Introduction
The SLC25A13 gene on chromosome 7q21.3 was
cloned, whilst its protein product, CITRIN, was designated in 1999
by Kobayashi et al (1).
This breakthrough finding opened up a new research field on citrin
deficiency (CD) and laid the foundation for subsequent
investigation into this autosomal recessive disorder. Subsequently,
citrin was found to be the liver-type aspartate/glutamate carrier
isoform 2 (AGC2) (2,3). The laboratory and clinical
characteristics of this CD, whether molecular (4–12),
biochemical (13,14), medical imaging (15), hepatohistological (16–18), metabolomic (19,20), behavioral (21), therapeutic (22–26) or epidemiological (27–31), have been increasingly depicted,
while patients with CD have been diagnosed not only in Asia
(32–42), but also in Europe (43–46) and North America (47–49). Currently, CD has developed into a
worldwide panethnic disease entity encompassing at least three
age-dependent clinical phenotypes, i.e. neonatal intrahepatic
cholestasis caused by citrin deficiency (NICCD) in neonates or
infants, adult-onset type 2 citrullinemia (CTLN2) in adolescents
and adults, as well as failure to thrive and dyslipidemia caused by
citrin deficiency (FTTDCD), which was recently suggested to be a
novel CD phenotype between NICCD and CTLN2 (7,11,12,19,50,51).
Although considerable laboratory and clinical
progress has been made in CD research, the genotypic and phenotypic
characteristics of this disease entity remain far from being
completely clarified. To date, a total of 84 deleterious mutations
of the SLC25A13 gene have been reported (11,12,30,52–54), constituting valuable molecular
evidence for the definite diagnosis of patients with CD. However,
the pathogenicity of the majority of missense mutations was based
on conventional bioinformatics evidence and studies on their direct
functional effects are rather limited (10,12). Moreover, all phenotypic,
therapeutic and prognostic knowledge on patients with CD has been
gained through the clinical management of such patients, both
pediatric and adult, and knowledge on the effects of
SLC25A13 mutations on CD fetuses remains limited (55), constituting a novel issue of
perinatal medicine. In the present study, an infant with NICCD was
diagnosed, who harbored a novel deleterious SLC25A13
mutation and demonstrated inspissated bile syndrome (IBS) along
with multiple congenital anomalies of the digestive system. We
herein report the molecular and clinical characteristics of this
case of NICCD.
Subjects and methods
Subjects and ethics
The research subjects in the present study were a
female infant (C0218) suspected to have NICCD and her parents. The
clinical findings of this family were described as a case report.
The majority of the data were collected at our clinical practice or
from the laboratory and imaging databases at our own hospital, with
partial biochemical or imaging results from the medical records in
another hospital, which were provided by the parents of the patient
at the time of her referral.
This study was carried out after written informed
consent was obtained from the parents of the infant prior to their
enrollment in the present study. For screening analysis of the
novel mutation, 60 used blood samples (with 120 SLC25A13
alleles) for health examinations were collected as the controls.
This study was approved by the Committee for Medical Ethics, the
First Affiliated Hospital, Jinan University, Guangzhou, China,
adhering to the World Medical Association Declaration of Helsinki
(WMADH 2008), which was adopted by the 59th WMA General Assembly,
Seoul, Korea, in October 2008.
Molecular diagnosis of CD
Genomic DNA was extracted from peripheral blood
samples collected from the subjects. Four high-frequency mutations
of the SLC25A13 gene, i.e., c.851_854delGTAT,
c.1638_1660dup, c.615+5G>A and IVS16ins3kb, were screened by
PCR/long and accurate (LA)-PCR and PCR-restriction fragment length
polymorphism (RFLP) analysis. Subsequently, all 18 exons and their
flanking genomic sequences were amplified by PCR/LA-PCR, and the
amplified products were then sequenced, as described in our
previous studies (7–9,11,15).
RT-PCR, cDNA cloning and sequencing
As previously described by our group (9,11),
EDTA anticoagulant peripheral blood samples were collected, the
lymphocytes were isolated with lymphocyte separation medium (LSM,
MP) and then homogenized immediately in RNAiso Plus (Takara Bio
Inc., Otsu, Japan) to extract the total mRNA following the
manufacturer’s instructions. Subsequently, the SLC25A13
transcripts were reverse-transcribed and amplified by PCR, and the
purified products were cloned into the pSIMPLE-18 EcoRV/BAP
Vector (Takara Bio Inc.) and transformed into DH5α Escherichia
coli competent cells. To examine the co-segregation of the 2
SLC25A13 variations detected in the family, only the cDNA
clones containing exons 1 and 8 together were selected for further
sequencing analysis, since the 2 variations occurred in these 2
exons, respectively. The sequencing results of the cDNA clones were
aligned with the SLC25A13 mRNA sequence to judge the
parental origins of the 2 variations.
Screening of the novel missense variation
in controls
A nested PCR-RFLP procedure was performed in the
present study for the screening for the novel missense variation in
the control individuals. The nucleotide sequences of the forward
and reverse primers were as follows: 5′-TCACTCATTCCAGT GCCTTG-3′
(IVS6F) and 5′-CAATGCCGCAAAGGCAA CTG-3′ (IVS8B) in the first PCR;
and 5′-GAGTTTGTTC TGGCAGCACAG-3′ (Ex8F) and 5′-TATTTCAGTATAG
CCTTCAGTTTGG-3′ (Ex8R) in the second. The temperature profile was
94°C for 5 min followed by 40 cycles (20 cycles in the second PCR)
of 94°C for 30 sec, 59°C for 40 sec and 72°C for 1.0 min, and a
final extension step at 72°C for 10 min. The restriction
endonuclease for RFLP analysis was Hpy188I (New England Biolabs
Inc., Ipswich, MA, USA). For frequency calculation of the
variation, the mutated allele number was divided by the total
allele number of the SLC25A13 gene in all controls and then
the quotient was amplified by 100%.
Bioinformatics analysis
The conservative property of the amino acid affected
by the novel missense mutation was surveyed as described in our
previous publication (11).
Briefly, using a comparative alignment software of
Genetyx® version 7.1 (Genetyx Co., Tokyo, Japan), the
amino acid sequences of human citrin and aralar were aligned with
those in the homologous proteins from 10 different eukaryotic
species, including chimpanzee, dog, mouse, rat, chicken, Xenopus
tropicalis, macaque, Caenorhabditis elegans, opossum and
cow. The amino acid sequences of the homologous proteins were
collected from ENSEMBL at http://www.ensembl.org/index.html. Moreover, 2 online
tools, MutationTaster (http://mutationtaster.org/MutationTaster/index.html)
and PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/), were used in
this study to evaluate the pathogenic potential of the novel
missense mutation. In the first software, a probability value close
to 1 indicates a high ‘security’ of the prediction and in the
second software used, the mutation with a probabilistic score
>0.85 is classified as ‘probably damaging’, while a score
>0.15 is classified as ‘possibly damaging’, as recently
described (12).
Functional effects of the novel missense
mutation
A yeast model with a disruption of the agc1
gene, which is highly homologous to human SLC25A13, was
applied in the present study to evaluate the functional effects of
the novel missense mutation. The diploid agc1-disrupted
yeast strain, BYagc1Δ, was the same as the one used in our
previous publication (12). The
normal citrin-encoding cDNA sequence was amplified and recombined
into the expression vector, pYX212 (Novagen Inc., Madison, WI,
USA), to form the plasmid, pYX212-citrin. The novel missense
mutation was introduced into the wild-type cDNA by
overlap-extension PCR, and the generated variants were cloned into
pYX212 to constitute the plasmid pYX212-mutant. Subsequently,
transformation of the BYagc1Δ strain with the empty plasmid
pYX212 (vector), pYX212-mutant and pYX212-citrin control (citrin)
was carried out, and the transformed strains were cultured in SA
medium with acetate as the unique carbon source. The growth
abilities of the transformed strains were examined after 96 h of
culture by measuring the OD600, and the data were
analyzed with one-way ANOVA followed by the Bonferroni method to
compare the differences in the mean values among the different
groups, with a value of P<0.05 considered to indicate a
statistically significant difference. All raw data were
logarithmically transformed in the case of non-homogeneity of
variance prior to statistical comparison.
Results
Case report
A 7-month-old female infant was referred to our
hospital due to prolonged jaundice for approximately 4 months and
growth retardation for 1.5 months. Her jaundice (yellow skin and
sclera) drew the attention of her parents at the age of 3.3 months.
A liver function test at the local hospital revealed elevated
cholestatic indices, including gamma-glutamyl transpeptidase (GGT),
direct bilirubin (Dbil) and total bile acid (TBA) (Table I). Due to the prolonged jaundice
and unresolved laboratory abnormalities, the infant was referred to
another hospital at the age of 4 months, where magnetic resonance
cholangiopancreatography revealed the dilatation of common hepatic
and bile ducts. Laparoscopy was thus performed when the infant was
aged 4.5 months, and an intraoperative cholangiography displayed
biliary tree dilatation and filling defect in the common bile duct,
along with an accessory hepatic duct (AHD) joining the cystic duct
(Fig. 1A). A cholecystectomy was
subsequently performed, an intrabiliary thick plug in the color of
dark green was removed, and bile duct irrigation and
choledochostomy with T-tube drainage was carried out. Pathological
analysis of the plug confirmed the diagnosis of inspissated bile
syndrome (IBS). Thereafter, the infant’s jaundice subsided and the
laboratory indices gradually improved (Table I); the infant was discharged at
the age of 5 months. Half a month later, nevertheless, a physical
examination revealed that her body weight was 5.34 kg (−3.6 SD),
her length was 59 cm (−3.2 SD) and her head circumference was 39 cm
(−2.5 SD). Another anthropometric test at the age of 6 months
revealed a weight of 6.0 kg (−2.5 SD) and a length of 62.0 cm (−2.2
SD). The growth retardation continued in the following month, and
the infant was referred to our hospital at the age of 7 months for
further evaluation, following the removal of the drainage
T-tube.
 | Table IDynamic alterations of the
biochemical indices in the infant with citrin deficiency. |
Table I
Dynamic alterations of the
biochemical indices in the infant with citrin deficiency.
| Biochemical
indices | 3.3 M | 3.5 M | 4.0 M | 4.3 M | 4.5 Ma | 4.6 M | 4.7 M | 4.8 M | 5.0 M | 5.5 M | 5.8 M | 7.0 Mb | 11 M |
|---|
| ALT (5–40 U/l) | 49 | 44 | 100 | 138 | 198 | 99 | 137 | 83 | 58 | 40 | 55 | 36 | 27 |
| AST (5–40 U/l) | 66 | 58 | 88 | 118 | 124 | 75 | 113 | 62 | 50 | 37 | 67 | 37 | 45 |
| GGT (8–50 U/l) | 444 | 411 | 537 | 532 | 388 | 313 | 465 | 508 | - | 364 | 134 | 318 | 34 |
| ALP (20–500
U/l) | 618 | 588 | 1133 | 1209 | 1332 | 1011 | 1428 | 944 | 643 | 174 | 426 | 420 | 399 |
| TP (60.0–83.0
g/l) | 56.9 | 54.9 | 61.2 | 56.1 | 50.0 | 56.8 | 60.8 | 68.5 | 73.5 | - | 61.6 | 68.7 | 68.6 |
| Alb (35.0–55.0
g/l) | 40.2 | 39.7 | 41.7 | 39.4 | 34.6 | 40.5 | 43.5 | 45.4 | 49.0 | - | 45.5 | 50.0 | 51.1 |
| Glb (20.0–30.0
g/l) | 16.7 | 15.2 | 19.5 | 16.7 | 15.4 | 16.3 | 17.3 | 23.1 | 24.5 | - | 16.1 | 18.7 | 17.5 |
| Tbil (2–19
μmol/l) | 67.7 | 59.2 | 123.6 | 149.8 | 204.5 | 148.3 | 135.9 | 67.3 | 45.4 | 20.3 | 13.2 | 3.9 | 5.5 |
| Dbil (0–6
μmol/l) | 53.8 | 48.7 | 96.3 | 118.4 | 161.6 | 120.1 | 110.8 | 57.4 | 40.1 | 13.0 | 9.4 | 1.8 | 1.4 |
| Ibil (2.56–20.9
μmol/l) | 13.9 | 10.5 | 27.3 | 31.4 | 42.9 | 28.2 | 25.1 | 9.9 | 5.3 | 7.3 | 3.8 | 2.1 | 4.1 |
| TBA (0–10
μmol/l) | 157.5 | 152.8 | 135.1 | 112.3 | 173.3 | 26.6 | 26.9 | 5.5 | 4.7 | 49.33 | 8.7 | 7.3 | 2.1 |
The infant was born to a non-consanguineous couple
after 37 weeks of uneventful gestation with a birth weight of 2.25
kg. On the first day after birth, the infant was admitted to
hospital due to vomiting and respiratory distress. Contrast imaging
of the upper digestive tract revealed the existence of esophageal
atresia (EA) (Fig. 1C), which was
then resolved by a gastroesophagostomy under general anesthesia.
The couple had experienced 2 pregnancies prior to this one, but
both were aborted in the first trimester. Both parents appeared
healthy, without any clinical symptoms or signs of CTLN2. There was
no known family history of any genetic disease.
Physical examination at referral revealed a weight
of 6.25 kg (−2.6 SD), a length of 63.0 cm (−3.3 SD) and a head
circumference of 42 cm (−1.0 SD). There was no dysmorphic
appearance, only a slightly chubby face. There was no evidence of
jaundice (yellow skin and sclera) and there were no visible
petechiae or ecchymoses. No pallor or cyanosis of the lips were
observed. There was no tachypnea or dyspnea, and no stridor,
wheezes, crackles or crepitus could be heard on auscultation of the
both lungs. The heart sounds were normal without audible murmurs or
arrhythmia. Upon abdominal inspection, no dilated veins or
abdominal distention were observed. The liver was palpated with a
soft edge 2 cm below the right costal margin in the mid-clavicular
line. Her spleen was not palpable. A neurological examination
revealed slightly reduced muscle tone. There was no neck stiffness,
knee reflex was normal and there was no positivity for Brudzinski’s
or Kernig’s sign.
Following biochemical analysis, no abnormal liver
function index was observed, although the serum GGT level was
elevated, suggesting the existence of cholestasis, as shown in
Table I. Taking into
consideration her prolonged jaundice, growth retardation and chubby
face, and the 2 first-trimester miscarriages of her mother,
SLC25A13 gene analysis was performed on the family to
evaluate the possibility of a diagnosis of CD. A lactose-free and
MCT-enriched therapeutic formula was subsequently introduced, while
a diet rich in protein and lipids was also encouraged. A clinical
following-up 4 months later revealed that the weight of the infant
was 7.9 kg (−1.6 SD), her length was 70 cm (−1.5 SD) and her head
circumference was 44 cm (−0.8 SD), along with a marked improvement
(complete recovery) of the cholestatic indices, GGT, TBA and Dbil
(Table I).
SLC25A13 genotypic characteristics of the
family
High-frequency mutation screening did not reveal any
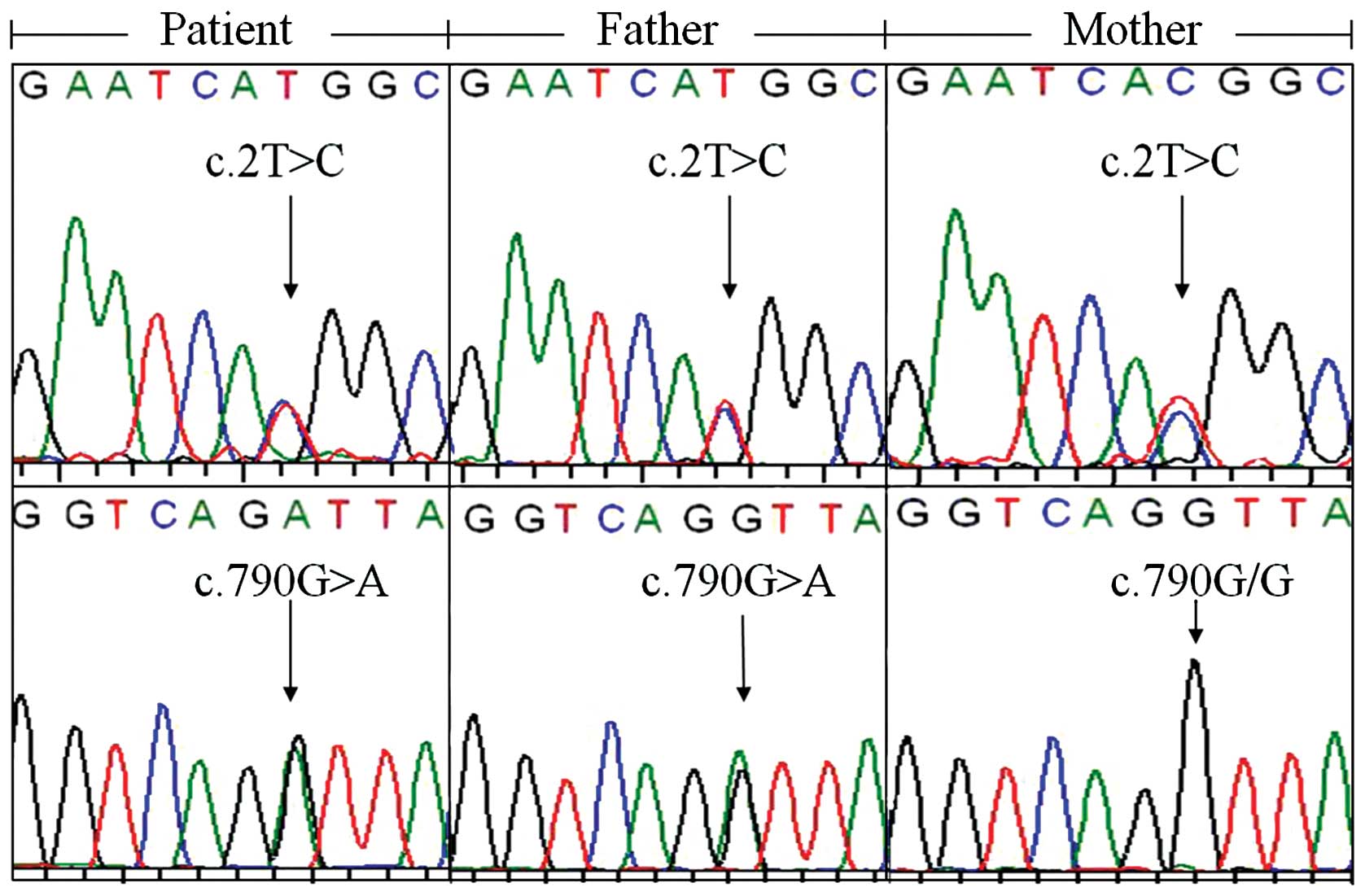
SLC25A13 mutation. However, direct DNA sequencing revealed
that both the infant and her father harbored c.2T>C and
c.790G>A (p.V264I) variations, while the mother was only a
carrier of c.2T>C (Fig. 2). To
the best of our knowledge, c.790G>A is a novel SLC25A13
variation that has not been previously reported. Following
SLC25A13 cDNA cloning analysis, from a total of 27 clones
from the infant, 7 were found to harbor c.2T>C, another 18
carried c.790G>A, 1 had neither, and the remaining clone had
both variations. Analysis of the cDNA clones from the father
revealed similar characteristics. The total 29 cDNA clones
consisted of 9 clones with c.2T>C, 13 with c.790G>A, 3 with
neither, and 4 with both variations. These findings clearly
indicated that the 2 variations were both biallelic, not only in
the infant displaying clinical characteristics, but also in her
father who did not show any symptoms or signs of CD to date.
Bioinformatic evidence of the
pathogenicity of the novel mutation
Using the newly-developed nest PCR-RFLP protocol
(Fig. 3), c.790G>A (p.V264I)
was screened in 60 control samples and no carrier was found,
indicating a frequency of <1%. The comparative alignment of the
amino acid sequences of the homologous protein in a diversity of
species (Fig. 4) documented the
highly conservative property of the valine at codon 264 affected by
this mutation. The probability value of >0.9999 upon
MutationTaster analysis strongly indicated its deleterious nature,
but according to the results produced by Polyphen-2 analysis, this
mutation was predicted to be benign with a score as low as 0.001.
This discrepancy necessitated conducting functional analysis for
this novel missense mutation.
Effect of the novel mutation on the AGC2
function of citrin protein
As illustrated in Fig.
5, the growth ability of the BYagc1Δ yeast transformed
with the mutant plasmid (p.V264I) was significantly reduced
(P<0.01) in comparison with that transformed with the plasmid
pYX212-citrin (citrin). However, when compared with the empty
plasmid group (vector), the growth ability in the mutant group
(p.V264I) was still higher (P<0.01). These findings indicated
that the p.V264I mutation reduced, but did not eliminate the AGC2
function of citrin protein.
Discussion
In previous studies, the c.2T>C variation
frequency was found to be 3.0% (6/200) in a Chinese (9), and approximately 2.8% (85/3074) in a
Thai population (31), both
suggesting that it may be a single-nucleotide polymorphism (SNP) of
the SLC25A13 gene. This initiation codon variation gave rise
to a citrin molecule lacking the first 34 amino acid residues, and
this truncated protein lost the ability to localize within the
mitochondrial membrane, thereby leading to an almost complete loss
of AGC2 function (10). Although
the frequency of this deleterious SNP has been proven to be rather
high, a homozygous variation (a patient with CD) has yet to be
identified. A possible explanation for this issue is the homozygote
lethality. In the present study, the two spontaneous miscarriages
of the mother in the first trimester may reflect the high
pathogenicity of this SNP. It was very likely that the two aborted
fetuses (possibly both with homozygous SNP) could not survive
through an uneventful pregnancy. As regards the c.790G>A
mutation, in addition to the bioinformatic evidence supporting its
disease-causing characteristic, the functional analysis in this
study provided direct eukaryotic evidence further solidifying its
pathogenicity. However, this missense mutation caused the
reduction, but not the elimination of AGC2 function of citrin
protein, as illustrated in Fig.
5. This is the most likely explanation for the survival of this
infant through the entire pregnancy, and her delivery as a full
term baby. This novel c.790G>A mutation, along with the
c.2T>C variation, constituted reliable diagnostic evidence for
NICCD in the infant, and further expanded the mutation spectrum of
the SLC25A13 gene.
The term IBS, also known as bile plug syndrome, was
used to indicate patients with prolonged jaundice in whom normal
extrahepatic bile ducts containing inspissated bile were found at
surgery for presumed extrahepatic biliary atresia (56). Although cholestasis has been
reported as a characteristic histological characteristic of NICCD
(17), such a large bile plug
causing obstruction of the common bile duct as in this infant has
not been reported previously in patients with NICCD. The
contributing factors for IBS included Rh and ABO incompatibility,
blood transfusion, parenteral nutrition in pre-term infants,
diuretic medication, bowel dysfunction and disseminated
intravascular coagulation (57).
None of these factors was found in this infant, and CD may be a
very likely contributing etiology for her IBS. Actually,
canalicular bile secretion involves a series of ATP-binding
cassette transporters as export pumps for bile salts and other
organic solutes (58), and among
these, the bile salt export pump (BSEP), a pump noteworthy in
particular, transports bile acids across the apical membrane and
constitutes the major determinant and driving force for the
generation of bile flow (59).
Since CD causes energy shortage in the liver (12,24), the function of these transporters,
including BSEP, would thereby be affected inevitably in this infant
with NICCD, causing deficit of the major driving force, disturbing
her generation of bile flow, giving rise to intra- and extrahepatic
cholestasis, and finally resulting in IBS formation.
Accessory bile ducts are rare congenital anomalies
of the primitive foregut bud during the development of the biliary
tract before 5 weeks of gestational age; apart from their
importance to the radiologist and to the biliary and hepatic
transplant surgeon, these anomalies may be associated with
congenital lesions elsewhere (60). In the present study, the infant
with NICCD with AHD also suffered from EA, another malformation
originated from the division of the primitive foregut into ventral
respiratory and dorsal esophageal parts during the 4th week of
embryonic life (61). Although
the underlying mechanisms remain unknown, EA has been recognized a
multifactorial complex disease with the involvement of genetic and
environmental factors, and has been reported to be associated with
some single-gene disorders, such as Feingold syndrome, CHARGE,
anophthalmia-oesophageal-genital (AEG) and Opitz G syndrome
(61,62). To the best of our knowledge,
congenital anomalies of the digestive system in patients with CD
have been rarely reported, although a Chinese infant with NICCD
with congenital biliary atresia, confirmed by abdominal laparoscopy
and liver biopsy, was previoulsy reported (54). In the present study, we reported
the concurrent existence of AHD and EA in an infant with NICCD.
These findings suggest that the SLC25A13 mutation may be an
additional contributing genetic factor leading to congenital
anomalies of the primitive foregut during the early stage of fetal
development, although more molecular, embryonic and histological
evidence is required in order to address this issue.
Non-penetrance refers to the lack of clinical signs
and symptoms in genetically affected individuals. This phenomenon
is not uncommon, not only in Mendelian disorders inherited as
autosomal dominant traits (63,64), but also in some autosomal
recessive diseases, such as Brown-Vialetto-Van Laere syndrome
(65) and Wolfram syndrome
(66). Whether or not CD
penetrance is complete has remained an unresolved issue for years
(27). However, there have been
several reports on adult individuals harboring the biallelic but
non-penetrant mutations of the SLC25A13 gene, at least at
the age these individuals were when these mutations were reported.
Two adult siblings had been definitely diagnosed as being
homozygous for the c.851_854del4 mutation, one demonstrating the
typical clinical and laboratory manifestations of CTLN2, while the
other did not (67). In addition,
a girl with the SLC25A13 genotype
c.851_854del4/c.1799_1800insA displayed characteristics of NICCD,
but her mother heterozygous for the mutations c.1799_1800insA and
IVS16ins3kb did not display any symptoms of CTLN2, as it recently
demonstrated by a Japanese group (68). In the present study, we reported
similar findings. The lack of CTLN2 phenotypic characteristics in
the father, who had the same genotype as that of the infant with
NICCD, lent further support to the concept that CD penetrance may
be incomplete, and suggested the existence of other environmental,
genetic or epigenetic factors that may modulate the onset of
CTLN2.
In conclusion, in this study, we reported two
individuals in the same family both harboring the same biallelic
variations, but demonstrating markedly different phenotypic
features. The infant had IBS and multiple anomalies of the
digestive system, while the father appeared healthy.
Bioinformatically and functionally, the c.790G>A variation
proved to be a novel pathogenic mutation of the SLC25A13
gene. These findings further enrich the clinical and molecular
spectrum of NICCD, and suggest the existence of CD non-penetrance
and the possible involvement of SLC25A13 in primitive
foregut development during early embryonic life.
Acknowledgements
The authors would like to thank all research
subjects for their kind cooperation in providing blood samples and
clinical data. The present study was financially supported by
grants from the Innovation Fund of Jinan University (No. 21612430)
and the National Natural Science Foundation of China (Nos. 81070279
and 81270957).
Abbreviations:
|
CD
|
citrin deficiency
|
|
NICCD
|
neonatal intrahepatic cholestasis
caused by citrin deficiency
|
|
CTLN2
|
adult-onset type 2 citrullinemia
|
|
FTTDCD
|
failure to thrive and dyslipidemia
caused by citrin deficiency
|
|
AGC2
|
aspartate/glutamate carrier isoform
2
|
|
GGT
|
gamma-glutamyl transpeptidase
|
|
Dbil
|
direct bilirubin
|
|
TBA
|
total bile acid
|
References
|
1
|
Kobayashi K, Sinasac DS, Iijima M, et al:
The gene mutated in adult-onset type II citrullinaemia encodes a
putative mitochondrial carrier protein. Nat Genet. 22:159–163.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Palmieri F: The mitochondrial transporter
family SLC25: identification, properties and physiopathology. Mol
Aspects Med. 34:465–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Palmieri F: Mitochondrial transporters of
the SLC25 family and associated diseases: a review. J Inherit Metab
Dis. 37:565–575. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tokuhara D, Iijima M, Tamamori A, et al:
Novel diagnostic approach to citrin deficiency: analysis of citrin
protein in lymphocytes. Mol Genet Metab. 90:30–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xing YZ, Qiu WJ, Ye J, et al: Studies on
the clinical manifestation and SLC25A13 gene mutation of Chinese
patients with neonatal intrahepatic cholestasis caused by citrin
deficiency. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 27:180–185.
2010.(In Chinese).
|
|
6
|
Fu HY, Zhang SR, Wang XH, et al: The
mutation spectrum of the SLC25A13 gene in Chinese infants with
intrahepatic cholestasis and aminoacidemia. J Gastroenterol.
46:510–518. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song YZ, Deng M, Chen FP, et al: Genotypic
and phenotypic features of citrin deficiency: five-year experience
in a Chinese pediatric center. Int J Mol Med. 28:33–40.
2011.PubMed/NCBI
|
|
8
|
Lin WX, Zhang ZH, Deng M, Cai XR and Song
YZ: Multiple ovarian antral follicles in a preterm infant with
neonatal intrahepatic cholestasis caused by citrin deficiency: a
clinical, genetic and transcriptional analysis. Gene. 505:269–275.
2012. View Article : Google Scholar
|
|
9
|
Zhang ZH, Lin WX, Deng M, Zhao XJ and Song
YZ: Molecular analysis of SLC25A13 gene in human peripheral
blood lymphocytes: marked transcript diversity, and the feasibility
of cDNA cloning as a diagnostic tool for citrin deficiency. Gene.
511:227–234. 2012.
|
|
10
|
Wongkittichote P, Tungpradabkul S,
Wattanasirichaigoon D and Jensen LT: Prediction of the functional
effect of novel SLC25A13 variants using a S.
cerevisiae model of AGC2 deficiency. J Inherit Metab Dis.
36:821–830. 2013.PubMed/NCBI
|
|
11
|
Song YZ, Zhang ZH, Lin WX, et al:
SLC25A13 gene analysis in citrin deficiency: sixteen novel
mutations in East Asian patients, and the mutation distribution in
a large pediatric cohort in China. PLoS One. 8:e745442013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang ZH, Lin WX, Deng M, et al: Clinical,
molecular and functional investigation on an infant with neonatal
intrahepatic cholestasis caused by citrin deficiency (NICCD). PLoS
One. 9:e892672014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagasaka H, Okano Y, Tsukahara H, et al:
Sustaining hypercitrullinemia, hypercholesterolemia and augmented
oxidative stress in Japanese children with aspartate/glutamate
carrier isoform 2-citrin-deficiency even during the silent period.
Mol Genet Metab. 97:21–26. 2009. View Article : Google Scholar
|
|
14
|
Wang JS, Wang XH, Zheng YJ, et al:
Biochemical characteristics of neonatal cholestasis induced by
citrin deficiency. World J Gastroenterol. 18:5601–5607. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song YZ, Li BX, Chen FP, et al: Neonatal
intrahepatic cholestasis caused by citrin deficiency: clinical and
laboratory investigation of 13 subjects in mainland of China. Dig
Liver Dis. 41:683–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Komatsu M, Yazaki M, Tanaka N, et al:
Citrin deficiency as a cause of chronic liver disorder mimicking
non-alcoholic fatty liver disease. J Hepatol. 49:810–820. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kimura A, Kage M, Nagata I, et al:
Histological findings in the livers of patients with neonatal
intrahepatic cholestasis caused by citrin deficiency. Hepatol Res.
40:295–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang GY, Cheng ZM and Liu KS: Neonatal
intrahepatic cholestasis caused by citrin deficiency: a
histopathologic study of 10 cases. Zhonghua Bing Li Xue Za Zhi.
41:452–455. 2012.(In Chinese).
|
|
19
|
Saheki T, Inoue K, Ono H, et al:
Metabolomic analysis reveals hepatic metabolite perturbations in
citrin/mitochondrial glycerol-3-phosphate dehydrogenase
double-knockout mice, a model of human citrin deficiency. Mol Genet
Metab. 104:492–500. 2011. View Article : Google Scholar
|
|
20
|
Kuhara T, Ohse M, Inoue Y and Cooper AJ: A
GC/MS-based metabolomic approach for diagnosing citrin deficiency.
Anal Bioanal Chem. 400:1881–1894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okano Y, Kobayashi K, Ihara K, et al:
Fatigue and quality of life in citrin deficiency during adaptation
and compensation stage. Mol Genet Metab. 109:9–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shigeta T, Kasahara M, Kimura T, et al:
Liver transplantation for an infant with neonatal intrahepatic
cholestasis caused by citrin deficiency using heterozygote living
donor. Pediatr Transplant. 14:E86–E88. 2010. View Article : Google Scholar
|
|
23
|
Yazaki M, Ikeda S, Kobayashi K and Saheki
T: Therapeutic approaches for patients with adult-onset type II
citrullinemia (CTLN2): effectiveness of treatment with
low-carbohydrate diet and sodium pyruvate. Rinsho Shinkeigaku.
50:844–847. 2010.(In Japanese).
|
|
24
|
Hayasaka K, Numakura C, Toyota K and
Kimura T: Treatment with lactose (galactose)-restricted and
medium-chain triglyceride-supplemented formula for neonatal
intrahepatic cholestasis caused by citrin deficiency. JIMD Rep.
2:37–44. 2012. View Article : Google Scholar
|
|
25
|
Saheki T, Inoue K, Ono H, et al: Effects
of supplementation on food intake, body weight and hepatic
metabolites in the citrin/mitochondrial glycerol-3-phosphate
dehydrogenase double-knockout mouse model of human citrin
deficiency. Mol Genet Metab. 107:322–329. 2012. View Article : Google Scholar
|
|
26
|
Yazaki M, Kinoshita M, Ogawa S, et al: A
73-year-old patient with adult-onset type II citrullinemia
successfully treated by sodium pyruvate and arginine. Clin Neurol
Neurosurg. 115:1542–1545. 2013.PubMed/NCBI
|
|
27
|
Lu YB, Kobayashi K, Ushikai M, et al:
Frequency and distribution in East Asia of 12 mutations identified
in the SLC25A13 gene of Japanese patients with citrin
deficiency. J Hum Genet. 50:338–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kikuchi A, Arai-Ichinoi N, Sakamoto O, et
al: Simple and rapid genetic testing for citrin deficiency by
screening 11 prevalent mutations in SLC25A13. Mol Genet
Metab. 105:553–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Treepongkaruna S, Jitraruch S, Kodcharin
P, et al: Neonatal intrahepatic cholestasis caused by citrin
deficiency: prevalence and SLC25A13 mutations among Thai
infants. BMC Gastroenterol. 12:1412012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen R, Wang XH, Fu HY, et al: Different
regional distribution of SLC25A13 mutations in Chinese
patients with neonatal intrahepatic cholestasis. World J
Gastroenterol. 19:4545–4551. 2013.PubMed/NCBI
|
|
31
|
Wongkittichote P, Sukasem C, Kikuchi A, et
al: Screening of SLC25A13 mutation in the Thai population.
World J Gastroenterol. 19:7735–7742. 2013.
|
|
32
|
Luder AS, Tabata A, Iijima M, Kobayashi K
and Mandel H: Citrullinaemia type 2 outside East Asia: Israeli
experience. J Inherit Metab Dis. 29:S592006.
|
|
33
|
Yeh JN, Jeng YM, Chen HL, Ni YH, Hwu WL
and Chang MH: Hepatic steatosis and neonatal intrahepatic
cholestasis caused by citrin deficiency (NICCD) in Taiwanese
infants. J Pediatr. 148:642–646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohura T, Kobayashi K, Tazawa Y, et al:
Clinical pictures of 75 patients with neonatal intrahepatic
cholestasis caused by citrin deficiency (NICCD). J Inherit Metab
Dis. 30:139–144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ko JM, Kim GH, Kim JH, et al: Six cases of
citrin deficiency in Korea. Int J Mol Med. 20:809–815.
2007.PubMed/NCBI
|
|
36
|
Chew HB, Ngu LH, Zabedah MY, et al:
Neonatal intrahepatic cholestasis associated with citrin deficiency
(NICCD): a case series of 11 Malaysian patients. J Inherit Metab
Dis. 33:S489–S495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee BH, Jin HY, Kim GH, Choi JH and Yoo
HW: Nonalcoholic fatty liver disease in 2 siblings with adult-onset
type II citrullinemia. J Pediatr Gastroenterol Nutr. 50:682–685.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ngu HL, Zabedah MY and Kobayashi K:
Neonatal intrahepatic cholestasis caused by citrin deficiency
(NICCD) in three Malay children. Malays J Pathol. 32:53–57.
2010.PubMed/NCBI
|
|
39
|
Thong MK, Boey CC, Sheng JS, Ushikai M and
Kobayashi K: Neonatal intrahepatic cholestasis caused by citrin
deficiency in two Malaysian siblings: outcome at one year of life.
Singapore Med J. 51:e12–e14. 2010.PubMed/NCBI
|
|
40
|
Lin JT, Hsiao KJ, Chen CY, et al: High
resolution melting analysis for the detection of SLC25A13
gene mutations in Taiwan. Clin Chim Acta. 412:460–465. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen ST, Su YN, Ni YH, et al: Diagnosis of
neonatal intrahepatic cholestasis caused by citrin deficiency using
high-resolution melting analysis and a clinical scoring system. J
Pediatr. 161:626–631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takahashi Y, Koyama S, Tanaka H, et al: An
elderly Japanese patient with adult-onset type II citrullinemia
with a novel D493G mutation in the SLC25A13 gene. Intern
Med. 51:2131–2134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hutchin T, Preece MA, Kobayashi K, et al:
Neonatal intrahepatic cholestasis caused by citrin deficiency
(NICCD) in an European patient. J Inherit Metab Dis.
29:S1122006.
|
|
44
|
Hutchin T, Preece MA, Hendriksz C, et al:
Neonatal intrahepatic cholestasis caused by citrin deficiency
(NICCD) as a cause of liver disease in infants in the UK. J Inherit
Metab Dis. 32:S151–S155. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fiermonte G, Parisi G, Martinelli D, et
al: A new Caucasian case of neonatal intrahepatic cholestasis
caused by citrin deficiency (NICCD): a clinical, molecular, and
functional study. Mol Genet Metab. 104:501–506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vitoria I, Dalmau J, Ribes C, et al:
Citrin deficiency in a Romanian child living in Spain highlights
the worldwide distribution of this defect and illustrates the value
of nutritional therapy. Mol Genet Metab. 110:181–183. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dimmock D, Kobayashi K, Iijima M, et al:
Citrin deficiency: a novel cause of failure to thrive that responds
to a high-protein, low-carbohydrate diet. Pediatrics.
119:e773–e777. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dimmock D, Maranda B, Dionisi-Vici C, et
al: Citrin deficiency, a perplexing global disorder. Mol Genet
Metab. 96:44–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wong LJ, Dimmock D, Geraghty MT, et al:
Utility of oligonucleotide array-based comparative genomic
hybridization for detection of target gene deletions. Clin Chem.
54:1141–1148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kobayashi K, Saheki T and Song YZ: Citrin
Deficiency. GeneReviews™ (Internet). Pagon RA, Bird TD, Dolan CR,
Stephens K and Adam MP: Seattle (WA): University of Washington,
Seattle; 1993–2005 Sep 16. (updated 2012 Jan 05).
|
|
51
|
Woo HI, Park HD and Lee YW: Molecular
genetics of citrullinemia types I and II. Clin Chim Acta. 431:1–8.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tabata A, Sheng JS, Ushikai M, et al:
Identification of 13 novel mutations including a retrotransposal
insertion in SLC25A13 gene and frequency of 30 mutations
found in patients with citrin deficiency. J Hum Genet. 53:534–545.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wen P, Wang G, Chen Z, et al: Clinical
investigation and mutation analysis of a child with citrin
deficiency complicated with purpura, convulsive seizures and
methioninemia. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 30:649–653.
2013.(In Chinese).
|
|
54
|
Tong F, Yang JB, Huang XL, Zhou XL and
Yang RL: A case of neonatal intrahepatic cholestasis caused by
citrin deficiency complicated with congenital biliary atresia.
Zhonghua Er Ke Za Zhi. 51:863–865. 2013.(In Chinese).
|
|
55
|
Zhao XJ, Tang XM, Zha QB, et al: Prenatal
diagnosis of citrin deficiency in a Chinese family with a fatal
proband. Tohoku J Exp Med. 225:273–276. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Desmet VJ and Roskams TA: Cholestatic
syndromes of infancy and childhood. Hepatology, a textbook of liver
disease. Zakim D and Boyer TD: 4th edition. Saunders; Philadelphia:
pp. 1481–1536. 2003
|
|
57
|
Heaton ND, Davenport M and Howard ER:
Intraluminal biliary obstruction. Arch Dis Child. 66:1395–1398.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Boyer JL: Bile formation and secretion.
Compr Physiol. 3:1035–1078. 2013.PubMed/NCBI
|
|
59
|
Suchy FJ and Ananthanarayanan M: Bile salt
excretory pump: biology and pathobiology. J Pediatr Gastroenterol
Nutr. 43:S10–S16. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sherlock S and Dooley J: Cysts and
congenital biliary abnormalities. Diseases of the liver and biliary
system. Sherlock S and Dooley J: 11th edition. Victoria
(Australia): Blackwell Publishing Asia; pp. 583–596. 2005
|
|
61
|
de Jong EM, Felix JF, de Klein A and
Tibboel D: Etiology of esophageal atresia and tracheoesophageal
fistula: ‘mind the gap’. Curr Gastroenterol Rep. 12:215–222.
2010.
|
|
62
|
Felix JF, de Jong EM, Torfs CP, de Klein
A, Rottier RJ and Tibboel D: Genetic and environmental factors in
the etiology of esophageal atresia and/or tracheoesophageal
fistula: an overview of the current concepts. Birth Defects Res A
Clin Mol Teratol. 85:747–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dworschak GC, Draaken M, Hilger A, et al:
An incompletely penetrant novel MAFB (p.Ser56Phe) variant in
autosomal dominant multicentric carpotarsal osteolysis syndrome.
Int J Mol Med. 32:174–178. 2013.
|
|
64
|
Jéru I, Charmion S, Cochet E, et al:
Involvement of the same TNFR1 residue in mendelian and
multifactorial inflammatory disorders. PLoS One.
8:e697572013.PubMed/NCBI
|
|
65
|
Dezfouli MA, Yadegari S, Nafissi S and
Elahi E: Four novel C20orf54 mutations identified in
Brown-Vialetto-Van Laere syndrome patients. J Hum Genet.
57:613–617. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Boutzios G, Livadas S, Marinakis E, Opie
N, Economou F and Diamanti-Kandarakis E: Endocrine and metabolic
aspects of the Wolfram syndrome. Endocrine. 40:10–13. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Imamura Y, Kobayashi K, Shibatou T, et al:
Effectiveness of carbohydrate-restricted diet and arginine granules
therapy for adult-onset type II citrullinemia: a case report of
siblings showing homozygous SLC25A13 mutation with and without the
disease. Hepatol Res. 26:68–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Yagi M, Kosunoki N, Lee T, Kikuchi A, Kure
S and Takeshima Y: A Japanese family with citrin deficiency: a
daughter with NICCD and a mother without symptoms of CTLN2. In: The
3rd Asian Congress for Inherited Metabolic Diseases (ACIMD)/The
55th Annual Meeting of The Japanese Society for inherited metabolic
diseases (JSIMD): Program Book; Japan. 2013 Nov 27–29; Chiba: pp.
P149
|