Introduction
Centella asiatica (L.) Urb. (Apiaceae), also
known as pegaga and gotu kola, has been used as a medicine in
tropical regions. This plant contains pharmacologically active
compounds including various pentacyclic triterpene derivatives,
such as centelloids (1–3). The Centella asiatica (C.
asiatica) extract contains four major triterpenoids, namely
asiatic acid, madecassic acid, asiaticoside and madecassoside, and
this mixture is commercially marketed as a titrated extract of
Centella asiatica (TECA). The C. asiatica extract is
used as an anti-microbial, anti-oxidative and anticancer agent, as
well as a therapeutic agent in the various processes of wound
healing, such as coagulation, inflammation, cell migration and
proliferation as well as scar formation and remodeling (4–9).
Following reports of the wound-healing properties of C.
asiatica in various studies, it has been used in skin cell
development and therapy. The major components of the skin are
collagen types I and III, which play a key role in wound healing
and are directly related to skin aging (10). Indeed, the C. asiatica
extract can promote an increase in both fibronectin and collagen
synthesis by 20–35% in skin fibroblasts (7,10–12). Furthermore, the C. asiatica
extract plays an important role in the process of anti-oxidation by
reducing the activity of reactive oxygen species (ROS), and thus
prevents hydrogen peroxide (H2O2)-induced
senescence in normal human dermal fibroblasts (NHDFs) (13–15).
Similar to ROS, ultraviolet (UV) radiation targets
the skin and continues to induce skin aging and cancer. Among the
three types of UV light (UVA, UVB and UVC), the UVB light only
penetrates into the epidermis and, therefore, UVB radiation at a
high dose can elicit severe skin damage. UV radiation stimulates
several biological processes in the skin, which include adaptive,
inflammatory and immunological reactions. Following UV irradiation,
adaptive responses are induced in the form of stratum
corneum thickening, pigmentation and epidermal hyperplasia
(16,17). UV exposure mediates an
inflammatory response, which is manifested as erythema and redness,
and is followed by the induction of apoptosis in keratinocytes
(16,18). Therefore, chronic UV irradiation
results in skin photoaging, which is characterized by irregular
pigmentation, dryness of the skin, wrinkling and elastosis
(18,19). Also, UV radiation activates
multiple signaling cascades, such as p38 mitogen-activated protein
kinase (MAPK), Jun N-terminal kinase (JNK), extracellular
signal-regulated kinase 1/2 (ERK1/2) and the NFκB pathways in skin
cells (19).
A preliminary study suggested that the C.
asiatica extract can serve as a potential natural protectant
against UVB damage in NHDFs (20). However, the cellular mechanisms
underlying the photoprotective effect of TECA against UV
irradiation have yet to be studied. This is the first report to
elucidate the cellular mechanisms of TECA-mediated photoprotection
against UV through the investigation of microRNA (miRNA) expression
profiling changes in NHDFs.
Materials and methods
Cell culture
The NHDF cells were purchased from Lonza (Basel,
Switzerland) and grown in DMEM media (Gibco-Invitrogen Life
Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum
(FBS; Sigma-Aldrich, St. Louis, MO, USA) with
penicillin/streptomycin in a humidified chamber at 37°C under 5%
CO2. Cells (4×103) were seeded into 96-well
plates a day before UVB exposure and treatment with the C.
asiatica extract. For RNA purification, 7×105 cells
were seeded into 60-mm dishes.
UVB irradiation and TECA treatment
Prior to UVB irradiation, cells were pre-treated
with control dimethylsulfoxide (DMSO; Sigma-Aldrich) or TECA (Bayer
HealthCare, Berlin, Germany) for 4 h. Cells were washed with PBS
twice and then exposed to 100 mJ/cm2 UVB without
covering the 96-well plates or 60-mm dishes, so that the UVB light
was not filtered. Following irradiation, the cells were cultured
for 24 h in DMEM media containing 10% FBS with DMSO or TECA.
RNA purification and qualification
NHDF cells exposed to UVB with or without TECA were
collected, and then total-RNA, including mRNAs, small RNAs and
miRNAs, was extracted and purified from each cell pellet using
TRIzol reagent (Invitrogen Life Technologies) according to the
manufacturer’s protocol. The integrity of each RNA sample was
verified using an Agilent 2100 Bioanalyzer® (Agilent
Technologies, Santa Clara, CA, USA). The quality and concentration
of each RNA sample were determined using MaestroNano®, a
micro-volume spectrophotometer (Maestrogen, Las Vegas, NV, USA).
RNA quality parameters for the miRNA microarray analysis were:
A260/280 and A260/A230 values >1.8 and an RNA integrity number
(RIN) >8.0.
Microarray analysis of miRNA
expression
The miRNA profiling analysis was performed using the
SurePrint G3 Human v16 miRNA 8×60K (Agilent Technologies) that
contained probes for 1,205 and 144 human viral miRNAs. The
qualified RNA samples (100 ng) were first dephosphorylated using
calf intestinal alkaline phosphatase (CIP) at 37°C for 30 min.
Next, DMSO was added to each sample, and the samples were incubated
at 100°C for 10 min and immediately transferred to an ice-water
bath. The dephosphorylated RNA samples were labeled with cyanine
3-pCp using T4 RNA ligase by incubation at 16°C for 2 h. After the
labeling reaction, the samples were completely dried using a vacuum
concentrator at 55°C for 4 h. The dried samples were treated with
GE Blocking Agent (Agilent Technologies) and hybridized to the
probes on the microarray at 55°C with a constant rotation at 20 rpm
in the Agilent Microarray Hybridization Chamber (Agilent
Technologies) for 20 h. The microarray slide was washed and scanned
using the Agilent scanner to obtain the microarray image. The
numerical data for the miRNA profiles were extracted from the image
using the Feature Extraction program (Agilent Technologies). These
data were analyzed with the aid of the GeneSpring GX software
version 7.3 (Agilent Technologies).
Classification of miRNAs
Among the total miRNAs probed on the microarray, 866
human miRNAs were selected for further analysis. The miRNAs whose
flags were present in at least one sample were filtered and applied
to the fold-change analysis. The fold-change analysis was conducted
to select miRNAs whose expression changed by a factor of 1.2-fold
or more between the following two groups: UVB-exposed and
DMSO-treated NHDF control cells and UVB-exposed and 50 μg/ml
TECA-treated NHDFs.
Bioinformatic analysis of miRNAs
Changes in miRNA expression of 1.2-fold and more
between the two groups were selected, and their putative cellular
target genes were determined using MicroCosm Target version 5
(www.ebi.ac.uk/enright-srv/microcosm/thdoc/targets/v5/).
Using a gene ontology (GO) analysis tool, AmiGO (amigo.geneontology.org/cgi-bin/amigo/browse.cgi), the
target genes were categorized into the following four groups:
aging, apoptosis, cell proliferation and skin development. Further
GO analysis for miRNA target genes that were also identified by
cross-linking and Argonaute (Ago) immunoprecipitation coupled with
high-throughput sequencing (CLIP-Seq) data was performed using
starBase web-based bioinformatics tools (starBase.sysu.edu.cn) (21).
Results
TECA treatment inhibits the decrease in
cell viability caused by UVB irradiation in NHDFs
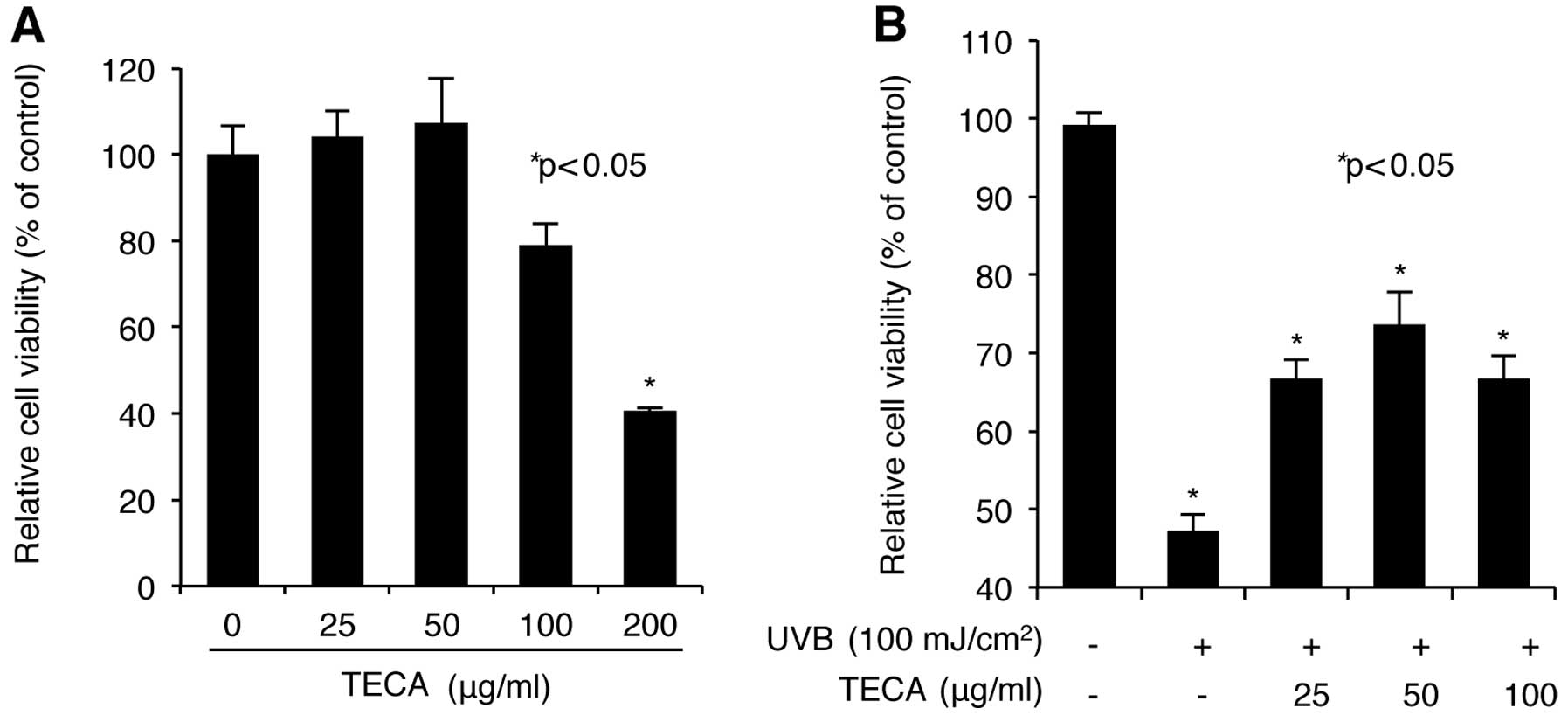
We first screened for the dose range of TECA that is
cytotoxic to NHDF cells. NHDF cells were treated with a series of
four concentrations of TECA (25, 50, 100 and 200 μg/ml) for 24 h,
and the WST-1-based cellular toxicity assay was used to determine
the level of cell viability. As shown in Fig. 1A, low doses (up to 50 μg/ml) of
TECA increased cell viability slightly; however, relatively high
doses (100 and 200 μg/ml) of TECA decreased cell viability. In
particular, although 100 μg/ml of TECA exhibited a low toxicity on
NHDF cells, 200 μg/ml of TECA largely decreased cell viability.
Therefore, TECA concentrations of 25, 50 and 100 μg/ml were chosen
for further experiments. Next, we investigated the protective
effect of TECA treatment against UVB-mediated damage of NHDFs. A
day before UVB irradiation, NHDF cells were seeded and incubated in
96-well plates. The cells were then pre-treated with TECA, at the
indicated concentration, for 4 h. The cells were then washed with
PBS and exposed to 100 mJ/cm2 of UVB without putting any
protective covers on the microplates. Following UVB irradiation,
the cells were incubated with TECA, at the indicated concentration,
for 24 h. Cell viability was determined using the WST-1 assay,
which revealed that treatment with TECA, in the range of 25 and 50
μg/ml, markedly restored the UVB-mediated loss of cell viability in
NHDFs to the normal status in a dose-dependent manner (Fig. 2B). Treatment with 100 μg/ml of
TECA did not increase cell viability more than the 50 μg/ml of
TECA, which can be attributed to the cytotoxic effect of 100 μg/ml
TECA on NHDF cells. Therefore, TECA displayed a protective effect
against UVB-mediated loss of cell survival observed in NHDF
cells.
The protective role of TECA in
UVB-induced NHDF damage is reflected as miRNA expression profiling
changes
Since miRNA is an important small non-coding RNA
molecule that regulates development, differentiation, proliferation
and apoptosis (22–25), we determined the protective effect
of TECA against UVB-induced cell damage through a miRNA expression
profiling analysis. Total-RNAs were purified from UVB-irradiated
NHDF control cells and from 50 μg/ml of TECA-stimulated and
UVB-irradiated NHDF cells, after which miRNA microarrays were
performed using the Agilent SurePrint G3 Human v16 miRNA 8×60K, as
described in Materials and methods. A total of 1,205 human miRNAs,
including 144 human viral miRNAs, were selected to analyze the
miRNA profiles. The human miRNAs whose flags were present in at
least one sample were continuously filtered to obtain more defined
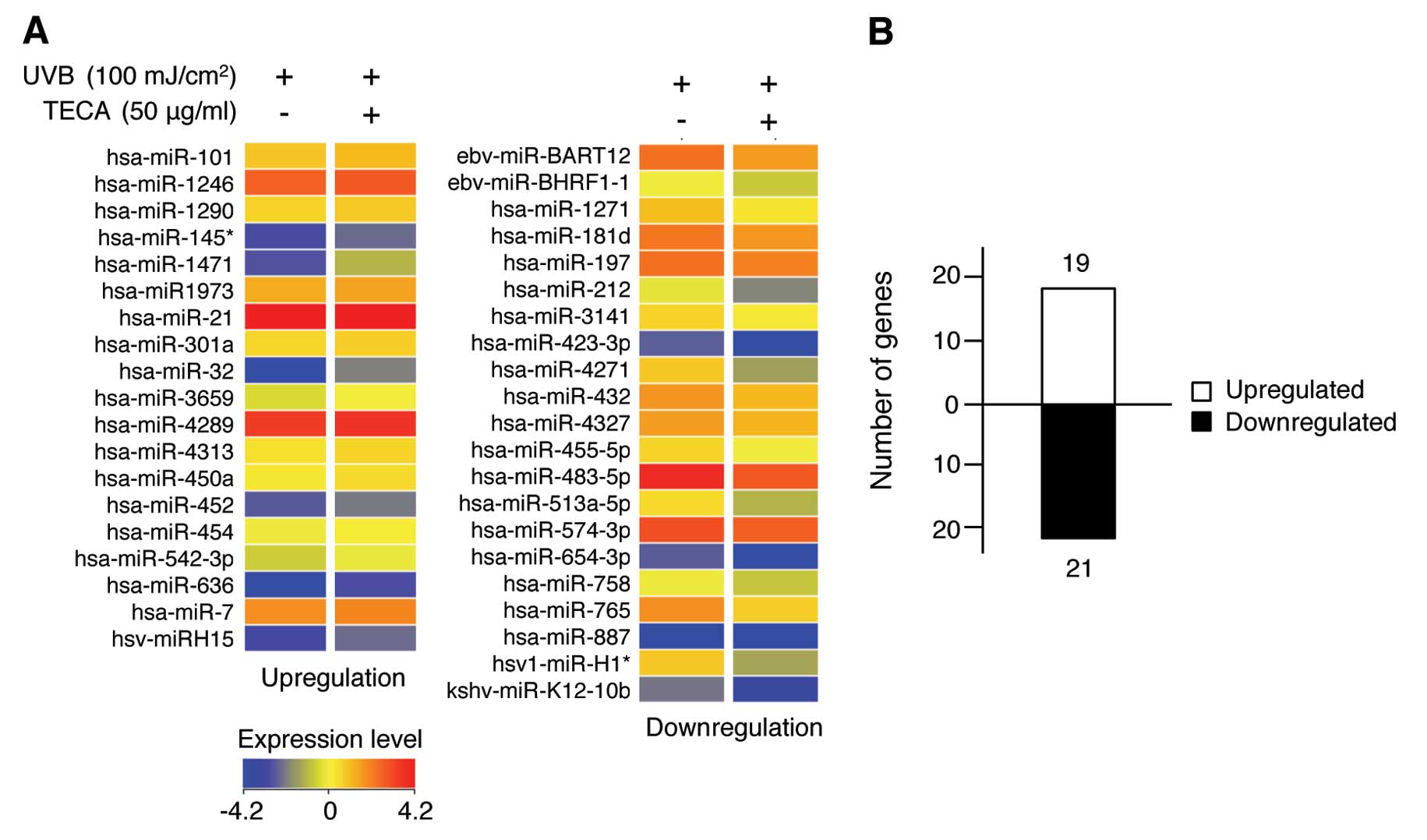
data using the Agilent GeneSpring software. As shown in Fig. 2A, a total of 40 human miRNAs were
differentially expressed following stimulation with TECA in
UVB-irradiated NHDF cells compared to cells exposed to UVB alone.
Upregulated miRNAs are shown in the left panel and downregulated
miRNAs are shown in the right panel of Fig. 2A. The color bar displaying altered
fluorescence intensity corresponds to miRNAs that were either
upregulated (red colors) or downregulated (blue color) by TECA
stimulation. The full list of the 40 miRNAs whose expression was
altered by TECA is listed in Table
I. The asterisk following the name indicates non-functional
miRNA or passenger strand that is released from the miRNA duplex
(26). Recent studies suggest
that miRNA* may offer potential opportunities for
contributing to the regulation network (27). As shown in Fig. 2B, 19 miRNAs were upregulated and
21 miRNAs were downregulated under the experimental conditions.
Although the majority of miRNAs did not show significant changes in
expression, treatment with TECA did affect certain miRNA expression
levels in NHDFs. These differentially expressed miRNAs may be
involved in specific mechanisms of TECA-mediated cellular responses
during the inhibition of UVB-induced cell damage in NHDFs.
 | Table I.miRNAs altered by TECA in UVB-exposed
NHDF cells. |
Table I.
miRNAs altered by TECA in UVB-exposed
NHDF cells.
Upregulated
| Downregulated
|
|---|
| miR name | FC | Chromosome | miR name | FC | Chromosome |
|---|
| hsa-miR-101 | 1.24 | Chr1 | ebv-miR-BART12 | 1.47 | - |
| hsa-miR-1246 | 1.21 | Chr2 |
ebv-miR-BHRF1-1 | 1.24 | - |
| hsa-miR-1290 | 1.20 | Chr1 | hsa-miR-1271 | 1.39 | Chr5 |
| hsa-miR-145* | 1.27 | Chr5 | hsa-miR-181d | 1.34 | Chr19 |
| hsa-miR-1471 | 2.08 | Chr2 | hsa-miR-197 | 1.20 | Chr1 |
| hsa-miR-1973 | 1.25 | Chr4 | hsa-miR-212 | 1.67 | Chr17 |
| hsa-miR-21 | 1.21 | Chr17 | hsa-miR-3141 | 1.21 | Chr5 |
| hsa-miR-301a | 1.21 | Chr17 | hsa-miR-423-3p | 2.01 | Chr17 |
| hsa-miR-32 | 2.65 | Chr9 | hsa-miR-4271 | 2.38 | Chr3 |
| hsa-miR-3659 | 1.28 | Chr1 | hsa-miR-432 | 1.35 | Chr14 |
| hsa-miR-4286 | 1.24 | Chr8 | hsa-miR-4327 | 1.22 | Chr21 |
| hsa-miR-4313 | 1.27 | Chr15 | hsa-miR-455-5p | 1.35 | Chr9 |
| hsa-miR-450a | 1.29 | ChrX | hsa-miR-483-5p | 1.71 | Chr11 |
| hsa-miR-452 | 1.23 | ChrX |
hsa-miR-513a-5p | 1.81 | ChrX |
| hsa-miR-454 | 1.29 | Chr17 | hsa-miR-574-3p | 1.20 | Chr4 |
| hsa-miR-542-3p | 1.25 | ChrX | hsa-miR-654-5p | 1.79 | Chr14 |
| hsa-miR-636 | 1.83 | Chr17 | hsa-miR-758 | 1.25 | Chr14 |
| hsa-miR-7 | 1.20 | Chr9 | hsa-miR-765 | 1.75 | Chr1 |
| hsv1-miR-H15 | 1.39 | - | hsa-miR-887 | 1.24 | Chgr5 |
| | | hsv1-miR-H1* | 2.35 | - |
| | |
kshv-miR-K12-10b | 1.51 | - |
Bioinformatic analysis of TECA-specific
miRNAs and their putative target genes in UVB-induced damage in
NHDF cells
miRNA expression profiling suggested a protective
role of TECA against UVB-induced damage that may be dependent on
the regulation of TECA-specific miRNA expression. These results
further highlight the significance of the altered miRNA expression
in light of the photoprotective property of TECA in UVB-induced
damage of NHDFs. Since the cellular functions of miRNAs are
mediated by controlling their target gene expression (28), we analyzed the cellular meaning of
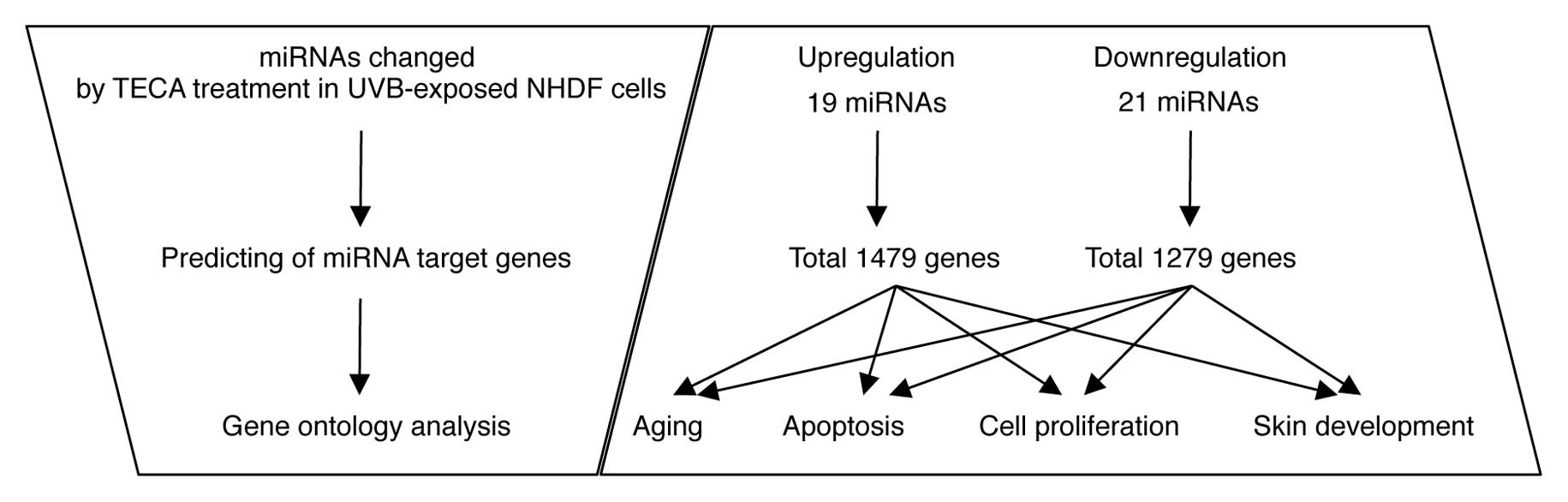
the TECA-dependent miRNA expression changes by sorting them
according to three independent criteria (Fig. 3): i) the putative target genes of
the differentially expressed miRNAs; ii) the cellular functions of
the target genes; and iii) the target genes involved in
TECA-mediated protective properties. First, using the miRBase
Target Database tool, Microcosm, we identified approximately 2,758
potential targets for all miRNA, excluding human viral miRNAs. A
total of 1,479 genes were identified as potential target genes for
the upregulated miRNAs and 1,279 genes were identified as potential
target genes for the downregulated miRNAs. Next, we identified the
potential target genes involved in TECA-mediated protective
properties against UVB damage, such as aging, apoptosis, cell
proliferation and skin development. Using a GO web-based tool,
AmiGO, we arranged the gene information in four classes: a total of
388 genes in aging, 3,824 genes in apoptosis, 3,148 genes in cell
proliferation and 399 genes in skin development were identified
(data not shown). We then compared these genes with the putative
target genes (1,479 genes corresponding to upregulated miRNAs and
1,279 genes corresponding to downregulated miRNAs), and the
overlapping genes in the two groups are listed in Tables II and III. Some of the miRNAs were potentially
targeted by more than one miRNA, since a single miRNA may target a
number of mRNAs, and, conversely, a single mRNA target may be
modulated by several miRNAs (29).
 | Table II.Predicted targets of miRNAs
exhibiting an upregulation in response to TECA in UVB-exposed NHDF
cells. |
Table II.
Predicted targets of miRNAs
exhibiting an upregulation in response to TECA in UVB-exposed NHDF
cells.
| Function of target
genes
|
|---|
| miRNA name | Aging | Apoptosis | Cell
proliferation | Skin
development |
|---|
| hsa-miR-21 | TBX2, PTEN, LRP2,
MSH2, PDCD4 | ARHGEF12, BCL7,
CCR7, FASLG, KRIT1, LRP2, MAP3K1, NTF3, PDCD4, PTEN, RHOB, SKI,
TIAM1, UBE2D3, SATB1, ACVR1C | DDX11, FGF1,
GATAD2B, IL12A, JAG1, KRIT1, LRP6, PBRM1, PELI1, PITX2, SKI, SPRY1,
TBX2, TGFB1 | - |
| hsa-miR-32 | HCN2, NOX4, PER2,
TWIST1, ADRB1 | ACTC1, ARHGEF17,
BCL2L11, BTG2, GATA6, HAND2, HIPK3, ITGA6, ITGAV, KIF1B, LYST,
MAP2K4, RAD21, SGK3, TRAF3, TRIO, TWIST1, UBE2Z, ADRB1, CDK5R1,
GP1, JMY, NR4A3, SNF1LK | BTG2, CDC27,
CDCA7L, CDKN1C, FOSL2, GATA2, MS4A2, NKX2-3, NOX4, PAX3, PCAF,
PTPRK, TACC2, TGIF1, TOB2, RAP1B, SOX11, BMPR2, TSC1, ZEB2 | BCL11B, COL1A2 |
| hsa-miR-101 | FOS, TIAM2, ADRB1,
LRP2 | ARHGEF3, DUSP1,
JAK2, MSX1, PHLDA1, PROK2, RAC1, SCN2A, SGK1, TGFBR1, USP47,
UBE2D3, ADRB1, CDKR1, GPI, DDIT4, MITF, PRKCE, TCF7L2, ROBO2, GJA1,
NEUROD1, PRKAA1, | CDH5, CEBPA, DLG5,
ELF5, EMP1, GNB1, HRB, JAK2, NDFIP1, PDS5B, PTGS2, RXRB, SOX9,
TAL1, TGFA, TGFBR1, UBE2A, RAP1B, SOX11, FZD6, LRP2, PTCH1 | - |
| hsa-miR-7 | - | AMBRA1, COL2A1,
CTSB, FNDC4, GLI3, HELLS, OGT, PRMT2, PSME3, RAF1, RB1, SNCA,
SORT1, VDAC1, SATB1, DDIT4, JMY, NR4A3, PHF17 | CONT8, CUL5, EGFR,
IRS1, PAX6, UHRF1, SATB1, IRS2 | COL2A1 |
| hsa-miR-301a | TP63, WNT1, LRRK2,
LRP2 | DLC1, TP63, WNT1,
TP63, APPL1, FXR1, SOX4, ROBO2 | DLEC1, ESR1, BMPR2,
TSC1, USP28, ZEB2, EREG, FOSL1, HOXA3, IMPDH1, INSIG1, IRF1,
JARID2, LRRK2, NR3C2, PPARG, TBC1D8, WNT28, FZD6, LRP2 | TP63, WNT10A,
EDA |
| hsa-miR-452 | IGF2BP2, TIMP3 | ERBB4, VEGFA,
IGF1 | BMI1, CDKN1B, EPS8,
ERBB4, IGF2BP2, LAMC1, MAB21L2, MAPRE1, MXD1, NPPC, PURA, RPA1,
TIMP2, VEGFA, PTPRJ, IRS2 | - |
| hsa-miR-636 | SOCS3 | ARF6, GRIK2, ITSN1,
PCGF2, PKN2, PROC, RPS6KA2, RTN3, SENP1, SFRP2, SOCS3, MITF, PRKCE,
TCF7L2, SNF1LK, TGFBR2, ZAK | BCAT1, EMX2, LIFR,
SSR1, TRAF5, MITF, TOB1 | - |
| hsa-miR-454 | LRRK2, LRP2 | NELL1, APPL1, FXR1,
SOX4, ROBO2, SIX4, ARHGEF4, BTG1, PAK6, POU4F1, PRKAA2, RASA1,
RNF216, RNF41, RNUX3, SLTM, SOS2, SPHK2, TP53INP1, TRIM2, GJA1,
NEUROD1, PRKAA1, SYNGAP1, IGF1, ZAK | NRP1, TNF, BMPR2,
TSC1, USP28, ZEB2, BTG1, EREG, FOSL1, HOXA3, IMPDH1, INSIG1, IRF1,
JARID2, LRRK2, NR3C2, PPARG, RUNX3, TBC1D8, WNT2B, FZD6, LRP2 | EDA |
 | Table III.Predicted targets of miRNAs
exhibiting a downregulation in response to TECA in UVB-exposed NHDF
cells. |
Table III.
Predicted targets of miRNAs
exhibiting a downregulation in response to TECA in UVB-exposed NHDF
cells.
| Function of target
genes
|
|---|
| miRNA name | Aging | Apoptosis | Cell
proliferation | Skin
development |
|---|
| hsa-miR-197 | - | CECR2, CTNNA1,
CYLD, TNFRSF21, RASA1, HIPK2, GP1 | FZD3, IGFBP3, TAL1,
FBXW7, HIPK2, PDGFRA | - |
| hsa-miR-212 | CTGF | ARHGEF11, CTGF,
DYRK2, EP300, FOXA1, FOXO3, GDF5, ISL1, KCNMA1, MAPK3, MAPT, RASA1,
SGK3, RASA1 | CTGF, EGR1, HHIP,
ISL1, RB1, SALL1, SPRY1, ZEB2, HBEGF, SOX11 | - |
| hsa-miR-432 | DLD, MNT | ADAR, CHAC1,
DAB2IP, HOXA5, IL7, MNT, PAX8, PLK3, SORT1, HIPK2 | CCDC88A, E2F3, IL7,
MNT, FBXW7, HIPK2 | - |
| hsa-miR-181d | ADRBK1, PRKCD, PAI,
SIRT1, TIMP3 | ANKRD13C, ATM,
BAG4, BCL2L11, BIRC6, CARD11, CBX4, DEPDC6, GATA6, HEY2, HSP90B1,
IL1A, INSL3, ITSN1, NOCTCH, PAWR, PDCD6IP, PHLDA1, PRKCD, RAD21,
RNF34, RPS6KA3, SIRT1, TGFBR1, TNF, TNFAIP1, UBE2B, TRIM2, USP47,
CCNG1 | ATM, BIRC6, CARD11,
CDON, GATA6, HEY2, IGF2BP2, IL1A, ING5, INSL3, IRS2, LRRC32, MCC,
NR6A1, PAWR, PDAP1, PLAU, PRDM4, PROX1, RBBP7, SERPINE1, SPRK2,
TNF, S1PR1, KRAS, TNS3 | - |
|
hsa-miR-513a-5p | CDK6, CHEK2, GRB2,
HMGCR, SERP1 | BNIP3L, CHEK2,
ECE1, EYA1, MAPK7, NOD2, PHLDB3, PPARGC1A, RAG1, XIAP, TRIM2,
USP47 | ASH2L, ATF3, CDK6,
DDX11, EHF, EPS8, EYA1, MAGI2, NOD2, PCAF, PDS5B, PURA, SMAD2,
TBX19, VSX2, S1PR1, KRAS | - |
| hsa-miR-455-5p | LRP2, SOCS3 | ETS1, FZD5, GABRB2,
KPNA1, SOCS3, TJP1, GP1 | CDC2L5, FZD5, IRF2,
KDR, LRP2, NCK2, PTPRJ, PDGFRA, SOX11 | - |
| hsa-miR-574-3p | - | CUL2, RXRA | CUL2, RXRA | - |
| hsa-miR-654-5p | BBC3, DBH | ARAF, BBC3, WNT11,
KPNB1 | DBH, EFNB1, ELLN,
TIMP2, WNT11, IRS1, MTSS1 | - |
| hsa-miR-758 | JUN | BCL11B, BMP7,
RABEP1 | BCL11B, BMP7, IGF1,
JUN, STAT5B | BCL11B |
| hsa-miR-765 | LMNA, TIMP3 | EGLN2, LMNA,
OSM | CDK2, CSF1R, OSM,
TXLNA, GPC3 | - |
| hsa-miR-1271 | CASP2, DDIT3,
EDNRA, MAP2K1 | ALK, AHR, DDIT3,
DOCK1, EDNRA, EPHA3, FOXO1, FOXQ1, MBD4, OGT, PLAGL1, PRKCE, PROK2,
PSME4, RALB, STK17A, TNFSF13B, TRIB3, TXNDC1, CCNG1, SORT1,
KPNB1 | AHR, CD164, DIXDC1,
EDNRA, FRAP1, FRS2, FYN, HOOK3, LAMC1, LIPG, MAB21L2, MAP2K1, MED1,
MYO16, NEUROD4, PGGT1B, TACC1, TNFSF13B, TXNDC1, TNS3, HBEGF, KRAS,
IRS1, MTSS1, GPC3 | - |
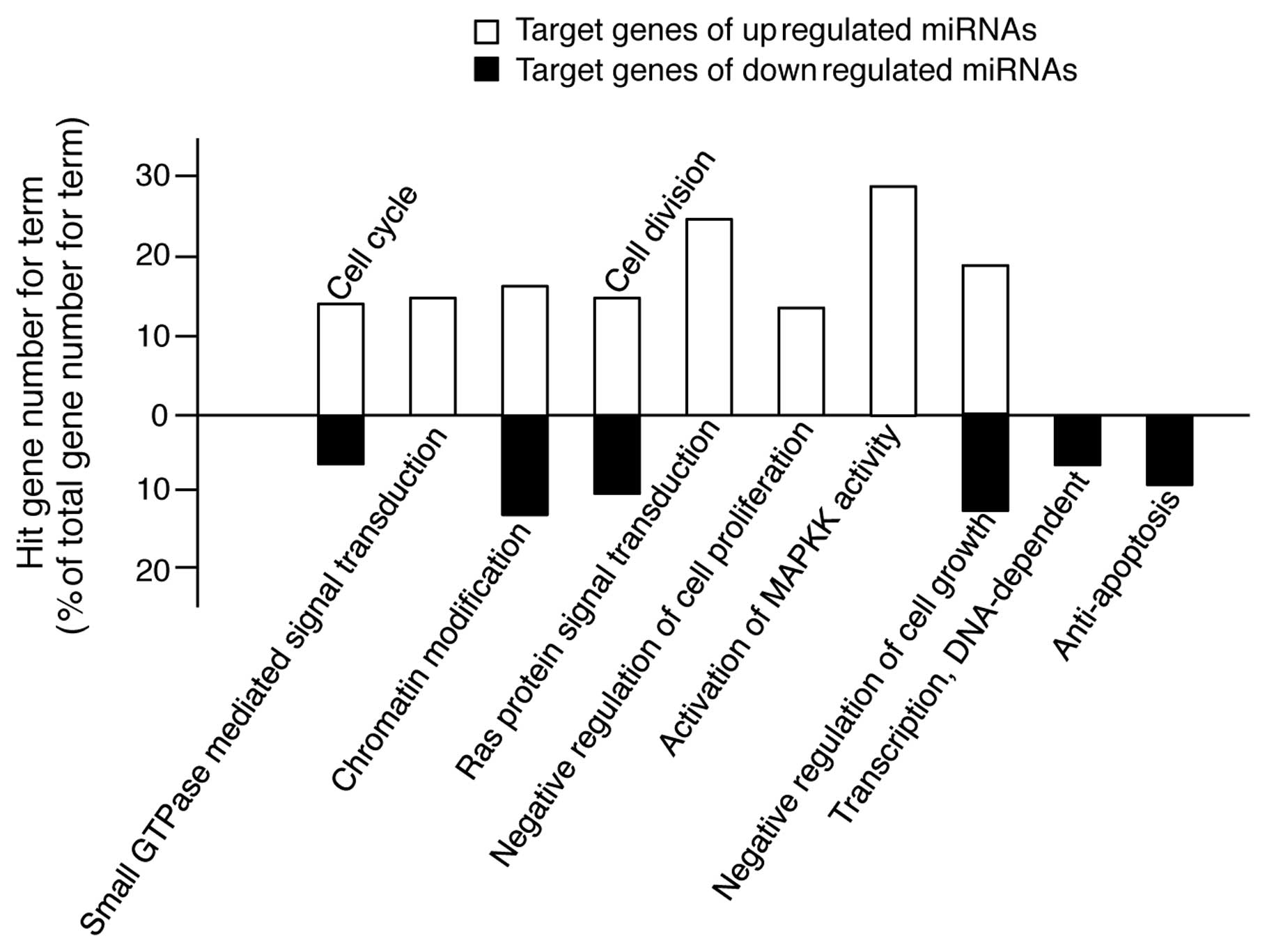
The GO terms in Tables
II and III cover a relatively
wide range of cellular processes. For example, the GO term of
apoptosis encompasses all the genes involved in apoptosis-promoting
and apoptosis-inhibiting processes. Therefore, for a more accurate
analysis, we rearranged the results shown in Tables II and III into a subset of GO terms, such as
positive or negative regulation of the cell cycle, cell division,
cell proliferation, cell growth and apoptosis, GTPase-, Ras-,
MAPKK-mediated signal transduction and DNA-dependent transcription.
As shown in Fig. 4, the target
genes of the upregulated miRNAs are involved in promoting processes
of cell proliferation; however, the target genes of the
downregulated miRNAs are involved in inhibiting processes of
apoptosis. Therefore, these findings suggest that TECA-mediated
protective effects against UVB-induced damage in NHDFs is related
to the changes in expression of specific miRNAs involved in cell
proliferation and apoptosis.
Discussion
The present study demonstrated that TECA exhibits a
protective effect against UVB-mediated damage in NHDF cells via
regulation of miRNA expression. The cytotoxicity and viability
assay revealed that a low dose of TECA (25 and 50 μg/ml) is not
toxic to NHDF cells, and UVB-mediated loss of cell viability is
recovered by stimulation with low doses of TECA. Also, TECA
stimulation of NHDF cells changes their miRNA expression profiles,
and the differentially expressed miRNAs may have potential
anti-apoptotic properties, as revealed by a bioinformatic analysis
of their putative target genes and GO analysis of the target genes.
Therefore, TECA-mediated changes in miRNA expression may regulate
the anti-proliferative effect of UVB irradiation on NHDF cells.
The bioinformatic analysis shown in Fig. 4 may represent the paradoxical
roles of TECA in NHDF cell proliferation, since some of the target
genes of the upregulated miRNAs were functionally associated with
positive regulation of the cell cycle and cell division and with
negative regulation of cell growth, thus suggesting that the
TECA-mediated upregulation of miRNAs can inhibit cell
proliferation. However, these results may be due to the residual
cytotoxicity of UVB irradiation in the system used. The fact that
treatment with 50 μg/ml TECA did not completely restore the
UVB-induced loss of cell viability to the normal status indicates
that the anti-proliferative effect of UVB remained, although at a
low level, in the experiments shown in Fig. 1B. Of note, the target genes of the
miRNAs that were downregulated by TECA were functionally related to
anti-apoptosis, negative regulation of cell growth, cell cycle and
cell division. These results demonstrate that the downregulated
miRNAs can functionally induce pathways of anti-apoptosis and cell
proliferation.
Additionally, the target genes of the miRNAs
upregulated by TECA were shown to be involved in small
GTPase-mediated signal transduction and Ras protein signal
transduction. These results indicate that the TECA-mediated
anti-apoptotic effect against UVB-mediated NHDF damage can be
mediated by inhibiting the small GTPase- and Ras-mediated signaling
pathways via upregulation of miRNAs that target the genes involved
in the above mechanisms. Rac1 is a small Rho GTPase, which is a key
transducer of proliferation and apoptosis in various cells,
including NHDFs (30–32). Rac1 has primarily been found to
induce NHDF proliferation via phosphorylation of the oncogene c-myc
(32). However, the pro-apoptotic
roles of Rac1 have been reported in previous studies. Rac1 induces
apoptosis via JNK in epithelial cells (33). Also, Rac1 stimulates apoptosis
through the activation of trivalent chromium in human dermal
fibroblasts (34). Furthermore,
Rac1 is necessary for the apoptotic process induced by UV
irradiation in Rat-2 fibroblasts, suggesting a stimulatory role of
Rac1 in apoptosis caused by perturbation of homeostasis (35). Although Ras proteins are known as
oncogenes, their pro-apoptotic function has also been reported. UV
irradiation induces apoptosis via the activation of Ha-ras and via
increasing the phosphorylation of Raf-1 and subsequently activating
c-Jun and other AP-1 proteins (36). The R-Ras protein promotes
apoptosis that is caused by growth factor deprivation in Rat-1
fibroblasts. Furthermore, in response to stress, the GTP-bound Ras
activates MEKKSEK-SARK-c-JUN and induces apoptosis (37–39). Therefore, there is a strong
possibility that the protective effect of TECA treatment in
UVB-irradiated NHDFs can be induced by upregulation of specific
miRNAs that inhibit the signal transduction mediated by small
GTPases and Ras.
We also showed that the majority of the target genes
of the miRNAs upregulated by TECA were involved in the activation
of MAPKK activity. MAPKK is a kinase that phosphorylates a MAPK,
such as p38, JNK and ERK1/2. UV irradiation of skin cells including
keratinocytes, melanocytes and dermal fibroblasts can regulate cell
fate via the activation of MAPK-mediated signaling pathways.
UV-activated p38 MAPK and JNK in skin cells have been shown to be
involved in both cell survival and cell death pathways (40–43). However, the ERK1/2 pathway has
been implicated in generating anti-apoptotic signals in skin cells
(44). Also, UV irradiation did
not increase p38 and JNK protein synthesis in the cells, but rather
increased the level of phosphorylated p38 activity (45,46). These results indicate that UV
irradiation causes phosphorylation-mediated activation of p38 MAPK,
JNK and ERK1/2 in skin cells; however, its effects on cells can be
observed in both apoptosis and cell survival. Therefore, the reason
that the target genes of upregulated miRNAs are highly involved in
the activation of MAPKK activity is the possibility that the
UVB-protective properties of TECA can be mediated through the
regulation of biphasic MAPK responses.
In conclusion, we determined for the first time that
TECA treatment of UVB-exposed NHDF cells causes a photoprotective
effect via a change in miRNA expression. The cellular mechanisms
underlying the photoprotective effect of TECA against UV
irradiation remain unknown; however, our study provides substantial
evidence of the role of TECA as a chemoprotective agent against
UVB-mediated damage in human dermal fibroblasts. Although further
studies must be performed to verify the predicted miRNA targets
identified in this study, our results suggest that characterization
of TECA-specific miRNA changes may provide a useful approach to
understanding cellular responses to TECA in UVB-induced NHDF
damage.
Acknowledgements
We thank all the members of our
research group for their support and advice during this study. This
study was supported by the Ministry of Education, Science and
Technology (grant 20110028646 to S.A.) of the Republic of
Korea.
References
|
1.
|
CD ColdrenP HashimJM AliSK OhAJ SinskeyC
RhaGene expression changes in the human fibroblast induced by
Centella asiatica triterpenoidsPlanta
Med69725732200310.1055/s-2003-4279114531023
|
|
2.
|
B BrinkhausM LindnerD SchuppanEG
HahnChemical, pharmacological and clinical profile of the East
Asian medical plant Centella
asiaticaPhytomedicine7427448200010.1016/S0944-7113(00)80065-311081995
|
|
3.
|
JT JamesIA DuberyPentacyclic triterpenoids
from the medicinal herb, Centella asiatica (L.)
UrbanMolecules1439223941200910.3390/molecules1410392219924039
|
|
4.
|
G JayashreeG Kurup MuraleedharaS
SudarslalVB JacobAnti-oxidant activity of Centella asiatica
on lymphoma-bearing miceFitoterapia744314342003
|
|
5.
|
TD BabuG KuttanJ PadikkalaCytotoxic and
anti-tumour properties of certain taxa of Umbelliferae with special
reference to Centella asiatica (L.) UrbanJ
Ethnopharmacol485357199510.1016/0378-8741(95)01284-K8569247
|
|
6.
|
FX MaquartF ChastangA SimeonP BirembautP
GilleryY WegrowskiTriterpenes from Centella asiatica
stimulate extracellular matrix accumulation in rat experimental
woundsEur J Dermatol92892961999
|
|
7.
|
F BonteM DumasC ChaudagneA
MeybeckInfluence of asiatic acid, madecassic acid, and asiaticoside
on human collagen I synthesisPlanta
Med60133135199410.1055/s-2006-9594348202564
|
|
8.
|
MR CesaroneG BelcaroMT De SanctisEffects
of the total triterpenic fraction of Centella asiatica in
venous hypertensive microangiopathy: a prospective,
placebo-controlled, randomized trialAngiology52Suppl 215182001
|
|
9.
|
L IncandelaMR CesaroneM CacchioTotal
triterpenic fraction of Centella asiatica in chronic venous
insufficiency and in high-perfusion microangiopathyAngiology52Suppl
29132001
|
|
10.
|
F BonteM DumasC ChaudagneA
MeybeckComparative activity of asiaticoside and madecassoside on
type I and III collagen synthesis by cultured human fibroblastsAnn
Pharm Fr5338421995(In French).
|
|
11.
|
R TenniG ZanaboniMP De AgostiniA RossiC
BendottiG CettaEffect of the triterpenoid fraction of Centella
asiatica on macromolecules of the connective matrix in human
skin fibroblast culturesItal J Biochem3769771988
|
|
12.
|
FX MaquartG BellonP GilleryY WegrowskiJP
BorelStimulation of collagen synthesis in fibroblast cultures by a
triterpene extracted from Centella asiaticaConnect Tissue
Res24107120199010.3109/030082090091524272354631
|
|
13.
|
T OttE FritzA PolleA
SchutzendubelCharacterisation of antioxidative systems in the
ectomycorrhiza-building basidiomycete Paxillus involutus
(Bartsch) Fr. and its reaction to cadmiumFEMS Microbiol
Ecol42359366200210.1111/j.1574-6941.2002.tb01025.x19709295
|
|
14.
|
RA MustafaA Abdul HamidS MohamedFA
BakarTotal phenolic compounds, flavonoids, and radical scavenging
activity of 21 selected tropical plantsJ Food
Sci75C28C35201010.1111/j.1750-3841.2009.01401.x20492146
|
|
15.
|
YJ KimHJ ChaKH NamY YoonH LeeS
AnCentella asiatica extracts modulate hydrogen
peroxide-induced senescence in human dermal fibroblastsExp
Dermatol209981003201110.1111/j.1600-0625.2011.01388.x
|
|
16.
|
GJ ClydesdaleGW DandieHK MullerUltraviolet
light induced injury: immunological and inflammatory effectsImmunol
Cell Biol79547568200110.1046/j.1440-1711.2001.01047.x11903614
|
|
17.
|
A HennessyC OhJ ReesB DiffeyThe
photoadaptive response to ultraviolet exposure in human skin using
ultraviolet spectrophotometryPhotodermatol Photoimmunol
Photomed21229233200510.1111/j.1600-0781.2005.00170.x16149934
|
|
18.
|
Y MatsumuraHN AnanthaswamyToxic effects of
ultraviolet radiation on the skinToxicol Appl
Pharmacol195298308200410.1016/j.taap.2003.08.01915020192
|
|
19.
|
V MuthusamyTJ PivaThe UV response of the
skin: a review of the MAPK, NFkappaB and TNFalpha signal
transduction pathwaysArch Dermatol
Res302517201010.1007/s00403-009-0994-y19756672
|
|
20.
|
P HashimH SidekMH HelanA SaberyUD
PalanisamyM IlhamTriterpene composition and bioactivities of
Centella
asiaticaMolecules1613101322201110.3390/molecules1602131021278681
|
|
21.
|
JH YangJH LiP ShaoH ZhouYQ ChenLH
QustarBase: a database for exploring microRNA-mRNA interaction maps
from Argonaute CLIP-Seq and Degradome-Seq dataNucleic Acids
Res39D202D209201110.1093/nar/gkq105621037263
|
|
22.
|
V AmbrosRC LeeIdentification of microRNAs
and other tiny noncoding RNAs by cDNA cloningMethods Mol
Biol265131158200415103073
|
|
23.
|
AM ChengMW ByromJ SheltonLP FordAntisense
inhibition of human miRNAs and indications for an involvement of
miRNA in cell growth and apoptosisNucleic Acids
Res3312901297200510.1093/nar/gki20015741182
|
|
24.
|
JF ChenEM MandelJM ThomsonThe role of
microRNA-1 and microRNA-133 in skeletal muscle proliferation and
differentiationNat Genet38228233200610.1038/ng172516380711
|
|
25.
|
WJ ChoJM ShinJS KimmiR-372 regulates cell
cycle and apoptosis of ags human gastric cancer cell line through
direct regulation of LATS2Mol
Cells28521527200910.1007/s10059-009-0158-019937137
|
|
26.
|
AS O’TooleS MillerN HainesMC ZinkMJ
SerraComprehensive thermodynamic analysis of 3’ double-nucleotide
overhangs neighboring Watson-Crick terminal base pairsNucleic Acids
Res34333833442006
|
|
27.
|
L GuoZ LuThe fate of miRNA*
strand through evolutionary analysis: implication for degradation
as merely carrier strand or potential regulatory molecule?PLoS
One5e11387201020613982
|
|
28.
|
RS PillaiSN BhattacharyyaW
FilipowiczRepression of protein synthesis by miRNAs: how many
mechanisms?Trends Cell
Biol17118126200710.1016/j.tcb.2006.12.00717197185
|
|
29.
|
B JohnAJ EnrightA AravinT TuschlC SanderDS
MarksHuman MicroRNA targetsPLoS
Biol2e363200410.1371/journal.pbio.0020363
|
|
30.
|
AB JaffeA HallRho GTPases: biochemistry
and biologyAnnu Rev Cell Dev
Biol21247269200510.1146/annurev.cellbio.21.020604.15072116212495
|
|
31.
|
L WangY ZhengCell type-specific functions
of Rho GTPases revealed by gene targeting in miceTrends Cell
Biol175864200710.1016/j.tcb.2006.11.00917161947
|
|
32.
|
E NikolovaV MitevN ZhelevCF DeroanneY
PoumayThe small Rho GTPase Rac1 controls normal human dermal
fibroblasts proliferation with phosphorylation of the oncoprotein
c-mycBiochem Biophys Res
Commun359834839200710.1016/j.bbrc.2007.05.21417568564
|
|
33.
|
S JinRM RayLR JohnsonRac1 mediates
intestinal epithelial cell apoptosis via JNKAm J Physiol
Gastrointest Liver
Physiol291G1137G1147200610.1152/ajpgi.00031.200616798728
|
|
34.
|
E RudolfM CervinkaTrivalent chromium
activates Rac-1 and Src and induces switch in the cell death mode
in human dermal fibroblastsToxicol
Lett188236242200910.1016/j.toxlet.2009.04.01919406221
|
|
35.
|
YW EomMH YooCH WooImplication of the small
GTPase Rac1 in the apoptosis induced by UV in Rat-2
fibroblastsBiochem Biophys Res
Commun285825829200110.1006/bbrc.2001.523311453667
|
|
36.
|
Y DevaryRA GottliebT SmealM KarinThe
mammalian ultraviolet response is triggered by activation of Src
tyrosine
kinasesCell7110811091199210.1016/S0092-8674(05)80058-31473146
|
|
37.
|
MT RamirezVP SahXL ZhaoJJ HunterKR ChienJH
BrownThe MEKK-JNK pathway is stimulated by alpha1-adrenergic
receptor and ras activation and is associated with in vitro and in
vivo cardiac hypertrophyJ Biol
Chem2721405714061199710.1074/jbc.272.22.140579162028
|
|
38.
|
I SanchezRT HughesBJ MayerRole of SAPK/ERK
kinase-1 in the stress-activated pathway regulating transcription
factor c-JunNature372794798199410.1038/372794a07997269
|
|
39.
|
M YanT DaiJC DeakActivation of
stress-activated protein kinase by MEKK1 phosphorylation of its
activator SEK1Nature372798800199410.1038/372798a07997270
|
|
40.
|
N ChouinardK ValerieM RouabhiaJ
HuotUVB-mediated activation of p38 mitogen-activated protein kinase
enhances resistance of normal human keratinocytes to apoptosis by
stabilizing cytoplasmic p53Biochem
J365133145200210.1042/BJ2002007212071847
|
|
41.
|
J HildesheimRT AwwadAJ Fornace Jrp38
Mitogen-activated protein kinase inhibitor protects the epidermis
against the acute damaging effects of ultraviolet irradiation by
blocking apoptosis and inflammatory responsesJ Invest
Dermatol122497502200410.1111/j.1523-1747.2004.22229.x
|
|
42.
|
YR ChenX WangD TempletonRJ DavisTH TanThe
role of c-Jun N-terminal kinase (JNK) in apoptosis induced by
ultraviolet C and gamma radiation. Duration of JNK activation may
determine cell death and proliferationJ Biol
Chem2713192931936199610.1074/jbc.271.50.319298943238
|
|
43.
|
R WisdomRS JohnsonC Moorec-Jun regulates
cell cycle progression and apoptosis by distinct mechanismsEMBO
J18188197199910.1093/emboj/18.1.1889878062
|
|
44.
|
YY HeJL HuangCF ChignellDelayed and
sustained activation of extracellular signal-regulated kinase in
human keratinocytes by UVA: implications in carcinogenesisJ Biol
Chem2795386753874200410.1074/jbc.M40578120015471881
|
|
45.
|
JW ChoK ParkGR KweonCurcumin inhibits the
expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT)
by inhibiting activation of AP-1: p38 MAP kinase and JNK as
potential upstream targetsExp Mol
Med37186192200510.1038/emm.2005.2516000872
|
|
46.
|
AL KimJM LabasiY ZhuRole of p38 MAPK in
UVB-induced inflammatory responses in the skin of SKH-1 hairless
miceJ Invest
Dermatol12413181325200510.1111/j.0022-202X.2005.23747.x15955110
|