Introduction
The morphological study of the petrous bone is a
significant challenge due the complex structure of the bone and its
inaccessibility during surgery, as well as the difficulty in
preparing qualified specimens. For an inexperienced surgeon, it is
necessary to practice with dissections and specimens, as well as to
learn from papers and atlases, which presents considerable
difficulties. As computing power has developed, computer technology
has played an increasingly important role in a variety of research
areas; for example, with the most advanced computer technology, it
would be possible to generate a virtual model of a dinosaur
appearing identical to a real dinosaur (1). The present is thus an appropriate
time for conventional methods of surgical training and anatomical
learning to be changed accordingly.
Numerous studies (2–4) have
reported the use of computer-aided three-dimensional (3D)
reconstruction based on serial stained celloidin sections, such as
for the morphological study of the inner ear; however, due to the
limitations of hardware and software, only parts of sections have
been employed, with the sampling of every fifth to 10th section. It
is still not possible to generate a 3D reconstruction of the
petrous bone with high-resolution computed tomography (CT)
(5) or magnetic resonance imaging
(MRI) (6). Although micro-CT
(7,8) and magnetic resonance microscopy (MRM)
(9) have already been used for the
3D reconstruction of the mammalian inner ear, the resolution is too
low to inspect detailed structures with precision.
For studies on the petrous bone, the majority of
previous reports have focused on a specific part or local
structure. There is thus a requirement for a comprehensive and
qualified 3D model for the integral structure of petrous bone to be
made for the convenience of further study of the relationship
between the external and internal portions. On this basis, the aim
of the present study was to use serial unstained celloidin sections
to reconstruct a comprehensive and qualified 3D model of the
petrous bone, with a special focus on the complicated and porous
structure of the skull base.
Materials and methods
Celloidin sections without staining
The present study utilized cadavers from the
Department of Anatomy, Histology and Embryology of Tianjin Medical
University (Tianjin, China). Signed informed consent had been
provided for the donation of the cadavers for research purposes,
and the study was approved by the Ethics Committee of Tianjin
Medical University.
The experimental material was from eight adult
cadavers fixed with formalin (four males and four females) with an
age range of between 56 and 81 years (mean age, 69.4 years). The
temporal bones were removed according to the general standards. The
specimens were fixed in formalin, decalcified in chlorhydric acid
(Tianjin Ting Da Xi Gui Chemical Reagent Factory, Tianjin, China)
and embedded in celloidin. Following being hardened in ethanol, the
celloidin blocks (Tianjin Da Mao Chemical Reagent Factory, Tianjin,
China) were trimmed to the shape of standard cuboids and mounted on
a plastic block provided with a sliding microtome (American Optical
Sliding Microtome 860; Reichert Technologies, Depew, NY, USA). The
petrous bones were serially sectioned in the horizontal plane at a
thickness of 40 μm and preserved in 60% ethanol. A right petrous
bone was randomly selected for digitization and reconstruction. The
right petrous bone originated from a 75-year-old male with no
history of otological disease. A total of 487 successive celloidin
sections of the petrous bone without staining were finally obtained
for the computer demonstration.
Digitization of unstained sections
Each unstained section was spread on a glass slide
measuring 45×60 mm, immersed in 60% ethanol and finally covered by
another thin slide of the same size. A digital camera (DC) with a
12-megapixel charge-coupled device (Canon PowerShot A2100 IS; Canon
Inc., Tokyo, Japan) was used to capture the images. The focus of
the DC, as well as the locations of the DC and the section, were
adjusted to ensure that the entire images were exactly covered by
the viewfinder. The setup then remained the same for the rest of
the procedure. The highest resolution and best definition of the DC
were used to generate the images, which were then saved in the
digital JPEG picture format. The refined structure was observed
through anatomic microscopy (Olympus SZX7 Zoom Stereo Microscope;
Olympus Corp., Tokyo, Japan). When the rupture of a section was
encountered, the next intact section took its place.
Reconstruction
The subsequent 3D reconstruction procedure consisted
of several steps, which were all accomplished with
Amira® software (version 5.4.1; Template Graphics
Software, Inc., San Diego, CA, USA) on a high-capability computer
(Hasee X86; Shenzhen Hasee Computer Co., Ltd., Beijing, China). The
first step was image alignment. As the available sections had no
previous embedded fixed markers, the images of the sections were
aligned manually following digitization by comparing, translating
and rotating adjacent slides with respect to one another and by
superimposing one over the next in Adobe Photoshop (version 6.0;
Adobe Systems, Inc., San Jose, CA). The frame of reference for the
alignment was the outline border of each slide and the regular
structures of the petrous bone.
The second vital step was segmentation. Once the
digitized sections were imported into Amira, the anatomical
structures of interest were extracted using the software
segmentation tools. The structures of interest included the bone
labyrinth, internal carotid artery (ICA) canal, internal jugular
vein (IJV) canal, sigmoid sinus (SS), inferior petrosal sinus
(IPS), glossopharyngeal meatus, vagal meatus, internal acoustic
meatus (IAM), facial nerve canal, greater superficial petrosal
nerve, vestibular aqueduct (VA), extraosseous portion of the
endolymphatic sac (ES), round and oval window, processus
cochleariformis and pyramidal eminence. When the names and colors
of the objects were set, the components of the petrous bone were
segmented (Fig. 1). The majority
of the segmentation work was performed manually by combining the
‘Blow tool’ and ‘Brush’ functions.
The final procedure of the 3D reconstruction
followed the segmentation step. Polygonal 3D surface models of the
segmented structures were generated, and the surface simplification
algorithm of the Amira software was applied to smooth the model by
eliminating redundant vertices and reducing the number of triangles
in each model. The 3D models were shown in Amira as shaded smooth
surfaces with control over rotation and transparency.
Results
Establishment of petrous bone 3D
model
A comprehensive 3D model of integral petrous bone
was established on the basis of a complete set images of the serial
sections. As shown in Fig. 2, the
contour of the detailed structure was clearly imaged with high
precision, including the posterior ampullar nerve, the branch of
the IPS and the ES. It was possible to display different parts of
the structure in different ways using outlining, shading, lining,
pointing, transparency and contrasting patterns. It was also
possible to determine which parts would be displayed or hidden,
which could be useful in providing insights into the complex
structure of human petrous bone. Of note, the structure of the
locations labeled in Fig. 2 (such
as locations 1–5, 11, 17, 22 and 23) can only be observed in
celloidin slices, rather than through the use of CT and MRI, which
strongly confirms the advantages of our model compared with
conventional imaging methods. Under anatomic microscopy, the
details of the refined structure could be observed clearly in an
enlarged view. As shown in Fig. 3,
enlargement of the components inside of the cochlea revealed the
two partitive membranes and three lumen.
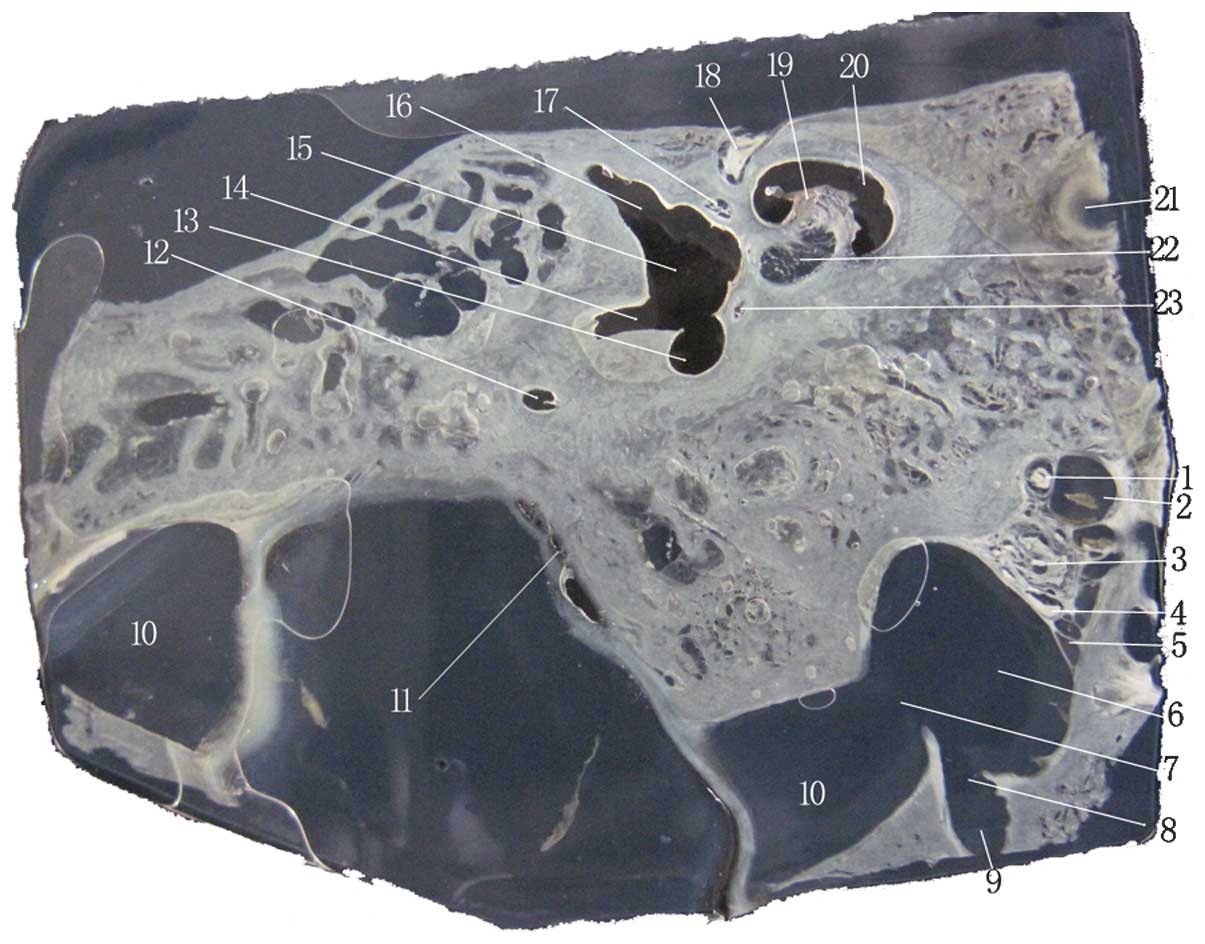 | Figure 2Image of an unstained celloidin
section. 1, glossopharyngeal nerve; 2, IPS; 3, vagal nerve; 4,
accessory nerve; 5, branch of the IPS draining into the IJV; 6,
IJV; 7, SS draining into the IJV; 8, junction of the condylar canal
and the IJV; 9, condylar canal; 10, SS; 11, extraosseous portion of
endolymphatic sac; 12, posterior semicircular canal; 13, common
crus; 14, opening of the crus simplex; 15, vestibule; 16,
ampullated end of the superior semicircular canal, which
corresponds with the vestibule; 17, superior vestibular nerve; 18,
labyrinth part of the facial nerve; 19, modiolus; 20, cochlea; 21,
internal carotid artery; 22, cochlear nerve and inferior vestibular
nerve; 23, posterior ampullar nerve. IJV, internal jugular vein;
SS, sigmoid sinus; IPS, inferior petrosal sinus. |
Association between VA, ES and the bony
labrynth
The petrous bone with its surface landmarkers could
be observed posteriorly, anteromedially (Fig. 4) or inferiorly through the 3D
model. All reconstructed structures could be represented
individually or jointly and rotated continuously in various planes.
The mutual positional relationship among the three semicircular
canals, cochlea, vestibule, round window, oval window, VA and ES is
shown in Fig. 5. The three
semicircular canals communicate with the vestibule via five
openings, one of which is formed by the union of the non-ampullated
ends of the superior and posterior canals, known as the ‘common
crus’. The spiral cochlea began at the vestibule and made
two-and-a-half turns around the cone-shaped modiolus for
distribution of the cochlear nerve. It lay between the internal
auditory canal and the tympanic cavity, inferiorly associated with
the jugular vein. The VA coursed upward from the vestibule, bent
near the isthmus portion, and then coursed downward and widened on
approach to the external aperture. The ES extended through the
distal VA and out the external aperture of the aqueduct to
terminate in the epidural space of the posterior cranial fossa.
 | Figure 4Anteromedial view of the petrous bone
model. (A) Semi-transparent view of the petrous bone. (B) Following
complete transparency of the petrous bone. 1, sigmoid sinus; 2,
extraosseous portion of the endolymphatic sac; 3, glossopharyngeal
meatus; 4, vagal meatus; 5, internal jugular vein canal; 6,
condylar canal; 7, branch of the IPS; 8, IPS; 9, internal carotid
artery canal; 10, tensor tympanic muscle; 11, greater superficial
petrosal nerve; 12, geniculate ganglion; 13, cochlea; 14, facial
nerve canal; 15, superior semicircular canal; 16, internal acoustic
meatus. IPS, inferior petrosal sinus. |
 | Figure 5Association among the VA, ES and bony
labyrinth. (A) Inferior view. 1, superior semicircular canal; 2,
posterior semicircular canal; 3, lateral semicircular canal; 4,
extraosseous portion of the ES; 5, external aperture of the VA; 6,
distal VA; 7, vestibule; 8, cochlea; 9, proximal VA; 10, isthmus of
the VA; 11, crus commune. (B) Anterolateral view. 1, vestibule; 2,
oral window; 3, apex of the cochlea; 4, cochlea; 5, round window;
6, ampulla of the posterior semicircular canal; 7, VA; 8,
extraosseous portion of the ES; 9, posterior semicircular canal;
10, crus simplex; 11, lateral semicircular canal; 12, superior
semicircular canal; 13, ampulla of the superior semicircular canal;
14, ampulla of the lateral semicircular canal. (C) Superior view.
1, distal VA; 2, isthmus of the VA; 3, proximal VA; 4, crus
commune; 5, crus simplex; 6, vestibule; 7, cochlea; 8, ampulla of
the superior semicircular canal; 9, lateral semicircular canal; 10,
superior semicircular canal; 11, posterior semicircular canal. VA,
vestibular aqueduct; ES, endolymphatic sac. |
Association between the ICA canan, IJV
canal, SS, IPS, glossopharyngeal meatus and vagal meatus
When the ICA entered the canal in the petrous
portion of the temporal bone, it first ascended a short distance
and then curved forward and medially. The ICA can be observed
adjacent to the basal turn of the cochlea (Fig. 4B). The Jugular foramen (JF) is an
important bony channel of the posterior fossa. The dura overlying
the intrajugular compartment formed two perforations. One of these
perforations was the glossopharyngeal meatus, through which the
glossopharyngeal nerve passes; the other was the vagal meatus,
through which the vagus and accessory nerves pass. The SS is the
largest source of venous drainage into the JF (10). The IPS formed a multi-channel
confluence that emptied into the IJV between the glossopharyngeal
and vagus nerves in 1–3 roots. The internal opening of the condylar
canal was located at the posteromedial margin of the IJV.
Association between the IAM and facial
nerve canal
The internal auditory meatus is a canal in the
petrous bone that carries nerves, i.e. cranial nerves VII and VIII,
from inside the skull towards the inner ear. Near the bottom of the
IAM, openings exist for three different canals (Fig. 4). The anterosuperior canal
transmits the facial nerve and is separated from the
posterosuperior section, which transmits the superior vestibular
nerve, by Bill’s bar. The cochlear nerve runs antero-inferiorly and
the inferior vestibular nerve runs postero-inferiorly. The facial
canal is a Z-shaped canal running through the temporal bone from
the fundus of the IAM to the stylomastoid foramen. The greater
superficial petrosal nerve can be observed extending anteriorly
over the basal turn of the cochlea and the ICA. The tensor tympani
muscle was observed to turn laterally along the cochleariform
process, which was located at the anterior part of the horizontal
segment of the facial nerve.
Discussion
The process of learning the surgical anatomy of the
petrous bone is challenging due to the small but complex structure
of the bone. The rapid development of computer technology has led
to the potential for its use in modeling and simulating the human
anatomy. The first computer-generated 3D model of the temporal bone
was produced by Antunez et al in 1980 (11). To create this model, images were
captured of each histological section and the images were then
transferred to a computer graphics tablet. From this, the computer
generated surface-rendered 3D models of the ES and the
endolymphatic duct. In 1989, Lutz et al (12) examined 60 sagittal histologic
sections of a normal left temporal bone, and data were entered into
a computer for the 3D reconstruction of a temporal bone. Over the
past two decades, there have been marked improvements in temporal
bone reconstruction. In 2001, Zieliński and Słoniewski (1) created a virtual, 3D computer model of
the petrous bone based on 1-mm tomographic X-ray slices. One year
later Page et al (13)
highlighted the feasibility of creating 3D images of the petrous
bone from a routine CT examination. Bernardo et al (14) designed a 3D surgical simulator
known as interactive virtual dissection, which allowed the user to
drill progressively deeper into the petrous bone and to identify
crucial structures. Tang et al (15) evaluated the application of a
virtual reality system in the construction of 3D petrous bone. The
Digital Imaging and Communications in Medicine data of the CT
performed for 15 adult cadaver heads were transferred to and
reconstructed in the Destroscope virtual reality system. The use of
3D imaging enabled the illustration of the distinct spatial
relationship of anatomic structures associated with the petrous
bone. A limitation of these previous studies, however, was that
they showed only a few of the internal structures of the petrous
bone and their mutual relationships. There remains a lack of a 3D
model that can be used to observe all the structures of the petrous
bone, and to make an association between superficial and deep
structures.
In this study, a 3D model of the petrous bone, which
systematically displayed the detailed structures of the bone
(including soft tissue and spatial locations), was generated. This
is likely to aid the understanding of otologists with regard to the
spatial relationships of temporal bone structures. Transparency
allowed the visualization of substructures such as the bony
labyrinth ‘inside’ the hard petrous pyramid. All reconstructed
structures could be represented individually or jointly and rotated
continuously in any plane (Fig.
5). Since the reconstructed images of the intratemporal bone
can be viewed from all surgical angles and numerous spatial
relationships can be accurately observed, the model may provide a
guide for otosurgery and enhance medical education. Previously,
focus was placed on the generation of 3D models for a structure or
part of a structure inside the petrous bone. Li et al
(2), for example, sliced the right
ear of a male cadaver (14 years old) with celloidin, and then
modeled the bone structures with Amira software and studied the
connection between the structures. There is, however, a lack of
comprehensive description based on a 3D model depicting the inside
structures of the petrous bone, including the IJV canal, carotid
artery, glossopharyngeal channel, three semicircular canals and
internal auditory canal, which has largely limited the anatomical
understanding of this region. On this basis, the present study has
provided a unique comprehensive presentation of the 3D structure
inside the petrous bone, and thus may enhance the understanding of
the intrinsic connection between different parts of the bone.
The methods of acquiring digital information from
mammals include micro-CT, MRM and orthogonal-plane fluorescence
optical sectioning microscopy (OPFOS) (16). Previous studies have predominantly
adopted technologies such as CT and MRI, which have resulted in
thick slices (10 and 86 μm, respectively) at low resolution, as
well as the loss of structural information due to the gray scaling
strategy (9,17–24).
Consequently, this has impaired the accuracy of reconstruction for
the fine structures of the inner ear. Although comparatively high
image resolution can be obtained with OPFOS, there is a decline in
image quality due to the dimensions of the human inner ear, which
make it difficult to describe the precise configuration in the
petrous bone. Images of celloidin sections are therefore the best
2D digital material for reference. In the present study, 40-μm,
serial, unstained collodion slices of the petrous bone, which
showed the inner ear, were utilized for the reconstruction of 3D
models on this basis. These efforts largely avoided the loss of
refined structural information about the inner ear, and ensured the
consistency in morphology between the modeled VA and the real
structure. Furthermore, technologies such as CT and MRI are largely
limited to partial structures inside petrous bone, while certain
other regions, such as the ES, glossopharyngeal channel and the
bottom of internal auditory canal, can only be observed and thus
modeled on the basis of the collodion films.
In previous studies, the sampling of every fifth to
10th section stained has typically been applied, producing
considerable error (25,26). As described by Rother et al
(25), when specimens were
prepared in the typical manner at 20 μm, and only every fifth to
10th section was scanned, the obtained measurements had an error of
±0.1–0.2 mm in a strictly vertical plane. The use of every fifth to
10th section for 3D reconstruction thus results in the loss of
considerable amounts of vital information regarding these tiny
structures, which evidently affects the precision of the 3D model.
In the present study, all the intact sections were employed and any
missing sections were replaced with adjacent ones. Additionally, in
the segmentation procedure, the boundaries of the sections that
replaced the missing ones were moved to the middle of the two
adjacent ones, thereby further reducing the error.
A process with fewer steps is likely to lead to a
smaller error margin. A complicated staining procedure could damage
some of the celloidin sections, and unpredictable distortion could
occur during dehydration, clearing or the other procedures
(26). In the present study, any
potential causes of error were avoided by using unstained sections,
which also reduced laboratory workload to a great extent.
Furthermore, in the images of the unstained sections, the small
structures of the petrous bone were distinct enough for precise
segmentation, as described earlier in the study (Fig. 1).
It is worth noting that, despite the significant
advantages of our comprehensive model of the complete petrous bone
structure, the model also exhibits limitations, such as poor
resolution and potential error in the sampling procedure; however,
these limitations do not affect the essential findings of the
study. Efforts will be made to improve the quality and
reliability.
Acknowledgements
This study was supported by a science grant from
Tianjin Medical University (no. 052-200010-ky).
References
|
1
|
Zieliński P and Słoniewski P: Virtual
modelling of the surgical anatomy of the petrous bone. Folia
Morphol (Warsz). 60:343–346. 2001.
|
|
2
|
Li PM, Wang H, Northrop C, Merchant SN and
Nadol JB Jr: Anatomy of the round window and hook region of the
cochlea with implications for cochlear implantation and other
endocochlear surgical procedures. Otol Neurotol. 28:641–648. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morita N, Kariya S, Farajzadeh Deroee A,
et al: Membranous labyrinth volumes in normal ears and Ménière
disease: a three-dimensional reconstruction study. Laryngoscope.
119:2216–2220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shimizu S, Cureoglu S, Yoda S, Suzuki M
and Paparella MM: Blockage of longitudinal flow in Meniere’s
disease: A human temporal bone study. Acta Otolaryngol.
131:263–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jager L, Bonell H, Liebl M, et al: CT of
the normal temporal bone: comparison of multi- and single-detector
row CT. Radiology. 235:133–141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miguéis A, Melo Freitas P and Cordeiro M:
Anatomic evaluation of the membranous labyrinth by imaging: 3D-MRI
volume-rendered reconstructions. Rev Laryngol Otol Rhinol (Bord).
128:37–40. 2007.
|
|
7
|
Uzun H, Curthoys IS and Jones AS: A new
approach to visualizing the membranous structures of the inner
ear-high resolution X-ray micro-tomography. Acta Otolaryngol.
127:568–573. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mukherjee P, Uzun-Coruhlu H, Curthoys IS,
Jones AS, Bradshaw AP and Pohl DV: Three-dimensional analysis of
the vestibular end organs in relation to the stapes footplate and
piston placement. Otol Neurotol. 32:367–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lane JI, Witte RJ, Henson OW, Driscoll CL,
Camp J and Robb RA: Imaging microscopy of the middle and inner ear.
Part II MR microscopy. Clin Anat. 18:409–415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ayanzen RH, Bird CR, Keller PJ, et al:
Cerebral MR venography: normal anatomy and potential diagnostic
pitfalls. Am J Neuroradio. 21:74–78. 2000.
|
|
11
|
Antunez JC, Galey FR, Linthicum FH and
McCann GD: Computer-aided and graphic reconstruction of the human
endolymphatic duct and sac: a method for comparing Meniere’s and
non-Meniere’s disease cases. Ann Otol Rhinol Laryngol Suppl.
89:23–32. 1980.PubMed/NCBI
|
|
12
|
Lutz C, Takagi A, Janecka IP and Sando I:
Three-dimensional computer reconstruction of a temporal bone.
Otolaryngol Head Neck Surg. 101:522–526. 1989.PubMed/NCBI
|
|
13
|
Page C, Taha F and Le Gars D:
Three-dimensional imaging of the petrous bone for the middle fossa
approach to the internal acoustic meatus: an experimental study.
Surg Radiol Anat. 24:388–392. 2002.
|
|
14
|
Bernardo A, Preul MC, Zabramski JM and
Spetzler RF: A three-dimensional interactive virtual dissection
model to simulate transpetrous surgical avenues. Neurosurgery.
52:499–505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang K, Mo DP and Bao SD: Application of
virtual reality technique in construction of 3-dimensional petrous
bone model. Zhonghua Shi Yan Wai Ke Za Zhi. 26:794–795. 2009.(In
Chinese).
|
|
16
|
Hofman R, Segenhout JM, Albers FW and Wit
HP: The relationship of the round window membrane to the cochlear
aqueduct shown in three-dimensional imaging. Hear Res. 209:19–23.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vogel U: New approach for 3D imaging and
geometry modeling of the human inner ear. ORL J Otorhinolaryngol
Relat. 61:259–267. 1999. View Article : Google Scholar
|
|
18
|
Chan LL, Manolidis S, Taber KH and Hayman
LA: In vivo measurements of temporal bone on reconstructed clinical
high-resolution computed tomography scans. Laryngoscope.
110:1375–1378. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghiz AF, Salt AN, DeMott JE, et al:
Quantitative anatomy of the round window and cochlear aqueduct in
guinea pigs. Hear Res. 162:105–112. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klingebiel R, Thieme N, Kivelitz D, et al:
Three-dimensional imaging of the inner ear by volume-rendered
reconstructions of magnetic resonance data. Arch Otolaryngol Head
Neck Surg. 128:549–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pettit K, Henson MM, Henson OW, Gewalt SL
and Salt AN: Quantitative anatomy of the guinea pig endolymphatic
sac. Hear Res. 174:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Voie A: Imaging the intact guinea pig
tympanic bulla by orthogonal-plane fluorescence optical sectioning
microscopy. Hear Res. 171:119–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Santi PA, Blair A, Bohne BA, Lukkes J and
Nietfeld J: The digital cytocochleogram. Hear Res. 192:75–82. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lane JI, Witte RJ, Driscoll CL, Camp JJ
and Robb RA: Imaging microscopy of the middle and inner ear: Part
I: CT microscopy. Clin Anat. 17:607–612. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rother T, Schröck-Pauli C, Karmody CS and
Bachor E: 3-D reconstruction of she vestibular endorgans in
pediatric temporal bones. Hear Res. 185:22–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li SF, Zhang TY and Wang ZM: An approach
for precise three-dimensional modeling of the human inner ear. ORL
J Otorhinalaryngal Relat Spec. 68:302–310. 2006. View Article : Google Scholar
|



















