Introduction
Tumor-associated antigen receptor-binding cancer
antigen expressed on SiSo cells (RCAS1) is a type-II membrane
protein, expressed in estrogen target organs as well as several
other tissues such as the brain, liver and kidney tissues (1). Previous studies have shown that
RCAS1 is expressed in a variety of malignancies, such as breast,
ovarian, prostate and hepatocellular carcinoma (2–5).
The RCAS1 protein acts as a ligand for a putative receptor present
in various human cells, including normal peripheral lymphocytes,
such as T, B and natural killer (NK) cells. RCAS1 inhibits the
growth of receptor-expressing cells both in vitro and in
vivo and induces apoptotic cell death through the activation of
caspase-3 and the collapse of mitochondrial transmembrane potential
(6,7). Therefore, it has been generally
accepted that RCAS1 is involved in the immune escape of tumor
cells. Immunohistochemical studies of tissue samples have revealed
that RCAS1 expression significantly correlates with poor overall
survival in patients with a variety of malignancies (8–10).
However, the possible mechanism behind the RCAS1-induced cell
apoptosis has not yet been elucidated.
In our previous studies, we induced the expression
and purification of glutathione S-transferase (GST)-RCAS1 fusion
protein (11), and reported that
RCAS1 promoted the tumor growth and metastasis of 4T1 murine
mammary carcinoma cells (12). In
this study, we used the recombinant adenovirus RCAS1 (Ad-RCAS1) and
GST-RCAS1 fusion protein, to investigate the apoptosis of T
lymphocytes and immune cells derived from leukemia cell lines
induced by RCAS1. We detected the expression of the RCAS1 receptor
(RCAS1R) in the cell lines, and investigated the mechanism behind
the apoptosis induced by RCAS1. We used immunohistochemical
analysis to examine the expression of this molecule in human breast
tissues.
Materials and methods
Reagents
Lipofectamine 2000 was obtained from Invitrogen
(Carlsbad, CA, USA). GST monoclonal antibody (MoAb) was purchased
from Kangchen Biotech Co. (Shanghai, China). The B-PER GST Fusion
Purification kit was purchased from Pierce Biotechnology (Rockford,
IL, USA). Mouse GAPDH MoAb, donkey anti-rabbit IgG (H+L)
horseradish peroxidase (HRP)-, donkey anti-goat IgG (H+L) HRP- and
goat anti-mouse IgG (H+L) HRP-conjugated antibodies were all from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Goat polyclonal
antibodies (PoAbs) to RCAS1 (N18 and C20; sc-23396 and sc-23394,
respectively; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
rabbit PoAbs to glycogen synthase kinase 3β (GSK3β) and
phospho-Ser9-GSK3β (BioVision Technologies, Exton, PA, USA) were
commercially purchased. RCAS1 primer was synthesized by Shanghai
Sangon Biological Engineering Technology and Services Co., Ltd.
(Shanghai, China). This study was carried out in accordance with
the Declaration of Helsinki. All the data presented and materials
used in this manuscript were approved by the Ethics Committee of
the Children’s Hospital of Zhejiang University School of Medicine,
Hangzhou, China.
Cell culture and treatment
A293 (human embryonic kidney), HeLa
(adenocarcinoma), K562 (chronic myelogenous leukemia), Jurkat
(acute T cell leukemia), THP1 (acute monocytic leukemia) and E003
(acute B cell leukemia) cells were from the American Type Culture
Collection (Manassas, VA, USA). All the cells were grown in
RPMI-1640 supplemented with 10% (v/v) fetal calf serum, 4.5 g/l
D-glucose, non-essential amino acids (100 μM each), 100 U/ml
penicillin, 100 μg/ml streptomycin and 2 mM glutamine at
37°C in a 5% CO2 atmosphere. HeLa cells were transfected
with the Ad-RCAS1 and Ad-LacZ vectors, using Lipofectamine 2000
according to the manufacturer’s instructions. Following
transfection, RT-PCR and western blot analysis were carried out to
determine the expression of RCAS1, β-actin or GAPDH, and the
effectiveness of the assay was determined by the negative control.
K562, Jurkat, THP1 and E003 cells were cultured in 6-well flat
bottom plates (5×105 cells/ well), and then Ad-RCAS1
[5×107 plaque forming units (PFU), multiplicity of
infection (MOI) 100] and Ad-LacZ (5×107 PFU, MOI 100)
were added to the medium. After 24 h of culture, the cells were
harvested for apoptosis assay.
Amplification of recombinant adenovirus
Ad-RCAS1 and Ad-LacZ
Recombinant adenovirus Ad-RCAS1 and Ad-LacZ were
gifts from Professor Cao (Second Military Medical University,
Shanghai, China). For recombinant adenovirus multiplication, A293
cells with 80% fusion were replenished with fresh medium and
subsequently infected with 100 μl of Ad-RCAS1 or Ad-LacZ.
After the cytopathic effect was achieved (approximately 24 to 72
h), the cells were collected and washed 3 times with PBS, then
metered in a total volume of 200 μl. After 6 freeze-thaw
cycles, the cells were centrifuged at 5,000 rpm at 4°C for 15 min,
and the virus was released into the supernatants (approximately 200
μl). Following amplification, all viruses were mixed and
filtered through a Gelman syringe filter (0.2 μm) for the
experiments. Virus titer (PFU/ml) was determined by a 50% tissue
culture infective dose (TCID50) assay (13).
Isolation and culture of T cells and
CD4+ T cells
Peripheral blood was obtained from volunteers (Blood
Center, Zhejiang, China). Peripheral blood mononuclear cells
(PBMCs) were obtained by Percoll density gradient centrifugation
using lymphocyte separation medium (Henxin Bio Co., Shanghai,
China) from human peripheral blood. T cells (1×108) were
purified using MACS Columns (Miltenyi Biotec). CD4+ T
cells and CD4− T cells were acquired. The PBMCs and
CD4+ T cells were then cultured in RPMI-1640 in 6-well
flat bottom plates (Orange Scientific), supplemented with 200 U/ml
interleukin (IL)-2. The old medium was replaced with fresh medium
every 2 days. On day 7, the cells were treated with 1 μg/ml
phytohemagglutinin (PHA), then cultured for 24 h. On day 8, the
cells were randomly divided into 3 groups: controls, Ad-RCAS1 and
Ad-LacZ; after 24 h of culture, the cells were harvested for
apoptosis assay (propidium iodide and Annexin V staining).
Apoptosis assay
The cells were washed, resuspended in staining
buffer, and stained with propidium iodide and Annexin V, according
to the manufacturer’s instructions. Stained cells were analyzed
using a FACSCalibur (Becton-Dickinson). The Annexin V-FITC
Apoptosis Detection kit was purchased from BioVision
Technologies.
RT-PCR analysis
Total RNA was extracted from the cultured cells
using TRIzol reagent (Dingguo Biotech Co., Beijing, China). Total
RNA (1 μg) from each sample was reverse transcribed using a
Reverse Transcription System kit (MBI Fermentas, Vilnius,
Lithuania) in a total volume of 20 μl. cDNA synthesized from
mRNA was quantified by PCR using specific primers as follows: RCAS1
sense, 5′-GCGAATTCTT ATGAAAGTTTCACACC-3′ and antisense, 5′-GTGGATCC
ATGGCCATCACCCAGTTT-3′, with a predicted PCR product of 642 bp;
β-actin sense, 5′-CCTAGAAGCATTT GCGGTGG-3′ and antisense,
5′-GAGCTACGAGCTT GACG-3′, with an expected PCR product of 402 bp.
Cycling conditions were 30 sec of denaturation at 94°C, 30 sec of
annealing at 56–60°C and 60 sec of extension at 72°C. The relative
amount of mRNA was determined using Quantity One software (Bio-Rad,
Hercules, CA, USA).
Western blot analysis
Cells were lysed in lysis buffer (Cell Signaling
Technology, Inc.) for 30 min on ice. A BCA kit (Cat. no. 23225;
Pierce Biotechnology) was then used to determine the concentration
of the lysates. Cell extracts were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene difluoride membranes. The blot was probed with RCAS1
(C20 and N18), GSK3β and phosphorylated GSK3β (phGSK3β) PoAbs and
GAPDH MoAb. Signals were developed using an enhanced
chemiluminescence detection reagent. The relative protein level was
determined with band intensities compared with GAPDH using the
Quantity One software (Bio-Rad).
Receptor detection
The binding of GST-RCAS1 to RCAS1 receptor
(RCAS1R)-expressing cells was determined by fluorescence-activated
cell sorting (FACS) analysis. Briefly, after 3 washes with PBS, the
K562, Jurkat or PHA-treated Jurkat cells (3×105) were
incubated in 1 ml PBS, with the addition of 10 μl GST-RCAS1
(10 μg) fusion protein, GST protein (10 μg) and PBS,
respectively, and incubated for 30 min at 4°C. After 3 washes, GST
MoAb was added, followed by incubation for 30 min at room
temperature. After 3 washes, the cells were labeled with anti-mouse
IgG conjugated with fluorescein isothiocyanate (FITC) and incubated
for 10 min at room temperature in the dark. After another 3 washes,
the cells were resuspended in a final volume of 1 ml of PBS and
acquired on a FACSCalibur (Becton-Dickinson). The experiments were
repeated on three separate occasions and the percentage and
fluorescence intensity of FITC-positive cells were detected by
FACS.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
K562 and Jurkat cells were cultured in 96-well
plates at a density of 1×104 cells/well in RMPI-1640
supplemented with 5% fetal calf serum (FCS) in the absence or
presence of Ad-RCAS1 or Ad-LacZ
(0.625×105–40×105 PFU, MOI 12.5–400). After
48 h of incubation, 20 μl of MTT (5 mg/ml) (Sigma) were
added to each well. The mixture was incubated for an additional 4 h
and then centrifuged at 3,000 rpm at 4°C for 10 min, and the
supernatant was discarded. The precipitate was dissolved in 150
μl of DMSO and the absorbance values were read at 570 nm
with a microplate reader (Molecular Devices, Palo Alto, CA,
USA).
Co-immunoprecipitation
Cell lysates of K562 cells transfected with Ad-RCAS1
(300 μg/tube) were mixed with RCAS1 PoAb, control or GSK3β
PoAb, and incubated for 6 h. Then a 3X reaction volume of Protein G
Plus Agarose (60 μl) was added, followed by incubation for
another 6 h. The cells were then washed 3 times in PBS, followed by
the addition of the buffer (120 μl), and subjected to
western blot analysis.
Histological examination of breast
tissue
Different breast tissues for histological
examination were collected from the Women’s Hospital School of
Medicine, Zhejiang University. Immunohistochemical analysis was
performed by Chaoying Bio Co. (Shanxi, China). Slides
(5-μm-thick) were deparaffinized, rehydrated and rinsed in
distilled water. Endogenous peroxidase activity was blocked by 10
min of incubation in 3% H2O2 at room
temperature. The slides were then rinsed and immersed in boiling
citrate buffer (pH 6.0) in a microwave oven with 3 changes of
buffer every 5 min. For the immunolocalization of RCAS1, the slides
were treated with the goat PoAbs to RCAS1 (N18 and C20; dilution
1:100, 1:50) in a moist chamber overnight. The slides were
subsequently rinsed in TBS buffer (pH 7.6) and incubated with
secondary antibody [donkey anti-goat IgG (H+L) HRP] (dilution
1:500, 1:250) for 45 min at 37°C. Visualization was performed using
3,3′-diaminobenzidine (DAB) as a chromogen for 10 min at room
temperature. The sections were counterstained with hematoxylin and
mounted in Glycergel. We assumed that positive reactivity for RCAS1
occurred when we observed a brown staining pattern.
Statistical analysis
All experiments were repeated 2 or 3 times.
Statistical analysis was carried out using the Student’s t-test. A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Identification of recombinant adenovirus
Ad-RCAS1
To determine whether RCAS1 expression affects
cellular apoptosis in vitro, we amplified recombinant
adenovirus Ad-RCAS1. Ad-LacZ was amplified as the control. In this
study, we amplified 5 ml Ad-RCAS1 and Ad-LacZ, respectively. TCID50
assay showed that Ad-RCAS1 and Ad-LacZ titers were
4.8×109 and 4.2×109 PFU/ml, respectively,
then adjusted to a final titer of 4×109 PFU/ml. HeLa
cells were then transfected with Ad-RCAS1 or Ad-LacZ. The
expression of RCAS1 was identified by RT-PCR and western blot
analysis (Fig. 1A–C).
Semi-quantitative RT-PCR analysis showed that the mRNA expression
of RCAS1 in the HeLa cells transfected with Ad-RCAS1 was higher
than that in the HeLa cells transfected with Ad-LacZ or in the
parental HeLa cells. Simultaneously, the density of the 32 kDa
bands detected by western blot analysis using PoAb to RCAS1 (C20
and N18) was relatively higher in the cell lysates of the HeLa
cells transfected with Ad-RCAS1 than that in the cell lysates of
the HeLa cells transfected with Ad-LacZ or in the parental HeLa
cells.
Detection of apoptosis in T lymphocytes
and immune cells derived from leukemia cell lines following
treatment with RCAS1
To investigate whether RCAS1 can induced the
apoptosis of T cells, we examined the percentage of apoptotic cells
by propidium iodide and Annexin V staining. Twenty-four hours after
the transfection of T cells isolated from peripheral blood with
Ad-RCAS1, Ad-LacZ or the blank control, a small number of apoptotic
cells was observed and no significant differences were detected in
the T cells transfected with Ad-RCAS1 compared with those
transfected with Ad-LacZ or the blank control (data not shown).
After the T cells were stimulated with IL-2 (200 IU/ml) and PHA (1
μg/ml), we found that the apoptotic value of Ad-RCAS1 in the
activated T cells was higher than that of the Ad-LacZ or the blank
control group (Annexin V-positive cells 46.26, 12.18 and 2.56%,
respectively) (Fig. 2A). We
examined the effect of RCAS1 on activated CD4+ T cell
apoptosis. As shown in Fig. 2B,
an increased number of apoptotic cells was observed in the
activated CD4+ T cells transfected with Ad-RCAS1
compared with those transfected with Ad-LacZ or the blank control
(Annexin V-positive cells 94.8, 56.7 and 23.2%, respectively).
These results suggest that RCAS1 overexpression induces the
apoptosis of activated T cells and CD4+ T cells.
Furthermore, using the same method, we examined
whether RCAS1 affects the apoptosis of immune cells derived from
leukemia cell lines, such as Jurkat, K562, THP1 and E003 cells.
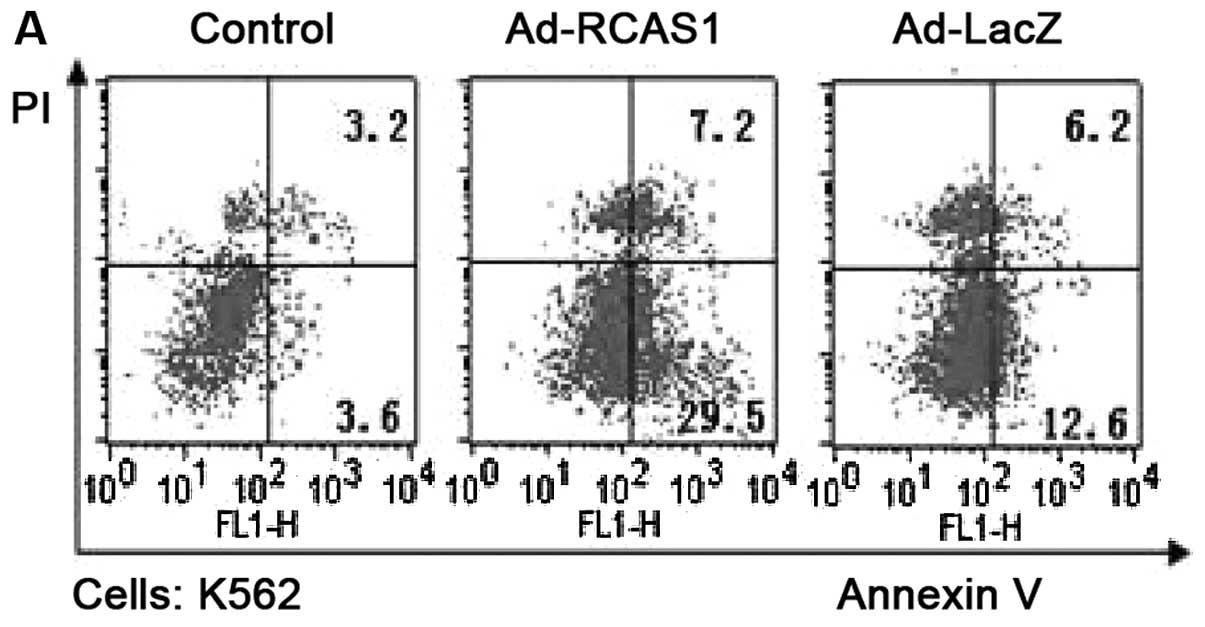
K562 cells exhibited higher levels of apoptosis following Ad-RCAS1
treatment, compared with the cells treated with Ad-LacZ or the
blank control (Annexin V-positive cells 36.7, 18.8 and 6.8%,
respectively) (Fig. 3A). However,
RCAS1 had no effect on the apoptosis of THP1 and E003 cells (data
not shown). For the Jurkat cells (acute T cell leukemia), the
apoptotic value of Ad-RCAS1, Ad-LacZ and the blank control group
was 31.2, 20.2 and 13.2%, respectively (Fig. 3B). The Ad-RCAS1 group had the
highest rate of apoptosis, whereas the blank control group had the
lowest rate. As shown in Fig. 3C,
an increased number of apoptotic cells was observed in the Jurkat
cells stimulated with PHA (1 μg/ml), and the difference in
the number of apoptotic cells between the Ad-RCAS1 and Ad-LacZ or
the blank control group was significant.
RCAS1 receptor detection
In our previous study, we induced the expression and
purification of GST-RCAS1 fusion protein (11). In this study, using the GST-RCAS1
fusion protein, we examined whether RCAS1R is expressed on the cell
membrane of Jurkat, K562, THP1 and E003 cells. In the K562 cells,
RCAS1R expression was detected and there was a significant
difference between the GST-RCAS1 group and the GST or control
group; however, the percentage of FITC-positive cells was not very
high (Fig. 4A). Of note, a low
expression of RCAS1R was detected in the THP1, E003 (data not
shown) and Jurkat cells (Fig.
4B). As shown in Fig. 4C,
RCAS1 expression in the Jurkat cells co-cultured with GST-RCAS1
increased significantly following stimulation with PHA (1
μg/ml) compared with the cells co-cultured with GST or the
blank control (FITC-positive cells 43.98, 1.59 and 4.07%,
respectively). These results suggest that RCAS1R is not expressed
in Jurkat cells; however, following stimulation with PHA, a
moderate upregulation of its expression can be induced.
RCAS1 inhibits K562 and Jurkat cell
proliferation
We used MTT assay to examine whether RCAS1 affects
the proliferation of K562 and Jurkat cells. Fig. 5A and B shows the inhibitory effect
of RCAS1 on the proliferation of K562 and Jurkat cells at 48 h
post-transfection at different MOIs of Ad-RCAS1 and Ad-LacZ. There
was a significant difference in the cell viability between Ad-RCAS1
and Ad-LacZ in a MOI-dependent manner, indicating that RCAS1
inhibited K562 and Jurkat cell growth in vitro.
Correlation between overexpression of
RCAS1 and GSK3β or phGSK3β
Using western blot analysis and
co-immunoprecipitation, we examined whether GSK3β plays a role in
the signaling pathway of cell apoptosis induced by RCAS1. When the
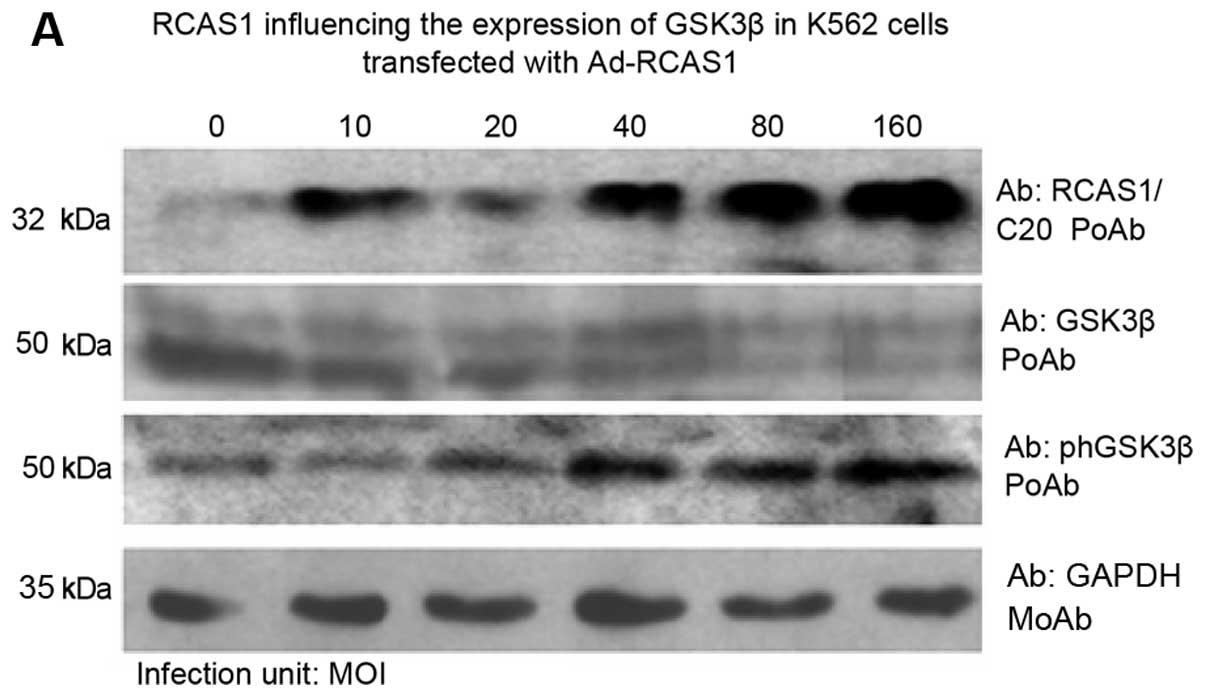
MOI of Ad-RCAS1 was doubled in the K562 cells, the expression of
RCAS1 in the K562 cells increased, but the expression of GSK3β
decreased, and the expression of phGSK3β increased (Fig. 6A). No significant difference was
observed in the K562 cells transfected with Ad-LacZ (Fig 6B), indicating that the
overexpression of RCAS1 in the K562 cells resulted in the altered
expression of GSK3β and phGSK3β. Our data suggest that the
expression of RCAS1 negatively correlates with the expression of
GSK3β, but positively correlates with the expression of phGSK3β.
Furthermore, we determined whether RCAS1 binds to GSK3β, leading to
the phosphorylation of GSK3β by using co-immunoprecipitation. RCAS1
was not detected in the immunoprecipitates of K562 cells
transfected with Ad-RCAS1, which were precipitated with anti-GKS3β
and anti-phGKS3β antibody (Fig.
7). This result demonstrates that RCAS1 does not directly bind
to GSK3β.
RCAS1 expression identified as a brown
staining pattern in breast cancer specimens
In our previous study, we reported that RCAS1
promoted tumor growth and metastasis in 4T1 murine mammary
carcinoma cells (12). In this
study, we collected different types of breast tissue from the
Women’s Hospital School of Medicine, Zhejiang University for
histological examination. For the immunolocalization of RCAS1 the
specimens were treated RCAS1 (N18 and C20) PoAb.
Immunohistochemical analysis confirmed that the breast cancer
tissue expressed RCAS1; however, the normal breast tissue did not
express RCAS1 (Fig. 8).
Discussion
RCAS1 is a type-II membrane protein, expressed in
estrogen target organs, as well as in various malignancies.
Nakashima et al (6) showed
that RCAS1 was a transmembrane molecule, and that activated T cells
expressed a putative RCAS1R, and apoptosis was induced after the
binding between RCAS1 and RCAS1R. However, other studies have
provided conflicting results without showing any significant
correlation. Engelsberg el al (14) showed that RCAS1 was predominantly
located on Golgi and that RCAS1 was not able to induce apoptosis,
also confirmed by the study of Reimer et al (15). In our study, we constructed the
GST-RCAS1 fusion protein and recombinant adenovirus Ad-RCAS1 to
examine the biological function of RCAS1 in vitro in
inducing the apoptosis of immune cells.
To determine whether RCAS1 expression affects cell
apoptosis in vitro, we amplified the recombinant adenovirus
Ad-RCAS1. Prior to its use, Ad-RCAS1 was analyzed by RT-PCR and
western blot analysis. HeLa cells are a cervical adenocarcinoma
cell line, which does not express RCAS1 (6). In our study, HeLa cells were
transfected with Ad-RCAS1, thus inducing the expression of RCAS1 in
the HeLa cells. Cells were also transfected with Ad-LacZ as the
controls. Using RT-PCR, we found that RCAS1 was expressed in the
HeLa cells transfected with Ad-RCAS1, the HeLa cells transfected
with Ad-LacZ and in the parental HeLa cells; this results is
contradictory to the results of other studies (6). Semi-quantitative RT-PCR analysis
showed that the mRNA expression of RCAS1 in the HeLa cells
transfected with Ad-RCAS1 was higher than that in the HeLa cells
transfected with Ad-LacZ or in the parental HeLa cells; this
difference was statistically significant. Simultaneously, 32 kDa
bands were detected by western blot analysis using RCAS1 PoAb (C20
and N18) in the 3 groups of HeLa cells, but the density of the
bands was upregulated in the HeLa cells transfected with Ad-RCAS1
compared to the HeLa cells transfected with Ad-LacZ or in the
parental HeLa cells.
After amplifing recombinant adenovirus Ad-RCAS1 and
Ad-LacZ, we determined and corrected the virus titer. Due to the
biological effects of adenovirus on cells, a consistent
concentration should be used for experiments. The virus titer
(PFU/ml) was determined by TCID50 assay. Ad-RCAS1 and Ad-LacZ titer
were then adjusted to the same concentration.
In our study, we separated peripheral blood
lymphocytes from human peripheral blood as the cellular model to
investigate whether RCAS1 induces apoptosis. Unfortunately, we did
not observe any cell apoptosis. These results were consistent with
those of a previous study on RCAS1 and cell apoptosis (6); namely that RCAS1 does not induce the
apoptosis of naïve T cells. Of note, we observed a significant
difference in cell apoptosis between the Ad-RCAS1 group and the
Ad-LacZ or control group after the cells were stimulated with IL-2
and PHA. We also observed an increased number of apoptotic cells in
the activated CD4+ T cells transfected with Ad-RCAS1 and
the percentage of Annexin V-positive cells was >90%. These
results suggested that activated T cells and CD4+ T
cells overexpressed RCAS1 following transfection with RCAS1,
leading to the induction of apoptosis. In the activated
CD4+ T cells, the high number of apoptotic cells
(>90%) may be due to MACS.
Furthermore, using the same method, we also detected
the effects of RCAS1 on the apoptosis of immune cells derived from
leukemia cell lines, such as Jurkat, K562, THP1 and E003 cells. We
observed an increased apoptosis of K562 cells in the RCAS1 group
compared with the other groups. However, RCAS1 had no effect on the
apoptosis of THP1 and E003 cells, and a low number of apoptotic
cells was observed in the Jurkat cells transfected with RCAS1.
Following treatment with PHA, the overexpression of RCAS1
significantly increased the apoptosis of Jurkat cells. In order to
elucidate the mechanism behind these phenomenon, we examined
whether the receptor expression differed in these cells. In our
previous study, we induced the expression and purification of
GST-RCAS1 fusion protein (11);
in this study, we used this GST-RCAS1 fusion protein to detect
RCAS1R expression in these cells. Of note, RCAS1R expression was
detected in the K562 cells, but low expression levels of RCAS1R
were detected in the THP1, E003 and Jurkat cells. RCAS1 expression
in the Jurkat cells increased significantly following stimulation
with PHA. These results suggest that RCAS1 does not induce the
apoptosis of THP1, E003 and Jurkat cells which do not express
RCAS1R; however, RCAS1 induces the apoptosis of K562 and
PHA-treated Jurkat cells which express RCAS1R. Thus, RCAS1 may be a
possible mechanism involved in the induction of cell apoptosis by
binding to RCAS1R. The other mechanisms involved remain to be
elucidated.
We also used MTT assay to examine the inhibitory
effect of RCAS1 on the proliferation of K562 and Jurkat cells. In
this study, there was a significant difference in cell viability
between the RCAS1 group and other groups in a MOI-dependent manner,
indicating that RCAS1 inhibited K562 and Jurkat cell growth in
vitro. This result were consistent with the result of cell
apoptosis.
Consisting of 2 isoforms, GSK3α and GSK3β, GSK3 was
originally considered to be inexorably linked to glycogen
metabolism (16,17). Increasing knowledge has altered
the status of GSK3β to that of a broadly influential enzyme that is
a crucial regulator of a nubmer of cellular functions, including
cellular structure, growth, motility and apoptosis (18). GSK3β activity was significantly
reduced by the phosphorylation of the N-terminal serine, Ser9, in
GSK3β, and Ser21 in GSK3α. One of the isoforms, GSK3β, has been
reported to be crucial to cell survival, including the attenuation
of apoptosis and NF-κB activation (19). GSK3 regulates Toll-like receptor
(TLR)-mediated inflammatory response by differentially affecting
the nuclear amounts of the transcription factors, NF-κB subunit p65
and cAMP response element-binding protein (CREB), interacting with
the co-activator CREB-binding protein (CBP) (20). In this study, we detected GSK3β
activity in the K562 cells transfected with Ad-RCAS1 and Ad-LacZ.
The upregulation of phGSK3β was detected in the K562 cells
transfected with RCAS1, whereas total GSK3β downregulated,
suggesting that the overexpression of RCAS1 results in the
phosphorylation of GSK3β by a certain pathway.
Co-immunoprecipitation assay showed that RCAS1 did not directly
bind to GSK3β, indicating that RCAS1 possibly interacted with the
protein located upstream of GSK3β. The inhibition of the GSK3β
activity and thus the inhibiton of the NF-κB signaling pathway may
be involved in the apoptosis of K562 cells induced by RCAS1,
although the precise mechanism involved needs to be further
elucidated.
A number of previous studies have linked the
exposure to RCAS1 as an important factor associated with cancer
(21,22). We used immunohistochemical
analysis to evaluate RCAS1 expression in breast tissues. RCAS1
expression was observed in the breast cancer tissues, but not in
normal breast tissue, suggesting that RCAS1 expression is an
important event associated with cancer.
In conclusion, we demonstrate that RCAS1 induces the
apoptosis of activated T cells, K562 cells and PHA-activated Jurkat
cells. The possible mechanism involved may be that the expression
of RCAS1R can be induced. RCAS1 inhibits the growth of Jurkat and
K562 cells, and is expressed in breast cancer tissues. In addition,
the downregulation of GSK3β may play a certain role in the cell
growth inhibition by RCAS1. Our data provide insight into the
mechanism through which tumor cells escape from immune surveillance
and provide a strategy for the therapeutic intervention of
cancer.
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (nos.
30100163 and 30328011), and the National Key Basic Research Program
of China (2004CB518802).
References
|
1
|
Tsuchiya F, Ikeda K, Tsutsumi O, et al:
Molecular cloning and characterization of mouse EBAG9, homolog of a
human cancer associated surface antigen: expression and regulation
by estrogen. Biochem Biophys Res Commun. 284:2–10. 2001. View Article : Google Scholar
|
|
2
|
Suzuki T, Inoue S, Kawabata W, et al:
EBAG9/RCAS1 in human breast carcinoma: a possible factor in
endocrine-immune interactions. Br J Cancer. 85:1731–1737. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akahira JI, Aoki M, Suzuki T, et al:
Expression of EBAG9/RCAS1 is associated with advanced disease in
human epithelial ovarian cancer. Br J Cancer. 90:2197–2202. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahashi S, Urano T, Tsuchiya F, et al:
EBAG9/RCAS1 expression and its prognostic significance in prostatic
cancer. Int J Cancer. 106:310–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoki T, Inoue S, Imamura H, et al:
EBAG9/RCAS1 expression in hepatocellular carcinoma: correlation
with tumour dedifferentiation and proliferation. Eur J Cancer.
39:1552–1561. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nakashima M, Sonoda K and Watanabe T:
Inhibition of cell growth and induction of apoptotic cell death by
the human tumor-associated antigen RCAS1. Nat Med. 5:938–942. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsushima T, Nakashima M, Oshima K, et
al: Receptor binding cancer antigen expressed on SiSo cells, a
novel regulator of apoptosis of erythroid progenitor cells. Blood.
98:313–321. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sonoda K, Miyamoto S, Yamazaki A,
Kobayashi H, Nakashima M, Mekada E and Wake N: Biologic
significance of receptor-binding cancer antigen expressed on SiSo
cells (RCAS1) as a pivotal regulator of tumor growth through
angiogenesis in human uterine cancer. Cancer. 110:1979–1990. 2007.
View Article : Google Scholar
|
|
9
|
Oshikiri T, Miyamoto M, Morita T, et al:
Tumor-associated antigen recognized by the 22-1-1 monoclonal
antibody encourages colorectal cancer progression under the scanty
CD8+ T cells. Clin Cancer Res. 12:411–416. 2006.
View Article : Google Scholar
|
|
10
|
Chatterjee M, Mohapatra S, Ionan A, et al:
Diagnostic markers of ovarian cancer by high-throughput antigen
cloning and detection on arrays. Cancer Res. 66:1181–1190. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong XJ, Shen FP and Wang QQ: Construction
of recombinant GST-RCAS1 fusion gene and its expression in E.
Coli. Zhejiang Da Xue Xue Bao Yi Xue Ban. 35:377–383. 2006.(In
Chinese).
|
|
12
|
Hong X, Liu Y, Hu G, et al: EBAG9 inducing
hyporesponsiveness of T cells promotes tumor growth and metastasis
in 4T1 murine mammary carcinoma. Cancer Sci. 100:961–969. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reed LJ and Muench HA: A simple method for
estimating fifty percent endpoint. Am J Hyg. 27:493–497. 1938.
|
|
14
|
Engelsberg A, Hermosilla R, Karsten U,
Schülein R, Dörken B and Rehm A: The Golgi protein RCAS1 controls
cell surface expression of tumor-associated O-linked glycan
antigens. J Biol Chem. 278:22998–23007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reimer TA, Anagnostopoulos I, Erdmann B,
et al: Reevaluation of the 22-1-1 antibody and its putative
antigen, EBAG9/RCAS1, as a tumor marker. BMC Cancer. 5:472005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Embi N, Rylatt DB and Cohen P: Glycogen
synthase kinase-3 from rabbit skeletal muscle. Separation from
cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J
Biochem. 107:519–527. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Woodgett JR and Cohen P: Multisite
phosphorylation of glycogen synthase. Molecular basis for the
substrate specificity of glycogen synthase kinase-3 and casein
kinase-II (glycogen synthase kinase-5). Biochim Biophys Acta.
788:339–347. 1984.
|
|
18
|
Jope RS and Johnson GV: The glamour and
gloom of glycogen synthase kinase-3. Trends Biochem Sci. 29:95–102.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin
O and Woodgett JR: Requirement for glycogen synthase kinase-3beta
in cell survival and NF-kappaB activation. Nature. 406:86–90. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin M, Rehani K, Jope RS and Michalek
SM: Toll-like receptor-mediated cytokine production is
differentially regulated by glycogen synthase kinase 3. Nat
Immunol. 6:777–784. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dutsch-Wicherek M and Wicherek L: The
association of RCAS1 serum concentration with the reversibility or
irreversibility of the process of immune cytotoxic activity
restriction during normal menstrual cycle, cancer relapse, and
surgical treatment for various types of squamous cell carcinomas
and adenocarcinomas. Am J Reprod Immunol. 59:266–275. 2008.
|
|
22
|
Dutsch-Wicherek M, Tomaszewska R, Lazar A,
Wicherek L and Skladzien J: The association between RCAS1
expression in laryngeal and pharyngeal cancer and its healthy
stroma with cancer relapse. BMC Cancer. 28:9–35. 2009.PubMed/NCBI
|