Introduction
Vascular endothelial cells, the natural barrier
between the blood and tissue, secrete various vasoactive
substances, cytokines and neurotransmitters, including nitric oxide
(NO), interleukins (ILs), adhesion molecules, endothelin and tissue
factors (1–3). These substances participate in and
influence the organic movement of substances, blood coagulation,
immune responses and other essential activities. Previous studies
have shown that oxidative stress injury in vascular endothelial
cells is the cause of various vascular diseases, including
atherosclerosis (AS), diabetes and hypertension (4,5).
Oxidative stress generates reactive oxygen species (ROS), such as
the superoxide anion (•O2−), the hydroxyl
radical (•OH) and hydrogen peroxide
(H2O2). ROS bind to the nuclear receptor of
vascular endothelial and smooth muscle cells as a ligand, or
directly regulate gene expression as signaling molecules. These
functions enhance the adhesion and migration of monocytes to the
tunica intima, which is important during the development of AS
(6,7).
NO is a neurotransmitter secreted by vascular
endothelial cells, which can inhibit monocyte-macrophage and
platelet adhesion, decrease the monolayer permeability of vascular
endothelial cells and reduce vascular endothelial cell and smooth
muscle cell proliferation (8,9).
Previous studies have shown that 30% of NO in the blood is derived
from endothelial nitric oxide synthase (eNOS) genes expressed by
vascular endothelial cells. NO diffuses to nearby endothelial and
smooth muscle cells, activates soluble guanosine monophosphate
cyclase within the cytoplasm, decomposes guanosine triphosphate and
generates cyclic guanosine monophosphate (cGMP), which results in
biological effects, including vasodilation, that play an important
role in AS (10).
Xin Mai Jia (XMJ) is a traditional Chinese medicine
(TCM) used in the treatment of AS that can effectively inhibit AS
occurrence and development (11).
XMJ is prepared using the following raw materials based on weight:
Functional red kojic rice powder, 10–35%; kudzu flavonoid powder,
1–10%; soybean isoflavone powder, 1–8%; bamboo leaf flavone powder,
1–8%; resveratrol powder, 1–8%; hawthorn powder, 1–6%;
Gastrodia powder, 1–6%; Auricularia auricula powder,
1–30%; hippocampus powder, 0.1–0.2%; astaxanthin powder,
0.008–0.04%; menthol powder, 0.1–0.3%; and resistant starch,
20–50%. To investigate the inhibitory mechanism of XMJ on the
occurrence and development of AS, the present study evaluated the
effects of intervention with the ultrafiltration extract from XMJ
on a human umbilical vein endothelial cell (HUVEC) injury model
induced by H2O2. The effect on the NO-cGMP
signaling pathway, caused by the inhibition of the
H2O2-induced injury, was also
investigated.
Materials and methods
Drugs and chemicals
XMJ crude drugs were purchased from Beijing Tong Ren
Tang Co., Ltd. (Beijing, China). Phospho-eNOS-3 antibodies were
purchased from Beijing Boaosen Biotechnology Co., Ltd. (BS-3447R;
Beijing, China), horseradish peroxidase-labeled goat anti-rabbit
IgG (heavy and light chains) antibody was purchased from Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd. (PV-9003; Beijing,
China) and Cy3-labeled goat anti-rabbit IgG (heavy and light chain)
antibodies were purchased from Biyuntian Institute of Biotechnology
(P0183; China). Lovastatin, H2O2, diethyl
carbonate, XTT, phenazine methosulfate (PMS), diethylpyrocarbonate
and avian myeloblastosis virus were purchased from Sigma-Aldrich
(St. Louis, MO, USA). Zhibituo was purchased from Chengdu Diao
Jiuhong Pharmaceutical Factory (Chengdu, China). Hematoxylin and
eosin (HE) and Coomassie Brilliant Blue stains were purchased from
Changsha Lixin Biotechnology Co., Ltd. (Changsha, China). Kits for
the detection of malondialdehyde (MDA), superoxide dismutase (SOD),
NO, interleukin (IL)-1, IL-6, intracellular adhesion molecule
(ICAM)-1, vascular adhesion molecule (VCAM)-1, matrix
metalloproteinase (MMP)-2, tissue inhibitor of metalloproteinase
(TIMP)-2 and nuclear factor (NF)-κB were purchased from R&D
Systems (Minneapolis, MN, USA). Other reagents were analytically
pure and made in China.
Cell experiment protocol
HUVECs were obtained from HUVEC cell lines purchased
from Acti Corp. (Irvine, CA, USA) and were routinely maintained in
phenol-red containing Dulbecco’s modified Eagle’s medium (Gibco
Construction, LLC, Cleveland, TN, USA), which was supplemented with
15% newborn calf serum, 100 U/ml penicillin and 0.1 mg/ml
phytomycin, in a 37°C incubator in an atmosphere of 5%
CO2. The third generation of HUVECs were used in the
study. The cells were randomly divided into eight groups and
incubated for 24 h with the corresponding drugs. The first group of
cells were incubated in Kreb’s solution and were classified as the
blank control group (n=6). The second group of cells were incubated
in 500 mg/l XMJ and were classified as the XMJ control group (n=6).
The third group of cells were incubated in 200 μmol/l
H2O2 and were classified as the model group
(n=6). The fourth group of cells were incubated in 1 μmol/l
lovastatin and 200 μmol/l H2O2 and were
classified as the lovastatin group (n=6). The fifth group of cells
were incubated in 50 μmol/l zhibituo and 200 μmol/l
H2O2 and were classified as the zhibituo
group (n=6). The sixth group of cells were incubated in 25 μmol/l
XMJ and 200 μmol/l H2O2 and were classified
as the low-dose XMJ group (n=6). The seventh group of cells were
incubated in 50 μmol/l XMJ and 200 μmol/l
H2O2 and were classified as the middle-dose
XMJ group (n=6). Finally, the eighth group of cells were incubated
in 50 μmol/l XMJ and 200 μmol/l H2O2 and were
classified as the high-dose XMJ group (n=6). Following the
individual treatments, the cultured cells were obtained for the
subsequent tests.
Ultrafiltration membrane extract
preparation for XMJ
In total, 1,000 g XMJ crude drugs were placed in a
container filled with 6,000 ml water, which was heated in a
microwave oven for 1 h at 1,000 W. A four-gauze filter was used to
obtain one type of liquid medicine. Next, 6,000 ml water was added
to the container again prior to repeating the aforementioned
procedure. Following the production of two types of liquid
medicine, the liquid was filtered with sterile absorbent cotton.
Ultrafiltration technology of refining XMJ was then used for water
decoction under 0.5 kPa/m3 at 25°C and 100
l/h/m2. The 5,000 ml filtrate was condensed to 1,000 ml,
which was equivalent to 1 ml liquid medicine containing 1 g XMJ.
Finally, the mother liquor was labeled and stored in a refrigerator
at 4°C prior to use.
HUVEC staining
Cell growth on the wall of the bottle was observed
in each experimental group. The supernatant in the 96-well plate
was discarded and the cells were washed three times with
phosphate-buffered saline (PBS; pH 7.4) for 1 min for each
repetition. Next, the cells were soaked in 95% ethanol for 20 min
and washed with PBS twice at 1 min for each repetition. Hematoxylin
was used to dye the cells for 2–3 min and then the cells were
washed with pure water. The cells were observed under a microscope
(Olympus Corp., Tokyo, Japan). For deeply-stained nuclei, a 1%
solution of hydrochloric acid and alcohol was used to dilute the
cells into dichroic for 5 sec. The cells were washed with pure
water and placed in 70% alcohol for dehydration for 10 min and then
in 90% alcohol for 10 min. The cells were washed with distilled
water and dyed using alcoholic eosin for 2–3 min. Finally, the
cells were dried and mounted on slides using neutral gum.
HUVEC activity detection
When the concentration of the cells growing on the
walls of the bottles in each experimental group reached
106 cells/cm2, the following steps were
undertaken. The cells were washed twice with PBS, 0.25% trypsin was
added and the flasks were shaken to remove the cells from the wall
of the bottle with the aid of nozzle-pipe blowing. Thiophene azole
blue solution (1 g/l; 37°C; volume fraction, 5%) was added to each
well and 10-μl samples were obtained from each well and added to an
automatic cell counting board (Countstar). The viability rate,
average compactness and aggregation rate of the cells in each group
were measured using a Countstar automatic cell-counting
instrument.
HUVEC monolayer permeability
determination
According previous studies (12,13),
the osmotic reflection coefficient (σ) and the endothelial
monolayer filtration coefficient (Kf; μl/min/cm2/kPa)
were calculated according to the following formulas: Kf = total
water flux (Jv)/(ΔP−σ·Δπ); σ = 1−CF/CP; π
(kPa) = C(mOsm/l) × 2.6(kPa/l/mOsm); where Jv
(μl·min−1·cm−2)=V/S·min; CP was
the upper chamber white protein concentration; CF was
the inferior vena albumin concentration; C was the albumin milli
seepage quantity concentration; ΔP was the perfusion pressure; and
π was the colloid osmotic pressure.
HUVEC supernatant fluid biochemical
indicator detection
Cells were seeded into six-well plates at 2 ml per
well in order for the samples to be treated as a group, according
to the aforementioned method. Cell supernatant fluid biochemical
indicators were detected. Culture fluids were obtained separately
to determine the SOD, MDA and NO concentration, as described in the
kits.
ELISA
Cells were seeded into six-well plates at 2 ml per
well in order for the samples to be treated as a group, according
to the aforementioned method. Following the instructions on the
ELISA kit, cell supernatant fluids were obtained from the wells and
the optical density (OD) value of each well was measured at a
wavelength of 450 nm. The acquired absorbance value of the OD was
regarded as the ordinate and the concentration of the standard
liquid was considered as the abscissa. A curve was constructed,
from which the curve equation was calculated. By substituting the
OD values of the samples into the equation of the standard curve,
IL-1, IL-6, ICAM-1, VCAM-1, MMP-2, TIMP-2 and NF-κB levels were
calculated.
Western blot analysis
As previously described (14), the supernatant in the 96-well
plates was discarded and the eNOS protein content of each
experimental group of adhered HUVECs was assayed. Pre-cooled cell
lysates were added, proteins were obtained and the protein
concentration was detected using the bicinchoninic acid method.
Immunohistochemical method
Cells were soaked in 95% ethanol for 20 min, washed
with PBS twice for 1 min per wash and sealed with animal serum. An
appropriate dilution (1:400) of phospho-eNOS-3-endothelial
antibodies was added and the cells were incubated overnight at 4°C.
Next, horseradish peroxidase-labeled goat anti-rabbit IgG
antibodies were added. Mayer’s hematoxylin was used to stain the
cells for a second time, and the cells were dehydrated and dried
using gradient alcohol. Transparent xylene was also added and the
cells were mounted on slides using neutral gum. Finally, the cells
were observed under a microscope and images were obtained. The
results were processed and analyzed using analysis software for
OD.
Immunofluorescence
The supernatant in the 96-well plates was discarded
and 0.01 mol/l PBS (pH 7.4) was added dropwise into the specimen
sheet to be tested, which was discarded after 10 min. To keep the
specimen wet to a certain extent, a l:200 phospo-eNOS-3-endothelial
antibody dilution was added and the cells were incubated overnight
at 4°C. The membranes were washed with Tris-buffered saline
Tween-20 three times and a dilution (l:l,000) of Cy3-labeled goat
anti-rabbit IgG antibodies were added and used to completely cover
the specimens. The specimens were placed into an enamel box with a
lid and incubated for 30 min. The slides were removed from the
enamel box and placed on the slide shelves. Next, the slides were
washed with 0.01 mol/l PBS (pH 7.4) and soaked in three water jars
containing 0.01 mol/l PBS (pH 7.4). The specimens were processed
and analyzed using analysis software for OD.
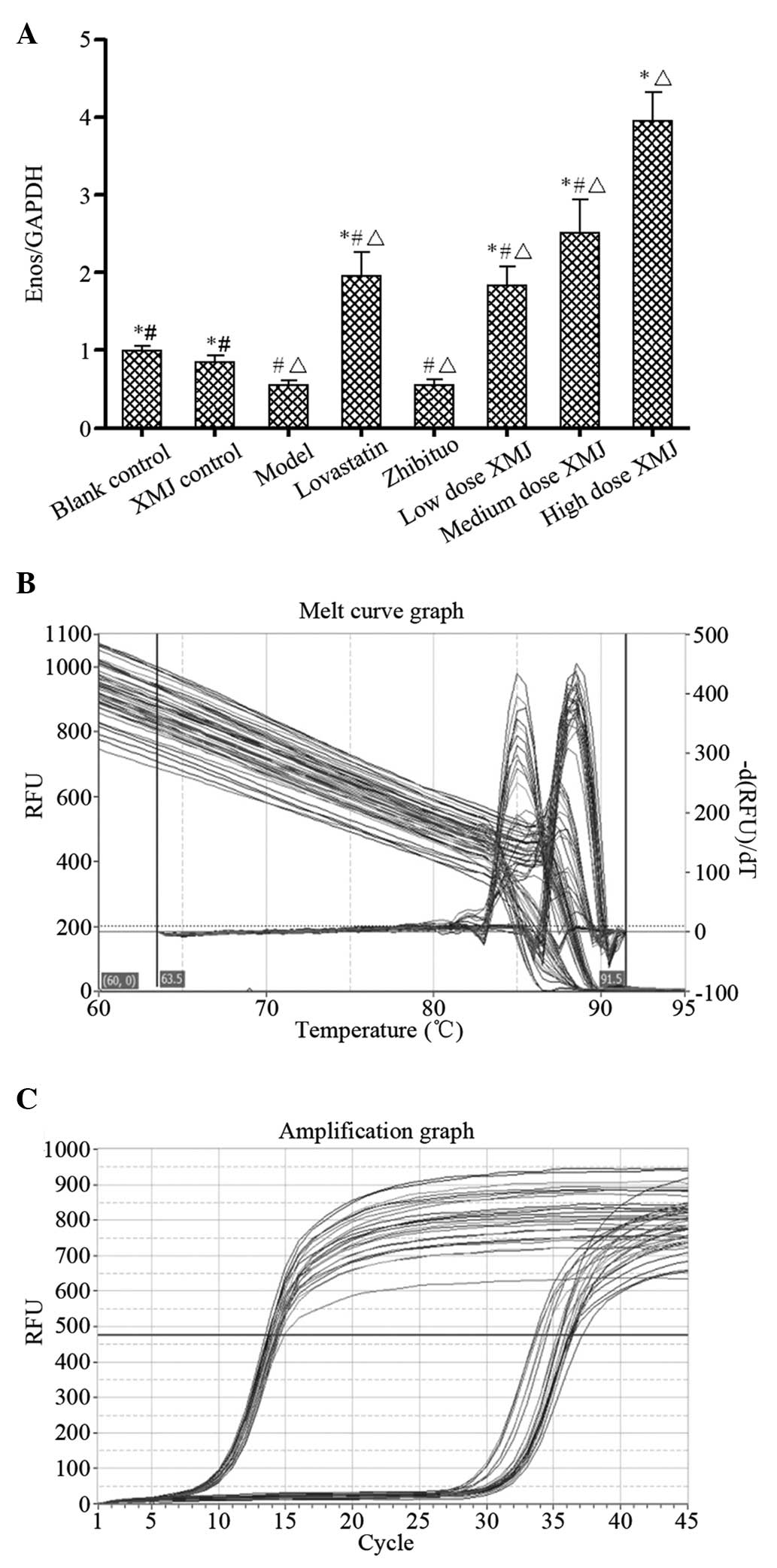
Quantitative polymerase chain reaction
(qPCR)
Total cellular RNA was extracted using a TRIzol
reagent kit. The primers were synthesized by Takara Biotechnology
Dalian Co., Ltd. (Dalian, China) and the forward and reverse primer
sequences (5′-3′) were as follows: e1, GGGACCACATAGGTGTCTGC; and
e2, CCAGCACAGCTACAGTGAGG. The 10-μl reaction system was composed of
5 μl SYBR Premix Taq TM (2X) reaction liquid, 0.25 μl PCR
forward primer (10 μM), 0.25 μl PCR reverse primer (10 μM), 0.5 μl
cDNA template and 4 μl deionized water. The recorded temperatures
of the melting curve ranged between 60 and 95°C. Following the
reaction, the PCR samples were processed separately by agarose gel
electrophoresis to verify whether the fragments had been amplified.
When the qPCR reaction had been completed, the data were collected
and analyzed using the computer analysis software, PikoReal
Software 2.1 (Thermo Fisher Scientific, Waltham, MA, USA). The
corresponding Ct value was calculated after adjusting the baseline
cycle threshold according to the requirements of the software.
Statistical analysis
All data are expressed as the mean ± standard error.
One-way analysis of variance and the Student-Newman-Keuls test for
multiple comparisons were used to compare the data among the
various groups. Statistical analysis was performed using the SPSS
13.0 statistical software (SPSS, Inc., Chicago, IL, USA) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Protective effect of XMJ on HUVEC injury
induced by H2O2 observed under a
microscope
HUVECs stained with HE were observed under a
microscope (magnification, ×400) and the observations were as
follows. HUVEC apoptosis was significantly reduced in the high-dose
XMJ group. Cytoplasmic staining was relatively uniform and the
metachromatic particles of the nuclei were not marked. The cells
were arranged closely and their morphology was normal. Evident
differences were identified when comparing these cells with the
model group. Higher-dose XMJ demonstrated a significant protective
effect on the HUVEC injury induced by H2O2.
Lovastatin and zhibituo also exhibited marked protective effects,
however, their protective effects with regard to morphology were
relatively weaker when compared with the high-dose XMJ group. The
protective effects of the low- and middle-dose XMJ groups were
significantly weaker than that of the high-dose XMJ group,
indicating the dependence of the protective effect on XMJ dosage
(Fig. 1).
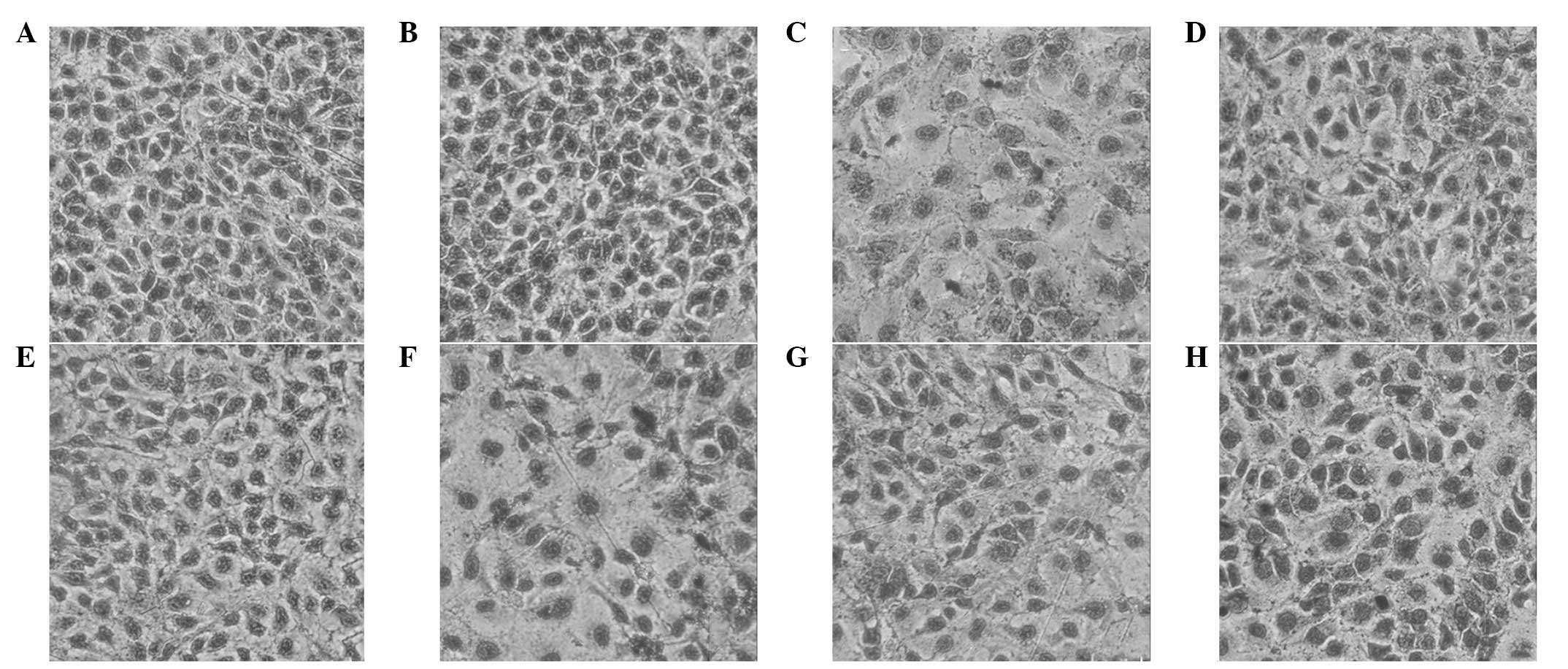 | Figure 1Microscopic images showing the
protective effect of XMJ on HUVEC injury induced by
H2O2 (magnification, ×400; HE stain) in the
(A) blank control, (B) XMJ control, (C) model, (D) lovastatin, (E)
zhibituo, (F) low-dose XMJ, (G) medium-dose XMJ and (H) high-dose
XMJ groups. XMJ, Xin Mai Jia; HUVEC, human umbilical vein
endothelial cell; HE, hematoxylin and eosin;
H2O2, hydrogen peroxide. |
Effect of XMJ on HUVEC activity
In the high-dose XMJ group, the rate of HUVEC
activity was 89.54%, the average degree of compaction was 0.77 and
the speed rate of polymerization was 60.83%. In the model group,
the activity rate of the HUVECs was 54.13%, the average degree of
compaction was 0.78 and the speed rate of polymerization was
52.52%. These results exhibited statistically significant
differences (P<0.05). The significant increase in the HUVEC
activity rate in the high-dose XMJ group demonstrated that a high
dosage of XMJ exhibits a significant protective effect on the
reduction in the activity rate of injured HUVECs induced by
H2O2. Lovastatin and zhibituo also
demonstrated marked protective effects on the
H2O2-induced reduction in the activity rate
of the HUVECs, and when compared with the model group, a
significant difference (P<0.05) was observed. However, when
compared with the high-dose XMJ group, the differences in the
activity rate of the HUVECs in the lovastatin and zhibituo groups
were not statistically significant. The protective effects of the
low- and middle-dose XMJ groups on the activity rate of the HUVECs
induced by H2O2 were significantly weaker
than that of the high-dose XMJ group, confirming that the effect
was dependent on XMJ dosage. However, the differences in the
average degree of compaction and the speed rate of polymerization
in the low-, middle- and high-dose XMJ groups were not
statistically significant (P>0.05; Fig. 2).
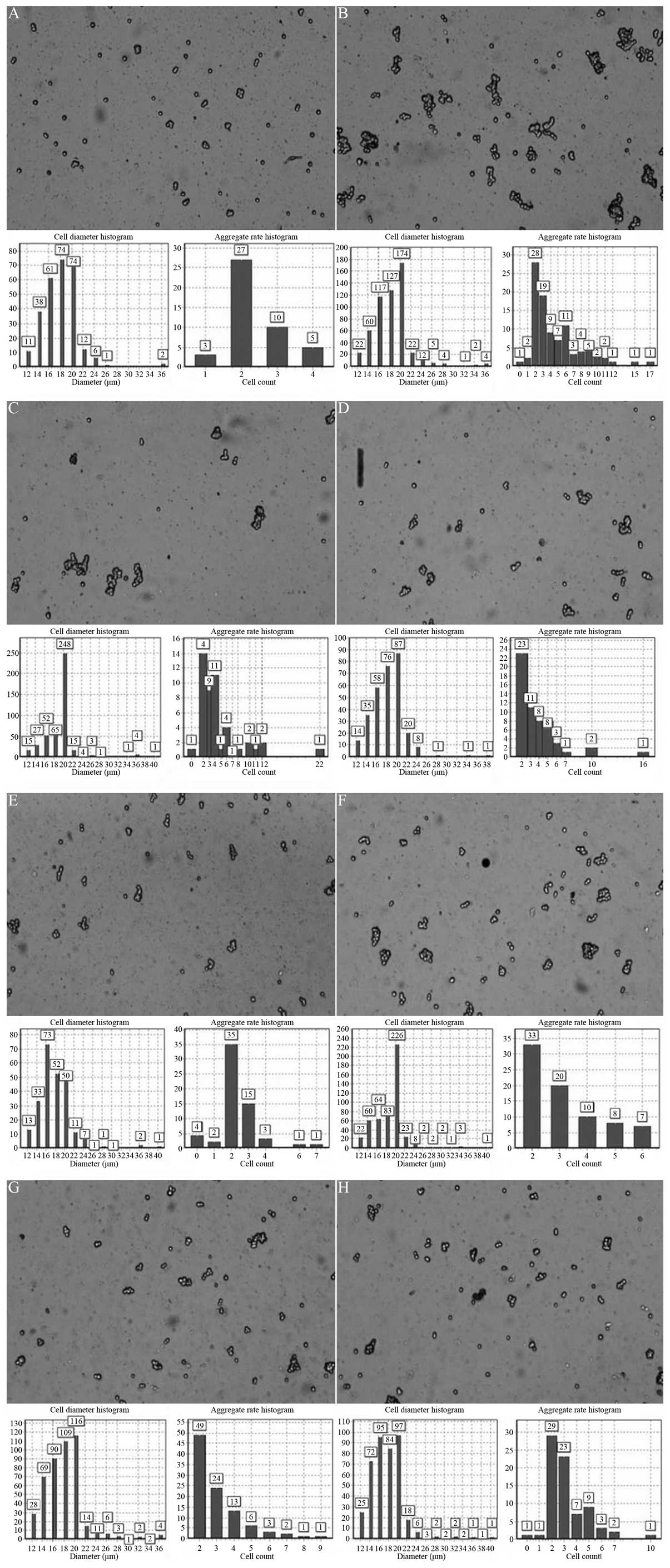 | Figure 2Effect of XMJ on the activity levels
of HUVECs in the (A) blank control, (B) XMJ control, (C) model, (D)
lovastatin, (E) zhibituo, (F) low-dose XMJ, (G) medium-dose XMJ and
(H) high-dose XMJ groups, as determined by a Countstar automatic
cell counter. XMJ, Xin Mai Jia; HUVECs, human umbilical vein
endothelial cells; H2O2, hydrogen
peroxide. |
Protective effect of XMJ on the reduction
of monolayer permeability in HUVEC injury induced by
H2O2
In the high-dose XMJ group, the Jv of the HUVECs was
29.43±7.53 μl/min/cm2, the Kf was 12.43±2.24
μl/min/cm2/kPa and the σ was 0.59±0.08. In the model
group, the Jv of the HUVECs was 44.47±8.56 μl/min/cm2,
the Kf was 17.66±3.43 μl/min/cm2/kPa and the σ was
0.29±0.03. Compared with the model group, the Jv of the HUVECs in
the high-dose XMJ group significantly decreased. Statistically
significant differences (P<0.05) were also identified in the Kf,
which markedly decreased in the high-dose XMJ group, as well as the
σ, which significantly increased. These results indicated that
high-dose XMJ demonstrated marked protective effects on the
reduction in HUVEC monolayer permeability induced by
H2O2. Lovastatin and zhibituo also exhibited
evident protective effects and statistically significant
differences (P<0.05) were observed when compared with the model
group. However, the protective effects of high-dose XMJ were more
significant than those of lovastatin and zhibituo (P<0.05). The
protective effects of low- and middle-dose XMJ, based on the by
H2O2-induced decrease in the permeability of
the HUVEC monolayer, were significantly weaker than that of
high-dose XMJ (P<0.05), thus, the protective effects were
dependent on the dose of XMJ (Table
I).
 | Table IProtective effects of XMJ on the
H2O2-induced decrease in the permeability of
the HUVEC monolayer (n=6; mean ± SE). |
Table I
Protective effects of XMJ on the
H2O2-induced decrease in the permeability of
the HUVEC monolayer (n=6; mean ± SE).
| Group | Jv
(μl/min/cm2) | Kf
(μl/min/cm2/Kpa) | σ |
|---|
| Blank control | 27.26±4.63a,b | 11.34±2.87a,b | 0.54±0.07a,b |
| XMJ control | 28.43±5.35a,b | 11.54±2.89a,b | 0.55±0.07a,b |
| Model | 44.47±8.56b,c | 17.66±3.43b,c | 0.29±0.03b,c |
| Lovastatin | 33.45±6.49a,b,c | 14.46±2.98a,b,c | 0.38±0.07a,b,c |
| Zhibituo | 35.32±5.12a,b,c | 14.64±2.39a,b,c | 0.43±0.06a,b,c |
| Low-dose XMJ | 34.34±5.43a,b,c | 14.54±2.32a,b,c | 0.41±0.08a,b,c |
| Medium-dose
XMJ | 30.12±5.67a,b,c | 12.28±2.13a,b,c | 0.46±0.08a,b,c |
| High-dose XMJ | 29.43±7.53a,c | 12.43±2.24a,c | 0.59±0.08a,c |
Effect of XMJ on the concentration of SOD
and MDA in the supernatant of HUVECs induced by
H2O2
In the high-dose XMJ group, the concentrations of
SOD and MDA were 18.21±1.39 U/ml and 1.37±0.26 nmol/ml,
respectively. In the model group, the concentration of SOD was
6.35±0.87 U/ml and the concentration of MDA was 1.84±0.26 nmol/ml.
Compared with the model group, statistically significant
differences (P<0.05) were observed in the high-dose XMJ group
with regard to SOD concentration, which significantly increased,
and MDA, which significantly decreased. Lovastatin and zhibituo
also exhibited marked suppressive effects on the decrease of
supernatant SOD levels and the increase of supernatant MDA levels
in the HUVEC injury model induced by H2O2.
The results were statistically significant (P<0.05) when
compared with the model group, however, when compared with the
high-dose XMJ group, the differences in HUVEC supernatant SOD and
MDA concentrations in the lovastatin and zhibituo groups exhibited
no statistically significant differences (P>0.05). The
suppressive effects of low- and middle-dose XMJ on the decrease in
supernatant SOD concentration and on the increase in supernatant
MDA concentration were significantly weaker than that of high-dose
XMJ (P<0.05), thus, the effects were dependent on XMJ dosage
(Fig. 3).
Effect of XMJ on the level of supernatant
cytokines in the HUVEC injury model induced by
H2O2
In the middle-dose XMJ group, the levels of
cytokines were as follows: ICAM-1, 36.33±7.32 ng/l; VCAM-1,
36.66±1.58 μg/l; IL-1, 14.75±2.04 ng/l; IL-6, 6.05±0.84 ng/l;
MMP-2, 0.671±0.07 μg/l; and TIMP-2, 2605.99±222.17 pg/l. In the
model group, ICAM-1 was 71.18±6.67 ng/l, VCAM-1 was 44.81±2.09
μg/l, IL-1 was 18.34±2.14 ng/l, IL-6 was 7.24±0.92 ng/l, MMP-2 was
0.608±0.07 μg/l and TIMP-2 was 1739.31±254.39 pg/l. No significant
difference (P>0.05) was identified between the model and
middle-dose XMJ groups. The levels of ICAM-1, VCAM-1, IL-1 and IL-6
decreased, while the levels of MMP-2 and TIMP-2 increased.
Lovastatin and zhibituo demonstrated marked suppressive effects on
the H2O2-induced increase in ICAM-1, VCAM-1,
IL-1 and IL-6 levels and on the decrease in MMP-2 and TIMP-2
levels, which were all statistically significant (P<0.05) when
compared with the model group. Compared with the middle-dose XMJ
group, the levels of supernatant cytokines in the lovastatin with
zhibituo groups exhibited no statistically significant differences
(P>0.05). The suppressive effects of low-dose XMJ on the
increase in ICAM-1, VCAM-1, IL-1 and IL-6 levels and on the
decrease in MMP-2 and TIMP-2 levels in the HUVEC injury model
induced by H2O2 were significantly weaker
when compared with the middle-dose XMJ group (P<0.05). However,
the suppressive effects of high-dose XMJ were weaker than those of
middle-dose XMJ; the reason is yet to be determined (Table II).
 | Table IIEffect of XMJ on the levels of
supernatant cytokines in HUVECs induced by
H2O2 (n=6; mean ± SE). |
Table II
Effect of XMJ on the levels of
supernatant cytokines in HUVECs induced by
H2O2 (n=6; mean ± SE).
| Group | ICAM-1 (ng/l) | VCAM-1 (μg/l) | IL-1 (ng/l) | IL-6 (ng/l) | MMP-2 (μg/l) | TIMP-2 (pg/l) |
|---|
| Blank control | 28.91±3.65a,b | 36.49±1.22a,b | 14.23±1.25a,b | 5.93±0.45a,b | 0.622±0.09a,b |
1925.88±236.12a,b |
| XMJ control | 36.33±4.39a,b | 38.78±2.39a,b | 13.93±1.36a,b | 6.51±0.65a,b | 0.647±0.07a,b |
2084.74±268.99a,b |
| Model | 71.18±6.67b,c | 44.81±2.09b,c | 18.34±2.14b,c | 7.24±0.92b,c | 0.608±0.07b,c |
1739.31±254.39b,c |
| Lovastatin | 38.55±4.16a,c | 35.84±1.96a,c | 14.63±1.57a,c | 6.05±0.78a,c | 0.683±0.08a,c |
1742.06±156.36a,c |
| Zhibituo | 34.10±4.12a,c | 36.49±2.68a,c | 14.79±1.69a,c | 6.19±0.65a,c | 0.622±0.07a,c |
1475.53±124.82a,c |
| Low-dose XMJ | 62.78±5.47a,b,c | 38.94±2.47a,b,c | 15.49±2.31a,b,c | 6.48±0.77a,b,c | 0.612±0.06a,b,c |
2137.27±213.29a,b,c |
| Medium-dose
XMJ | 36.33±7.32a,c | 36.66±1.58a,c | 14.75±2.04a,c | 6.05±0.84a,c | 0.671±0.07a,c |
2605.99±222.17a,c |
| High-dose XMJ | 39.29±4.57a,b,c | 43.38±2.39a,b,c | 16.07±2.55a,b,c | 7.21±0.98a,b,c | 0.643±0.07a,b,c |
1579.37±123.47a,b,c |
Effect of XMJ on the level of NF-κB in
the supernatant of HUVECs induced by
H2O2
In the high-dose XMJ group, the concentration of
NF-κB was 65.84±10.32 ng/l, whereas the level in the model group
was 200.46±25.68 ng/l. Compared with the model group, the
significant decrease in NF-κB concentration in the high-dose XMJ
group was statistically significant (P<0.05). Lovastatin and
zhibituo exhibited marked suppressive effects on the increase in
supernatant NF-κB concentration in the HUVEC injury model induced
by H2O2, which were statistically significant
(P<0.05) when compared with the model group. However, when
compared with the high-dose XMJ group, the differences in NF-κB
concentration in the lovastatin and zhibituo groups were not
statistically significant (P>0.05). The suppressive effects of
the low- and middle-dose XMJ groups on the increase in NF-κB
concentration in the HUVECs induced by H2O2
were significantly weaker when compared with the high-dose XMJ
group (P<0.05), thus, the effects were dependent on the dose of
XMJ (Fig. 4).
Effect of XMJ on the level of NO in the
supernatant of HUVECs induced by H2O2
In the high-dose XMJ group, the concentration of NO
was 22.58±2.58 μmol/l, while in the model group, the concentration
was 11.21±1.11 μmol/l. The increase in NO concentration in the
high-dose XMJ group exhibited a statistically significant
difference (P<0.05) when compared with the model group.
Lovastatin and zhibituo exhibited marked suppressive effects on the
decrease in NO concentration in HUVECs induced by
H2O2, which revealed statistically
significant differences (P<0.05) when compared with the model
group. However, the effect of the high-dose XMJ was more
significant than that of lovastatin and zhibituo. The suppressive
effects of low- and middle-dose XMJ on the
H2O2-induced decrease in NO levels in the
HUVECs were significantly weaker than that of high-dose XMJ
(P<0.05), indicating that the effects were dependent on the dose
of XMJ (Fig. 5).
Detection of eNOS concentration using
western blot analysis
The concentration of eNOS/β-actin was 1.75±0.22 in
the high-dose XMJ group and 0.65±0.11 in the model group. The
significant increase in eNOS/β-actin levels observed in the
high-dose XMJ group was statistically significant (P<0.05) when
compared with the model group. Lovastatin and zhibituo exhibited
marked suppressive effects on the decrease in eNOS concentration in
the HUVECs induced by H2O2, which was
statistically significant (P<0.05) when compared with the model
group. The suppressive effect of high-dose XMJ on the decrease in
eNOS content was more significant than those of lovastatin and
zhibituo (P<0.05). In addition, the levels of eNOS/β-actin in
the low- and middle-dose XMJ groups were significantly weaker than
that of the high-dose XMJ group (P<0.05), indicating that the
effects were dependent on XMJ dosage (Fig. 6).
Determination of eNOS protein
concentration using immunohistochemistry
In the high-dose XMJ group, the degree of eNOS
staining saturation was 7.23±0.29%, the chromaticity was
42.55±3.27%, the grayscale value was 254.33±11.39%, the red value
was 153.98±12.97%, the green value was 178.98±15.23% and the blue
value was 165.38±11.27%. In the model group, the degree of eNOS
staining saturation was 16.55±0.67%, the chromaticity was
33.78±2.17, the grayscale value was 189.46±12.54, the red value was
136.59±11.22, the green value was 154.78±13.24 and the blue value
was 144.29±12.67. A significant difference (P<0.05) was observed
in the eNOS coloration index between the high-dose XMJ and model
groups. Lovastatin and zhibituo exhibited significant effects on
the eNOS coloration index of the HUVEC model induced by
H2O2, which were statistically significant
(P<0.05) when compared with the model group. The eNOS staining
indicators in the high-dose XMJ group were greater than those in
the lovastatin and zhibituo groups (P<0.05). The effect of low-
and middle-dose XMJ on the eNOS coloration index in the HUVECs
induced by H2O2 were markedly weaker than
that of the high-dose XMJ group, indicating XMJ dose dependence
(Fig. 7).
 | Figure 7Immunohistochemical analysis
(immunohistochemistry, Mayer’s hematoxylin counterstain) was used
to detect the eNOS protein content in HUVECs (magnification, ×400)
in the (A) blank control, (B) XMJ control, (C) model, (D)
lovastatin, (E) zhibituo, (F) low-dose XMJ, (G) medium-dose XMJ and
(H) high-dose XMJ groups. XMJ, Xin Mai Jia; HUVECs, human umbilical
vein endothelial cells; eNOS, endothelial nitric oxide
synthase. |
Determination of eNOS content using
immunofluorescence
Confocal fluorescence tomography was performed on
immunofluorescence cells with a laser scanning confocal microscope.
The 32 facets of each cell were scanned and the fluorescence
intensity within the cells was detected using fluorescence
quantitative analysis software. The eNOS protein was predominantly
expressed in the cytoplasm of the HUVECs. The positive signals
presented yellowish-green spotlight, with diffused distribution.
The fluorescence intensity values of the eNOS protein were
178.33±11.26 in the high-dose XMJ group and 65.27±4.66 in the model
group, which exhibited a statistically significant difference
(P<0.05). Lovastatin and zhibituo significantly increased
(P<0.05) the fluorescence intensity of the eNOS protein in the
HUVECs induced by H2O2. However, the
fluorescence intensity of the eNOS protein in the high-dose XMJ
group was greater than those in the lovastatin and zhibituo groups
(P<0.05) The fluorescence intensities of the eNOS protein in the
HUVECs treated with low- and middle-dose XMJ were significantly
weaker than that of high-dose XMJ (P<0.05), indicating XMJ dose
dependence (Fig. 8).
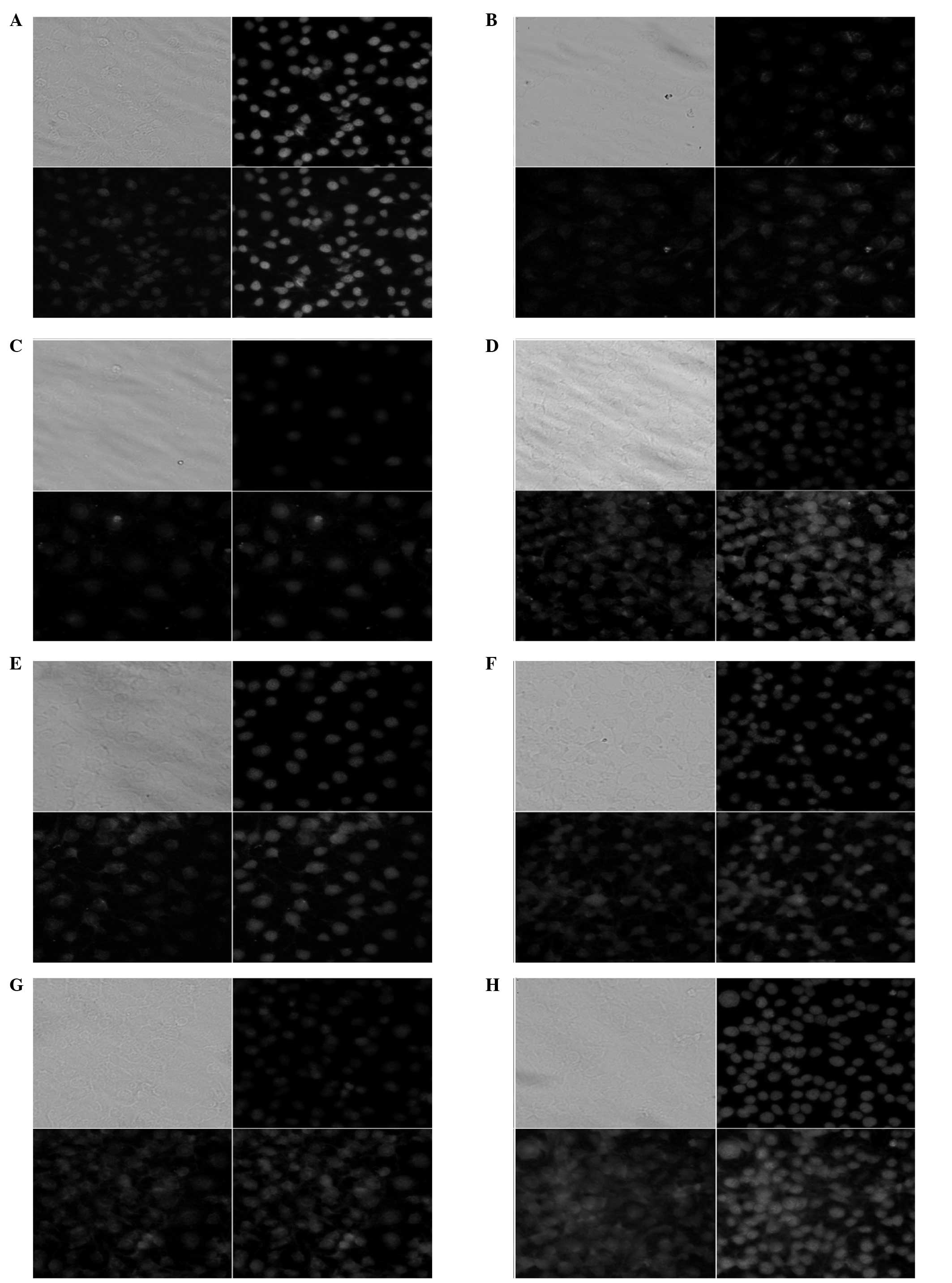 | Figure 8Immunofluorescence analysis was used
to detect the levels of eNOS in HUVECs (magnification, ×400) in the
(A) blank control, (B) XMJ control, (C) model, (D) lovastatin, (E)
zhibituo, (F) low-dose XMJ, (G) medium-dose XMJ and (H) high-dose
XMJ groups. XMJ, Xin Mai Jia; HUVECs, human umbilical vein
endothelial cells; eNOS, endothelial nitric oxide synthase. |
Detection of eNOS gene expression levels
using fluorescence qPCR
Fluorescence intensity values of eNOS gene
expression were 3.96±0.36 in the high-dose XMJ group and 0.55±0.77
in the model group; the results exhibited a statistically
significant difference (P<0.05). Lovastatin and zhibituo
significantly increased (P<0.05) the fluorescence intensity of
eNOS gene expression in the HUVECs induced by
H2O2 when compared with the model group. The
fluorescence intensity of eNOS gene expression in the high-dose XMJ
group was greater than those of the lovastatin and zhibituo groups
(P<0.05). The fluorescence intensities of eNOS gene expression
in the low- and middle-dose XMJ groups were significantly weaker
than that of the high-dose XMJ group (P<0.05), thus, the effects
were dependent on XMJ dosage (Fig.
9).
Discussion
NO in the body results from the activation of the
N-methyl-D-aspartate receptor and the catalysis of NOS. NOS widely
exists in the nervous system, internally and externally. NOS
isoenzymes are classified into three subtypes: Neuronal NOS (nNOS),
eNOS and immune-NOS. nNOS and eNOS have Ca2+-dependent structural
expressions, which exist in normal physiological state (15). NF-κB, an important transcription
factor, regulates the expression of various genes involved in
inflammation and immune processes, and is closely associated with
several important pathophysiologies, including cell proliferation,
transformation and apoptosis (16). In the development of AS, the
expression of NF-κB, induced by a variety of pathogenic factors,
increases, stimulating the increased secretion of IL-1, IL-6,
ICAM-1, VCAM-1 and other inflammatory cytokines. In addition, NF-κB
inhibits eNOS expression, reduces NO release and decreases normal
vasomotion function. As a secondary intracellular messenger in
HUVECs, NO increases the concentration of cGMP via the cGMP
pathway. This phenomenon can influence ion channels or
phosphodiesterase activity, activate cGMP-dependent protein kinase,
activate cycloxygenase, protein kinase C and iron regulatory
proteins, stimulate the expression of the early gene response or
inhibit NF-κB and other non-cGMP pathways.
The majority of studies show that XMJ exhibits a
marked protective effect on HUVEC injury induced by
H2O2. This protective effect is dependent on
the dose of XMJ (17,18). However, by studying the effect that
XMJ has on the level of supernatant cytokines in HUVECs induced by
H2O2, the protective effects of low- and
middle-dose XMJ were shown to be dependent on XMJ dosage to a
certain extent, whereas the suppressive effects of high-dose XMJ
were weaker than those of middle-dose XMJ. In the high-dose XMJ
group, cytokine levels were as follows: ICAM-1, 39.29±4.57 ng/l;
VCAM-1, 43.38±2.39 μg/l; IL-1, 16.07±2.55 ng/l; IL-6, 7.21±0.98
ng/l; MMP-2, 0.643±0.07 μg/l; and TIMP-2, 1579.37±123.47 pg/l. This
variation may possibly be attributed to the disproportion between
the secretion and expression levels of the cytokines within the
HUVEC supernatant, however, the specific reasons require further
investigation.
MMP-2 belongs to the MMP family, which requires
Ca2+, Zn2+ and other metal ions as cofactors
for protein hydrolysis. MMP-2 consists of five functional domains.
Firstly, a hydrophobic signaling peptide sequence. Secondly, the
pre-peptide region, which functions in maintaining the stability of
the proenzyme, as the MMP-2 zymogen is activated when the region is
cut off by an exogenous enzyme. Thirdly, the catalytic activity
area, which is a Zn2+ binding site that plays a crucial
role in enzyme catalysis. Fourthly, the hinge region that contains
abundant proline residues. Finally, there is the carboxyl terminal
region, which is associated with the specificity of the enzyme
substrate. The MMP-2 gene, which consists of 13 exons and 12
introns, is located on the 16q21 human chromosome. The total length
of the structural gene is 27 kb, which differs from other MMPs due
to the MMP-2 gene 5′ flanking sequence that contains two GC boxes
instead of two TATA sequences, which promotes the subregion
(19). MMP-2 can decompose the
stromal component among the cells and type IV collagen, which is
the main component of the basement membrane (20–22).
Previous studies have reported that the expression of MMP-2
increases during early AS pathogenesis. However, following drug
intervention, MMP-2 expression decreases, which confirmed the
increase in MMP-2 to be an iconic indicator of AS (23–29).
However, experimental data have produced contradictory results. In
the present study, the level of MMP-2 in the model group was
0.608±0.07 μg/l, which was a marked decrease when compared with the
blank control group, whereas the level of MMP-2 in the drug groups
increased significantly. This difference may be associated with the
secretion and disproportionate expression levels of cytokines in
the supernatant fluid of the HUVECs, however, if XMJ is able to
increase the levels of MMP-2, then the degradation of matrix
proteins increases. This difference may be relevant with the
ablation of the AS plaque. In the present study, the level of
TIMP-2 in the drug groups increased, which may be attributable to a
feedback mechanism from the body.
In order to determine whether XMJ itself exhibits
harmful effects on the HUVEC model, which may have significantly
affected the experimental data, a control group of XMJ drug media
was used in the experiment. The results demonstrated that XMJ
itself presents no harm to the HUVECs and does not affect the
experimental results. Lovastatin and zhibituo are chemical drugs
and TCM commonly used for the treatment of AS in clinical practice.
Lovastatin and zhibituo were selected as the control drugs to
ascertain the degree of the protective effect of XMJ on HUVEC
injury induced by H2O2. The majority of the
results indicated that the protective effects of XMJ on HUVEC
injury induced by H2O2 were greater than
those of lovastatin and zhibituo. However, the differences in the
levels of SOD, MDA and NF-κB in the HUVEC supernatant among the
high-dose XMJ, lovastatin and zhibituo groups presented no
statistical significance (P>0.05). In addition, the difference
in the levels of cytokines in the HUVEC supernatant among the
middle-dose XMJ, lovastatin and zhibituo groups was not
statistically significant (P>0.05). We hypothesize that these
observations may have resulted from the dilution of cytokines in
the HUVEC supernatant fluid or may be associated with the level of
secretion. In conclusion, Xin Mai Jia prevents atherosclerosis and
is superior to routine anti-atherosclerosis drugs such as
lovastatin and Zhibituo.
Acknowledgements
The study was supported by a grant from the Major
Research Projects of the Department of Science and Technology of
Henan Province, China (no. 121100910300).
References
|
1
|
Aliev G, Li Y, Palacios HH and Obrenovich
ME: Oxidative stress induced mitochondrial DNA deletion as a
hallmark for the drug development in the context of the
cerebrovascular diseases. Recent Pat Cardiovasc Drug Discov.
6:222–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kotsovolis G and Kallaras K: The role of
endothelium and endogenous vasoactive substances in sepsis.
Hippokratia. 14:88–93. 2010.PubMed/NCBI
|
|
3
|
Czyzewska-Buczyńska A and Witkiewicz W:
Role of mast cells in the pathogenesis of atherosclerosis. Przegl
Lek. 68:171–174. 2011.(In Polish).
|
|
4
|
Petrofsky JS: The effect of
type-2-diabetes-related vascular endothelial dysfunction on skin
physiology and activities of daily living. J Diabetes Sci Technol.
5:657–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu R and Wu S, Cao G, Wang W, Liu K and
Wu S: Transfection of human hepatocyte growth factor gene inhibits
advancing pulmonary arterial hypertension induced by shunt flow in
a rabbit model. Transplant Proc. 45:705–712. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perez-Herrera A, Rangel-Zuñiga OA,
Delgado-Lista J, et al: The antioxidants in oils heated at frying
temperature, whether natural or added, could protect against
postprandial oxidative stress in obese people. Food Chem.
138:2250–2259. 2013. View Article : Google Scholar
|
|
7
|
Razavi SM, Gholamin S, Eskandari A, et al:
Red grape seed extract improves lipid profiles and decreases
oxidized low-density lipoprotein in patients with mild
hyperlipidemia. J Med Food. 16:255–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rocha BS, Gago B, Pereira C, et al:
Dietary nitrite in nitric oxide biology: a redox interplay with
implications for pathophysiology and therapeutics. Curr Drug
Targets. 12:1351–1363. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wan J, Yin Y, Sun R, et al: Protective
effect of the ultra-filtration extract from Xin Mai Jia on human
aortic smooth muscle cell injury induced by hydrogen peroxide. Exp
Ther Med. 7:11–16. 2014.PubMed/NCBI
|
|
10
|
Wilcox JN, Subramanian RR, Sundell CL, et
al: Expression of multiple isoforms of nitric oxide synthase in
normal and atherosclerotic vessels. Arterioscler Thromb Vasc Biol.
17:2479–2488. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan GR, Wan J, Dong XH, et al: The
preparative method of a kind of supplement food with the effect of
adjusting blood-fat and antagonism to artherosclerosis. China
patent 201010536001. (In Chinese).
|
|
12
|
Postlethwaite AE, Snyderman R and Kang AH:
The chemotatic attraction of human fibroblasts to a
lymphocyte-derived factor. J Exp Med. 144:1188–1203. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ding ZQ, Li SH and Wu ZL: The effect of
platelet activating factor on endothelial monolayer permeability by
extracorporeal perfusion. Di Er Jun Yi Da Xue Xue Bao. 14:101–106.
1993.(In Chinese).
|
|
14
|
Ni L, Li T, Liu B, et al: The protective
effect of Bcl-xl overexpression against oxidative stress-induced
vascular endothelial cell injury and the role of the Akt/eNOS
pathway. Int J Mol Sci. 14:22149–22162. 2013. View Article : Google Scholar
|
|
15
|
Das T, Bhattacharya S, Biswas A, Gupta SD
and Gomes A and Gomes A: Inhibition of leukemic U937 cell growth by
induction of apoptosis, cell cycle arrest and suppression of VEGF,
MMP-2 and MMP-9 activities by cytotoxin protein NN-32 purified from
Indian spectacled cobra (Naja naja) venom. Toxicon. 65:1–4. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JH, Shim JW, Choi YJ, Heo K and Yang
K: The combination of sorafenib and radiation preferentially
inhibits breast cancer stem cells by suppressing HIF-1α expression.
Oncol Rep. 29:917–924. 2013.PubMed/NCBI
|
|
17
|
Shahlaee A, Farahanchi A, Javadi S, Delfan
B and Dehpour AR: Sucrose-induced analgesia in mice: role of nitric
oxide and opioid receptor-mediated system. Indian J Pharmacol.
45:593–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han C, Zhao Q and Lu B: The role of nitric
oxide signaling in food intake; insights from the inner
mitochondrial membrane peptidase 2 mutant mice. Redox Biol.
1:498–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasaki T, Nakamura K, Sasada K, et al:
Matrix metalloproteinase-2 deficiency impairs aortic
atherosclerotic calcification in ApoE-deficient mice.
Atherosclerosis. 227:43–50. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeong YJ, Cho HJ, Whang K, et al: Melittin
has an inhibitory effect on TNF-α-induced migration of human aortic
smooth muscle cells by blocking the MMP-9 expression. Food Chem
Toxicol. 50:3996–4002. 2012.
|
|
21
|
Xu YZ, Zhao KJ, Yang ZG, et al: Decreased
plasma decorin levels following acute ischemic stroke: correlation
with MMP-2 and differential expression in TOAST subtypes. Mol Med
Rep. 6:1319–1324. 2012.
|
|
22
|
Kang SW, Kim MS, Kim HS, et al: Celastrol
attenuates adipokine resistin-associated matrix interaction and
migration of vascular smooth muscle cells. J Cell Biochem.
114:398–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miksztowicz V, Siseles N, Fernandez
Machulsky N, Schreier L and Berg G: Increase in MMP-2 activity in
overweight and obese women is associated with menopausal status.
Climacteric. 15:602–606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soumyarani VS and Jayakumari N:
Oxidatively modified high density lipoprotein promotes inflammatory
response in human monocytes-macrophages by enhanced production of
ROS, TNF-α, MMP-9, and MMP-2. Mol Cell Biochem. 366:277–285.
2012.PubMed/NCBI
|
|
25
|
Karki R, Jeon ER and Kim DW: Magnoliae
Cortex inhibits intimal thickening of carotid artery through
modulation of proliferation and migration of vascular smooth muscle
cells. Food Chem Toxicol. 50:634–640. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ibanez B, Giannarelli C, Cimmino G, et al:
Recombinant HDL (Milano) exerts greater anti-inflammatory and
plaque stabilizing properties than HDL (wild-type).
Atherosclerosis. 220:72–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Butoi ED, Gan AM, Manduteanu I, et al:
Cross talk between smooth muscle cells and monocytes/activated
monocytes via CX3CL1/CX3CR1 axis augments expression of
pro-atherogenic molecules. Biochim Biophys Acta. 1813:2026–2035.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng XW, Song H, Sasaki T, et al:
Angiotensin type 1 receptor blocker reduces intimal
neovascularization and plaque growth in apolipoprotein E-deficient
mice. Hypertension. 57:981–989. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yi L, Chen CY, Jin X, et al: Differential
suppression of intracellular reactive oxygen species-mediated
signaling pathway in vascular endothelial cells by several
subclasses of flavonoids. Biochimie. 94:2035–2044. 2012. View Article : Google Scholar
|