Introduction
Type 1 diabetes is an insulin-dependent, autoimmune
disorder characterized by the destruction of insulin-producing
β-cells (1). Islet
transplantation involves the transplantation of pancreatic islets
from the pancreas of a donor to another individual. It has proven
to be an effective method for the treatment of type 1 diabetes, as
well as for patients with diabetic nephropathy, retinopathy and
other complications (2,3). However, successful islet
transplantation is hampered by immune rejection, as well as the
shortage of donor islets (4).
Stem cells possess the ability to differentiate into functional
insulin-producing cells (5),
suggesting that these cells are a promising source for obtaining a
sufficient number of islet cells.
Studies have indicated that bone marrow mesenchymal
stem cells (BMSCs) (6) and
embryonic stem cells (ESCs) (7)
can differentiate into insulin-producing cells and can used in
transplantion therapy for type 1 diabetes. However, ESCs can not be
widely applied in clinical practice due to the ethical issues that
are provoked by their use. To date, the study of mesenchymal stem
cells (MSCs) has mainly focused on BMSCs. However, the invasiveness
of the bone marrow aspiration procedure and the age-dependent
degradation of the quantity and quality of BMSCs limit their
clinical potential (8,9).
Human umbilical cord Wharton’s jelly is a new source
of MSCs that exhibit a high degree of self-renewal capacity and
multi-differentiation potential. Human umbilical cord Wharton’s
jelly-derived mesenchymal stem cells (HUMSCs) have a wider range of
collection sources than BMSCs (10) or ESCs (11), and can be easily collected with
fewer ethical constraints. As an alternative source of MSCs, HUMSCs
have promising clinical application prospects. However, the immune
rejection problems associated with their use need to be solved
before they can be considered for successful transplantation. AS
previously demonstrated, MSCs can suppress lymphocyte proliferation
induced by phytohemagglutinin (PHA) and that these cells are not
restricted by major histocompatibility complex (MHC) (12). An allograft has been demonstrated
to minimize the risk of rejection following transplantation, even
between unmatched individuals (12). These unique immunological
properties of MSCs increase their potential for use in the organ
transplantion and for the prevention of rejection, as well as for
the treatment of autoimmune disease.
Although the immunogenic behavior of BMSCs has been
characterized (13,14), the immunoregulatory properties of
HUMSCs have not been fully defined. Interferon-γ (IFN-γ) activates
and promotes lymphocyte function as a positive immune regulator in
immune rejection. In this study, we investigated the immunological
characteristics of HUMSCs and their effects on lymphocyte
proliferation and the secretion of IFN-γ, and explored whether
direct cell-to-cell interactions and soluble factors, such as IFN-γ
are important for balancing HUMSC-mediated immune regulation. We
transplanted HUMSCs into diabetic rats to determine whether these
cells can colonize in vivo and differentiate into pancreatic
β-cells, and examined whether the hyperglycemia of diabetic rats
can be improved by HUMSC transplantation.
Materials and methods
Cell culture
Ethical approval was obtained from the Institutional
Review Board of Shantou University Medical College, Shantou, China.
Human umbilical cords from consenting patients (full-term caesarian
sections) were collected immediately into a sterilized 50 ml tube,
washed with phosphate-buffered saline (PBS) and cut into small
2–3-cm-thick sections. After dissecting the arteries and veins, the
remaining tissue, the Wharton’s jelly, was diced into smaller
fragments and transferred to a 75 cm2 flask in DMEM/F12
(Sigma-Aldrich, St. Louis, MO, USA) culture medium supplemented
with 10% fetal bovine serum (FBS; Gibco, Sydney, Australia), 100
μg/ml penicillin/streptomycin (Shanghai Bioscience, Shangai,
China), 1 g/ml amphotericin B (Gilead Sciences, Inc., San Dimas,
CA, USA), 5 ng/ml epidermal growth factor (EGF; Invitrogen Life
Technologies, Carlsbad, CA, USA) and 5 ng/ml basic fibroblast
growth factor (bFGF; Sigma-Aldrich). The cultures were left
undisturbed for 5–7 days at 37°C, 5% CO2 to allow the
migration of cells from the explants, after which the medium was
replaced.
Phenotypic characterization of
HUMSCs
Approximately 1×106 HUMSCs at passage 3
were dispersed with trypsin and resuspended in PBS containing
phycoerythrin (PE)-conjugated antibodies against CD40, CD40L, CD80
and CD86 (BD Biosciences, Franklin Lakes, NJ, USA) for 60 min at
4°C. The cells were washed 3 times with PBS and incubated with
PE-conjugated rabbit anti-mouse IgG (Santa Cruz Biotechnology,
Inc., Santa Cruz, USA) or FITC-conjugated goat anti-rat IgG (Santa
Cruz Biotechnology) for 30 min at room temperature. After 3 washes,
the cells were resuspended in 0.5 ml PBS and analyzed by flow
cytometry with the use of Epics XL flow cytometer (Beckman Coulter,
Brea, CA, USA).
Lymphocyte proliferation assay
Human peripheral blood lymphocytes (PBMCs) were
isolated from healthy donors by Ficoll-Paque (1.077 g/ml) density
gradient centrifugation. The cell concentration was adjusted to
1×106/ml with RPMI-1640 medium (Gibco, Carlsbad, CA,
USA) supplemented with 10% FBS. HUMSCs at passage 3 were harvested
and adjusted to 1×103 cells/ml, 1×104
cells/ml, or 1×105 cells/ml in L-DMEM containing 10%
FBS. A 100 μl suspension of HUMSCs was plated into 96-well plates.
The plates were incubated for 72 h at 37°C, 5% CO2.
After the cells reached 70–80% confluence, the medium was removed
and 100 μl of fresh medium containing 2.5 μl of mitomycin C (1
μg/μl; Sigma-Aldrich) were added for 30 min at 37°C to mitotically
inactivate the HUMSCs. After the medium was removed, the
inactivated HUMSCs were washed twice with PBS. HUMSCs were
resuspended in 100 μl of lymphocyte medium (RPMI-1640 containing
10% FBS), co-cultured with 1×105 cells/l PBMC, and
stimulated by PHA (10 mg/l) (Sigma-Aldrich) for 72 h at 37°C, 5%
CO2. The cells were divided into the following groups:
PBMCs + PHA (positive control); HUMSCs (1×105) + PBMCs +
PHA; HUMSCs (1×104) + PBMCs + PHA; and HUMSCs
(1×103) + PBMCs + PHA. Three ratios of HUMSCs to PBMCs
were used: 1:1, 1:10 and 1:100. Each trial was repeated in
triplicate. The CCK-8 kit (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was used to assess the immunomodulatory impact of
HUMSCs on PBMCs following stimulation with PHA. The procedure was
carried out according to the manufacturer’s instructions. The
inhibitory effects of HUMSCs on lymphocyte proliferation were
evaluated by comparing the optical density (OD) in cells
co-cultured with inactivated HUMSCs with the OD of lymphocytes
cultured alone.
ELISA
Passage 3 HUMSCs were trypsinized, the
concentrations adjusted to 1×105 cells/ml, and the cells
were plated (1 ml) in 24-well plates. After the cells reached 70%
confluence, 10 μl mitomycin-C (1 μg/μl) were added into each well.
Following incubation for 1 h at 37°C, 5% CO2, the medium
was removed and the cells were washed twice with PBS. PBMCs
(1×105) in 1 ml of lymphocyte medium were added and
co-cultured in the presence of 10 μl of PHA for 72 h at 37°C, 5%
CO2. The groups were as follows: PBMCs + PHA (positive
control); unstimulated PBMCs (negative control); and HUMSCs
(1×105 cells) + PBMCs + PHA. The supernatants of each
group were collected and IFN-γ expression was evaluated using the
ELISA detection kit according to the manufacturer’s instructions
(Invitrogen).
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from the HUMSCs. In addition,
we extracted the pancreas of the diabetic rats after HUMSC
transplantation, as well as the pancreas of diabetic rats without
HUMSC transplantation using TRIzol reagent (Invitrogen) according
to the manufacturer’s instructions. cDNA was prepared using the
Prime Script RT Reagent kit (Takara Bio, Inc., Shiga, Japan). cDNA
samples were analyzed by quantitative PCR using SYBR premix
(Takara) in an ABI 7300 system. The primers used for qRT-PCR
analyses were as follows: human pancreatic and duodenal homeobox 1
(PDX1; 148 bp) forward, 5′-ttcacgagccagtatgaccttcac-3′ and reverse,
5′-gaagacagacctgggatgcaca-3′; human insulin (221 bp) forward,
5′-acccagccgcagcctttgtg-3′ and reverse, 5′-ttccacaatgcc
acgcttctgc-3′; human glucagon (161 bp) forward, 5′-cagagctta
ggacacagagcacatc-3′ and reverse, 5′-acgttgccagctgccttgta-3′; HLA-I
(293 bp) forward, 5′-gcagacacggaatgtgaagg-3′ and reverse,
5′-gtaggctctcaactgctccg-3′; HLA-DR (350 bp) forward,
5′-tcttgtctgttctgcctcactc-3′ and reverse,
5′-ttccaggttggctttgtcc-3′; and β-actin (396 bp) forward,
5′-tggcaccacaccttctacaatgagc-3′ and reverse,
5′-gcacagcttctccttaatgtcacgc-3′.
Adenoviral expansion and infection
The E1-deleted adenovirus (serotype 5) carrying the
CMV promoter/EGFP hybrid gene was purchased from Vector Gene
Technology Company (Beijing, China). For amplification of the
adenoviruses, 1×108 infection units/ml (IU/ml) of
viruses was added into a 10-cm dish pre-seeded with
1×106 Ad293 cells (Stratagene, La Jolla, CA, USA)
overnight. Following incubation for 30–48 h, the cells were
harvested by scraping and centrifugation at 3,000 rpm for 10 min
while the supernatant was saved for the following round of virus
amplification. The harvested cells underwent 4 freeze/thaw cycles
and were centrifuged at 12,000 × g for 10 min to obtain cell
lysates. Serial dilutions of the supernatant and cell lysates were
used to transduce Ad293 cells in a 96-well plate pre-seeded with
5,000 cells overnight. The viral titers (IU/ml) were determined by
counting the EGFP-positive cells under a fluorescence microscope
after 30 h of culture. HUMSCs at passages 3–5 were seeded at a
density of 1×105 cells/well in 6-well plates. Following
24 h of culture, the medium was replaced with 1 ml of serum-free
medium containing indicated adenoviruses at a multipicity of
infection (MOI) of 50 for 4 h.
Transplantation model
Ethical approval for the animal experiments was
obtained from the Institutional Review Board of Shantou University
Medical College. A total of 20 rats received an intraperitoneal
injection of streptozotocin (STZ, Sigma-Aldrich) at 70 mg/kg in
order to induce diabetes. Blood glucose levels were monitored every
3 days. Rats with blood glucose levels >16.7 mmol/l for 3
measurements were diagnosed with type 1 diabetes.
Transplantation and physiological
monitoring
The rats were divided into 3 groups, with 6–8
rats/group. After blood glucose spontaneously increased to 16.7
mmol/l, the rats were restrained and 5×106 HUMSCs
suspended in 0.1 ml of normal saline were injected through the tail
vein. The control group underwent the same procedure, but was only
injected with PBS. Body weight, blood glucose and serum insulin
levels were recorded before and after cell transplantation. Blood
was collected from the tail vein and blood glucose levels were
measured using a blood glucose meter (Bayer, Leverkusen,
Germany).
Statistical analysis
The results are expressed as the means ± standard
deviation (SD). The statistical significance of the differences was
assessed by the analysis of variance. In all comparisons, a value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of HUMSCs
The HUMSCs derived from Wharton’s Jelly grew as a
flat monolayer after being cultured in vitro for 7–10 days.
After 2 weeks, some adherent cells had dissociated around the
adherent tissue sections and were visible under an inverted
microscope (Fig. 1A). Cells
gradually multiplied and grew into a radial-like array around the
adherent tissue sections. After the HUMSCs were passaged, they
showed strong proliferative ability. These cells proliferated with
a doubling time of approximately 24 h (Fig. 1B), but this proliferation rate
decreased after the 9th passage (Fig.
1C). Ad293-EGFP was then transfected into the HUMSCs at
passages 3–5 for transplantation (Fig. 1D).
Immunological characteristics of
HUMSCs
Flow cytometry analysis revealed that the HUMSCs
expressed low levels of CD80, CD86, CD40 and CD40L (Fig. 2A). qRT-PCR indicated that HUMSCs
expressed the HLA-I gene (MHC-I), but not HLA-DR (MHC-II), which is
closely related to graft-versus-host disease (Fig. 2B). To investigate the mechanisms
responsible for the immunosuppressive effects mediated by HUMSCs,
we co-cultured PHA-stimulated human PBMCs with various
concentrations of HUMSC culture supernatant. The PHA-induced
proliferation of human PBMCs was significantly suppressed by
co-culture with different numbers of mitotically inactivated
HUMSCs. The OD value of each group was as follows: PBMCs + PHA
(positive control), 1.90±0.25; HUMSCs (1×105) + PBMCs +
PHA, 1.37±0.024 (P<0.05, n=3); HUMSCs (1×104) + PBMCs
+ PHA, 1.81±0.31 (P>0.05, n=3); HUMSCs (1×103) +
PBMCs + PHA, 1.71±0.28 (P>0.05, n=3); (Fig. 2C). ELISA analysis of the PBMCs
revealed that the secretion of IFN-γ was 12.88±4.22 IU/ml in the
absence of HUMSCs, but decreased to 9.48±1.98 IU/ml when the cells
were co-cultured with HUMSCs (P<0.05, n=3) (Fig. 2D).
Improvement of hyperglycemia of diabetic
rats and repair of damaged pancreatic cells by HUMSC
transplantation
STZ was used to induce diabetes in rats, with a
single administration of 70 mg/kg. After 9 days, the blood glucose
levels of the STZ-treated rats increased from the normal levels
(7.14±2.08 mM) to severe hyperglycemic levels (24.04±2.84 mM).
EGFP-positive HUMSCs were infused into diabetic rats on the 10th
day. To avoid the aggregation of these cells and to ensure
reproducible delivery, the cells were injected into the rats
through the caudal vein. The rat pancreas and liver sections were
analyzed by fluorescence microscopy 4 weeks after transplantation
to observe whether the HUMSCs colonized in vivo. Green
fluorescence was detected by laser scanning microscopy of frozen
sections of the rat pancreas and liver. The pancreas and liver of
the rats transplanted with HUMSCs stained positive for PDX1 and
glucagon, but the control groups showed negative staining (Fig. 3). The PDX1 and glucagon gene were
also detected in the pancreas of the group transplanted with
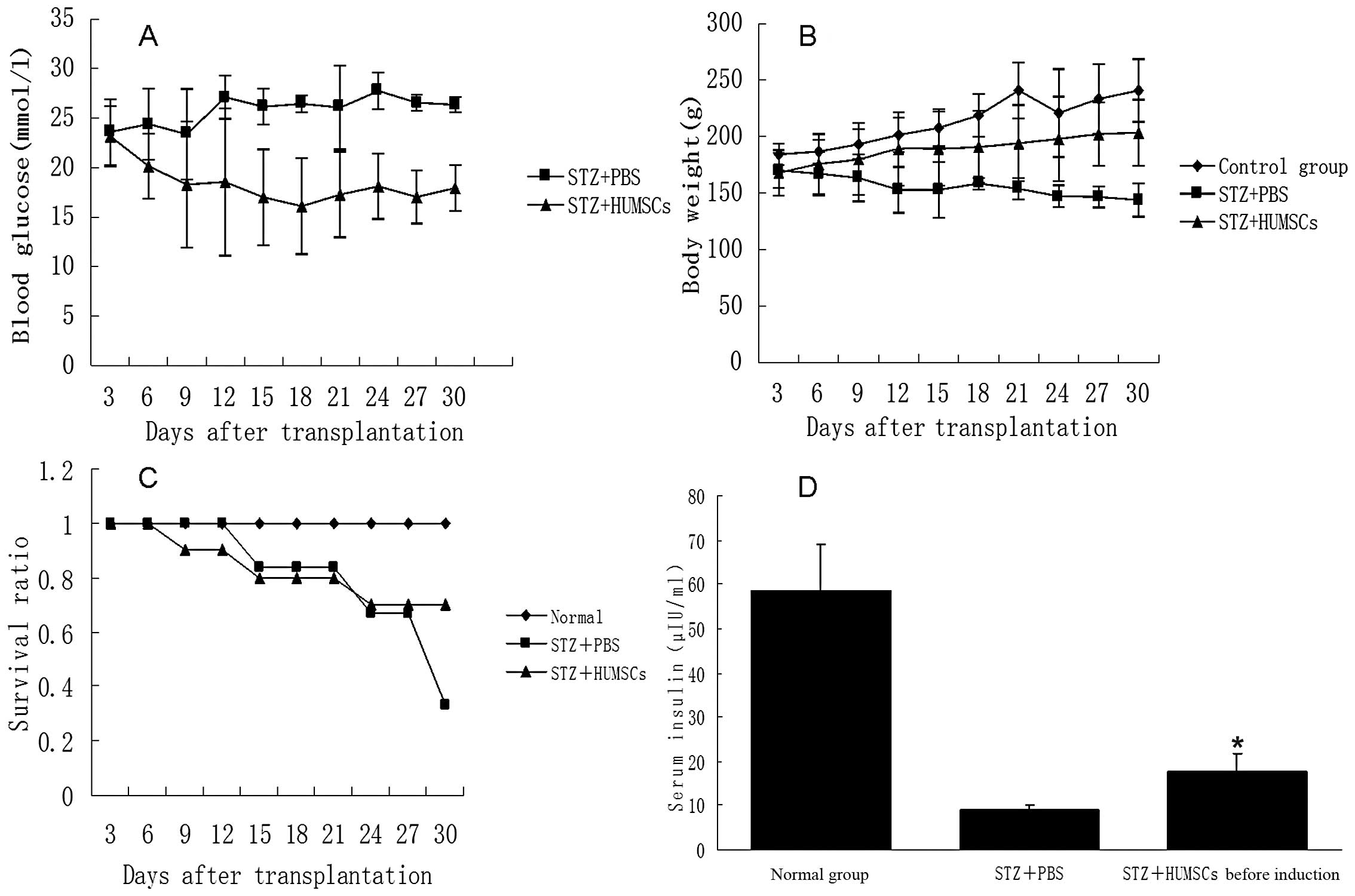
HUMSCs, although insulin expression was not detected (Fig. 4). Blood glucose levels in the
diabetic rats transplanted with HUMSCs (Fig. 5A) decreased significantly. Serum
insulin levels (Fig. 5D), body
weight (Fig. 5B) and the survival
ratio increased (Fig. 5C), and
islet repair was also improved (Fig.
6) compared with the diabetic rats not transplanted with
HUMSCs.
Discussion
MSCs can not only be derived from bone marrow, but
also from blood, spleen, amniotic fluid, placenta, tendon, adipose
tissue, synovial fluid, thymus cancellous bone, umbilical cord
blood, skin, pulp, lungs and umbilical cord (15–18). The umbilical cord, which is
considered a medical waste, can be easily obtained without
adversely affecting the donor or provoking any ethical issues.
Thus, it is an ideal source of cells for cell replacement therapy.
MSCs derived from human umbilical cords possess self-renewal and
multipotent differentiation potential. Previous studies have
demonstrated that HUMSCs can be induced to differentiate into nerve
cells (19), cardiomyocytes
(20), pancreatic islet cells
(5,21) and germ cells (22). Whether HUMSCs can colonize and
differentiate into islet cells in vivo, and whether these
are useful in the treatment of diabetes, requires further
research.
Challenges with immune rejection first need to be
addressed before HUMSC transplantation can be applied for the
treatment of type 1 diabetes. MHC-I functions to protect MSCs from
destruction by natural killer (NK) cells (23). The HLA-I antigen includes HLA-DR,
HLA-DP and HLA-DQ, with HLA-DR considered the most important for
allogeneic graft rejection. MHC-II can aid MSCs in escaping immune
recognition by CD4+ T cells (13). In this study, we found that HUMSCs
produce an immunosuppressive isoform of HLA-I, and do not express
HLA-DR (24). This indicates that
HUMSCs are a type of low immunogenic cell. Allogeneic transplant
rejection is mainly mediated by recipient T cells. Recent studies
have demonstrated that the excessive activation and proliferation
of T lymphocytes is one of the main reasons for graft-versus-host
disease (25). The full
activation of naïve T cells requires the synergy of the 2 types of
activation signals. When the first and second signals are bound by
the corresponding ligands, T cells proliferate to form functional
cell subsets. If T cells lack co-stimulatory signals, the first
signal of antigen recognition is unable to effectively activate
specific T cells, leading to the loss of T cell function. Thus, the
synergistic activation of the stimulatory molecules is essential
for normal T cell activation (26). Our results revealed that the
expression of immune response-related surface antigens, such as
CD40, CD40L, CD80, and CD86 is absent on HUMSCs, suggesting that
HUMSCs lack the second signal system. Therefore, our results
indicate that HUMSCs can escape the host immune attack in
vivo.
In recent years, a number of studies have focused on
the influence of MSC-regulated immune cells, particularly T cells.
MSCs can suppress the proliferation of lymphocytes (27,28). It has been suggested that the
immunomodulatory effects of MSCs are mainly exerted through the
following 2 mechanisms: direct contact between MSCs and T
lymphocytes, or soluble cytokines secreted by MSCs indirectly
inhibiting T lymphocytes (27–29). IFN, the earliest discovered
soluble cytokine, is mainly produced by activated T cells and NK
cells. In a previous study, following MSC stimulation, IFN-γ
secretion by Th1 cells decreased by 50%, while interleukin (IL)-4
secretion from Th2 cells increased significantly. This indicates
that MSCs may induce T lymphocyte differentiation into Th2 cells.
The decrease in IFN-γ secretion accompanied by the IL-4 increase
can result in a decrease in the Th1/Th2 ratio (30). Our results suggested that HUMSCs
(HUMSCs:PBMCs, 1:1) can inhibit the proliferation of T cells
activated by PHA and that the IFN-γ secretion by T cells is
decreased, indicating that HUMSCs may exert immunosuppressive
effects on T cells. Taken together, our results provide important
experimental evidence for the use of HUMSCs in in vivo
transplantation.
Adult stem cells, which include HUMSCs, exhibit two
important biological characteristics: first, injected adult stem
cells can migrate to the target affected areas in the body after
intravenous injection, and second, stem cells can be induced to
differentiate into appropriate cells required for the repair of
damaged tissue. This phenomenon is termed ‘site-specific
differentiation’ (31). We found
that HUMSCs can colonize in the liver and pancreas after
transplantation via caudal veins for 4 weeks, without immune
rejection. The colonized cells expressed glucagon and PDX1, which
are markers of pancreatic endocrine precursor cells, but did not
express insulin. These results suggest that HUMSCs can survive in
different parts of the body without immune rejection, but are
unable to differentiate into mature pancreatic β-cells. In
addition, hyperglycemia, body weight and the survival ratio
improved following the transplantation of HUMSCs into diabetic
rats. On the 9th day after transplantation, blood glucose levels in
the diabetic rats transplanted with HUMSCs were lower than those in
diabetic rats injected with only PBS (18.3±6.372 mmol/l vs.
23.16±3.055 mmol/l, respectively). Following transplantation, blood
glucose levels were maintained between 16.1 mmol/l and 18.5 mmol/l.
The body weight of the diabetic rats with injected with PBS
decreased rapidly, whereas the body weight of the rats transplanted
with HUMSCs decreased at a slower rate. In addition, survival
curves and serum insulin levels showed significant differences
between the control group and the group transplanted with HUMSCs.
Moreover, the pancreas of diabetic rats was repaired following
HUMSC transplantation. However, the mechanisms responsble for the
improvement of hyperglycemia remain unclear. We hypothesized that
three possible mechanisms may be responsible for the improvement of
hyperglycemia observed in this study. First, type 1 diabetes is an
immune-related disease, and immune therapy can improve the symptoms
of hyperglycemia. HUMSCs are a type of immunogenic cell, which also
exhibit immunomodulatory functions. HUMSCs can reduce damage to
islet cells by immune regulation. Second, HUMSCs are a type of
support cell that may promote the repair of partially damaged islet
cells to restore insulin secretion. Third, a portion of HUMSCs can
be differentiated into islet cells, which secrete some insulin.
However, the precise mechanism(s) involved required further
investigation.
In conclusion, we found that HUMSCs did not
stimulate the proliferation lymphocytes and did not induce
allogeneic or xenogeneic immune cell responses. qRT-PCR revealed
that the HUMSCs produced an immunosuppressive isoform of HLA-I, and
did not express HLA-DR. Flow cytometry revealed that the expression
of immune response-related surface antigens, such as CD40, CD40L,
CD80 and CD86 was absent on the HUMSCs. These results suggest that
HUMSCs may be tolerated in an allogeneic transplant. HUMSCs were
transplanted into diabetic rats, and these cells survived in the
liver and pancreas. The hyperglycemia of diabetic rats was improved
and the damage to pancreatic cells was partly reversed by HUMSC
transplantation. Hyperglycemic improvement may be related to the
immunomodulatory effects of HUMSCs, although the exact mechanisms
involved remain to be clarified.
Acknowledgments
The present study was supported by grants from the
Science and Technology Planning Project of Guangdong Province,
China (nos. 2010B031600273 and 2008B030301237), the National
Natural Science Foundation of China (no. 81070478), the Science and
Technology planning Project of Shantou, China (nos. E200900137 and
E201100373), the Foundation of the Department of Health, Guangdong
Province, China (no. B2010223) and the Science and Technology
Development Special Fund of Guangdong Province, China (no.
2012B031800443).
References
|
1
|
Atkinson MA and Eisenbarth GS: Type 1
diabetes: new perspectives on disease pathogenesis and treatment.
Lancet. 358:221–229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Noguchi H: Pancreatic islet
transplantation. World J Gastrointest Surg. 1:16–20. 2009.
View Article : Google Scholar
|
|
3
|
Matsumoto S: Islet cell transplantation
for Type 1 diabetes. J Diabetes. 2:16–22. 2010. View Article : Google Scholar
|
|
4
|
Shapiro AM, Lakey JR, Ryan EA, et al:
Islet transplantation in seven patients with type 1 diabetes
mellitus using a glucocorticoid-free immunostippressive regimen. N
Engl J Med. 343:230–238. 2000. View Article : Google Scholar
|
|
5
|
Wang HW, Lin LM, He HY, et al: Human
umbilical cord mesenchymal stem cells derived from Wharton’s jelly
differentiate into insulin-producing cells in vitro. Chin Med J.
124:1534–1539. 2011.
|
|
6
|
Jiang J, Au M, Lu K, et al: Generation of
insulin-producing islet-like clusters from human embryonic stem
cells. Stem Cells. 25:1940–1953. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karnieli O, Izhar-Prato Y, Bulvik S, et
al: Generation of insulin-producing cells from human bone marrow
mesenchymal stem cells by genetic manipulation. Stem Cells.
25:2837–2844. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mueller SM and Glowacki J: Age-related
decline in the osteogenic potential of human bone marrow cells
cultured in three-dimensional collagen sponges. J Cell Biochem.
82:583–590. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stenderup K, Justesen J, Clausen C and
Kassem M: Aging is associated with decreased maximal life span and
accelerated senescence of bone marrow stromal cells. Bone.
33:919–926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie QP, Huang H, Xu B, et al: Human bone
marrow mesenchymal stem cells differentiate into insulin-producing
cells upon microenvironmental manipulation in vitro.
Differentiation. 7:483–91. 2009.PubMed/NCBI
|
|
11
|
Shi Y: Generation of functional
insulin-producing cells from human embryonic stem cells in vitro.
Methods Mol Biol. 636:79–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Le Blanc K, Tammik L, Sundberg B, et al:
Mesenchymal stem cells inhibit and stimulate mixed lymphocyte
cultures and mitogenic responses independently of the major
histocompatibility complex. Scand J Immunol. 57:11–20. 2003.
|
|
13
|
Krampera M, Glennie S, Dyson J, et al:
Bone marrow mesenchymal stem cells inhibit the response of naive
and memory antigen-specific T cells to their cognate peptide.
Blood. 101:3722–3729. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meisel R, Zibert A, Laryea M, et al: Human
bone marrow stromal cells inhibit allogeneic T-cell responses by
indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood.
103:4619–4621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alhadlaq A and Mao JJ: Mesenchymal stem
cells: isolation and therapeutics. Stem Cells Dev. 13:436–438.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Le Blanc K and Pittenger M: Mesenchymal
stem cells: progress toward promise. Cytotherapy. 7:36–45.
2005.PubMed/NCBI
|
|
17
|
Beyer Nardi N and da Silva Meirelles L:
Mesenchymal stem cells: isolation, in vitro expansion and
characterization. Handb Exp Pharmacol. 174:249–282. 2006.
|
|
18
|
Wang HS, Hung SC, Peng ST, et al:
Mesenchymal stem cells in the Wharton’s jelly of the human
umbilical cord. Stem Cells. 22:1330–1337. 2004.
|
|
19
|
Ma L, Feng XY, Cui BL, et al: Human
umbilical cord Wharton’s Jelly-derived mesenchymal stem cells
differentiation into nerve-like cells. Chin Med J. 118:1987–1993.
2005.
|
|
20
|
Qian Q, Qian H, Zhang X, et al:
5-Azacytidine induces cardiac differentiation of human umbilical
cord-derived mesenchymal stem cells by activating extracellular
regulated kinase. Stem Cells Dev. 21:67–75. 2012. View Article : Google Scholar
|
|
21
|
He D, Wang J, Gao Y and Zhang Y:
Differentiation of PDX1 gene-modified human umbilical cord
mesenchymal stem cells into insulin-producing cells in
vitro. Int J Mol Med. 28:1019–1024. 2011.PubMed/NCBI
|
|
22
|
Huang P, Lin LM, Wu XY, et al:
Differentiation of human umbilical cord Wharton’s jelly-derived
mesenchymal stem cells into germ-like cells in vitro. J Cell
Biochem. 109:747–754. 2010.
|
|
23
|
Ghio M, Contini P, Negrini S, et al:
Soluble HLA-I-mediated secretion of TGF-beta1 by human NK cells and
consequent down-regulation of anti-tumor cytolytic activity. Eur J
Immunol. 39:3459–3468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stumpf AN, van der Meijden ED, van Bergen
CA, et al: Identification of 4 new HLA-DR-restricted minor
histocompatibility antigens as hematopoietic targets in antitumor
immunity. Blood. 114:3684–3692. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antin JH: Clinical practice. Long-term
care after hematopoietic-cell transplantation in adults. N Engl J
Med. 347:36–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leitner J, Kuschei W,
Grabmeier-Pfistershammer K, et al: T cell stimulator cells, an
efficient and versatile cellular system to assess the role of
costimulatory ligands in the activation of human T cells. J Immunol
Methods. 362:131–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi M, Liu ZW and Wang FS:
Immunomodulatory properties and therapeutic application of
mesenchymal stem cells. Clin Exp Immunol. 164:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sioud M: New insights into mesenchymal
stromal cell-mediated T-cell suppression through galectins. Scand J
Immunol. 73:79–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yagi H, Soto-Gutierrez A, Parekkadan B, et
al: Mesenchymal stem cells: Mechanisms of immunomodulation and
homing. Cell Transplant. 19:667–679. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aggarwal S and Pittenger MF: Human
mesenchymal stem cells modulate allogeneic immune cell responses.
Blood. 105:1815–1822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yano S, Kuroda S, Shichinohe H, et al:
Bone marrow stromal cell transplantation preserves gamma
aminobutyric acid receptor function in the injured spinal cord. J
Neurotrauma. 23:1682–1692. 2006. View Article : Google Scholar : PubMed/NCBI
|