Introduction
Glaucoma is a serious progressive disorder of the
eye that is found primarily in aged populations and causes a high
percentage of blindness (1–3).
Elevated intraocular pressure (IOP) is considered to be one of the
important factors in the initiation of glaucoma, leading to the
shrinkage of the optic nerve and the loss of retinal ganglion cells
(RGCs). Cell loss is irreversible; thus, self-recovery or
restoration of vision is not possible following blindness. Stem
cell transplantation currently offers one possibility to treat the
disease. However, for the RGC layer, transplantation is unable to
produce sufficiently high numbers of integrated cells or
specialized differentiation of the transplanted cells (1,4).
Therefore, neuroprotection during the progression of glaucoma
represents the main approach of the current therapies.
Investigating new neuroprotective agents for the eye is
critical.
A number of traditional Chinese medicines that are
known to be beneficial to the eye have been studied in animal
models of glaucoma. For example, Lycium barbarum Lynn
extracts were shown to exhibit neuroprotective effects on RGCs in
various diseases, including glaucoma (5,6).
Danshen (Salvia miltiorrhiza) has also been administered for
eye protection, particularly in aged populations (7,8).
Thus, it is highly possible that this extract exhibits
neuroprotective effects in the retina in progressive glaucoma.
Therefore, the aim of the present study was to examine the
potential neuroprotection of Salvia miltiorrhiza extracts on
the retina in a rat model of glaucoma.
Materials and methods
Ethics
The study was approved by the Ethics Committee of
Animal Research of the Department of Ophthalmology (Second Hospital
of Jilin University, Changchun, China). Animal care guidelines were
strictly followed for all experimental procedures in the study.
Animal model
In total, 30 Sprague Dawley rats (gender, male; age,
4–5 months) were obtained from the Animal Experiment Center (Second
Hospital of Jilin University) and maintained in conditions with a
normal light cycle. The animals were housed with three rats per
cage.
A total of 10 animals served as controls and were
anesthetized with 50 mg/kg ketamine and 10 mg/kg xylazine, but did
not undergo surgery (control group). For the glaucoma model,
following anesthesia, the rats were subjected to occlusion of
aqueous outflow by laser irradiation under a slit lamp. This
procedure was performed twice on the left eye, leaving the right
eye as the control.
For the 20 animals that underwent surgery, the
surrounding lateral lines of the eye were occluded with 60–70 laser
pulses at a power of 0.8 W (time duration of each pulse, 0.2 sec;
size, 60 μm in diameter; diode laser output, 532 nm). The
procedures were performed by two experienced surgeons and the model
had been previously validated in preliminary experiments.
IOP
IOP was examined constantly with an
Icare® Tonolab tonometer (Icare Finland Oy, Espoo,
Finland) in order to select the animals with successful aqueous
outflow occlusion. All 20 rats exhibited a clear IOP elevation as
early as 24 h after surgery, with a baseline value of ~20 mmHg and
an increase of 5–10 mmHg. The IOPs remained elevated for several
days.
Salvia miltiorrhiza extract feeding
The Salvia miltiorrhiza extracts were
obtained from Tianjin Tasly Pharmaceutical Co., Ltd. (Tianjin,
China) and were administered to rats orally at 1 g/kg/day, starting
1 week prior to the glaucoma model experiment. The IC50
value of the extracts was 178 g/kg, based on preliminary
experiments.
Of the 20 animals subjected to surgery, 10 animals
were treated with Salvia miltiorrhiza extract (treatment
group) and 10 animals were left untreated (glaucoma group).
FluoroGold™ (FG; Fluorochrome, Denver,
CO, USA) labeling for RGC quantification
Labeling of the rat retinas was performed one month
following surgery. Retrograde labeling on the superior colliculus
was achieved with the application of 5 μl 5% FG (Fluorochrome,
Inc., Denver, CO, USA) soaked in gel foam. After six days, the
animals were sacrificed and the retinas were obtained and mounted
flat for cell counting (double-blindly). A total of eight random
regions of the retina were selected for counting and six animals
from each group were used for this experiment.
Statistical analysis
Data are presented as the mean ± SD and were
analyzed with SPSS software, version 17.0 (SPSS, Inc., Chicago, IL,
USA). For statistical analyses, the t-test was performed and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Salvia miltiorrhiza extract does not
prevent IOP increase
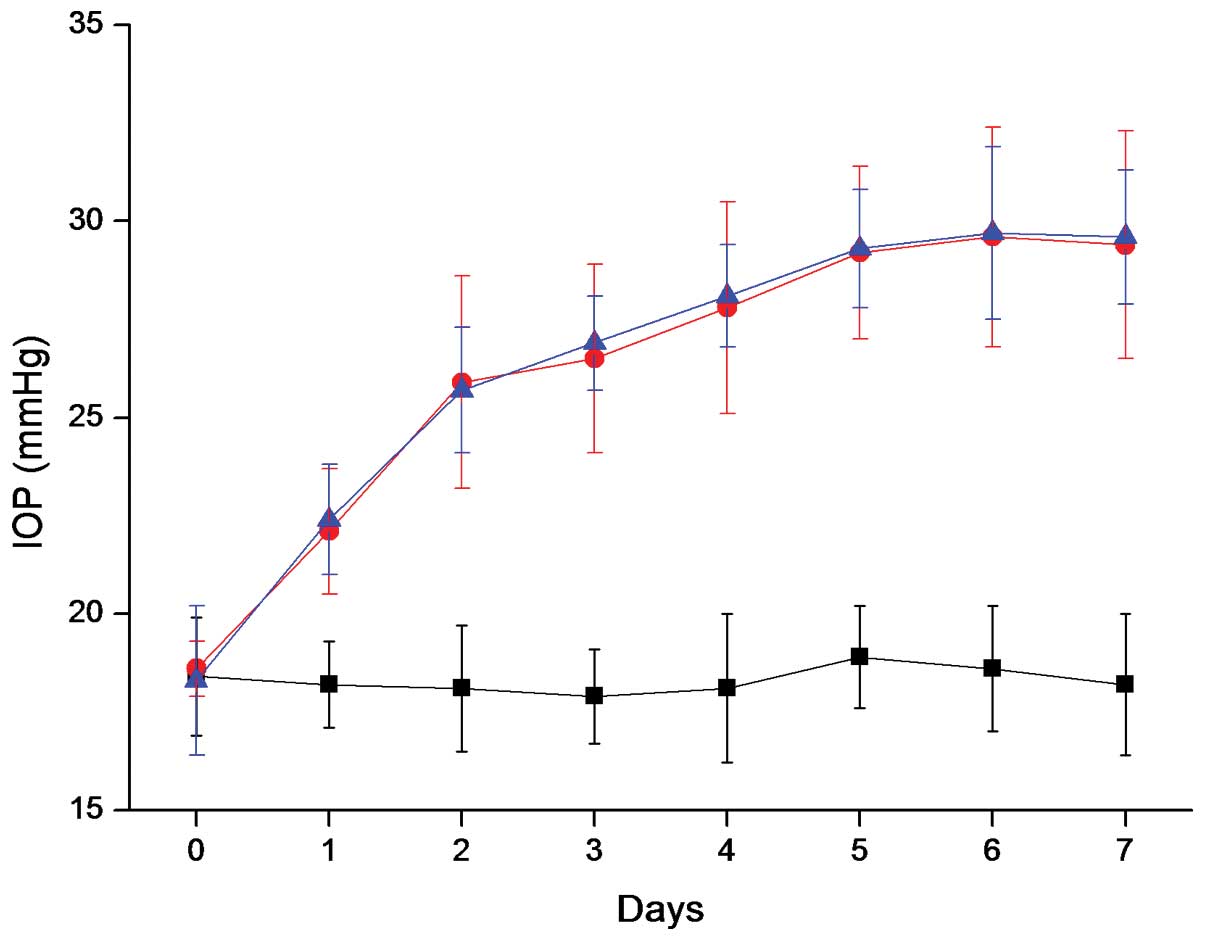
Results following surgery indicated that there was a
rapid and steady increase in IOP for 7 days in the left eyes of the
rats subjected to aqueous outflow occlusion, demonstrating the
success of the glaucoma model. However, the oral administration of
Salvia miltiorrhiza extract for one week did not prevent the
IOP increase in the treatment group (Fig. 1). This indicates that Salvia
miltiorrhiza extract does not change the physiological
circulation properties of the aqueous outflow and is unable to
decrease the IOP.
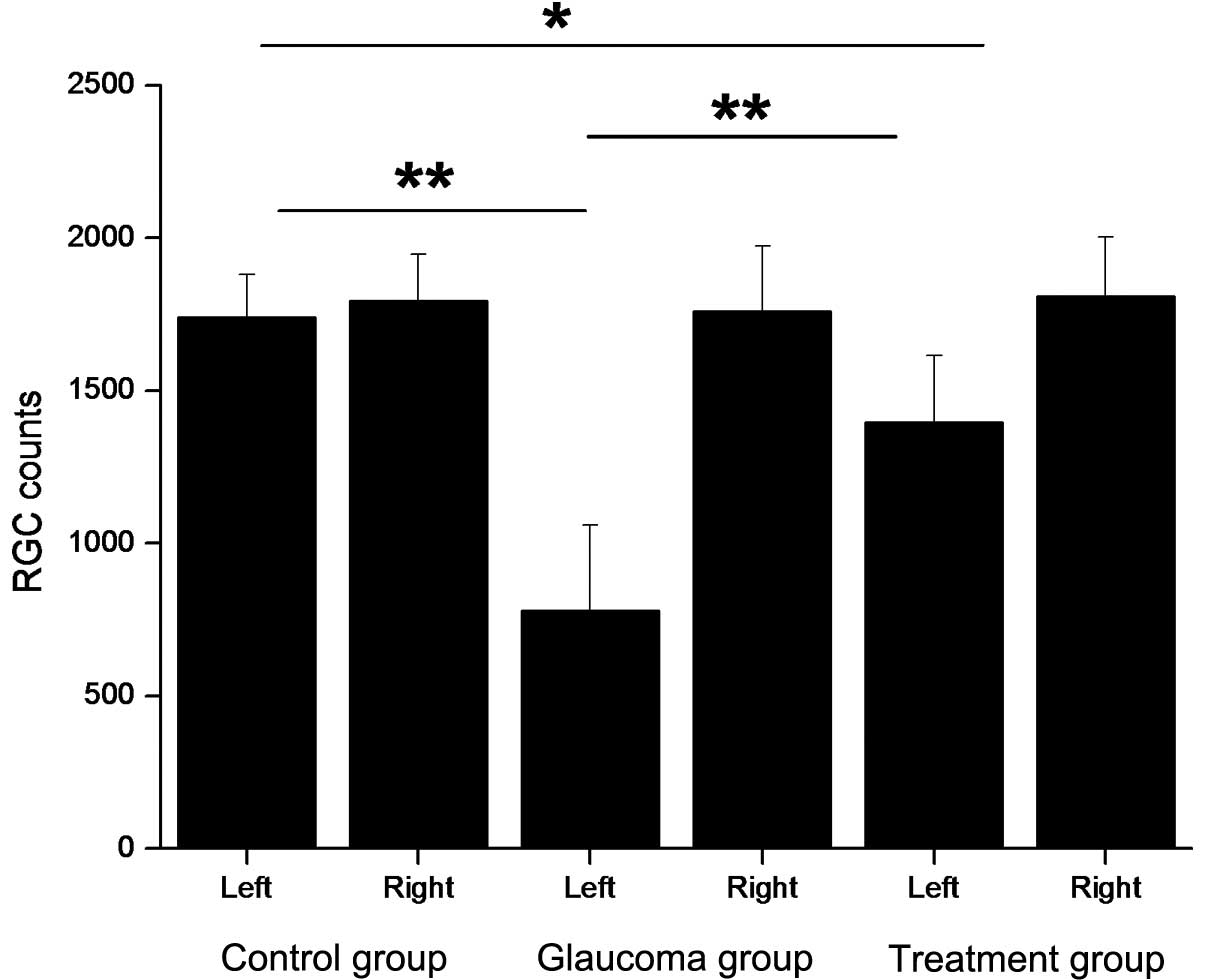
Salvia miltiorrhiza extract protects
RGCs
The results indicated that even with a rapid
increase in IOP, orally administered Salvia miltiorrhiza
extract protected against RGC loss 30 days following the IOP
increase. In the glaucoma group, the loss of RGCs in the left eye
was statistically significant (P<0.01), while in the treatment
group there was mild reduction in the number of RGCs in the left
eye (P<0.05), which was statistically significant from that in
the glaucoma group (P<0.01; Table
I and Fig. 2).
 | Table IOrally administration of Salvia
miltiorrhiza extract protects RGCs (counts per mm2,
mean ± SEM). |
Table I
Orally administration of Salvia
miltiorrhiza extract protects RGCs (counts per mm2,
mean ± SEM).
| Eye | Control group | Glaucoma group | Treatment group |
|---|
| Left | 1,740±140 | 780±280a | 1,396±220b,c |
| Right | 1,794±153 | 1,760±214 | 1,810±195 |
Discussion
Glaucoma-induced retinal degeneration and vision
loss is a severe problem in Asian countries, particularly in aged
populations. Various efforts have been made to improve the survival
of retinal neurons following an IOP increase, including infusion of
trophic factors, electrical stimulation and visual training
(9–11). Extracts from traditional medicine,
particularly herbal medicine, have been shown to improve the
survival of RGCs in various models of neuronal injury, including
glaucoma-induced cell loss (12,13).
The present study employed Salvia miltiorrhiza extract
feeding in a rat model of glaucoma to investigate the potential
protective effects of this extract in glaucoma. The results
demonstrated that the orally administered extract did not prevent
the IOP from increasing, but did protect the RGCs from
pressure-induced cell death.
Salvia miltiorrhiza (Danshen) has been widely
used in formulations and has demonstrated anti-ageing effects, as
well as neuroprotection (7,8). In
addition, Salvia miltiorrhiza, as part of a formulation, has
been used to reverse diabetes-induced retinopathy or
glaucoma-associated retinal degeneration (7,14,15).
The present study used Salvia miltiorrhiza extracts alone
and further demonstrated the protective functions of these extracts
on RGCs. Salvia miltiorrhiza is composed of numerous
bioactive components, including danshensu (DSS), protocatechuic
aldehyde, salvianolic acid B, cryptotanshinone and tanshinone. DSS
is considered to be one of the most important components and may be
partially responsible for the neuroprotective effects observed in
the retina (16). Future studies
are required to investigate the bioactive components of Salvia
miltiorrhiza and determine whether purified DSS at various
doses exhibits neuroprotective effects in glaucoma and other eye
diseases.
In summary, the present study has demonstrated the
neuroprotective effects of Salvia miltiorrhiza extracts on
RGCs in a rat model of glaucoma. Thus, Salvia miltiorrhiza
may be a new therapeutic agent for the clinical management of
glaucoma.
References
|
1
|
Worley A and Grimmer-Somers K: Risk
factors for glaucoma: what do they really mean? Aust J Prim Health.
17:233–239. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong VH, Bui BV and Vingrys AJ: Clinical
and experimental links between diabetes and glaucoma. Clin Exp
Optom. 94:4–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yanagi M, Kawasaki R, Wang JJ, Wong TY,
Crowston J and Kiuchi Y: Vascular risk factors in glaucoma: a
review. Clin Experiment Ophthalmol. 39:252–258. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayreh SS: Neovascular glaucoma. Prog
Retin Eye Res. 26:470–485. 2007. View Article : Google Scholar
|
|
5
|
Chan HC, Chang RC, Koon-Ching Ip A, et al:
Neuroprotective effects of Lycium barbarum Lynn on
protecting retinal ganglion cells in an ocular hypertension model
of glaucoma. Exp Neurol. 203:269–273. 2007.
|
|
6
|
Mi XS, Zhong JX, Chang RC and So KF:
Research advances on the usage of traditional Chinese medicine for
neuroprotection in glaucoma. J Integr Med. 11:233–240. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng TO: Cardiovascular effects of
Danshen. Int J Cardiol. 121:9–22. 2007. View Article : Google Scholar
|
|
8
|
Qin RA, Yao XX and Huang ZY: Effects of
compound danshen tablets on spatial cognition and expression of
brain beta-amyloid precursor protein in a rat model of Alzheimer’s
disease. J Tradit Chin Med. 32:63–66. 2012.PubMed/NCBI
|
|
9
|
Jensen RJ and Rizzo JF III: Responses of
ganglion cells to repetitive electrical stimulation of the retina.
J Neural Eng. 4:S1–S6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ko ML, Hu DN, Ritch R and Sharma SC: The
combined effect of brain-derived neurotrophic factor and a free
radical scavenger in experimental glaucoma. Invest Ophthalmol Vis
Sci. 41:2967–2971. 2000.PubMed/NCBI
|
|
11
|
Wilensky JT, Gieser DK, Mori MT,
Langenberg PW and Zeimer RC: Self-tonometry to manage patients with
glaucoma and apparently controlled intraocular pressure. Arch
Ophthalmol. 105:1072–1075. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhee DJ1, Katz LJ, Spaeth GL and Myers JS:
Complementary and alternative medicine for glaucoma. Surv
Ophthalmol. 46:43–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huynh TP1, Mann SN and Mandal NA:
Botanical compounds: effects on major eye diseases. Evid Based
Complement Alternat Med. 2013:5491742013.PubMed/NCBI
|
|
14
|
Yue KK, Lee KW, Chan KK, Leung KS, Leung
AW and Cheng CH: Danshen prevents the occurrence of oxidative
stress in the eye and aorta of diabetic rats without affecting the
hyperglycemic state. J Ethnopharmacol. 106:136–141. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Q, Zhang Y, Lin Z, et al: Danshen
extract 15,16-dihydrotanshinone I functions as a potential
modulator against metabolic syndrome through multi-target pathways.
J Steroid Biochem Mol Biol. 120:155–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qi HP, Wei SQ, Zhang LQ, et al: Preventive
effect of danshensu on selenite-induced cataractogenesis in
cultured rat lens. Clin Experiment Ophthalmol. 41:172–179. 2013.
View Article : Google Scholar : PubMed/NCBI
|