Introduction
Caffeic acid phenethyl ester (CAPE) is an important
active component of honeybee propolis extract and has been used in
traditional medicine for a number of years. CAPE is a polyphenol
that contains hydroxyl groups within a catechol ring, the molecular
formula of CAPE is C!17H16O4
(1,2)
(Fig. 1). It has been shown that
this active component of propolis possesses anti-inflammatory,
immunomodulatory, antineoplastic, antioxidant and wound-healing
properties (1–4). Inflammation is induced by the release
of chemical mediators from damaged tissue and migratory cells.
Mediators identified in the inflammatory process include biogenic
amines, metabolites of arachidonic acid (eicosanoids), platelet
aggregation factors, cytokines [interleukins (ILs) and tumor
necrosis factor-α (TNF-α)] and free oxygen radicals. These
substances are produced by inflammatory cells, such as
polymorphonuclear leukocytes (neutrophils, eosinophils and
basophils), endothelial cells, mast cells, macrophages, monocytes
and lymphocytes (5,6). CAPE inhibits cytokine and chemokine
production, the proliferation of T cells and lymphokine production,
and thus suppresses the inflammatory process. Specifically, CAPE is
a potent and a specific inhibitor of nuclear factor-κB (NF-κB)
activation, and this may provide the molecular basis for its
multiple anti-inflammatory and immunomodulatory activities
(2,7). The aim of this review is to highlight
the anti-inflammatory and immunomodulatory activities of CAPE,
focusing on the mechanisms of action (already identified)
underlying this activity.
Overview to the inflammatory response
Inflammation is an immunological response to
pathogens and damage that is initiated to protect the body, and
contributes to physiological and pathological processes, such as
wound healing and infection at the compromised site. The process is
accompanied by adhesion, migration and chemotaxis of leukocytes to
the inflammatory environment (6). In
response to tissue injury, a multifactorial network of chemical
signals initiates and maintains a host response designed to ‘heal’
the afflicted tissue. The first effectors recruited in the acute
inflammatory response are neutrophils. These are followed by
monocytes, which undergo differentiation in the tissue into
macrophages and migrate to the site of tissue injury under the
guidance of chemotactic factors (5,8). The
activated leukocytes provide proinflammatory cytokines, reactive
oxygen species (ROS) and matrix metalloproteinases to remove the
invading pathogen (9). The pathogens
and damaged tissue are then phagocytosed, and the inflammatory
process is eventually terminated when lipoxins start to overrule
the proinflammatory signals (10).
In general, IL-1 and TNF target the endothelium and initiate the
inflammatory mediator cascade following exposure to certain
stimuli, including infection, trauma, ischemia, immune-activated T
cells or toxins. The inflammatory cascade can be summarized as
follows: i) Activation of inflammatory cytokine-secreting cells,
and increases in the levels of proinflammatory cytokines, such as
IL-1, TNF-α and interferon-γ (IFN-γ); ii) activation/synthesis of
phospholipase A2, cyclooxygenase-2 (COX-2) and inducible nitric
oxide synthase (iNOS); increased endothelial adhesion molecules and
synthesis of chemokines; iii) increased platelet-activating factor
(PAF), leukotriene, prostanoid (prostaglandin E2) and NO levels,
neutrophil endothelial adhesion, and the migration and activation
of neutrophils; and iv) inflammation, tissue destruction and loss
of function (10–12).
Proinflammatory cytokines and signaling
pathways
Cytokines are polypeptide regulators of host
responses to infection, immune responses, inflammation and trauma.
Cytokine secretion by immune cells has a pivotal role in directing
the course of an inflammatory response. Cellular cytokine
production is regulated at transcriptional and translational levels
and via cell signaling (11).
Certain cytokines act to enhance the effects of the disease
(proinflammatory), whereas others are involved in reducing
inflammation and promoting healing (anti-inflammatory). Methods to
block potentially harmful cytokines, particularly during an
overwhelming infection, have been an area of particular interest.
Administration of the proinflammatory cytokines IL-1 and TNF-α to
humans can lead to fever, inflammation, tissue destruction and, in
certain cases, shock and mortality (9,12). TNF-α
is a ‘master regulator’ among cytokines and is responsible for
mediating the inflammatory responses and innate immunity. The major
pathways activated by TNF-α include caspases, NF-κB and
mitogen-activated protein kinases (MAPKs). Crosstalk between these
signaling pathways plays a role in determining the physiological
outcome of the responses to TNF-α (13). The network response is further
complicated by the phases associated with TNF-α signaling: In the
early phase, TNF-α signaling induces the expression of inflammatory
cytokines; this then initiates a secondary cytokine-mediated
cellular response that contributes to the biological activity of
TNF-α (14).
The transcription factor NF-κB plays a central role
in regulating inflammatory, immune and anti-apoptotic responses. It
is composed of homodimers and heterodimers of the Rel family of
proteins, including p65/RelA, RelB, c-Rel, p50/p105 and p52/p100
(15,16). The activation of inactive NF-κB
proteins existing in the cytoplasm is induced by numerous factors,
including inflammatory cytokines (IL-1 and TNF-α), bacterial
products and protein synthesis inhibitors (17); therefore, agents that can
downregulate the activation of NF-κB have potential for therapeutic
interventions, whereas the activation of NF-κB promotes
inflammation in animals. The binding of TNF-α to cell surface
receptors engages multiple signal transduction pathways, including
three groups of MAPKs: Extracellular-signal-regulated kinases,
c-Jun N-terminal kinases and p38 MAPKs. These MAPK signaling
pathways induce a secondary response by increasing the expression
of several inflammatory cytokines that contribute to the biological
activity of TNF-α. MAPKs, therefore, function both upstream and
downstream of signaling by TNF-α receptors (13,18). In
almost all cell types, the exposure of the cells to TNF-α induces
the activation of NF-κB and leads to the expression of a range of
genes associated with inflammation. NF-κB is a protein complex that
controls the transcription of DNA and is a central regulator of
cellular stress in all cell types in humans. NF-κB plays a key role
in regulating the immune response to infection and in acute and
chronic inflammation. The activation of NF-κB in rats can induce
the expression of IL-1β, which increases the expression of
proinflammatory molecules (17,19).
Anti-inflammatory effects of CAPE
The transcription factor NF-κB has a pivotal role in
a variety of physiological processes throughout the body, including
immune responses, cell proliferation and inflammation. NF-κB
elicits its effects by promoting the transcription of a range of
cytokines, enzymes, chemokines and antiapoptotic and cell growth
factors (20). Several in
vitro and in vivo studies have described diverse
biological activities of CAPE (at micromolar concentrations), such
as a specific inhibition of NF-κB and a suppression of the
lipoxygenase pathway of arachidonic acid metabolism during
inflammation (2–4). It has also been shown that CAPE acts to
suppress the NF-κB activation induced by ROS-generating agents in
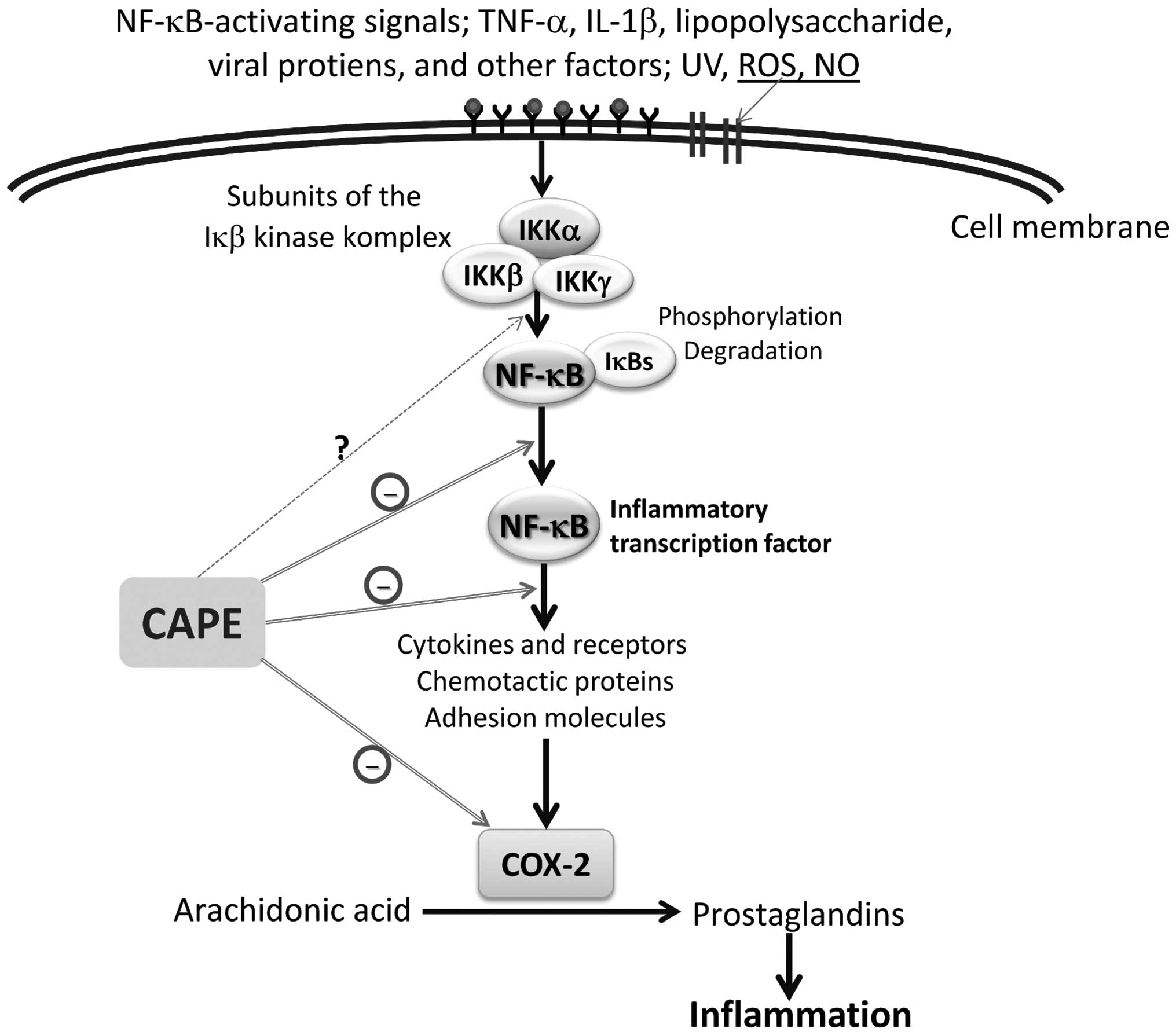
human histiocytic and coronary artery endothelial cells (2). It is believed that, rather than
preventing the degradation of κB inhibitor-α (IκB-α), CAPE
suppresses NF-κB activation by inhibiting the interaction between
NF-κB proteins and DNA (21)
(Fig. 2). Ilhan et al
(22) suggested that the
anti-inflammatory effect of CAPE is most likely due to the
inhibition of ROS production at the transcriptional level, through
the suppression of NF-κB activation, and the direct inhibition of
the catalytic activity of iNOS. Toyoda et al (23) reported that CAPE treatment inhibited
Helicobacter pylori-induced NF-κB activation via the
suppression of IκB-α degradation and the phosphorylation of p65 in
a gastric cancer cell line. Furthermore, the results clearly
demonstrated that the mRNA expression levels of inflammatory
factors known to be induced by NF-κB transcriptional activation,
such as TNF-α, IFN-γ, IL-2, IL-6, iNOS and KC, an IL-8 homologue
chemokine, were all significantly decreased by CAPE treatment in
the pyloric mucosa of H. pylori-infected Mongolian gerbils
(23,24). Colonization of gastric epithelial
cells with H. pylori induces NF-κB and results in the
increased production of the proinflammatory cytokines TNF-α, IL-1,
IL-6 and IL-8, all of which are regulated by NF-κB (25,26). It
has also been demonstrated that local administration of CAPE leads
to increased levels of leukocyte apoptosis and marked reductions in
the concentrations of leukocytes, neutrophils and monocytes in the
inflammatory site exudate. Furthermore, CAPE decreases the levels
of cytosolic IκB-α and increases the nuclear translocation of p65
(27).
CAPE possesses strong antioxidant, anti-inflammatory
and healing properties, and its effects on the wound healing have
been attributed to the inhibition of NF-κB (28,29).
Consistent with these two cited studies, Santos et al
(30) reported that treatment with
CAPE enhanced wound healing, particularly wound healing following
burns; decreased inflammatory parameters and oxidative damage; and
inhibited the activity of cyclooxygenase and lipooxygenase. Under
most inflammatory conditions, such as in thermal injury, NO
production is enhanced. In addition to performing histological and
biochemical analyses, Santos et al (30) evaluated the anti-cluster of
differentiation 68 (CD68) and NO levels, as well as myeloperoxidase
(MPO) activity. CAPE exhibited an anti-inflammatory action on rat
burn healing by reducing MPO activity, NO levels and the number of
CD68-positive cells (30). Khan
et al (31) demonstrated that
CAPE reduced neurovascular inflammation and protected the rat brain
following transient focal cerebral ischemia by downregulating NF-κB
and certain mediators, such as cytokines and iNOS (31).
CAPE classically exerts anti-inflammatory effects by
reducing prostaglandin and leukotriene synthesis. Furthermore, it
has been suggested that the anti-inflammatory action exhibited by
CAPE is a result of the inhibition of arachidonic acid release from
the cell membrane. As a consequence of this inhibition, the
activity of COX-1 and −2 and the activation of the COX-2 gene
expression are suppressed (32,33).
Recently, the protective effect of CAPE on a model of eccentric
exercise-induced muscle injury was investigated (34). The study results showed that
inflammatory skeletal muscle injury enhanced the expression of
COX-2 and iNOS, as well as the production of IL-1β and monocyte
chemoattractant protein-1 (MCP-1). It was proposed that these
pathological changes in the rats were suppressed by CAPE, which
blocked the NF-κB-dependent activation of the inflammatory response
(34). In another in vitro
study, CAPE significantly suppressed the levels of
lipopolysaccharide (LPS)-induced IL-1β, TNF-α and MCP-1 from a
macrophage cell line, RAW264.7 (35). Furthermore, in a recent study of
RAW264.7 murine macrophage in vivo models, CAPE reduced the
production of cytotoxic molecules, such as NO and peroxynitrite,
and thus suppressed the inflammatory responses that could have
resulted in cell damage and, potentially, cell death. According to
the study results, RAW264.7 cells under LPS/IFNγ stimulation
exhibited significantly improved viability following treatment with
CAPE, which also inhibited NO production in a similar manner to an
iNOS inhibitor. This indicated that CAPE exhibits therapeutic
potential in a variety of inflammatory disorders (36).
NF-κB signaling additionally has central roles in
precancerous chronic inflammation and cancer-induced inflammation
(37). CAPE acts to downregulate
inflammation by blocking NF-κB, and affects a variety of mediators,
including adhesion molecules, cytokines and iNOS. CAPE is a
well-documented inhibitor of NF-κB, which may be an action
mechanism for the CAPE-mediated anti-inflammatory and anticancer
effects (4,38). Although CAPE has been described to
conduct its anti-inflammatory activities by modulating different
inflammatory pathways, including inhibition of the transcription
factors NF-κB and signal transducer and activator of transcription
3 (acute-phase response factor), the compound has already been
evaluated for antitumor efficacy in numerous in vitro and
in vivo studies (39,40). Coimbra et al (41), for example, investigated the
antitumor efficacy of liposomal formulations of CAPE that are known
to interfere with inflammatory signaling pathways and have been
described to exert antitumor effects. Furthermore, CAPE has been
demonstrated to be selectively cytotoxic to cancer cells (42–44).
Previous studies found that CAPE could rapidly enter HL-60 cells
and induce glutathione depletion (42), mitochondrial dysfunction and
caspase-3 activation (43). In a
study by Park et al (45) it
was observed that CAPE suppressed the expression of phospholipase
D1 (PLD1) at the transcriptional level by preventing the binding of
NF-κB to the PLD1 promoter. This suggested that the CAPE-induced
suppression of matrix metalloproteinase-2 and invasion was mediated
by the downregulation of PLD1 by CAPE in glioma cells. Several
proposed molecular anti-inflammatory mechanisms of CAPE have been
suggested by in vivo and in vitro studies. Table I (22,23,30,34,35,46–50)
summarizes the anti-inflammatory effects of CAPE.
 | Table I.Potential mechanism underlying the
effects or progression pathways of CAPE in its
anti-inflammatory/immunomodulatory action. |
Table I.
Potential mechanism underlying the
effects or progression pathways of CAPE in its
anti-inflammatory/immunomodulatory action.
| Mechanism for the
effect or progression pathway(s) | In
vivo/in vitro | Cells/animals
used | Reported outcomes
(ref.) |
|---|
| Inhibiting ROS
production; suppressing NF-κB activation | In vivo, EAE
(animal model of MS) | Rats | Inhibited ROS
production (XO activity, levels of MDA); reduced infiltration of
inflammatory cells (22) |
| Suppressing
inflammation and ocular tissue damage | In vivo,
LPS-induced inflammation | Rats | Suppressed number
of inflammatory cells and MPO activity (46) |
| Inhibiting NF-κB
activation and mRNA expression; preventing degradation and
phosphorylation of p65; suppressed of p65 subunit | In vitro,
cell culture; in vivo, H. pylori-induced chronic
gastritis | AGS cells,
Mongolian gerbils | Inhibited NF-κB
activation by suppression of IκB-α degradation of IκB-α and
phosphorylation NF-κB p50; reduced mRNA expression of TNF-α, IL-2,
IL-6, iNOS and KC (23) |
| Inhibiting NF-κB
transcriptional activation | In
vitro | Jurkat, MT2 human
T-cell lines | Inhibited NF-κB
transcriptional activation induced by Tax (47) |
| Inhibiting
TNF-α-dependent NF-κB activation via direct inhibition of IKK as
well as activation of the Nrf2 pathway | In vitro,
cell culture | HCT116 (human
coloncarcinoma) cells | Inhibited NF-κB
activation by TNF-α and LPS, and directly inhibited IKK in HCT116
cells. Nrf2 activation is associated with the inhibition of the
NF-κB pathway (48) |
| Inhibiting the
inflammatory pathway | In vivo | Mice | Reduced NF-κB
activation and levels of COX-2 (49) |
| Inhibiting cytokine
and chemokine production associated with the NF-κB signaling
pathway | In vitro,
peripheral blood sampling | MoDCs | Inhibited cytokine
and chemokine production, IκB-α phosphorylation and NF-κB
activation in human MoDCs (50) |
| Inhibiting gene
expression of proinflammatory cytokines from LPS-stimulated
macrophages | In vitro,
cell culture | LPS-stimulated
RAW264.7 cells | Reduced mRNA
expression of MCP-1, TNF-α, IL-6 and IL-1β (35) |
| Blocking
NF-κB-dependent activation of the inflammatory responses | In vivo,
eccentric exercise-induced skeletal muscle injury | Rats | Suppressed high
COX-2 and iNOS expression and IL-1β and MCP-1 levels (34) |
| Anti-inflammatory
action on rat burn healing | In vivo,
burn injury | Rats | Reduced MPO
activity, NO levels and CD68 expression; improved wound healing
after burn (30) |
Immunomodulatory effects of CAPE
Although the mechanisms underling CAPE-induced NF-κB
inhibition have yet to be fully elucidated, the anti-inflammatory
and immunomodulatory effects of the compound have been demonstrated
in human and experimental models. Immunological studies have
indicated that CAPE strongly inhibits mitogen-induced T-cell
proliferation, lymphokine production and NF-κB activation (22,27,50).
Furthermore, CAPE has been shown to regulate the nuclear binding of
the NF-κB subunit p65/RelA, attenuate the expression of cytosolic
IκB-α and suppress the dephosphorylation and T-cell transcriptional
activity of nuclear factor of activated T cells (51). It has additionally been demonstrated
that CAPE can inhibit eicosanoid synthesis, and NF-κB activation
may be a causative factor for the increased expression of numerous
inflammatory genes in asthma (52,53).
NF-κB is expressed in the majority of cell types, and is known to
play a central role in immune and inflammatory responses, including
asthma. In a study by Jung et al (54) CAPE was found to be capable of
downregulating NF-κB activity and reducing the levels of eosinophil
peroxidase, indicating that CAPE could be considered as an adjuvant
therapy for patients with bronchial asthma. Consistent with these
observations, Choi et al (55) demonstrated the importance of NF-κB in
the pathogenesis of asthma in mice. In addition, Park et al
(56) showed that there was a
significant decrease in the cellularity of the spleen and thymus
and the thymus weight of mice treated with CAPE at a dose of 20
mg/kg. These results suggested that the treatment of CAPE directly
or indirectly caused the immune cells to decrease in cell number,
particularly T cells. A different study strongly indicated that the
anti-allergy effect of CAPE was a result of the suppression of IgE
levels occurring due to the inhibition of NF-κB activation and PAF
release (57). Furthermore, it has
been reported that CAPE suppresses the contraction of guinea-pig
trachea induced by histamine and adenosine. CAPE may therefore be
an effective therapeutic agent for allergic diseases (58).
Conclusion
In conclusion, CAPE, a compound recognized as the
active component of propolis extract, has anti-inflammatory,
antioxidant and immunomodulatory properties. Additionally, CAPE
inhibits the transcriptional activity of the COX-2 gene in
epithelial cells, iNOS gene expression and NO production in
macrophage cell lines, and suppresses eicosanoid synthesis and the
release of arachidonic acid from cell membranes. In accordance with
the above effects, it has been demonstrated that CAPE is a potent
and specific inhibitor of NF-κB, lipid peroxidation and
lipoxygenase. NF-κB therefore represents a potential target for
novel therapeutic agents developed to block the inflammatory
response in cases where this process has become chronic or
dysregulated. In addition, abnormalities in the NF-κB pathway are
frequently observed in a variety of types of human cancer. NF-κB
pathway activation is associated with the pathogenesis of chronic
inflammatory diseases, including asthma, atherosclerosis,
rheumatoid arthritis, inflammatory bowel disease and cancer
(59). Furthermore, CAPE
demonstrates potential health benefits for the prevention of
obesity and associated metabolic disorders and is a potential drug
candidate for ischemic stroke treatment due to its inhibition of
oxidative stress and inflammation, examples that illustrate how
clinically relevant it can be across a wide therapeutic window
(49,59). The findings described in this review
provide novel insights into the molecular mechanisms underlying the
immunomodulatory and anti-inflammatory activities of CAPE. Several
of the widely used anti-inflammatory agents inhibit the NF-κB
pathway, at least in part, as one of their targets. The effect of
CAPE in the treatment of inflammatory diseases may be mediated
through the inhibition of NF-κB activation, and it is believed that
CAPE is a safe, natural compound and a promising drug candidate for
anti-inflammation therapy.
Glossary
Abbreviations
Abbreviations:
|
CAPE
|
caffeic acid phenethyl ester
|
|
CD68
|
cluster of differentiation 68
|
|
COX-2
|
cyclooxygenase-2
|
|
EAE
|
experimental autoimmune
encephalomyelitis
|
|
IFN-γ
|
interferon-γ
|
|
IκB-α
|
κB inhibitor-α
|
|
IKK
|
IκB-kinase
|
|
IL-1
|
interleukin-1
|
|
iNOS
|
inducible nitric oxide synthase
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
MDA
|
malondialdehyde
|
|
MPO
|
myeloperoxidase
|
|
MoDC
|
monocyte-derived dendritic cell
|
|
MS
|
multiple sclerosis
|
|
NF-κB
|
nuclear factor κB
|
|
NO
|
nitric oxide
|
|
Nrf2
|
nuclear-factor-E2-related factor 2
|
|
PAF
|
platelet-activating factor
|
|
PLD1
|
phospholipase D1
|
|
ROS
|
reactive oxygen species
|
|
TNF-α
|
tumor necrosis factor-α
|
|
XO
|
xanthine oxidase
|
References
|
1
|
Sud'ina GF, Mirzoeva OK, Pushkareva MA,
Korshunova GA, Sumbatyan NV and Varfolomeev SD: Caffeic acid
phenethyl ester as a lipoxygenase inhibitor with antioxidant
properties. FEBS Lett. 329:21–24. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Natarajan K, Singh S, Burke TR Jr,
Grunberger D and Aggarwal BB: Caffeic acid phenethyl ester is a
potent and specific inhibitor of activation of nuclear
transcription factor NF-kappa B. In: Proc Natl Acad Sci USA. 93.
pp. 9090–9095. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koltuksuz U, Mutuş HM, Kutlu R, Ozyurt H,
Cetin S, Karaman A, Gürbüz N, Akyol O and Aydin NE: Effects of
caffeic acid phenethyl ester and epidermal growth factor on the
development of caustic esophageal stricture in rats. J Pediatr
Surg. 36:1504–1509. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akyol S, Ozturk G, Ginis Z, Armutcu F,
Yigitoglu MR and Akyol O: In vivo and in vitro antineoplastic
actions of caffeic acid phenethyl ester (CAPE): Therapeutic
perspectives. Nutr Cancer. 65:515–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Czermak BJ, Friedl HP and Ward PA:
Complement, cytokines, and adhesion molecule expression in
inflammatory reactions. In: Proc Assoc Am Physicians. 110. pp.
306–312. 1998; PubMed/NCBI
|
|
6
|
Ley K, Laudanna C, Cybulsky MI and
Nourshargh S: Getting to the site of inflammation: The leukocyte
adhesion cascade updated. Nat Rev Immunol. 7:678–689. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X, Pang J, Maffucci JA, Pade DS,
Newman RA, Kerwin SM, Bowman PD and Stavchansky S: Pharmacokinetics
of caffeic acid phenethyl ester and its catechol-ring fluorinated
derivative following intravenous administration to rats. Biopharm
Drug Dispos. 30:221–228. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eming SA, Krieg T and Davidson JM:
Inflammation in wound repair: Molecular and cellular mechanisms. J
Invest Dermatol. 127:514–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leder C, Ziegler M and Gawaz M: Modulating
immune responses and inflammation. Semin Thromb Hemost. 36:219–222.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stow JL and Murray RZ: Intracellular
trafficking and secretion of inflammatory cytokines. Cytokine
Growth Factor Rev. 24:227–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dinarello CA: Proinflammatory cytokines.
Chest. 118:503–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sabio G and Davis RJ: TNF and MAP kinase
signalling pathways. Semin Immunol. 26:237–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Janes KA, Gaudet S, Albeck JG, Nielsen UB,
Lauffenburger DA and Sorger PK: The response of human epithelial
cells to TNF involves an inducible autocrine cascade. Cell.
124:1225–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghosh S, May MJ and Kopp EB: NF-kappa B
and Rel proteins: Evolutionarily conserved mediators of immune
responses. Annu Rev Immunol. 16:225–260. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen F, Castranova V, Shi X and Demers LM:
New insights into the role of nuclear factor-kappaB, a ubiquitous
transcription factor in the initiation of diseases. Clin Chem.
45:7–17. 1999.PubMed/NCBI
|
|
17
|
Tak PP and Firestein GS: NF-kappaB: A key
role in inflammatory diseases. J Clin Invest. 107:7–11. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muller DN, Dechend R, Mervaala EM, Park
JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H and
Luft FC: NF-kappaB inhibition ameliorates angiotensin II-induced
inflammatory damage in rats. Hypertension. 35:193–201. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jura J, Wegrzyn P, Korostyński M, Guzik K,
Oczko-Wojciechowska M, Jarzab M, Kowalska M, Piechota M, Przewlocki
R and Koj A: Identification of interleukin-1 and
interleukin-6-responsive genes in human monocyte-derived
macrophages using microarrays. Biochim Biophys Acta. 1779:383–389.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bonizzi G and Karin M: The two NF-kappaB
activation pathways and their role in innate and adaptive immunity.
Trends Immunol. 25:280–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song YS, Park EH, Hur GM, Ryu YS, Lee YS,
Lee JY, Kim YM and Jin C: Caffeic acid phenethyl ester inhibits
nitric oxide synthase gene expression and enzyme activity. Cancer
Lett. 175:53–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ilhan A, Akyol O, Gurel A, Armutcu F, Iraz
M and Oztas E: Protective effects of caffeic acid phenethyl ester
against experimental allergic encephalomyelitis-induced oxidative
stress in rats. Free Radic Biol Med. 37:386–394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toyoda T, Tsukamoto T, Takasu S, Shi L,
Hirano N, Ban H, Kumagai T and Tatematsu M: Anti-inflammatory
effects of caffeic acid phenethyl ester (CAPE), a nuclear
factor-kappaB inhibitor, on Helicobacter pylori-induced gastritis
in Mongolian gerbils. Int J Cancer. 125:1786–1795. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naito Y and Yoshikawa T: Molecular and
cellular mechanisms involved in Helicobacter pylori-induced
inflammation and oxidative stress. Free Radic Biol Med. 33:323–336.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keates S, Hitti YS, Upton M and Kelly CP:
Helicobacter pylori infection activates NF-kappa B in gastric
epithelial cells. Gastroenterology. 113:1099–1109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aihara M, Tsuchimoto D, Takizawa H, Azuma
A, Wakebe H, Ohmoto Y, Imagawa K, Kikuchi M, Mukaida N and
Matsushima K: Mechanisms involved in Helicobacter pylori-induced
interleukin-8 production by a gastric cancer cell line, MKN45.
Infect Immun. 65:3218–3224. 1997.PubMed/NCBI
|
|
27
|
Orban Z, Mitsiades N, Burke TR Jr..Tsokos
M and Chrousos GP: Caffeic acid phenethyl ester induces leukocyte
apoptosis, modulates nuclear factor-kappa B and suppresses acute
inflammation. Neuroimmunomodulation. 7:99–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoşnuter M, Gürel A, Babucçu O, Armutcu F,
Kargı E and Işikdemir A: The effect of CAPE on lipid peroxidation
and nitric oxide levels in the plasma of rats following thermal
injury. Burns. 30:121–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Armutcu F, Gürel A, Hoşnuter M, Pabuçcu O
and Altnyazar C: Caffeic acid phenethyl ester improves oxidative
erythrocyte damage in a rat model of thermal injury. J Burn Care
Rehabil. 25:171–178. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
dos Santos JS and Monte-Alto-Costa A:
Caffeic acid phenethyl ester improves burn healing in rats through
anti-inflammatory and antioxidant effects. J Burn Care Res.
34:682–688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan M, Elango C, Ansari MA, Singh I and
Singh AK: Caffeic acid phenethyl ester reduces neurovascular
inflammation and protects rat brain following transient focal
cerebral ischemia. J Neurochem. 102:365–377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung WK, Choi I, Lee DY, et al: Caffeic
acid phenethyl ester protects mice from lethal endotoxin shock and
inhibits lipopolysaccharide-induced cyclooxygenase-2 and inducible
nitric oxide synthase expression in RAW 264.7 macrophages via the
p38/ERK and NF-kappaB pathways. Int J Biochem Cell Biol.
40:2572–2582. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KW, Chun KS, Lee JS, Kang KS, Surh YJ
and Lee HJ: Inhibition of cyclooxygenase-2 expression and
restoration of gap junction intercellular communication in
H-ras-transformed rat liver epithelial cells by caffeic acid
phenethyl ester. Ann NY Acad Sci. 1030:501–507. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen YC, Yen JC and Liou KT: Ameliorative
effects of caffeic acid phenethyl ester on an eccentric
exercise-induced skeletal muscle injury by down-regulating NF-κb
mediated inflammation. Pharmacology. 91:219–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Juman S, Yasui N, Ikeda K, Ueda A,
Sakanaka M, Negishi H and Miki T: Caffeic acid phenethyl ester
suppresses the production of pro-inflammatory cytokines in
hypertrophic adipocytes through lipopolysaccharide-stimulated
macrophages. Biol Pharm Bull. 35:1941–1946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kassim M, Mansor M, Kamalden TA,
Shariffuddin II, Hasan MS, Ong G, Sekaran SD, Suhaimi A, Al-Abd N
and Yusoff KM: Caffeic acid phenethyl ester (CAPE): Scavenger of
peroxynitrite in vitro and in sepsis models. Shock. 42:154–160.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Carrasco-Legleu CE, Márquez-Rosado L,
Fattel-Fazenda S, Arce-Popoca E, Pérez-Carreón JI and Villa-Treviño
S: Chemoprotective effect of caffeic acid phenethyl ester on
promotion in a medium-term rat hepatocarcinogenesis assay. Int J
Cancer. 108:488–492. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cao Q, Kaur C, Wu CY, Lu J and Ling EA:
Nuclear factor-kappa B regulates Notch signaling in production of
proinflammatory cytokines and nitric oxide in murine BV-2
microglial cells. Neuroscience. 192:140–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Omene CO, Wu J and Frenkel K: Caffeic Acid
Phenethyl Ester (CAPE) derived from propolis, a honeybee product,
inhibits growth of breast cancer stem cells. Invest New Drugs.
30:1279–1288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Coimbra M, Crielaard BJ, Storm G and
Schiffelers RM: Critical factors in the development of
tumor-targeted anti-inflammatory nanomedicines. J Control Release.
160:232–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen YJ, Shiao MS and Wang SY: The
antioxidant caffeic acid phenethyl ester induces apoptosis
associated with selective scavenging of hydrogen peroxide in human
leukemic HL-60 cells. Anticancer Drugs. 12:143–149. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Watabe M, Hishikawa K, Takayanagi A,
Shimizu N and Nakaki T: Caffeic acid phenethyl ester induces
apoptosis by inhibition of NFkappaB and activation of Fas in human
breast cancer MCF-7 cells. J Biol Chem. 279:6017–6026. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee YT, Don MJ, Hung PS, Shen YC, Lo YS,
Chang KW, Chen CF and Ho LK: Cytotoxicity of phenolic acid
phenethyl esters on oral cancer cells. Cancer Lett. 223:19–25.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park MH, Kang DW, Jung Y, Choi KY and Min
S: Caffeic acid phenethyl ester downregulates phospholipase D1 via
direct binding and inhibition of NFκB transactivation. Biochem
Biophys Res Commun. 442:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yilmaz A, Yildirim O, Tamer L, et al:
Effects of caffeic acid phenethyl ester on endotoxin-induced
uveitis in rats. Curr Eye Res. 30:755–762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shvarzbeyn J and Huleihel M: Effect of
propolis and caffeic acid phenethyl ester (CAPE) on NFκB activation
by HTLV-1 Tax. Antiviral Res. 90:108–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lee Y, Shin DH, Kim JH, et al: Caffeic
acid phenethyl ester-mediated Nrf2 activation and IkappaB kinase
inhibition are involved in NFkappaB inhibitory effect: Structural
analysis for NFkappa B inhibition. Eur J Pharmacol. 643:21–28.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bezerra RM, Veiga LF, Caetano AC, Rosalen
PL, Amaral ME, Palanch AC and de Alencar SM: Caffeic acid phenethyl
ester reduces the activation of the nuclear factor κB pathway by
high-fat diet-induced obesity in mice. Metabolism. 61:1606–1614.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wang LC, Lin YL, Liang YC, et al: The
effect of caffeic acid phenethyl ester on the functions of human
monocyte-derived dendritic cells. BMC Immunol. 10:392009.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Márquez N, Sancho R, Macho A, Calzado MA,
Fiebich BL and Muñoz E: Caffeic acid phenethyl ester inhibits
T-cell activation by targeting both nuclear factor of activated
T-cells and NF-kappaB transcription factors. J Pharmacol Exp Ther.
308:993–1001. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mirzoeva OK and Calder PC: The effect of
propolis and its components on eicosanoid production during the
inflammatory response. Prostaglandins Leukot Essent Fatty Acids.
55:441–449. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hart LA, Krishnan VL, Adcock IM, Barnes PJ
and Chung KF: Activation and localization of transcription factor,
nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med.
158:1585–1592. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jung WK, Lee DY, Choi YH, Yea SS, Choi I,
Park SG, Seo SK, Lee SW, Lee CM, Kim SK, et al: Caffeic acid
phenethyl ester attenuates allergic airway inflammation and
hyperresponsiveness in murine model of ovalbumin-induced asthma.
Life Sci. 82:797–805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Choi IW, Kim DK, Ko HM and Lee HK:
Administration of antisense phosphorothioate oligonucleotide to the
p65 subunit of NF-kappaB inhibits established asthmatic reaction in
mice. Int Immunopharmacol. 4:1817–1828. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park JH, Lee JK, Kim HS, Chung ST, Eom JH,
Kim KA, Chung SJ, Paik SY and Oh HY: Immunomodulatory effect of
caffeic acid phenethyl ester in Balb/c mice. Int Immunopharmacol.
4:429–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Park SG, Lee DY, Seo SK, Lee SW, Kim SK,
Jung WK, Kang MS, Choi YH, Yea SS, Choi I and Choi IW: Evaluation
of anti-allergic properties of caffeic acid phenethyl ester in a
murine model of systemic anaphylaxis. Toxicol Appl Pharmacol.
226:22–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Nader MA: Caffeic acid phenethyl ester
attenuates IgE-induced immediate allergic reaction.
Inflammopharmacology. 21:169–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yamamoto Y and Gaynor RB: Therapeutic
potential of inhibition of the NF-kappaB pathway in the treatment
of inflammation and cancer. J Clin Invest. 107:135–142. 2001.
View Article : Google Scholar : PubMed/NCBI
|