Introduction
Acute pancreatitis (AP) is a common disease of the
digestive system, characterized by acute, severe symptoms, a
variety of complications and a high rate of mortality (1,2).
Paralytic intestinal obstruction is an early clinical symptom of
AP, occurring in >80% of cases (3). The pathogenesis of pancreatitis is
considered to be associated with the inflammatory response and an
abnormal immune reaction (4,5),
which are caused by the activation of pancreatic enzymes,
fermentemia and numerous cytokines and inflammatory mediators
(6). The intestinal hypomotility
caused by the pathogenesis of pancreatitis may therefore lead to AP
(7). In the course of AP, the
intestinal mucosa barrier is damaged by the translocation of
intestinal bacteria and endotoxins (8–10).
AP aggravates the inflammatory reaction, interferes with the nerve
function of the gastrointestinal tract, compromises blood
circulation in the intestines and damages the intestinal mucosa.
This damage may result in an intestinal motility disorder,
flatulence, bowel dilatation and abdominal pressure, which may in
turn affect the gastrointestinal blood supply and increase
microcirculation (11,12). In addition, the large volume of
liquid exudation, damage to the intestinal mucosal barrier,
bacterial translocation and the absorption of toxins may aggravate
shock and systemic infection, resulting in mortality (13,14).
There is a close association between AP and infective pancreatic
necrosis (15,16).
However, the etiology of AP is complicated and the
appropriate therapy for the disease remains contested (2,17).
The application of acupuncture, when combined with Western
medicine, has been considered an effective therapy for AP (18). The majority of treatments for AP
have evident side effects (19),
which limit their use in the treatment of AP. Integrated
traditional Chinese medicine (ITCM) has been practiced for over
1,000 years and is widely used as an external treatment for AP,
with few side effects (20–22).
The present study reports a novel ITCM treatment that is an
effective therapy for AP. However, the mechanism underlying the
effect of ITCM in the treatment of AP remains unknown. The protein,
serine 1 (PRSS1) gene, which expresses cationic trypsinogen, is
associated with hereditary pancreatitis and characterized by acute
inflammation of the pancreas (23). SPINK1, which codes for serine
protease inhibitor Kazal-type 1, is an additional gene regarded as
a disease modifier of AP (24,25).
Thus, AP has been shown to be associated with the expression of
PRSS1 and serine peptidase inhibitor, Kazal type 1 (SPINK1)
(24,26,27).
The aim of the present study was to investigate whether ITCM may
ameliorate AP by regulating the expression of PRSS1 and SPINK1.
Materials and methods
Participants
The study protocols on human subjects and consent
documents were approved by the Human Research Ethics Committee of
Yantai Hospital of Traditional Chinese Medicine (Yantai, China). In
addition, written consent documents were obtained and the data were
collected with a Certificate of Confidentiality protecting patient
privacy. A total of 100 AP patients were recruited from the Yantai
Hospital of Traditional Chinese Medicine between March 2009 and
March 2014 in order to rule our or prevent any genetic differences.
All patients were unrelated, ethnic Han Chinese with similar
cultural and economic backgrounds.
AP patients were divided at random into two groups
(Table I). In total, 100 patients
(age range, 40–55 years) were examined who were reported to be
suffering with AP, but had no symptoms of other intestinal or
inflammatory diseases. All eligible cases had their diagnoses
confirmed by histological examination. The course of the disease
ranged between 3 h and three days. Of the 100 cases, 48 were
biliary in origin, 40 cases were due to overeating and overdrinking
and 12 cases were the result of unidentified causes. In 94 cases,
the patient was suffering from AP for the first time, while the
remaining six cases were recurrent.
 | Table IPatient characteristics prior to ITCM
treatment. |
Table I
Patient characteristics prior to ITCM
treatment.
| A, Male
patients |
|---|
|
|---|
|
Characteristics | Experiment
group | Control group | P-value |
|---|
| Cases (n) | 32 | 32 | >0.05a |
| Age (years) | 50.5±4.9 | 50.8±4.8 | >0.05b |
| BMI
(kg/m2) | 25.7±6.1 | 26.0±6.7 | >0.05b |
| Daily calorie
intake (kcal) | 2255±377 | 2188±409 | >0.05b |
| AP classification
(n) |
| Mild | 18 | 17 | >0.05a |
| Moderate | 8 | 8 | >0.05a |
| Severe | 6 | 7 | >0.05a |
|
| B, Female
patients | | | |
|
|
Characteristics | Experiment
group | Control group | P-value |
|
| Cases (n) | 18 | 18 | >0.05a |
| Age (years) | 46.9±6.7 | 48.1±6.9 | >0.05b |
| BMI
(kg/m2) | 25.1±5.8 | 25.7±5.5 | >0.05b |
| Daily calorie
intake (kcal) | 2033±249 | 2099±276 | >0.05b |
| AP classification
(n) |
| Mild | 8 | 8 | >0.05a |
| Moderate | 5 | 5 | >0.05a |
| Severe | 5 | 5 | >0.05a |
Diagnostic criteria and disease
classification
The diagnostic criteria for AP patients were in
accordance with a previous study (28), and were based upon the elevated
levels of pancreatic enzymes in the blood and urine. According to
the diagnostic criteria, patients presenting with the following
symptoms were considered to suffer from AP: Upper abdominal pain;
enhanced levels of lipase, examined using a Human Pancreatic Lipase
ELISA kit (Wuhan Jiacheng Biotechnology Co., Ltd., Wuhan, China);
ultrasonography (US) scans (GE LOCIQ 400; GE Healthcare, Milwaukee,
WI, USA) demonstrating dilation of the common bile duct or swelling
of the pancreas. Clinical manifestations of AP included sudden and
sharp cutting pains in upper abdomen, nausea and vomiting, fever,
several days without defecation, abdominal distension, dry and
bitter taste in the mouth, coated and dry or burned black tongue
and forceful sphygmus. All other abdominal diseases associated with
pancreatitis were ruled out. In addition, peripheral blood tests
revealed increases in the number of peripheral white blood cells,
neutrophils and serum amylase, while an increase in the level of
urine amylase was also observed. Diffuse enlargement of the
pancreas was observed in B-mode US and computed tomography (CT)
examinations. The bioactivities of serum and urine amylase were
analyzed in accordance with procedures described in previous
studies (29,30). Blood lipase levels were also
measured, since lipase is a more sensitive and specific indicator
of AP compared with other pancreatic enzymes. CT scanning is the
preferred method for confirming the image findings of AP.
AP can be classified as mild, moderate or severe,
according to the disease severity (31). Mild AP patients exhibit no organ
failure, nor any local or systemic complications. Moderate AP is
characterized by the presence of transient organ failure, while
severe AP patients exhibit persistent organ failure for >48
h.
Therapeutic methods
Experimental group patients were externally
administered with an ITCM preparation. The ITCM preparation was
composed and prepared in accordance with previous studies (32,33)
as follows: 30 g Chinese rhubarb, 15 g Citrus aurantium, 15
g magnolia bark, 10 g mirabilite, 10 g Pinellia ternata, 10
g Coptis chinensis, 15 g Scutellaria baicalensis, 10
g Gardenia, 15 g Radix Paeoniae Alba, 15 g tree peony bark,
15 g Radix Paeoniae Rubra, 15 g Rhizoma Corydalis, 10 g
Bupleurum and 10 g licorice. The materials were ground to a
powder using a high-pressure homogenizer (Jiangsu Makwell Machinery
Co., Ltd., Huai’an, China). These powders were dissolved in 500 ml
water with 200 g starch, and the mixture was concentrated and dried
to form pastes of ~5×5×1 cm. The pastes were applied to the
acupuncture points (18,34), Yishu, Zhongwan, Tianshu, Zusanli,
Guan Yuan and Pishu, twice daily. The dose of rhubarb and
mirabilite was adjusted according to the severity of the patients’
abdominal distention and pain. In addition, the composition of the
powder was modified according to patient symptoms, tongue coating
and pulse. The control group patients received acupuncture point
application with a placebo treatment, containing a mixture of
cornmeal, starch and flour.
Curative standard
A curative standard was defined in order to
categorize the efficacy of the ITCM treatment. The standard was
defined as the regression of symptoms, including abdominal pain and
distension, fever, vomiting and passing of feces or wind.
Furthermore, improvements to the patient abdominal muscle tension,
abdominal tenderness, rebound tenderness and bowel sound were
considered to indicate successful therapy. In addition, blood and
urine amylase levels, and routine blood and liver function tests,
were returned to normal, and the extent of pancreatic edema had
improved when compared with the previous abdominal B-mode US and CT
examinations. The following categories were used for defining the
therapeutic results: Excellent, reaching the aforementioned
standard in four days; effective, reaching the aforementioned
standard in seven days; inefficient, failing to reach the standard
after eight days.
Sample isolation
Sinker-assisted endoscopic submucosal dissection
(ESD) is an effective technique for the removal of minus
superficial pancreatic tissues (35); thus, this technique is the
preferred approach for obtaining surgical specimens from AP
patients. Ethical issues were considered to be very important. It
was a priority to ensure that patient participation was entirely
voluntary and that patient privacy was maintained. Experts
conducting the study were not to attempt to persuade participants
to donate any tissues if the participant was at all in doubt
regarding the safety or ethics of the study.
Potential mediating factors
A number of confounding factors have been reported
to be associated with an increased risk of AP (36–40).
A previous meta-analysis indicated that obesity was associated with
a higher risk of AP (41);
therefore, the body mass index (BMI) of the patients was measured
in the present study. Gender has also been regarded as an important
determinant of outcome in AP patients (42); thus, the data for the relative
female and male reactions to AP treatment were also surveyed.
Alcohol consumption can increase the risk of AP (43,44);
therefore, all the subjects were non-drinkers, having never
consumed alcohol, in order to avoid interference. To avoid
age-related issues (45,46), all individuals were aged between 40
and 55 years-old. All volunteers reported a food consumption
frequency of three meals per day, and the food consumption was
consistent to a healthy Nordic food index (47,48).
Calorie intake was calculated according to self-reported assessment
of daily calorie intake (49).
ELISA
The concentration of lipase was examined using an
ELISA, with lipase antibodies purchased from Wuhan Jiacheng
Biotechnology Co., Ltd. (Wuhan, China). Biopsy specimens were
ground using liquid nitrogen. The grounded specimens were diluted
by 1/200 in phosphate-buffered saline (PBS) and transferred to
antibody-coated wells. Following the manufacturer’s instructions,
all wells were washed three times with PBS, and IgG (Immuno
Pure® peroxidase-conjugated goat anti-human IgG; 21348;
Beijing Jiamay Biotechnology Ltd., Beijing, China) was added and
incubated for 30 min. After washing, 3,3′,5,5′-tetramethylbenzidine
(54827-17-7; Robiot Co., Ltd., Nanjing, China) was added and
cultured for 10 min for visualization. Finally, the absorbance of
the mixture was examined at 450 nm using a SM600 ELISA plate reader
(Shanghai Utrao Medical Instrument Co., Ltd., Shanghai, China). The
concentration of lipase was calculated according to a standard
calibrator curve.
Quantitative reverse transcription
polymerase chain reaction (qRT-PCR)
The mRNA expression levels of PRSS1 and SPINK1 were
assessed using qRT-PCR. RNA was extracted from the AP tissues using
a GenElute™ Mammalian Total RNA Miniprep kit (RTN10; Sigma-Aldrich
Trading Co., Ltd., Shanghai, China). Initial cDNA was amplified
from RNA using a random primer. The cDNA molecules were used as a
template for qRT-PCR of the PRSS1 and SPINK1 transcripts on a rapid
qTOWER 2.0 (Analytik Jena AG, Thuringia, Germany). The primers for
qRT-PCR were as follows: PRSS1 (GenBank no. BC103998.2) sense,
5′-AGGGGAATGAGCAGTTCATC-3′ and antisense,
5′-CACCAGAACTCAGAGTGTTG-3′; SPINK1 (GenBank no. NM003122.3), sense,
5′-GAAGAGACGTGG TAAGTGCG-3′ and antisense, 5′-CCATCAGTCCCACAG
ACAGGG-3′; GAPDH sense, 5′-CCCTTCATTGACCTCAAC TAC-3′ and antisense,
5′-CCACCTTCTTGATGTCATCAT-3′. GAPDH was used as an internal control.
All genes were amplified by 30 cycles of heating for 30 sec at
94°C, followed by 1 min at 60°C. The quality of the synthesized
cDNA was determined using GAPDH as the reference gene.
Protein expression levels of PRSS1 and
SPINK1
Protein expression levels of PRSS1 and SPINK1 were
determined by western blot analysis. The protein was isolated from
selected AP tissues and separated using 12% SDS-PAGE. The separated
protein was electrotransferred onto polyvinylidene fluoride
membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA), which
were blocked with 5% non-fat dry milk for 1 h, and then incubated
with a primary antibody: Mouse anti-human PRSS1 monoclonal antibody
(MAB3848; 1:5,000; R&D Systems China, Shanghai, China) and
mouse anti-human SPINK1 monoclonal antibody (70R-5308; 1:2,000;
Beijing Dakewei Bio-Technology Co, Ltd., Beijing, China). A
secondary antibody (anti-mouse horseradish peroxidase-conjugated;
cat. no. 201201; 1:10,000; Shanghai Guoyuan Biotechnology Co.,
Ltd., Shanghai, China) was added and chemiluminescence detection
performed using an kit from GE Healthcare (Piscataway, NJ,
USA).
Statistical analysis
The t-test and the χ2 test were used to
detect the statistical significance for the variables between the
experimental and control groups. Data were analyzed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA), and P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of AP patients
A total of 100 AP patients (64 male, 50.8±4.2 years;
36 female, 47.5±7.5 years) were recruited. Considering the
complicated causative factors for increasing the risk of AP, the
gender, age, BMI and daily calorie intake of the patients were not
contributing factors (Table I;
P>0.05). All these factors were excluded between the
experimental and control groups during the data collection. The
number of cases of each stage of AP were similar between the
experimental and control groups (Table
I).
Effects of ITCM on AP patients
Effects of ITCM on the health outcomes of AP
patients were investigated, as shown in Table I. All AP patients were diagnosed by
gastroenterologists. Following eight days of treatment with ITCM,
29 cases (26 mild, two modest and one severe case) exhibited
excellent results, 17 cases (11 modest and six severe) showed an
effective response and four cases (severe) exhibited inefficient
results. In the control group, one patient exhibited excellent
results, two cases showed an effective outcome, while all the other
cases resulted in an inefficient outcome. The excellent and
effective outcomes in the control group were hypothesized to be the
result of natural recovery since the onset of AP. The results
demonstrated that ITCM showed strong therapeutic results for AP. In
addition, all the symptoms of AP in the experimental group patients
were improved significantly when compared with those in the control
group (P<0.05; Table II). The
results suggested that ITCM was able to ameliorate AP; thus, ITCM
should be considered as a potential drug candidate for the
treatment of AP.
 | Table IIComparison of clinical
characteristics for AP patients prior to and following ITCM
treatment. |
Table II
Comparison of clinical
characteristics for AP patients prior to and following ITCM
treatment.
| Before
treatment | After
treatment |
|---|
|
|
|
|---|
| Clinical
characteristic | Experiment
group | Control group | P-value | Experiment
group | Control group | P-value |
|---|
| Upper abdominal
pain (n) | 50 | 50 | >0.05a | 4 | 47 | <0.05a |
| Findings of
ultrasonography (n) | 50 | 50 | >0.05a | 10 | 48 | <0.05a |
| Sudden and sharp
cutting pains in upper abdomen (n) | 50 | 50 | >0.05a | 8 | 47 | <0.05a |
| Abdominal
distension (n) | 50 | 50 | >0.05a | 6 | 49 | <0.05a |
| Dry and bitter
taste (n) | 50 | 50 | >0.05a | 3 | 46 | <0.05a |
| Coated and dry
tongue or burned black tongue (n) | 50 | 50 | >0.05a | 5 | 48 | <0.05a |
| Forceful sphygmus
(n) | 50 | 50 | >0.05a | 5 | 47 | <0.05a |
| Diffuse enlargement
of the pancreas (n) | 50 | 50 | >0.05a | 8 | 48 | <0.05a |
| White blood cells
(cells/ml) |
1.51×105 |
1.53×105 | >0.05b |
8.51×104 |
1.50×105 | <0.05b |
|
±42×104 |
±41×104 | |
±3.58×104 |
±40×104 | |
| Neutrophils
(%) | 68.2±5.2 | 68.8±5.5 | >0.05b | 55.7±4.7 | 67.3±5.8 | <0.05b |
| Lipase (pg/ml) | 326±76 | 318±81 | >0.05b | 207±57 | 320±71 | <0.05b |
| Serum amylase
(IU/l) | 965±307 | 952±297 | >0.05b | 709±286 | 943±255 | <0.05b |
| Urine amylase
(IU/l) | 1876±325 | 1866±298 | >0.05b | 1054±233 | 1834±311 | <0.05b |
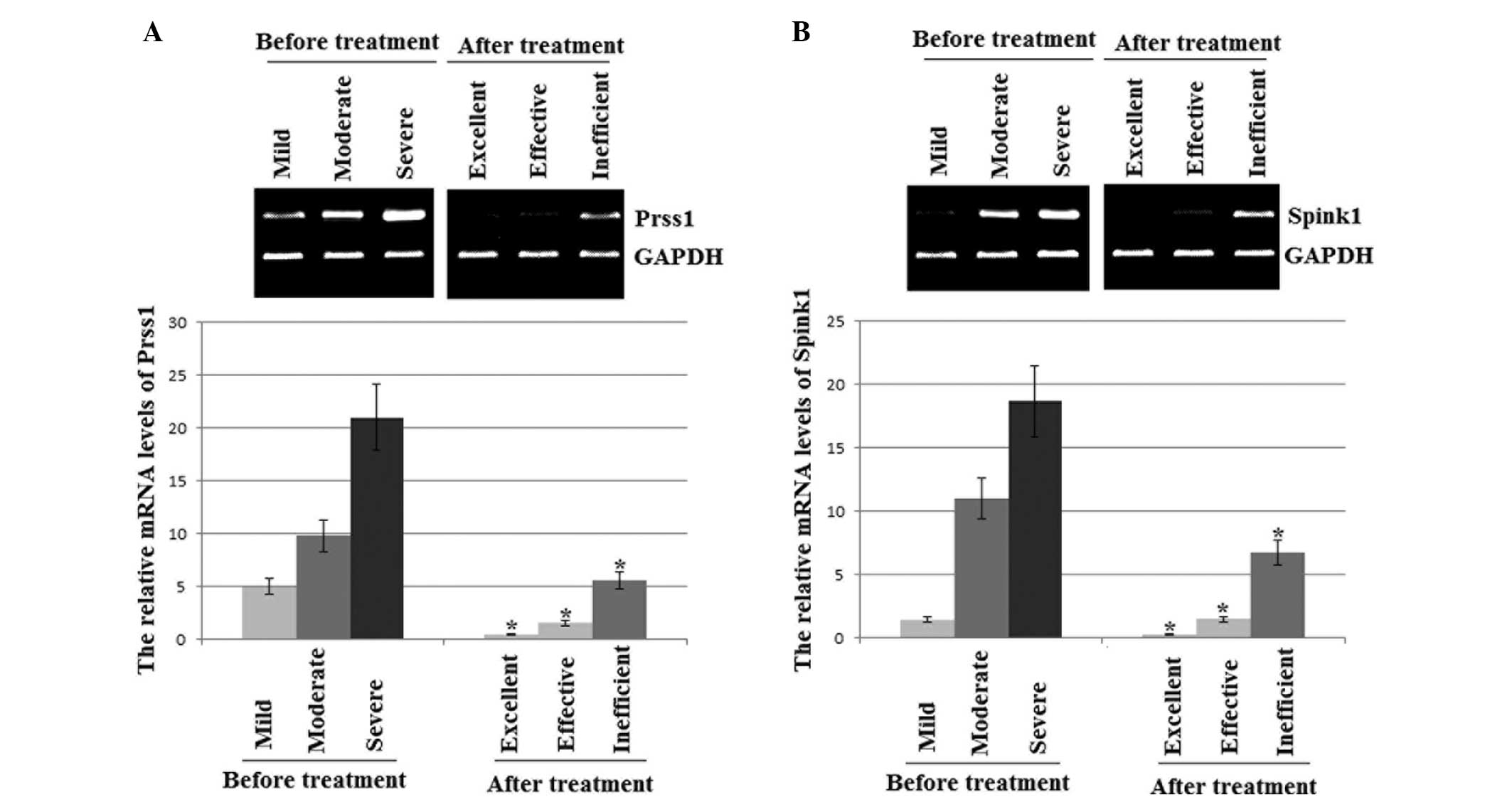
mRNA expression levels of PRSS1 and
SPINK1 in AP patients
The qRT-PCR results revealed that the mRNA
expression levels of PRSS1 and SPINK1 were lower in the AP patients
treated with ITCM than those prior to treatment. The levels of
PRSS1 and SPINK1 were lowest in the excellent outcome experimental
group patients, moderate in the effective result patients and
highest in the control and inefficient result patients (Fig. 1).
Protein expression levels of PRSS1 and
SPINK1 in AP patients
Levels of PRSS1 and SPINK1 proteins are known to be
closely associated with the development of AP. The protein
expression levels of PRSS1 and SPINK1 were at their highest prior
to treatment with ITCM (Fig. 2).
Following treatment with ITCM, the protein expression levels of
PRSS1 and SPINK1 were lowest in the excellent outcome patients,
moderate in the effective outcome patients and highest in the
control and inefficient result patients (Fig. 2). Thus, the results were consistent
with a previous study (50), which
reported that PRSS1 and SPINK1 were potential negative biomarkers
for the diagnosis and prognosis of AP.
Discussion
AP is the most common intestinal disease worldwide,
with a mortality rate as high as 36–50% for severe AP (20). Severe AP can cause serious
inflammation in other systems and patients may succumb suddenly to
the disease (14,51). Significant progress has been
reported in the treatment of AP by ITCM (21,52,53);
however, there remain a number of difficulties that hinder the
improvement of therapeutic efficacy. TCM methods are commonly used
in an integrative manner; thus, the present study aimed to assess
the efficacy of an ITCM approach in the therapy of AP.
However, the molecular mechanisms underlying the
pathogenesis of AP are not yet known, and a suitable biomarker for
AP must be identified in order to further investigate these
mechanisms. In certain cases, the levels of a number of important
proteins are undetectable in the serum, which may be affected by
the instability of human clinical and metabolic conditions. Thus,
only newly obtained AP specimens were considered for examination in
the present study.
Mutations in the human PRSS1 gene are associated
with pancreatitis and have provided insight into the pathogenesis
of the disease (54). PRSS1 is
widely reported to be associated with AP (27,55,56).
In addition, SPINK1 was originally identified as a trypsin
inhibitor and is strongly elevated in patients with pancreatitis,
where the level of elevation correlates with the disease severity
(57). This association between
SPINK1 and AP has also been widely reported (24,26,58–63).
Thus, the present study aimed to investigate whether the expression
levels of PRSS1 and SPINK1 were closely associated with the
development of AP.
The sinker-assisted ESD approach to sampling AP
patient tissues facilitated the detection of pancreatitis by
examination of mRNA levels. The mRNA expression levels were
examined initially, following which the protein levels were
determined. The mRNA and protein expression levels of PRSS1 and
SPINK1 demonstrated the same changed trend with the development of
AP (Fig. 1). Therefore, PRSS1 and
SPINK1 are potential combined adjuvant biomarkers for investigating
the mechanisms underlying the increased risk of AP, and PRSS1 and
SPINK1 should be considered as targets for drug therapy.
Furthermore, the protein expression levels of PRSS1 and SPINK1 were
lower on average in the experimental group when compared with the
control group (Fig. 2). Therefore,
the development of AP can be characterized by the levels of
predominant PRSS1 and SPINK1. Since the expression levels of PRSS1
and SPINK1 were higher in the AP patients of the control group, the
changing levels of these biomarkers may be the result of
inflammatory processes. The levels of PRSS1 and SPINK1 in the AP
patients treated with placebos were comparable to the levels prior
to treatment (data not shown).
The application of Chinese herbs to acupuncture
points is a type of external TCM. The treatment involves processing
the herbs into paste, powder or ointment and subsequently applying
them to corresponding acupuncture points (18,34).
The interactions among the drugs, main and collateral channels and
acupuncture points are involved in the treatment of AP. Medicine is
absorbed through the skin, but not the digestive system; thus, the
procedure is simple and safe to conduct, with no risk of an adverse
reaction. ITCM has been clinically observed to ameliorate a variety
of symptoms and conditions, including pain, abdominal distension,
constipation, inflammation, endotoxin absorption, enterogenic
infection and intestine failure. In addition, ITCM has been shown
to improve the levels of amylase in the blood and urine, improve
pancreatic blood circulation, promote the absorption of necrotic
tissue and gastrointestinal peristalsis and decrease a variety of
complications, improving the overall prognosis (64).
A number of important questions should be considered
in future studies investigating the effects of ITCM in the
treatment of AP. Firstly, the expression levels of PRSS1 and SPINK1
should be assessed in healthy control subjects to better understand
the mechanism underlying the therapeutic effects of ITCM. However,
it is difficult to recruit appropriate volunteers as the majority
of healthy subjects are wary of the potential side effects of
surgery based on sinker-assisted ESD techniques. Secondly, the
classification system for the various stages of AP requires
improvement via the design of a more precise scale.
In conclusion, the present study examined 100 AP
patients in order to investigate the effects of ITCM therapy on AP.
The results revealed that the development of AP was positively
associated with the expression levels of the biomarkers, PRSS1 and
SPINK1. Thus, PRSS1 and SPINK1 may be useful combined targets for
the treatment of AP. ITCM produced significant therapeutic results
for AP when compared with the placebo-treated control group, and
should be considered as a potential drug to be developed for the
treatment of AP. There are, however, limitations to the present
study, and the underlying mechanisms of AP should be investigated
further in future research.
References
|
1
|
Matta A, Tandra PK, Cichowski E and
Reddymasu SC: Acute necrotising pancreatitis: a late and fatal
complication of pancreaticoduodenal arterial embolisation. BMJ Case
Rep. bcr20142041972014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rana SS, Sharma V, Sharma R and Bhasin DK:
An unusual complication of acute necrotising pancreatitis detected
by endoscopic ultrasound. JOP. 15:276–277. 2014.PubMed/NCBI
|
|
3
|
Ambiru S, Furuyama N, Aono M, et al:
Hyperbaric oxygen therapy for the treatment of postoperative
paralytic ileus and adhesive intestinal obstruction associated with
abdominal surgery: experience with 626 patients.
Hepatogastroenterology. 54:1925–1929. 2007.
|
|
4
|
Sheikh I, Fontenot E, Waghray N, et al:
The role of nonsteroidal anti-inflammatory drugs in the prevention
of post endoscopic retrograde cholangiopancreatography
pancreatitis. JOP. 15:219–224. 2014.PubMed/NCBI
|
|
5
|
Sit M, Aktas G, Yilmaz EE, et al: Effects
of the inflammatory response on serum omentin levels in early acute
and chronic pancreatitis. Clin Ter. 165:e148–e152. 2014.PubMed/NCBI
|
|
6
|
Shalimov SA, Popov ON, Dubitskiĭ AE and
Lifshits IuZ: Direct thermometry of the pancreas in the
postoperative period. Vestn Khir Im I I Grek. 132:36–39. 1984.(In
Russian). PubMed/NCBI
|
|
7
|
Mascolo N, Izzo AA, Ligresti A, et al: The
endocannabinoid system and the molecular basis of paralytic ileus
in mice. FASEB J. 16:1973–1975. 2002.PubMed/NCBI
|
|
8
|
Elder AS, Saccone GT, Bersten AD and Dixon
DL: Evaluation of lung injury and respiratory mechanics in a rat
model of acute pancreatitis complicated with endotoxin.
Pancreatology. 12:240–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujita T: Endotoxin as a trigger of
alcoholic pancreatitis. Gastroenterology. 134:640–641. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verma R, Dhamija R, Ross SC, Batts DH and
Loehrke ME: Symbiotic bacteria induced necrotizing pancreatitis.
JOP. 11:474–476. 2010.PubMed/NCBI
|
|
11
|
Zhang X, Tian H, Wu C, et al: Effect of
baicalin on inflammatory mediator levels and microcirculation
disturbance in rats with severe acute pancreatitis. Pancreas.
38:732–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cuthbertson CM and Christophi C:
Disturbances of the microcirculation in acute pancreatitis. Br J
Surg. 93:518–530. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang XN, Guo J, Lin ZQ, et al: The study
on causes of death in fulminant pancreatitis at early stage and
late stage. Sichuan Da Xue Xue Bao Yi Xue Ban. 42:686–690. 2011.(In
Chinese). PubMed/NCBI
|
|
14
|
Yuan Z, Meyerholz DK, Twait EC, et al:
Systemic inflammation with multiorgan dysfunction is the cause of
death in murine ligation-induced acute pancreatitis. J Gastrointest
Surg. 15:1670–1678. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuji Y, Takahashi N, Fletcher JG, et al:
Subtraction color map of contrast-enhanced and unenhanced CT for
the prediction of pancreatic necrosis in early stage of acute
pancreatitis. AJR Am J Roentgenol. 202:W349–W356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garip G, Sarandöl E and Kaya E: Effects of
disease severity and necrosis on pancreatic dysfunction after acute
pancreatitis. World J Gastroenterol. 19:8065–8070. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Popa D: Treatment in severe acute
pancreatitis - still a reason of debate. J Med Life. 6:486–490.
2013.
|
|
18
|
Ge HY and Chen B: Clinical observation of
acute pancreatitis treated with acupoint application combined with
medicine. Zhongguo Zhen Jiu. 32:602–604. 2012.(In Chinese).
PubMed/NCBI
|
|
19
|
Bossi A, Romeo G and Pezzoli A:
Side-effects, structure, and H2-receptor antagonists. Lancet.
339:13661992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia Q and Deng LH: Hot issues on the
treatment of severe acute pancreatitis by integrated traditional
Chinese and Western medicine. Sichuan Da Xue Xue Bao Yi Xue Ban.
44:962–965. 2013.(In Chinese).
|
|
21
|
Wan MH, Yao J, Li J, et al: The
effectiveness of purgation and electroacupuncture in extrahepatic
bile duct stone complicated with acute biliary pancreatitis:
management of biliary stone pancreatitis through traditional
Chinese medicine. Pancreas. 40:483–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tang Y, Liao Y, Kawaguchi-Sakita N, et al:
Sinisan, a traditional Chinese medicine, attenuates experimental
chronic pancreatitis induced by trinitrobenzene sulfonic acid in
rats. J Hepatobiliary Pancreat Sci. 18:551–558. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Solomon S, Whitcomb DC and LaRusch J:
PRSS1-related hereditary pancreatitis. GeneReviews®.
Pagon RA, Adam MP, Ardinger HH, et al: University of Washington;
Seattle, WA: 2012
|
|
24
|
Sánchez-Ramírez CA, Flores-Martínez SE,
García-Zapién AG, et al: Screening of R122H and N29I mutations in
the PRSS1 gene and N34S mutation in the SPINK1 gene in Mexican
pediatric patients with acute and recurrent pancreatitis. Pancreas.
41:707–711. 2012.PubMed/NCBI
|
|
25
|
Tukiainen E, Kylänpää ML, Kemppainen E, et
al: Pancreatic secretory trypsin inhibitor (SPINK1) gene mutations
in patients with acute pancreatitis. Pancreas. 30:239–242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee YJ, Kim KM, Choi JH, et al: High
incidence of PRSS1 and SPINK1 mutations in Korean children with
acute recurrent and chronic pancreatitis. J Pediatr Gastroenterol
Nutr. 52:478–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sobczyńska-Tomaszewska A, Bak D, Oralewska
B, et al: Analysis of CFTR, SPINK1, PRSS1 and AAT mutations in
children with acute or chronic pancreatitis. J Pediatr
Gastroenterol Nutr. 43:299–306. 2006. View Article : Google Scholar
|
|
28
|
Kiriyama S, Gabata T, Takada T, et al: New
diagnostic criteria of acute pancreatitis. J Hepatobiliary Pancreat
Sci. 17:24–36. 2010. View Article : Google Scholar
|
|
29
|
Gubergrits N, Golubova O, Lukashevich G
and Fomenko P: Elevated serum amylase in patients with chronic
pancreatitis: acute attack or macroamylasemia? Pancreatology.
14:114–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wojtuń S and Gil J: Utility of amylase
serum and urine activity in acute biliary pancreatitis treated with
biliary sphincterotomy. Pol Merkur Lekarski. 22:381–384. 2007.(In
Polish).
|
|
31
|
Banks PA, Bollen TL, Dervenis C, et al;
Acute Pancreatitis Classification Working Group. Classification of
acute pancreatitis - 2012: revision of the Atlanta classification
and definitions by international consensus. Gut. 62:102–111. 2013.
View Article : Google Scholar
|
|
32
|
Li YC, Dong L, Jia A, Chang XM and Xue H:
Preparation of solid lipid nanoparticles loaded with traditional
Chinese medicine by high-pressure homogenization. Nan Fang Yi Ke Da
Xue Xue Bao. 26:541–544. 2006.(In Chinese). PubMed/NCBI
|
|
33
|
Zhuang WJ and Qian C: Treatment of
infected deciduous root canal with Samyan (traditional Chinese
medicine) paste: Clinical analysis. Shanghai Kou Qiang Yi Xue.
6:1851997.(In Chinese).
|
|
34
|
Xue QM, Li N, Xue P, Wang CW and Wen Q:
Therapeutic effects of electroacupuncture at ST36 acupoint on
sodium-taurocholate-induced severe acute pancreatitis. Chin J
Integr Med. 20:695–700. 2014. View Article : Google Scholar
|
|
35
|
Saito Y, Emura F, Matsuda T, et al: A new
sinker-assisted endoscopic submucosal dissection for colorectal
cancer. Gastrointest Endosc. 62:297–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao J, Liao Q, Zhao Y and Hu Y: Mortality
indicators and risk factors for intra-abdominal hypertension in
severe acute pancreatitis. Int Surg. 99:252–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vipperla K, Papachristou GI, Easler J, et
al: Risk of and factors associated with readmission after a
sentinel attack of acute pancreatitis. Clin Gastroenterol Hepatol.
May 9–2014.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zeng YB, Zhan XB, Guo XR, et al: Risk
factors for pancreatic infection in patients with severe acute
pancreatitis: an analysis of 163 cases. J Dig Dis. 15:377–385.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin J, Yu YH, Zhong M and Zhang GW:
Analyzing and identifying risk factors for acute pancreatitis with
different etiologies in pregnancy. J Matern Fetal Neonatal Med.
June 5;1–5. 2014.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tamasaki A, Nishimura Y, Kondo N, et al:
Risk factors for acute pancreatitis in patients with severe motor
and intellectual disabilities. Pediatr Int. 56:240–243. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hong S, Qiwen B, Ying J, Wei A and
Chaoyang T: Body mass index and the risk and prognosis of acute
pancreatitis: a meta-analysis. Eur J Gastroenterol Hepatol.
23:1136–1143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shen HN, Wang WC, Lu CL and Li CY: Effects
of gender on severity, management and outcome in acute biliary
pancreatitis. PLoS One. 8:e575042013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fu P, Yuan AH, Wang CH, Li X and Wu HY:
Alcohol-induced severe acute pancreatitis followed by hemolytic
uremic syndrome managed with continuous renal replacement therapy.
BMC Nephrol. 15:12014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nikkola J, Räty S, Laukkarinen J, et al:
Abstinence after first acute alcohol-associated pancreatitis
protects against recurrent pancreatitis and minimizes the risk of
pancreatic dysfunction. Alcohol Alcohol. 48:483–486. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Müller S, Kaiser H, Krüger B, et al:
Age-dependent effects of UCP2 deficiency on experimental acute
pancreatitis in mice. PLoS One. 9:e944942014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Okamura D, Starr ME, Lee EY, et al:
Age-dependent vulnerability to experimental acute pancreatitis is
associated with increased systemic inflammation and thrombosis.
Aging Cell. 11:760–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Akesson A, Andersen LF, Kristjánsdóttir
AG, et al: Health effects associated with foods characteristic of
the Nordic diet: a systematic literature review. Food Nutr Res.
572013.
|
|
48
|
Overby NC, Sonestedt E, Laaksonen DE and
Birgisdottir BE: Dietary fiber and the glycemic index: a background
paper for the Nordic Nutrition Recommendations 2012. Food Nutr Res.
57:2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Knaan T, Endevelt R and Singer P:
Evaluating the validity of the ‘categories method’: A new method
for self-report assessment of daily calorie intake: A pilot study.
Can J Diabetes. 37(Suppl 2): S2832013. View Article : Google Scholar
|
|
50
|
Pelaez-Luna M, Robles-Diaz G,
Canizales-Quinteros S and Tusie-Luna MT: PRSS1 and SPINK1 mutations
in idiopathic chronic and recurrent acute pancreatitis. World J
Gastroenterol. 20:11788–11792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roncati L, Gualandri G, Fortuni G and
Barbolini G: Sudden death and lipomatous infiltration of the heart
involved by fat necrosis resulting from acute pancreatitis.
Forensic Sci Int. 217:e19–e22. 2012. View Article : Google Scholar
|
|
52
|
Chen H, Li F, Jia JG, et al: Effects of
traditional Chinese medicine on intestinal mucosal permeability in
early phase of severe acute pancreatitis. Chin Med J (Engl).
123:1537–1542. 2010.
|
|
53
|
Zhang MJ, Zhang GL, Yuan WB, Ni J and
Huang LF: Treatment of abdominal compartment syndrome in severe
acute pancreatitis patients with traditional Chinese medicine.
World J Gastroenterol. 14:3574–3578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Athwal T, Huang W, Mukherjee R, et al:
Expression of human cationic trypsinogen (PRSS1) in murine acinar
cells promotes pancreatitis and apoptotic cell death. Cell Death
Dis. 5:e11652014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang J, Ohmuraya M, Suyama K, et al:
Relationship of strain-dependent susceptibility to experimentally
induced acute pancreatitis with regulation of Prss1 and Spink3
expression. Lab Invest. 90:654–664. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Corleto VD, Gambardella S, Gullotta F, et
al: New PRSS1 and common CFTR mutations in a child with acute
recurrent pancreatitis, could be considered an ‘Hereditary’ form of
pancreatitis? BMC Gastroenterol. 10:1192010. View Article : Google Scholar
|
|
57
|
Ohmuraya M and Yamamura K: Roles of serine
protease inhibitor Kazal type 1 (SPINK1) in pancreatic diseases.
Exp Anim. 60:433–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rai P, Sharma A, Gupta A and Aggarwal R:
Frequency of SPINK1 N34S mutation in acute and recurrent acute
pancreatitis. J Hepatobiliary Pancreat Sci. 21:663–668. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tremblay K, Dubois-Bouchard C, Brisson D
and Gaudet D: Association of CTRC and SPINK1 gene variants with
recurrent hospitalizations for pancreatitis or acute abdominal pain
in lipoprotein lipase deficiency. Front Genet. 5:902014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Terlizzi V, De Gregorio F, Sepe A, et al:
Brand new SPINK1 and CFTR mutations in a child with acute recurrent
pancreatitis: a case report. Minerva Pediatr. 65:669–672.
2013.PubMed/NCBI
|
|
61
|
Jøergensen MT, Brusgaard K, Novovic S, et
al: Is the SPINK1 variant p. N34S overrepresented in patients with
acute pancreatitis? Eur J Gastroenterol Hepatol. 24:309–315. 2012.
View Article : Google Scholar
|
|
62
|
Baudry C, Rebours V, Houillier P, et al:
Recurrent acute pancreatitis caused by association of a novel
mutation of the calcium-sensing receptor gene and a heterozygous
mutation of the SPINK1 gene. Pancreas. 39:420–421. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Aoun E, Muddana V, Papachristou GI and
Whitcomb DC: SPINK1 N34S is strongly associated with recurrent
acute pancreatitis but is not a risk factor for the first or
sentinel acute pancreatitis event. Am J Gastroenterol. 105:446–451.
2010. View Article : Google Scholar
|
|
64
|
Liu XB, Jiang JM, Huang ZW, et al:
Clinical study on the treatment of severe acute pancreatitis by
integrated traditional Chinese medicine and Western medicine. J
Sichuan University (medical science edition). 35:204–208. 2004.(In
Chinese).
|