Introduction
Dental caries is an infectious oral disease
prevalent across the world and it is associated with various
pathogenic microorganisms. Streptococcus mutans (S.
mutans) is considered a crucial pathogen in the pathogenesis of
dental caries (1). It is involved
in the development and establishment of cariogenic biofilms. The
major factors responsible for the cariogenicity of this pathogen
include its ability to produce glucosyltransferases (Gtfs),
synthesize insoluble glucans, generate acids and survive at low pH
values (2–5). Therefore, a previous study
hypothesized that disrupting the ability of S. mutans to
form acids and glucans may be an effective therapeutic approach for
the treatment of dental caries (6).
Despite advances in the development of anti-caries
chemotherapy, conventional therapeutic strategies are often unable
to control the progression of dental caries. It has been reported
that the use of natural products is one of the most successful
strategies for the discovery of new medicines (7). The herbaceous plants Polygonum
(Polygonaceae), Rhamnus (Rhamnaceae) and Senna
(Fabaceae) have been successfully used as traditional medicines in
East Asian countries (8,9). These herbs have demonstrated various
pharmacological effects; for example, Polygonaceae have the ability
to control dental diseases (10).
Furthermore, a previous study revealed that extracts from
Polygonaceae roots are able to inhibit the production of acid by
S. mutans, suggesting that they may be useful for the
treatment of dental caries (11).
Emodin (1,3,8-trihydroxy-6-methylanthraquinone) is a major active
component commonly present in these herbaceous plants. It has been
reported that emodin exhibits a wide range of biological activities
including antibacterial, anticancer, anti-inflammatory,
anti-diabetic and anti-oxidative activities (12–14).
Considering that emodin is the main component of Polygonaceae, it
may be meaningful to evaluate the biological effects of emodin on
dental caries. The aim of the present study was to examine the
effects of emodin on the growth, acid production and insoluble
glucan synthesis of S. mutans in vitro, and caries
development in vivo.
Materials and methods
Materials
Emodin with purity >98% was obtained from Xi’an
Tianxingjian Natural Bio-products Group (Xi’an, China). Emodin was
prepared in a phosphate buffer containing 15% (v/v) ethanol.
Appropriate solvent controls were included. Sodium fluoride was
purchased from Sigma (St. Louis, MO, USA). S. mutans ATCC
25175 was provided by the Shanghai Zhi Cheng Bio-Tech Co., Ltd.
(Shanghai, China). Cariogenic diet 2000 was purchased from Trophic
Animal Feed High-tech Co., Ltd. (Nantong, China). All other
chemicals used were of analytical grade and commercially
available.
Measurement of bacterial growth
Bacterial growth was established using previously
described methods with slight modifications (15,16).
Briefly, various concentrations of filter-sterilized emodin were
added to 0.95 ml tryptic soy broth containing 1% glucose. S.
mutans ATCC 25175 seed culture (0.1 ml) was inoculated into the
broth medium and incubated at 37°C. The optical density of the
culture was measured at 520 nm by a UV-2550 spectrophotometer
(Shimadzu Corporation, Kyoto, Japan) every 1 h for 24 h.
Measurement of the production of acid by
S. mutans ATCC 25175
The acid production assay was carried out using
previously described methods with slight modifications (15). Briefly, emodin was added to 0.95 ml
broth containing 1% glucose and the mixture was inoculated with
0.05 ml S. mutans ATCC 25175 seed culture. Following
incubation at 37°C for 24 h, the pH of the cultures was determined
using a pH meter (pHS-3C; Shanghai REX Instrument Factory,
Shanghai, China).
Measurement of insoluble glucan synthesis
by Gtfs
S. mutans TCC 25175 was cultured at 37°C for
24 h in tryptic soy broth. The culture supernatant was salted out
with solid ammonium sulfate to 70% saturation and agitated at 4°C
for 1 h. Following centrifugation at 13,500 × g for 20 min, the
precipitate was dialyzed against 10 mM potassium phosphate buffer
(pH 6.0). The solution of crude Gtfs was stored at −80°C until
required.
The reaction mixture, consisting of 0.025 ml of the
prepared solution of crude Gtfs and 0.175 ml emodin (final
concentrations: 0, 0.5, 1 and 2 mg/ml) in 0.8 ml of 0.0625 M
potassium phosphate buffer containing 12.5 μg/l sucrose and 0.25
μg/l sodium azide, was incubated at 37°C for 18 h. The insoluble
glucan was allowed to sediment, then washed with distilled water
and placed under ultrasonication for 6 sec. The absorbance was
examined using a UV spectrophotometer at 520 nm against a
blank.
Gtf B activity assay
The Gtf B enzyme was obtained from the supernatant
of the S. mutans ATCC 25175 culture and purified to almost
homogeneity by hydroxyapatite column chromatography using a
previously described method (17).
The enzymatic activity of Gtfs was examined by incorporating
[14C] glucose from labeled sucrose (China Isotope
Corporation, Beijing, China) into the glucans. The amount of Gtf B
enzyme added to every sample for all assays was equivalent to the
amount required to incorporate 1 μmol of glucose during the 4 h
reaction period. Purified Gtf B was mixed with different
concentrations of emodin (0.5, 1 and 2 mg/ml) and incubated with
[14C]-glucose-labeled-sucrose substrate (final
concentration, 100 mM sucrose). Ethanol (final concentration, 15%
v/v) was used as the control. Radiolabeled glucan was measured by
scintillation counting (17,18).
Animal study
Animal experiments were performed using previously
described methods (19,20). Briefly, pathogen-free male Wistar
rats (19 days of age; purchased from Kunming Medical University,
Kunming, China) were infected daily for five consecutive days with
a growing culture of S. mutans ATCC 25175. The rats, aged 25
days, were randomly divided into three groups (n=15) and their
teeth were treated topically using a camel hair brush, twice daily,
for five weeks as follows: i) vehicle control (15% ethanol); ii)
emodin 2 mg/ml; and iii) 250 ppm fluoride. The rats were placed in
individual cages and given cariogenic diet 2000 and 5% sucrose
water ad libitum. At the end of the five-week experimental
period, the rats were anesthetized and sacrificed. The lower left
jaw was aseptically removed, immerged in 5.0 ml sterile saline
solution and sonicated. The suspension was plated on blood agar and
on Mitis Salivarius agar plus streptomycin, to respectively
estimate the total number of cultivable microorganisms and S.
mutans populations. Smooth-surface and sulcal caries and their
severities (Ds, dentin exposed; Dm, 3/4 of the dentin affected; Dx,
whole dentin affected) were evaluated by means of Larson’s
modification of Keyes’ system (20). The caries score was determined
blindly with respect to the groups. All procedures were performed
in accordance with guidelines set for the use of experimental
animals by the local Committee of Kunming Medical College on Animal
Care and Use.
Statistical analysis
All values are expressed as mean ± standard error of
the mean. The data were analyzed using analysis of variance
followed by a Tukey-Kramer multiple comparison test using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
In vitro effect of emodin on the
cariogenic properties of S. mutans
In the present study, the antibacterial effect of
emodin on S. mutans was investigated. As shown in Fig. 1, growth of S. mutans ATCC
25175 was significantly reduced in the presence of emodin. This
effect was revealed to be concentration dependent.
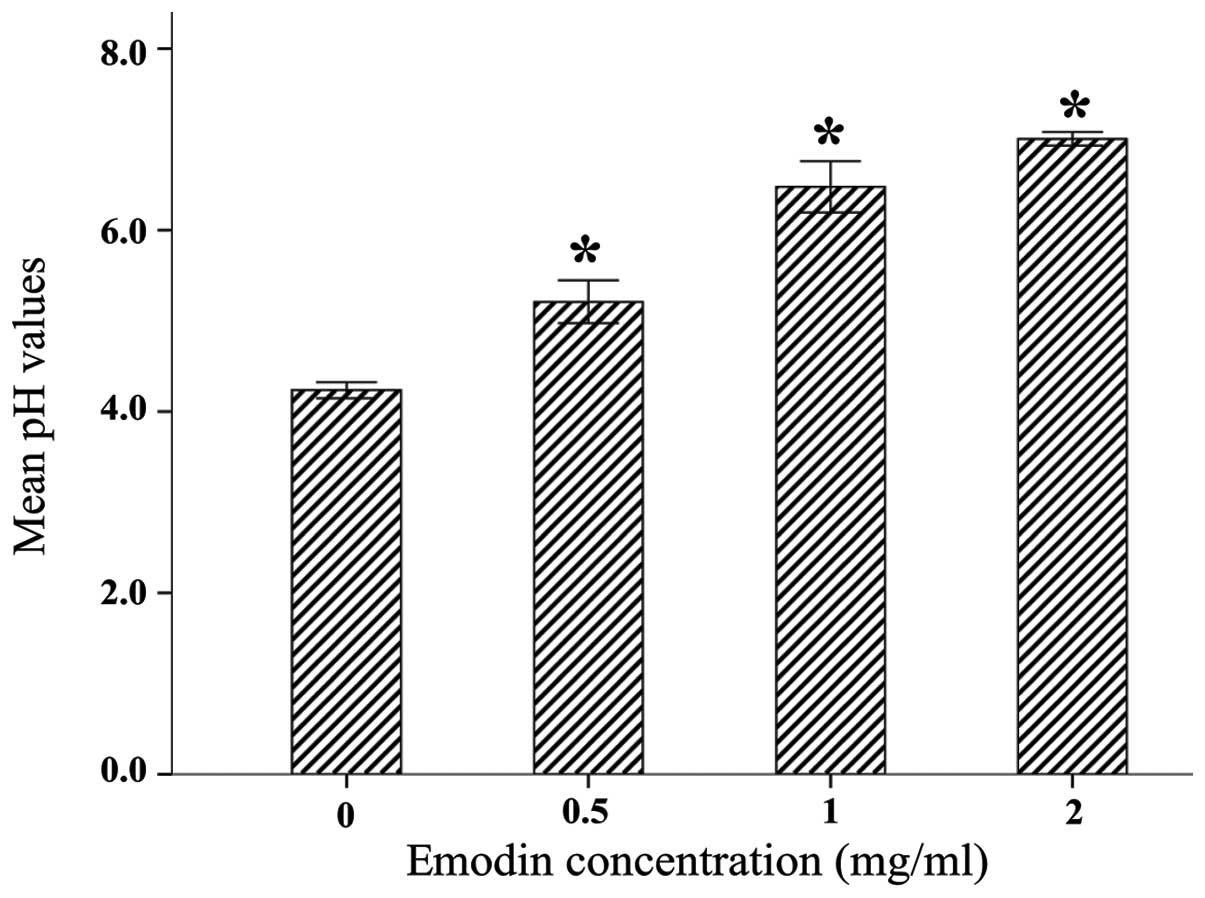
To determine the inhibitory effect of emodin on the
production of acid by S. mutans ATCC 25175, the cells were
treated with different concentrations of emodin and the pH was
measured. The addition of emodin did not change the color and pH
value of cultures prior to the growth of S. mutans ATCC
25175 (data not shown). As shown in Fig. 2, the production of acid by S.
mutans ATCC 25175 was significantly suppressed by emodin
compared with that in the control group.
Whether emodin may suppress insoluble glucan
synthesis by Gtfs was also examined. As shown in Fig. 3, a significant reduction of
insoluble glucan synthesis by crude Gtfs from S. mutans ATCC
25175 was demonstrated at concentrations >0.5 mg/ml emodin.
Furthermore, emodin reduced Gtf B activity to a notable extent.
Inhibitory effect of emodin on caries in
rats
In the animal experiment, the rats remained healthy
and gained weight during the five weeks of the experimental period.
No significant differences in weight gain were observed among the
groups (P>0.05, data not shown).
The effect of emodin on the total cultivable
microorganisms, viable S. mutans populations and percentage
of S. mutans recovered from the rat jaws (as calculated from
the S. mutans and total cultivable microorganism
populations) are shown in Table I.
The emodin-treated group demonstrated significantly lower total
microorganism counts compared with those in the vehicle control
group. However, the number and percentage of S. mutans in
the biofilms of rats treated with emodin did not differ
statistically from those of the vehicle control group.
 | Table IEffect of emodin on the oral
microbiota of rats following a five-week experiment. |
Table I
Effect of emodin on the oral
microbiota of rats following a five-week experiment.
| Group | Total
microorganisms (×104 cfu/ml) | S. mutans
ATCC 25175 (×104 cfu/ml) | S. mutans
ATCC 25175 (%) |
|---|
| Vehicle | 4.0a
(1.2) | 2.7a
(1.8) | 67.9a
(20.7) |
| 2 mg/ml emodin | 2.2b
(0.6) | 1.4a
(0.4) | 63.6a
(16.8) |
| 250 ppm
fluoride | 2.3b
(0.5) | 1.5a
(0.2) | 65.2a
(13.5) |
Tables II and
III show the incidence and
severity of smooth-surface and sulcal caries. In the present study,
250 ppm fluoride was used as a positive control. The 250 ppm
fluoride treatment revealed the lowest scores for incidence and
severity of smooth-surface and sulcal caries. Treatment with emodin
significantly reduced the incidence of smooth-surface and sulcal
caries compared with that of the vehicle control group.
Furthermore, the severity scores of smooth-surface and sulcal
caries were significantly lower in the group treated with emodin
than in the vehicle control group.
 | Table IIEffect of various treatments on
smooth-surface caries development (incidence and severity) in
rats. |
Table II
Effect of various treatments on
smooth-surface caries development (incidence and severity) in
rats.
| | Severity |
|---|
| |
|
|---|
| Group | Total smooth
surface | Ds | Dm | Dx |
|---|
| Vehicle | 67.2a
(6.6) | 40.8a
(7.8) | 16.8a
(6.1) | 6.2a
(6.4) |
| 2 mg/ml emodin | 42.6b
(5.8) | 24.7b
(6.9) | 5.4b
(7.1) | 2.2b
(0.7) |
| 250 ppm
fluoride | 22.7c
(2.5) | 18.6b
(7.6) | 0.9b
(0.5) | 0.2c
(0.3) |
 | Table IIIEffect of various treatments on
sulcal-surface caries development (incidence and severity) in
rats. |
Table III
Effect of various treatments on
sulcal-surface caries development (incidence and severity) in
rats.
| | Severity |
|---|
| |
|
|---|
| Group | Total sulcal
surface | Ds | Dm | Dx |
|---|
| Vehicle | 36.3a
(5.3) | 27.2a
(4.2) | 20.8a
(4.3) | 15.4a
(6.2) |
| 2 mg/ml emodin | 30.4b
(4.2) | 18.1b
(3.2) | 10.1b
(4.2) | 6.3b
(3.8) |
| 250 ppm
fluoride | 19.3c
(2.3) | 10.2c
(3.5) | 3.2c
(1.6) | 0.7c
(0.6) |
Discussion
Considering the high incidence rate of dental caries
and its detrimental effects in the oral cavity, the development of
novel strategies for its prevention and control are required.
Previous studies have demonstrated that natural products are
promising candidates for novel anticariogenic substances (7,21,22).
The present study revealed that emodin, a natural product,
interfered with key cariogenic factors of S. mutans, namely
the synthesis of insoluble glucans and production of acid in
vitro, and reduced the induction of caries in rats.
Emodin is a natural anthraquinone derived from the
roots and rhizomes of a number of plants including Rheum
undulatum (R. undulatum) and Polygonum cuspidatum (P.
cuspidatum). P. cuspidatum has demonstrated a broad
range antibacterial effects (10).
It has also been reported that the ethyl acetate fraction of P.
cuspidatum, which is composed of polydatin, resveratrol,
anthraglycoside B and emodin, is able to inhibit the glycolytic
acid production and Gtf activity of S. mutans and
Streptococcus sobrinus (11). The dichloromethane fraction from
R. undulatum, composed mainly of aloe-emodin, emodin,
chrysophanol and physcion, has revealed inhibitory effects on the
production of glycolytic acid by S. mutans on biofilms
(23). In the present study,
emodin markedly suppressed the production of acid and the synthesis
of insoluble glucan by S. mutans ATCC 25175. These results
suggest that emodin may be responsible for the anticariogenic
activity of R. undulatum and P. cuspidatum.
The synthesis of insoluble glucans is one of the
most important virulent properties of S. mutans (24,25).
Insoluble glucans promote the adhesive interaction of bacteria with
the tooth surface and contribute to the formation of dental
biofilms (26). Accordingly, the
current study examined whether emodin may inhibit the synthesis of
insoluble glucans by crude Gtfs. The results revealed that the
formation of insoluble glucans was significantly suppressed by
emodin. These data suggest that emodin may be a novel substance
capable of modulating the activities of these important dental
caries-related factors. S. mutans synthesizes insoluble
glucans from sucrose by the action of Gtfs. There are three types
of Gtfs in S. mutans: B, C and D. Among these, Gtfs B and C
are essential virulence factors of S. mutans (4,27).
Gtf B synthesizes primarily insoluble glucans, whereas Gtf C
synthesizes a mixture of insoluble and soluble glucans. The present
study revealed that Gtf B enzyme activity was reduced by emodin,
suggesting that emodin inhibits the synthesis of insoluble glucans
partly through the suppression of Gtf B activity.
Acid production is an important dental
caries-related factor of S. mutans (28–30).
In dental biofilms, S. mutans metabolizes sugars and
produces organic acids including lactic, propionic, formic and
butyric acids. A concentration of organic acids may demineralize
the tooth surface and thereby induce dental caries (2,3). In
the present study, emodin significantly reduced the level of acid
produced by S. mutans. This inhibitory activity of emodin
may be due to its effect on the bacterial membrane. Emodin has a
high affinity for phospholipid membranes and is able to disrupt the
hydrophobic interactions between hydrocarbon chains in phospholipid
bilayers (31). The inhibitory
effect of emodin on the production of acid by S. mutans may
occur through the disruption of the bacterial cell membrane and,
thus, the inhibition of the expression levels and activities of
specific proteins associated with sugar transport and
metabolism.
The inhibitory effect of emodin on the growth,
insoluble glucan synthesis and acid production of S. mutans
may be beneficial for the prevention of the formation of cariogenic
biofilms in vivo. Therefore, the present study further
examined the anti-caries activity of emodin using a rat model of
dental caries. The topical application of emodin reduced the
incidence and severity of carious lesions in rats without affecting
the percentage of S. mutans in the biofilms. In
smooth-surface caries, emodin effectively reduced the abundance and
severity of the caries, which was similar to that of the positive
control (250 ppm fluoride). However, the inhibitory effect of
emodin on sulcal caries was not as effective as the 250 ppm
fluoride control treatment. These results suggest that the
anti-caries mechanism of emodin may be attributed to multiple
inhibitory effects, including inhibition of the growth, insoluble
glucan and acid production of S. mutans. Furthermore, matrix
metalloproteinases are involved in the pathogenesis of dental
caries (32). Tetracycline-3, an
inhibitor of matrix metalloproteinases, is effective in the
prevention of dental caries (33).
Emodin has been demonstrated to inhibit the activity and expression
of matrix metalloproteinases in vitro and in vivo
(34,35). Thus, the anti-caries activity of
emodin may also be due to its inhibitory effect on matrix
metalloproteinases. Further study is required to investigate this
issue.
In summary, the results of the present study
revealed that emodin significantly attenuated the growth, acid
production and insoluble glucan synthesis of S. mutans in
vitro, and suppressed the development of dental caries in rats.
These results suggest that emodin may be a novel therapeutic agent
for the prevention and control of dental caries.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant No. 81360600) and the Science
and Technology Joint Special Fund of Yunnan Province
(2011FB220).
References
|
1
|
Loesche WJ: Role of Streptococcus
mutans in human dental decay. Microbiol Rev. 50:353–380.
1986.
|
|
2
|
Bowden GH: Microbiology of root surface
caries in humans. J Dent Res. 69:1205–1210. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belli WA and Marquis RE: Adaptation of
Streptococcus mutans and Enterococcus hirae to acid
stress in continuous culture. Appl Environ Microbiol. 57:1134–1138.
1991.
|
|
4
|
Yamashita Y, Bowen WH, Burne RA and
Kuramitsu HK: Role of the Streptococcus mutans gtf genes in
caries induction in the specific-pathogen-free rat model. Infect
Immun. 61:3811–3817. 1993.
|
|
5
|
Bowen WH: Do we need to be concerned about
dental caries in the coming millennium? Crit Rev Oral Biol Med.
13:126–131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Branco-de-Almeida LS, Murata RM, Franco
EM, et al: Effects of 7-epiclusianone on Streptococcus
mutans and caries development in rats. Planta Med. 77:40–45.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Badria FA and Zidan OA: Natural products
for dental caries prevention. J Med Food. 7:381–384. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Z, Miwa M, Nara K, et al: Patch
establishment and development of a clonal plant, Polygonum
cuspidatum, on Mount Fuji. Mol Ecol. 12:1361–1373. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park CS, Lee YC, Kim JD, Kim HM and Kim
CH: Inhibitory effects of Polygonum cuspidatum water extract
(PCWE) and its component resveratrol [correction of rasveratrol] on
acyl-coenzyme A-cholesterol acyltransferase activity for
cholesteryl ester synthesis in HepG2 cells. Vascul Pharmacol.
40:279–284. 2004.
|
|
10
|
Song JH, Kim SK, Chang KW, Han SK, Yi HK
and Jeon JG: In vitro inhibitory effects of Polygonum
cuspidatum on bacterial viability and virulence factors of
Streptococcus mutans and Streptococcus sobrinus. Arch
Oral Biol. 51:1131–1140. 2006. View Article : Google Scholar
|
|
11
|
Ban SH, Kwon YR, Pandit S, Lee YS, Yi HK
and Jeon JG: Effects of a bio-assay guided fraction from
Polygonum cuspidatum root on the viability, acid production
and glucosyltranferase of mutans streptococci. Fitoterapia.
81:30–34. 2010.PubMed/NCBI
|
|
12
|
Chang CH, Lin CC, Yang JJ, Namba T and
Hattori M: Anti-inflammatory effects of emodin from Ventilago
leiocarpa. Am J Chin Med. 24:139–142. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou M, Xu H, Pan L, Wen J, Guo Y and Chen
K: Emodin promotes atherosclerotic plaque stability in fat-fed
apolipoprotein E-deficient mice. Tohoku J Exp Med. 215:61–69. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tzeng TF, Lu HJ, Liou SS, Chang CJ and Liu
IM: Emodin protects against high-fat diet-induced obesity via
regulation of AMP-activated protein kinase pathways in white
adipose tissue. Planta Med. 78:943–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumoto M, Minami T, Sasaki H, Sobue S,
Hamada S and Ooshima T: Inhibitory effects of oolong tea extract on
caries-inducing properties of mutans streptococci. Caries Res.
33:441–445. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi BK, Kim KY, Yoo YJ, Oh SJ, Choi JH
and Kim CY: In vitro antimicrobial activity of a
chitooligosaccharide mixture against Actinobacillus
actinomycetemcomitans and Streptococcus mutans. Int J
Antimicrob Agents. 18:553–557. 2001. View Article : Google Scholar
|
|
17
|
Venkitaraman AR, Vacca-Smith AM, Kopec LK
and Bowen WH: Characterization of glucosyltransferaseB, GtfC, and
GtfD in solution and on the surface of hydroxyapatite. J Dent Res.
74:1695–1701. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Germaine GR, Schachtele CF and Chludzinski
AM: Rapid filter paper assay for the dextransucrase activity from
Streptococcus mutans. J Dent Res. 53:1355–1360. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bowen WH, Madison KM and Pearson SK:
Influence of desalivation in rats on incidence of caries in intact
cagemates. J Dent Res. 67:1316–1318. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koo H, Pearson SK, Scott-Anne K, et al:
Effects of apigenin and tt-farnesol on glucosyltransferase
activity, biofilm viability and caries development in rats. Oral
Microbiol Immunol. 17:337–343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gazzani G, Daglia M and Papetti A: Food
components with anticaries activity. Curr Opin Biotechnol.
23:153–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koo H, Schobel B, Scott-Anne K, et al:
Apigenin and tt-farnesol with fluoride effects on S. mutans
biofilms and dental caries. J Dent Res. 84:1016–1020. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JE, Kim HJ, Pandit S, Chang KW and
Jeon JG: Inhibitory effect of a bioactivity-guided fraction from
Rheum undulatum on the acid production of Streptococcus
mutans biofilms at sub-MIC levels. Fitoterapia. 82:352–356.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hotz P, Guggenheim B and Schmid R:
Carbohydrates in pooled dental plaque. Caries Res. 6:103–121. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schilling KM and Bowen WH: Glucans
synthesized in situ in experimental salivary pellicle
function as specific binding sites for Streptococcus mutans.
Infect Immun. 60:284–295. 1992.
|
|
26
|
Madison KM, Bowen WH, Pearson SK and
Falany JL: Enhancing the virulence of Streptococcus sobrinus
in rats. J Dent Res. 70:38–43. 1991. View Article : Google Scholar
|
|
27
|
Koo H, Xiao J, Klein MI and Jeon JG:
Exopolysaccharides produced by Streptococcus mutans
glucosyltransferases modulate the establishment of microcolonies
within multispecies biofilms. J Bacteriol. 192:3024–3032. 2010.
|
|
28
|
Harper DS and Loesche WJ: Growth and acid
tolerance of human dental plaque bacteria. Arch Oral Biol.
29:843–848. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bender GR, Thibodeau EA and Marquis RE:
Reduction of acidurance of streptococcal growth and glycolysis by
fluoride and gramicidin. J Dent Res. 64:90–95. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuramitsu HK: Virulence factors of mutans
streptococci: role of molecular genetics. Crit Rev Oral Biol Med.
4:159–176. 1993.PubMed/NCBI
|
|
31
|
Alves DS, Pérez-Fons L, Estepa A and Micol
V: Membrane-related effects underlying the biological activity of
the anthraquinones emodin and barbaloin. Biochem Pharmacol.
68:549–561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chaussain-Miller C, Fioretti F, Goldberg M
and Menashi S: The role of matrix metalloproteinases (MMPs) in
human caries. J Dent Res. 85:22–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sulkala M, Wahlgren J, Larmas M, et al:
The effects of MMP inhibitors on human salivary MMP activity and
caries progression in rats. J Dent Res. 80:1545–1549. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee J, Jung E, Lee J, Huh S, et al: Emodin
inhibits TNF α-induced MMP-1 expression through suppression of
activator protein-1 (AP-1). Life Sci. 79:2480–2485. 2006.
|
|
35
|
Wierzchacz C, Su E, Kolander J and
Gebhardt R: Differential inhibition of matrix metalloproteinases-2,
-9, and -13 activities by selected anthraquinones. Planta Med.
75:327–329. 2009. View Article : Google Scholar : PubMed/NCBI
|