evoked by peripheral nerve
stimulation. Methods. 16:161–167. 1953.
|
|
40
|
Merrill DR, Bikson M and Jefferys JG:
Electrical stimulation of excitable tissue: Design of efficacious
and safe protocols. J Neurosci Methods. 141:171–198. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dong W, Zhu C, Hu W, Xiao L and Huang Y:
Stretchable human-machine interface based on skin-conformal sEMG
electrodes with self-similar geometry. J Semicond. 39:0140012018.
View Article : Google Scholar
|
|
42
|
O'Suilleabhain PE, Frawley W, Giller C and
Dewey RB Jr: Tremor response to polarity, voltage, pulsewidth and
frequency of thalamic stimulation. Neurology. 60:786–790. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mooziraji FB and Shoaei O: A high power
efficient multi-waveform current stimulator used in implantable
neural stimulation. Analog Integr Circuits Signal Process.
86:459–469. 2016. View Article : Google Scholar
|
|
44
|
Shealy CN, Mortimer JT and Reswick JB:
Electrical inhibition of pain by stimulation of the dorsal columns:
Preliminary clinical report. Anesth Analg. 46:489–491. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cogan SF, Ludwig KA, Welle CG and Takmakov
P: Tissue damage thresholds during therapeutic electrical
stimulation. J Neural Eng. 13:210012016. View Article : Google Scholar
|
|
46
|
Levy RM: Device complication and failure
management in neuromodulation. Neuromodulation. 16:495–502. 2013.
View Article : Google Scholar : PubMed/NCBI
|