Introduction
Immunoglobulin G transport from the maternal
circulation to the fetus in the human placenta is crucial for the
immunity of the fetus and the newborn, whose own immune system is
maturing. This mechanism, which is not yet well understood,
requires IgG movement across two cell layers of the human placenta:
the syncytiotrophoblast and the fetal placental endothelium
(1,2). Transport across the
syncytiotrophoblast is thought to be mediated by endosomes
containing the MHC-related Fc receptor, FCGRT (also known as FcRn)
(3,4). An efficient transcytotic mechanism
to move the IgG from the apical to the basolateral surface of the
syncytiotrophoblast via the FCGRT-containing endosomes can readily
be envisioned, but has not yet been substantiated. The mechanisms
through which IgG crosses the fetal endothelium are much less
clear.
Low-affinity gamma immunoglobulin Fc region receptor
IIb (FCGR2B; also designated as FcγRIIb) is a negative regulator of
immune cell function that is encoded by a single FCGR2B
gene; the two most common isoforms, B1 (FCGR2B1) and B2 (FCGR2B2),
are generated by the alternative splicing of the corresponding mRNA
sequences (5). FCGR2B is
generally expressed on the surface of immune cells, e.g.,
neutrophils, B-lymphocytes and monocytes (6,7),
but is not expressed in any other endothelial cells of the adult
human body, apart from placental endothelial cells (8) and hepatic sinusoidal endothelial
cells (9). Human placental
endothelial cells abundantly and predominantly express FCGR2B2
(10–12). We previously identified an
FCGR2B2-defined, IgG-containing organelle (tentatively designated
as the FCGR2B2 compartment) in placental endothelial cells by
immunoelectron microscopy; the FCGR2B2 compartments did not overlap
with various marker proteins of well-recognized intracellular
organelles [e.g., caveolae, secretory granules and (early, late and
recycling) endosomes] (11).
These previous in vivo findings suggested that FCGR2B2
compartments mediate the transfer of IgG across the placental
endothelium, independent of caveolae. However, the molecular
mechanisms underlying the formation and intra cellular dynamics of
FCGR2B2 compartments and their IgG trafficking in placental
endothelial cells remain to be elucidated.
In this study, we performed bio-imaging analysis of
IgG trafficking of FCGR2B2 compartments using human umbilical vein
endothelial cells (HUVECs) transfected with a plasmid vector
containing enhanced GFP-tagged FCGR2B2 (pFCGR2B2-EGFP) as an in
vitro system for the analysis of FCGR2B2 expression in
placental endothelial cells. We also isolated FCGR2B2 compartments
from the human placenta and performed proteomic analysis of the
vesicles to identify the molecules involved in the regulation of
the FCGR2B2 compartment trafficking; we found that the Rab family
of proteins [RAS-related protein Rab family (RABs)] were associated
with FCGR2B2 compartments in placental endothelial cells. Among the
RABs, RAB3D was expressed predominantly in placental endothelial
cells. Furthermore, we generated small interfering RNAs (siRNAs)
targeting RAB3D to investigate the role of the RAB3D in the
FCGR2B2 compartment in our in vitro system.
Materials and methods
Sample collection
Human first-trimester placentas and full-term
placentas with umbilical cords from patients who provided informed
consent were obtained according to the protocols approved by the
Nippon Medical School Hospital Ethics Committee (Tokyo, Japan) and
the Jichi Medical University Ethics Committee (Tochigi, Japan).
Tissue samples were processed as soon as possible following
delivery (within 20 min).
Isolation of endothelial cells from the
human placenta
Human umbilical cords were processed to obtain the
HUVECs through collagenase digestion and subsequent magnetic bead
isolation (Dynabeads CD31; catalog no. DB11128; Invitrogen,
Carlsbad, CA, USA). The HUVECs were maintained with the endothelial
cell growth medium MV2 kit (catalog no. C-22121; PromoCell,
Heidelberg, Germany) at 37°C in a humidified incubator with 5%
CO2. The placental endothelial cells were isolated from
human placental tissues as described in a previous study (3). Dynabeads CD31 was used instead of
Dynabeads that were coated with QB-End/40 monoclonal antibody to
thrombomodulin.
Plasmid construction and transient
transfection by electroporation
The open reading frame of human FCGR2B2 cDNA
was amplified as previously described (12). The PCR product was inserted at the
HindIII and AgeI sites of a pEGFP-Hyg-N1 vector, as
previously described (13), which
was generated by modifying the commercially available
pDsRed-Monomer-Hyg-N1 vector (BD Biosciences Clontech, Mountain
View, CA, USA). These plasmids were designed to express EGFP fused
to the C-terminus of the FCGR2B2 protein avoiding the signal
peptide. The final construct was designated as pFCGR2B2-EGFP. A
pFCGR2B2 vector lacking EGFP was also constructed by the PCR method
and used to provide experimental foundations for the bio-imaging
analysis of IgG trafficking using HUVECs transfected with
pFCGR2B2-EGFP. As a mock control, cells were transfected in
parallel with vector pEGFP-N1.
For transfection, 100 μl HUVECs were
trypsinized, resus-pended with serum-free Opti-MEM (Invitrogen) at
a density of 1×106 cells/ml, and placed in 2 mm cuvettes
with 2 μg plasmid. Electroporation was performed using an
exponential wave pulse of 100 V and 500 μF using the Gene
Pulser Xcell electroporation system (Bio-Rad, Hercules, CA, USA).
The the cells were then replated with 200 μl medium and
cultured for up to 72 h. Validation of our plasmid construction and
transfection procedure was performed by transfection of the HUVECs
with pFCGR2B2-EGFP followed by immunocytochemistry.
Antibodies
Rabbit anti-human FCGR2B antibody directed against
the C-terminal cytoplasmic tail of human FCGR2B (MHPDALEEPDDQNRI)
and rabbit anti-human FCGRT antibody (DADLKDVNVIPATA) directed
against the C-terminal cytoplasmic tail of human FCGRT were
generated (Peptide Institute, Osaka, Japan). Rabbit anti-human
low-affinity immunoglobulin FCGR2A antibody directed against the
C-terminal cytoplasmic tail of human FCGR2A (YLTLPPNDHVNSNN) was
also generated and used to test the specificity of the anti-FCGR2B
antibody. Cos-7 cells (Cell Resource Center for Biomedical Research
Institute of Development, Aging and Cancer Tohoku University,
Miyagi, Japan) were transfected with a plasmid designed to
overexpress EGFP or EGFP-tagged FCGRT, FCGR1A, FCGR2A, FCGR2B1 or
FCGR2B2; the specificity of these affinity-purified antibodies was
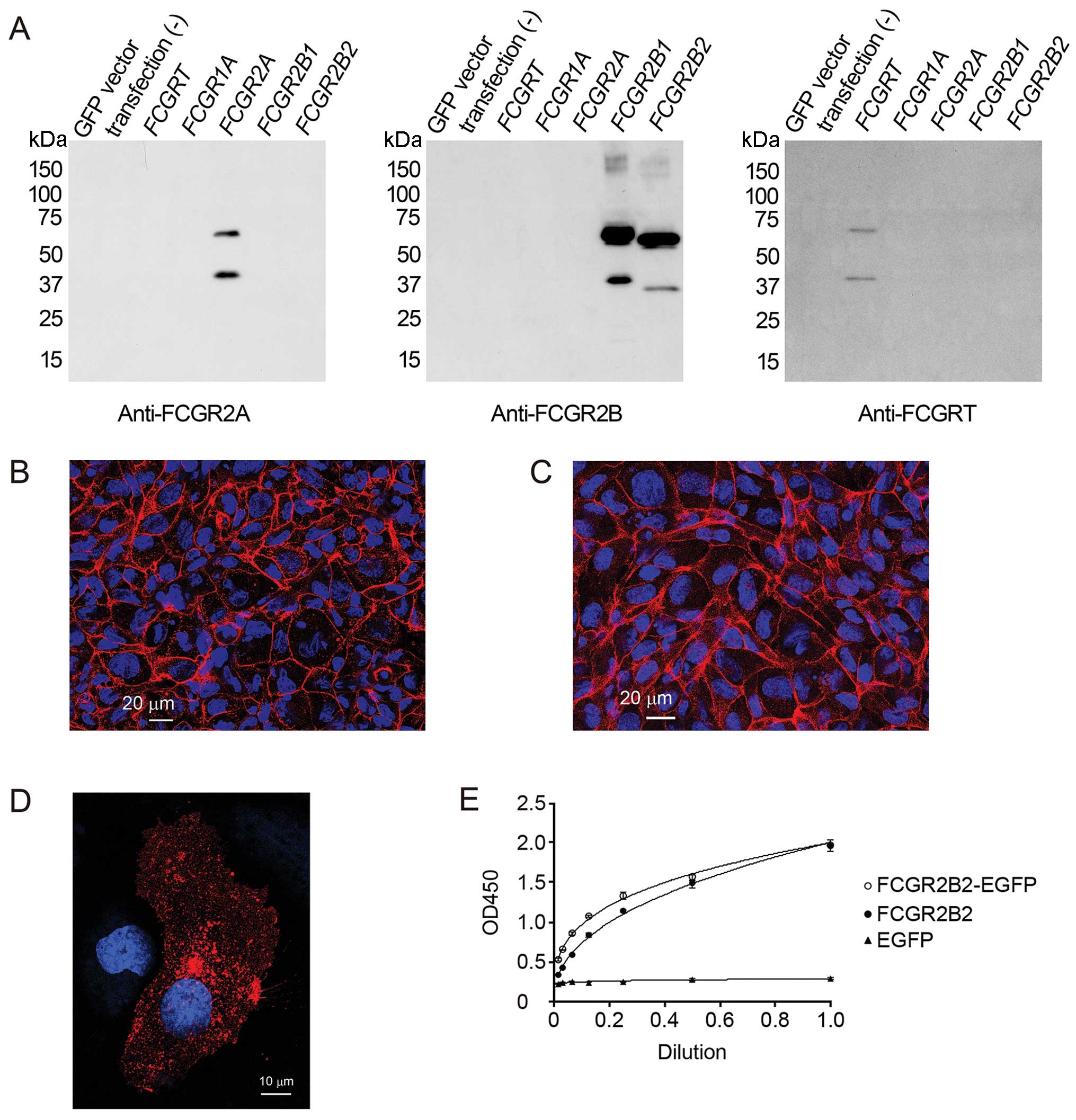
validated by western blot analysis (Fig. 1A). Rabbit anti-von Willebrand
factor antibody (catalog no. F3520; Sigma-Aldrich), mouse
anti-caveolin-1 antibody (catalog no. C-37120-150; BD Transduction
Laboratories, Franklin Lakes, NJ, USA), mouse anti-CD31 antibody
(catalog no. BBA7; R&D Systems, Minneapolis, MN, USA), mouse
anti-CD144 (clone 16B1; eBioscience, San Diego, CA, USA; CD144 also
known as VE-cadherin and CDH5), rabbit anti-human RAB1 antibody
(catalog no. FL-205), rabbit anti-human RAB3 antibody (catalog no.
FL-220) (both from Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), rabbit anti-human RAB3D (catalog no. 12320-1-AP; ProteinTech
Group, Chicago, IL, USA), mouse anti-human RAB11 antibody (catalog
no. 610656; BD Transduction Laboratories), mouse anti-human RAB33B
(catalog ID H00083452-M01; Abnova, Taipei, Taiwan), mouse
anti-human Ras-related protein Ral-A antibody (catalog no. 610221;
BD Transduction Laboratories), Alexa Fluor-labeled secondary
antibodies (Invitrogen), and HRP-conjugated secondary antibodies
(Jackson ImmunoResearch Laboratories, West Grove, PA, USA) were
obtained from commercial sources.
Immunocytochemistry
The cultured HUVECs were fixed with 4%
paraformaldehyde in PBS at room temperature for 2 h and
permeabilization was performed with 0.2% Triton X-100. For the
detection of caveolin-1, additional permeabilization was performed
with 0.5% sodium dodecyl sulfate (SDS). Dilutions for the primary
antibodies were 1:500 (FCGR2B), 1:200 (von Willebrand factor),
1:100 (caveolin-1), 1:500 (CD31), and 1:20 (CD144). Alexa
Fluor-labeled secondary antibodies were used at 1:200 dilutions.
Following incubation with the antibodies, the cells were subjected
to nuclear staining with 4',6-diamidino-2-phenylindole (DAPI) for
10 min and were mounted in ProLong Gold (Invitrogen). Fluorescence
images were collected using a BX60 microscope (Olympus, Tokyo,
Japan) equipped with a Spot RT SE6 CCD camera (Diagnostic
Instruments, Sterling Heights, MI, USA) and captured with the
MetaMorph image analysis system (Universal Imaging, Dowingtown, PA,
USA). The figures were compiled using Photoshop CS software (Adobe
Systems, Mountain View, CA, USA).
Binding affinity of FCGR2B2-EGFP for
human IgG
The binding property of FCGR2B2-EGFP for human IgG
was analyzed by enzyme-linked immunosorbent assay (ELISA), as
previously described (14,15).
Rabbit anti-human FCGR2B antibody was biotinylated using a Biotin
Labeling kit-NH2 (Dojindo Molecular Technologies,
Kumamoto, Japan). MaxiSorp 96 well microtiter plates (Nunc,
Roskilde, Denmark) were coated with 100 μg/ml human IgG
(catalog no. 31154; Thermo Fisher Scientific Pierce, Waltham, MA,
USA) at 4°C overnight. The plates were blocked with 5% non-fat dry
milk in PBS containing 0.05% Tween-20 (PBST) for 1 h at room
temperature and then incubated with serially diluted cell lysate
samples from the transfected HUVECs for 2 h at 37°C. After washing
with Tween-20-containing PBS, the plates were incubated with the
biotinylated anti-FCGR2B antibody at 1:1,000 dilutions for 2 h at
37°C. High Sensitivity Streptavidin HRP conjugates at 1:10,000
dilutions (catalog no. 21130; Thermo Fisher Scientific Pierce) was
added to each well followed by incubation for 1 h at 37°C. ELISA
was developed using 1-Step Ultra TMB-ELISA (Thermo Fisher
Scientific Pierce,). The absorbance of each well was measured at
450 nm using an iMark Microplate Absorbance Reader (Bio-Rad).
Incorporation of human IgG and
fluorescence imaging
We evaluated the ability of the
pFCGR2B2-EGFP-transfected HUVECs to incorporate IgG using a
modification of a previously described method (16,17). Alexa 555-human IgG and Alexa
633-human IgG were prepared using human IgG (Thermo Fisher
Scientific Pierce) and an Alexa Fluor protein labeling kit
(Invitrogen). Alexa 555-F(ab′)2 was generated by
digesting Alexa-human IgG using a Pierce F(ab′)2
Preparation kit (catalog no. 44988; Thermo Fisher Scientific
Pierce) and purified by chromatography and dialysis using the
methods recommended by the manufacturer.
Preliminary experiments of the cells exposed to
different concentrations of Alexa 555-IgG or Alexa
555-F(ab′)2 (0, 1, 10 and 100 μg/ml) indicate
that the minimum concentration of detectable fluorescence signals
in imaging analysis was 10 μg/ml. After 24 h transfection
with pFCGR2B2-EGFP or pEGFP-N1, HUVECs were washed with HBSS
(catalog no. H4891; Sigma-Aldrich) containing 10 mM HEPES (pH 7.4),
serum starved for 20 min, and then exposed to 10 μg/ml Alexa
555-IgG or equimolar Alexa 555-F(ab′)2 for 10 min. The
cells were immediately washed with PBS and incubated with fresh
medium for another 10 min. For quantitative image analysis of Alexa
555-IgG and Alexa 555-F(ab′)2, the cells were fixed with
4% paraformaldehyde, counterstained with DAPI, and mounted in
ProLong Gold. The signals were detected using a fluorescence
microscope (IX71; Olympus) or a confocal laser-scanning microscope
(LSM 710; Carl Zeiss, Jena, Germany).
The quantitative image analysis of the
pFCGR2B2-EGFP-transfected or mock-transfected HUVECs was performed
using the MetaMorph image analysis system as follows: using the
auto-fluorescence threshold on merged images, the cellular profiles
were demarcated as a region of interest. The area within the region
of interest occupied by the fluorescence signals of the
internalized Alexa 555-IgG or Alexa 555-F(ab')2 were
detected with an appropriate fluorescence threshold. Approximately
120 cells were used for this analysis. The figures were compiled
using Photoshop CS software.
The ability of IgG to incorporate from the
basolateral surface was evaluated as follows: the HUVECs
transfected with pFCGR2B2-EGFP were plated on Transwell membrane
inserts for 24-well plates (0.4 μm pores, catalog no.
353495, BD Falcon, Franklin Lakes, NJ, USA) at a density of
2×105 cells/insert and cultured for 24 h. Confluent
HUVECs were washed with HBSS containing 10 mM HEPES (pH 7.4) and
serum starved for 20 min. The Alexa 633-IgG (100 μg/ml) was
added to the wells of the lower chambers of the 24-well plates.
Following incubation for 60 min, the cells were immediately washed
and fixed. Super-resolution microscopy detection of FCGR2B2-EGFP
and Alexa 633-IgG incorporation was performed using a stimulated
emission-depletion (STED) microscope (TSC STED CW; Leica, Wetzlar,
Germany). Images were compiled using Photoshop CS software.
Transcytosis of human IgG
We evaluated the ability of the
pFCGR2B2-EGFP-transfected HUVECs to transcytose IgG using a
modification of a previously described method (18,19). Human IgG (Thermo Fisher Scientific
Pierce) was biotinylated using a Biotin Labeling
kit-NH2. The HUVECs transfected with pFCGR2B2-EGFP or
pEGFP-N1 were plated on Transwell membrane inserts for 24-well
plates at a density of 1×105 cells/insert and cultured
for 24–36 h.
To confirm the confluence of the polarized
monolayers, we validated the passage of Lucifer yellow and the
formation of cell-cell junctions (Fig. 1B and C). Before conducting IgG
transcytosis experiments, 100 μM Lucifer yellow (catalog no.
L-1177; Invitrogen-Molecular Probes, Eugene, OR, USA) was added to
the basolateral chamber of HUVECs growing on the culture inserts.
After 4 h, the concentration of Lucifer yellow of apical media in
the culture inserts was measured using a GloMax 96 microplate
luminometer (Promega, Madison, WI, USA). The integrity of the
monolayer was confirmed with <2% leakage of Lucifer yellow flux
observed in all the experiments. Immunofluorescence analysis
revealed that adherens junction (CD144) and adhesive molecule
(CD31) were expressed on cell to-cell contacts. These results
indicated that the HUVECs developed a confluent polarized
monolayer.
After testing the cell monolayer integrity, 15
μg/ml biotin-labeled human IgG were added to in the
basolateral donor chamber. After 24 h, transcytosed, biotin-labeled
human IgG in the apical receiver chamber was detected by western
blot analysis using high sensitivity streptavidin HRP conjugates at
1:10,000 dilutions (catalog no. 21130; Thermo Fisher Scientific
Pierce). Signal detection was carried out using Immobilon Western
HRP Substrate (Millipore, Billerica, MA, USA) and an LAS-4000
lumino-image analyzer (Fujifilm, Tokyo, Japan). Densitometric
analysis of the band intensities of the heavy chain of the
transcytosed, biotin-labeled IgG was quantitatively analyzed using
Multi Gauge software (GE Healthcare, Little Chalfont, UK).
Gene silencing
The siRNA-mediated knockdown of FCGRT and
RAB3D in the HUVECs was performed as follows: two distinct
types of siRNA duplexes for each target gene were designed using
siDirect (http://sidirect2.rnai.jp/) as
previously described (20), which
is based on an algorithm to increase the knockdown efficiency and
minimize off-target silencing. The designed siRNAs were synthesized
by Nippon EGT (Toyama, Japan). The transfection of the siRNAs was
performed using Lipofectamine 2000 (Invitrogen) according to the
manufacturer’s instructions. The efficacy of gene silencing at the
mRNA level was assessed after 6–72 h by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). The
sequences of the designed siRNAs were as follows: siRNAs for
FCGRT, siFCGRT-1 [21 nt guide (5′→3′): acuuuugacuguua
gugacGA; 21 nt passenger (5′→3′): gucacuaacagucaaaaguGG)] and
siFCGRT-2 [21 nt guide: uuuacauccuucaaa ucagCA; 21 nt
passenger: cugauuugaaggauguaaaUG)]; siRNAs for RAB3D,
siRAB3D-1 [21 nt guide: uaucaguagcaguuugaacAU; 21 nt
passenger: guucaaacugcuacugauaGG)] and siRAB3D-2 [21 nt
guide: acauugugaaggaaugagcCA; 21 nt passenger: gcucauuccuu
cacaauguCG)]; control siRNA [21 nt guide: uucuccgaacguguc acguTT;
21 nt passenger: acgugacacguucggagaaTT)]. Nucleotides shown by
uppercases indicate those of 3′-overhangs.
Western blot analysis
Proteins in the HUVECs were extracted using M-PER
Mammalian Protein Extraction Reagent (Thermo Fisher Scientific
Pierce) containing protease inhibitors. Proteins of isolated
FCGR2B2 compartments were directly extracted with SDS-containing
loading buffer. Dilutions for the primary antibodies were 1:5,000
(FCGR2B, Ral-A and ACTB), 1:250 (FCGRT), 1:200 (RAB1 and RAB3),
1:400 (RAB3D) and 1:1,000 (RAB11 and RAB33B). HRP-conjugated
secondary antibodies were diluted to 1:10,000. Signals were
detected using Immobilon reagent and visualized using a LAS-4000
Lumino image analyzer. The intensity of the visualized signals was
quantitatively analyzed using Multi Gauge software.
RT-qPCR
Total RNA was extracted from the samples using
Isogen reagent (Nippon Gene, Tokyo, Japan) according to the
manufacturer's instructions. cDNA synthesis was performed using a
PrimeScript RT reagent kit (Takara Bio, Shiga, Japan). For
conventional RT-PCR, ExTaq Hot Start Version (Takara Bio) was used
for amplification using gene-specific primers, some of which were
designed using DNASIS software (Hitachi, Tokyo, Japan).
The primers used were as follows: FCGR2B
forward, CCACTAATCCTGATGAGGCTGACA and reverse, CTC
AAATCCCAATGCAAGACAATG; FCGRT forward, CAC GCCTCGTCGTCACTAACA
and reverse, AGTAGCAAG ACACCGATGACGATTC; RAB1A forward,
TATGGGACA CAGCAGGCCAGG and reverse, ACGGAATTCCAAGGG AATCAGC;
RAB1B forward, TGAACCCCGAATATGAC TAC and reverse,
GTGTACGTGTCATCAGCAAA; RAB3A forward, CAGACCTGTTCTGACCTCAT
and reverse, CTTTATTGGGTGCGTGTAGT; RAB3B forward,
AGATGGTCCCAGTAATAGATACTC and reverse, GACTGTTCTCT AAGTCCCTGTAGT;
RAB3C forward, GCGACAAAATGT CAGAGAGT and reverse,
GAGGAGGAGTTTCCTTGAGT; RAB3D forward, TCTGGAACTATGGACCACAT
and reverse, CTCCTGGCTCTGAGGTTAAT; RAB2A forward,
CAGGTGTTGGTAAATCATGC and reverse, CACCGAA CTCTACACCAATAG;
RAB15 forward, ATCAAGACC TATGCCACATC and reverse,
CCACTGCCCAATATAA CTTC; RAB18 forward, TACCCC TCAGTAAGATTCCA
and reverse, TATTGCAAAGGTGGTCACTC; RAB35 forward,
CCAGGATGTGTTTCCTTAGA and reverse, AACTGCA GTGTGATCTGTGA;
RAB39A forward, ACGTCAAGTT ACAAGGGAAG and reverse,
TGTCTCTCGTCAAGATTGTG; ACTB forward, ATTGCCGACAGGATGCAGA and
reverse, GAGTACTTGCGCTCAGGAGGA; and GAPDH forward,
GCACCGTCAAGGCTGAGAAC and reverse, ATG GTGGTGAAGACGCCAGT. All
primers were synthesized by Nippon EGT. The PCR products were
separated by electrophoresis on a 2% agarose gel. Quantitative PCR
(qPCR) was performed using SYBR Premix ExTaq (Takara Bio) on an ABI
7300 apparatus (Applied Biosystems, Foster City, CA, USA).
GAPDH was used for the ormalization of gene expression.
Isolation of FCGR2B2 compartments from
the human placenta
FCGR2B2-containing vesicles were purified from
full-term placentas as follows: human placental terminal
villus-rich fractions were collected from full-term placentas as
previously described (12). The
terminal villus-rich fractions were resus-pended in homogenization
buffer (20 mM Tris-HCl pH 7.4, 5 mM MgCl2, 0.25 M
sucrose, 1 mM PMSF, 1 μM aprotinin and 1 μM pepstatin
A) and disrupted using a Bioruptor (Cell Disruption Bomb; Parr
Instrument, Moline, IL, USA). After 15 min of shaking under high
pressure at 1,300 psi on ice, cells in terminal villi were
disrupted by passage through a narrow valve. The collected
homogenate was centrifuged twice at 4°C, first at 600 x g for 10
min and then at 10,000 x g for 20 min. The supernatant was
transferred to ultracentrifuge tubes and centrifuged at 105,000 x g
for 1 h at 4°C. The pellets, representing the microsomal fraction,
were resuspended in phosphate-buffered saline (PBS) containing 5 mM
MgCl2 and 1% BSA. Magnetic Dynabeads M280 sheep
anti-rabbit IgG (catalog no. 112.04; Invitrogen) was used for
immunoprecipitation. The microsomal fraction was pre-incubated with
Dynabeads bound to the non-immune rabbit IgG whole molecule
(catalog no. 011-000-003; Jackson ImmunoResearch Laboratories) at
4°C overnight to reduce non-specific binding. To capture
FCGR2B2-containing vesicles, the pre-incubated microsomal fraction
was incubated with Dynabeads and either anti-FCGR2B antibody or the
non-immune rabbit IgG as a control at 4°C for 2 h. The beads were
then washed 5 times with PBS at room temperature and subjected to
the following procedures.
Transmission electron microscopy of
FCGR2B2 compartments
The FCGR2B2 compartments were fixed overnight at
room temperature with 2.5% glutaraldehyde and 2% paraformal-dehyde
in 0.1 M sodium cacodylate buffer, pH 7.4 containing 0.05%
CaCl2. The pellet was post-fixed with 2% osmium
tetroxide and 1.6% potassium ferrocyanide in 0.1 M sodium
cacodylate at room temperature for 30 min. The pellet was
dehydrated in ethanol and then embedded in epoxy resin. The
sections were electron stained with uranyl acetate and lead citrate
and examined under a transmission electron microscope (H-7600;
Hitachi) at 80 kV.
Two-dimensional differential in-gel
electrophoresis (2D-DIGE) and mass spectrometry
2D-DIGE was performed as previously described
(21). The isolated FCGR2B2
compartments were lysed with thiourea lysis buffer. As a control,
an equal volume of the control beads bound to the non-immune rabbit
IgG whole molecule was used. The proteins extracted from the
purified FCGR2B2 compartments and the control beads were labeled
with CyDye DIGE Fluor Cy3 and Cy5 minimal Dyes (GE Healthcare),
respectively. The CyDye-labeled proteins and non-labeled microsomal
proteins (as a spot reference) were then mixed and subjected to
2D-DIGE. The CyDye-labeled protein spots in the gel were visualized
using a Typhoon 9400 imager and analyzed using differential in-gel
analysis with DeCyder 2D Differential Analysis Software (both from
GE Healthcare). Protein spots that exhibited 2-fold higher
intensity in the FCGR2B2 compartments compared to the control were
selected as candidate FCGR2B2 compartment-associated proteins. All
proteins, including the non-labeled microsomal proteins, on the
same gel were then stained with SYPRO Ruby (Takara Bio) to
determine the positions of the FCGR2B2 compartment-associated
proteins. The eluted proteins were analyzed by liquid
chromatography-tandem mass spectrometry (LC-MS/MS) (LCQ DECA, XP
Plus; Thermo Finnigan, San Jose, CA, USA). Data were analyzed using
Mascot Search on the Matrix Science website (http://www.matrixscience.com/). The specificity of
trypsin digestion was used as the cutting enzyme, allowing 3 missed
cleavages. Peptide mass tolerance and fragment mass tolerance were
set to ±1.2 and ±0.6 Da, respectively. Carbamidomethyl of cysteine
was selected as the fixed modification and oxidation of methionine
was searched as the variable modification. Identity or extensive
homology is indicated for peptides with individual ion scores
>42 (p<0.05).
Statistical analysis
We conducted all the analyses using the SPSS
statistical software package (Windows version 20; IBM-SPSS). The
significance of between-group differences was assessed using the
Student's t-test or ANOVA with Dunnett’s post test, and p-values
<0.05 were considered to indicate statistically significant
differences.
Results
Expression and localization of FCGR2B2 in
pFCGR2B2- EGFP-transfected HUVECs
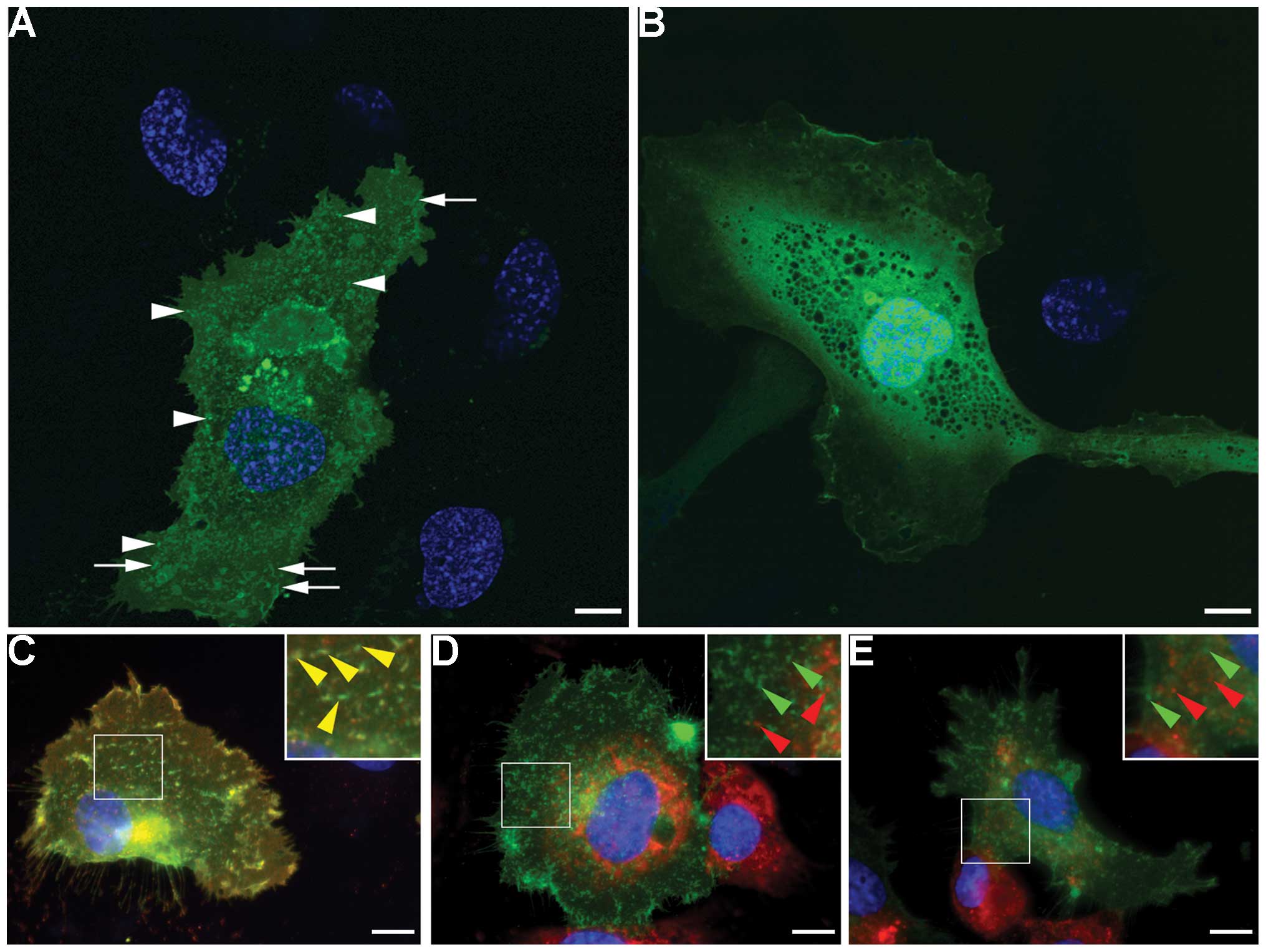
In the pFCGR2B2-EGFP-trans-fected HUVECs,
fluorescence signals indicating the expression of FCGR2B2
(FCGR2B2-EGFP signals) were transiently detected within 18-48 h
after electroporation. In the transfected cells, FCGR2B2-EGFP
signals were detected as intracellular vesicular and tubular
structures and were widely distributed in the cytoplasm (Fig. 2A). Some signals were also present
on the cell surface. The pFCGR2B2-EGFP-transfected HUVECs showed a
similar expression of FCGR2B2 in placental endothelial cells in
vivo as previously reported (11). By contrast, in the
mock-transfected pEGFP-N1 cells, the fluorescence signals showed a
diffuse distribution throughout the cytoplasm and the nucleus and
did not exhibit intracellular structure-specific localization
(Fig. 2B).
Subsequently, we wished to investigate whether the
localization of the FCGR2B2-EGFP reflected that of the endogenous
protein. In the pFCGR2B2-EGFP-transfected HUVECs, the majority of
the EGFP signals were co-localized with the Alexa Fluor 594
fluorescence signals detected with an anti-FCGR2B antibody
(Fig. 2C), thus suggesting that
the EGFP signals can be used to reliably monitor FCGR2B2 in the
transfected cells. Although an endogenous endothelial marker, von
Willebrand factor, and a caveola marker, caveolin-1, were present
in the FCGR2B2-EGFP-expressing HUVECs, they were not co-localized
with the FCGR2B2-EGFP signals (Fig.
2D and E). These results are consistent with those of previous
in vivo findings that the intracellular FCGR2B2 compartments
do not overlap with caveolae and secretory granules in the
placental endothelium (22). In
addition, in order to provide an experimental foundation for
bio-imaging analysis, we compared the subcellular localization and
IgG binding property of FCGR2B2-EGFP with those of FCGR2B2.
Immunocytochemistry revealed that the pFCGR2B2-EGFP-transfected
HUVECs showed a localization of FCGR2B2 similar to the
pFCGR2B2-transfected cells (Fig.
1D). We also examined the affinities of human IgG for
FCGR2B2-EGFP and FCGR2B2 by ELISA (Fig. 1E). FCGR2B2-EGFP showed an almost
identical affinity of human IgG for FCGR2B2. Taken together, these
results indicate that the pFCGR2B2-EGFP-transfected HUVECs are a
useful model for placental endothelial cells in vitro.
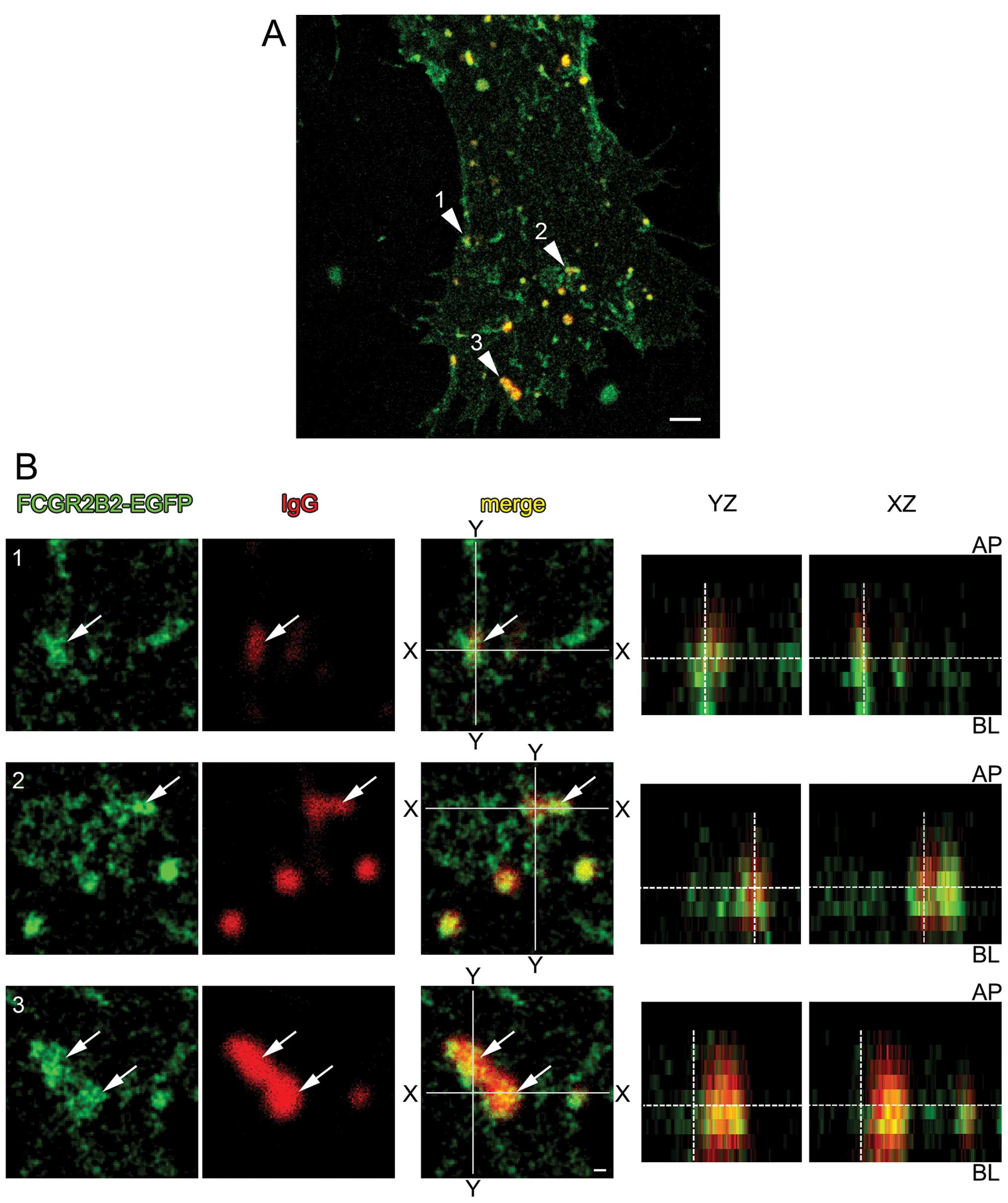
Human IgG incorporation from the
basolateral surface of FCGR2B2-EGFP-expressing cells
As maternal IgG is internalized from the basolateral
surface of the placental endothelium in vivo, we then
examined the subcellular IgG incorporation from the basolateral
surface of FCGR2B2-EGFP-expressing HUVECs by both STED and confocal
imaging. STED microscopy is a technique that uses the non-linear
de-excitation of fluorescent dyes to overcome the resolution limit
of standard confocal microscopes that is imposed by diffraction
(23). Confluent
pFCGR2B2-EGFP-transfected HUVECs that were cultured on Transwell
filters were exposed to Alexa Fluor 633-labeled IgG (Alexa 633-IgG)
from the basolateral surface. Super-resolution imaging with STED
and confocal imaging revealed that oval and tubular FCGR2B2-EGFP
signals were often found and frequently co-localized with Alexa
Fluor 633-IgG signals that were incorporated from the basolateral
surface (Fig. 3). By
three-dimensional analysis, it appeared that some FCGR2B2-EFGP
compartments extended or fused along the axis of apical-basal cell
polarity (Fig. 3B, YZ and
XZ).
Internalization and transcytosis of human
IgG in the FCGR2B2-EGFP-expressing cells
The ability of FCGR2B2-EGFP-expressing HUVECs to
incorporate human IgG was evaluated by monitoring the fluorescence
signals in the cells exposed to Alexa Fluor 555-labeled human IgG
(Alexa 555-IgG). In the cells, the majority of the internalized
Alexa 555-IgG signals were co-localized with the FCGR2B2-EGFP
signals (Fig. 4A–D). Internalized
Alexa 555-IgG was more easily detected as small dots of
fluorescence in the pFCGR2B2-EGFP-transfected cells than in the
EGFP (mock)-transfected cells (Fig.
4E). The ability of the FCGR2B2-EGFP-expressing cells to
incorporate IgG was evaluated by comparing the ratios of the Alexa
555-positive area to the total cellular area in the
pFCGR2B2-EGFP-transfected cells (n=47) and mock-transfected cells
(n=48); this ratio was significantly higher in the
pFCGR2B2-EGFP-transfected cells than in the mock-transfected cells
(P<0.01) (Fig. 4G).
Furthermore, to validate the Fc fragment-specific internalization,
FCGR2B2-EGFP-expressing cells were incubated with equimolar
concentrations of IgG or F(ab′)2 fragments (Fig. 4F). The ratios of the Alexa 555-IgG
and Alexa 555-labeled-F(ab′)2 fragment [Alexa
555-F(ab′)2] positive areas were measured in 43 and 48
cells, respectively. While the Alexa 555-F(ab′)2 was
detected in the FCGR2B2-EGFP-expressing cells (Fig. 4F), the ratio of Alexa
555-F(ab′)2 was significantly lower than the ratio of
Alexa 555-IgG (P<0.01) (Fig.
4H).
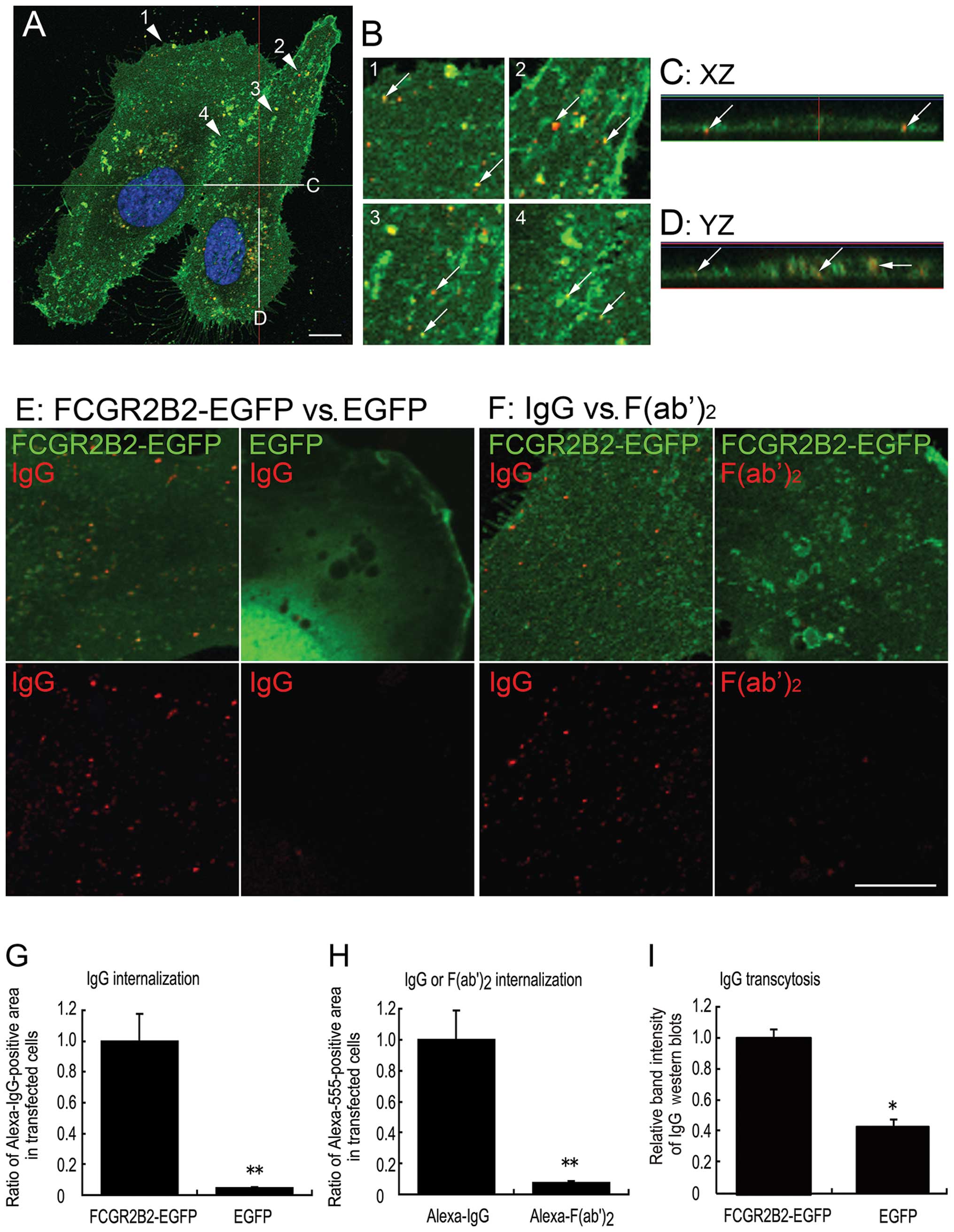 | Figure 4Internalization and transcytosis of
human IgG in FCGR2B2-EGFP-expressing cells. (A-D) Representative
confocal fluorescence images of internalized Alexa Fluor 555-IgG
(red) in FCGR2B2-EGFP (green)-expressing human umbilical vein
endothelial cells (HUVECs). (A) A confocal optical slice of 0.6
μm thickness at the position closest to the basal surface.
Nuclei were counterstained with 4′,6-diamidino-2-phenylindole
(DAPI) (blue). (B) Higher magnification images of the regions
indicated with the numbered arrowheads in (A). (C and D) Virtual
slices cut at the horizontal gray line denoted by (C and D) in (A).
In each virtual slice, the apical surface of the HUVECs is
indicated as the upper side. Internalized Alexa Fluor 555-IgG
signals appear as small dots in the cells. Co-localization of
internalized Alexa Fluor 555-IgG and FCGR2B2-EGFP signals is
frequently observed (arrows in B-D). (E) Higher magnification
confocal images of Alexa Fluor 555-IgG (red) in FCGR2B2-EGFP- and
EGFP (green)-expressing cells. Internalized Alexa Fluor 555-IgG
signals appear as small vesicular structures colocalized with
FCGR2B2-compartments in a pFCGR2B2-EGFP-transfected cell. By
contrast, in an EGFP (mock)-transfected cell, the level of
internalized Alexa Fluor 555-IgG is very low. (F) Higher
magnification confocal images of Alexa Fluor 555-IgG or Alexa Fluor
555-F(ab′)2 fragments (red) in FCGR2B2-EGFP-expressing
cells. The internalization of the Alexa Fluor
555-F(ab′)2 signal is obviously lower than that of Alexa
Fluor 555-IgG. Scale bars are 10 μm. (G and H)
Quantification of internalized Alexa Fluor 555-IgG or Alexa Fluor
555-F(ab′)2 signals. The area of intracellular Alexa
Fluor 555-IgG or Alexa Fluor 555-F(ab′)2 signals was
quantified. Values are the means ± SE [FCGR2B2-EGFP-expressing
cells, n=47; EGFP (mock)-expressing cells, n=48 (G)] and the mean ±
SE [Alexa 555-IgG-internalized cells, n=43; Alexa
555-F(ab′)2-internalized cells, n=48) (H)].
**p<0.01, Student’s t-test. Three or more experiments
were performed and yielded similar results. (I) Quantification of
transcytosed, biotin-labeled human IgG by western blot analysis.
The band intensities of the heavy chain of the transcytosed,
biotin-labeled IgG are significantly higher in
pFCGR2B2-EGFP-transfected cells than in EGFP (mock)-transfected
cells (*p<0.05, Student's t-test). The data are the
means from 3 measurements; error bars represent the means ± SD.
FCGR2B2, low-affinity immunoglobulin gamma Fc region receptor
IIb2. |
Subsequently, we aimed to investigate the ability of
the pFCGR2B2-EGFP-transfected HUVECs to transcytose IgG.
Biotin-labeled human IgG was added to in the basolateral donor
chamber. After 24 h, transcytosed, biotin-labeled human IgG in the
apical receiver chamber was detected by western blot analysis. The
band intensities of the heavy chain of the transcytosed,
biotin-labeled IgG were significantly higher in the
pFCGR2B2-EGFP-transfected cells than in the mock-transfected cells
(P<0.05) (Fig. 4I). Taken
together, these results suggest that the IgG internalization and
transcytosis observed in this study was caused by the enforced
expression of FCGR2B2 in the HUVECs and that it was an Fc
fragment-specific event.
IgG internalization and transcytosis in
FCGR2B2-EGFP-expressing cells that are FCGRT-deficient
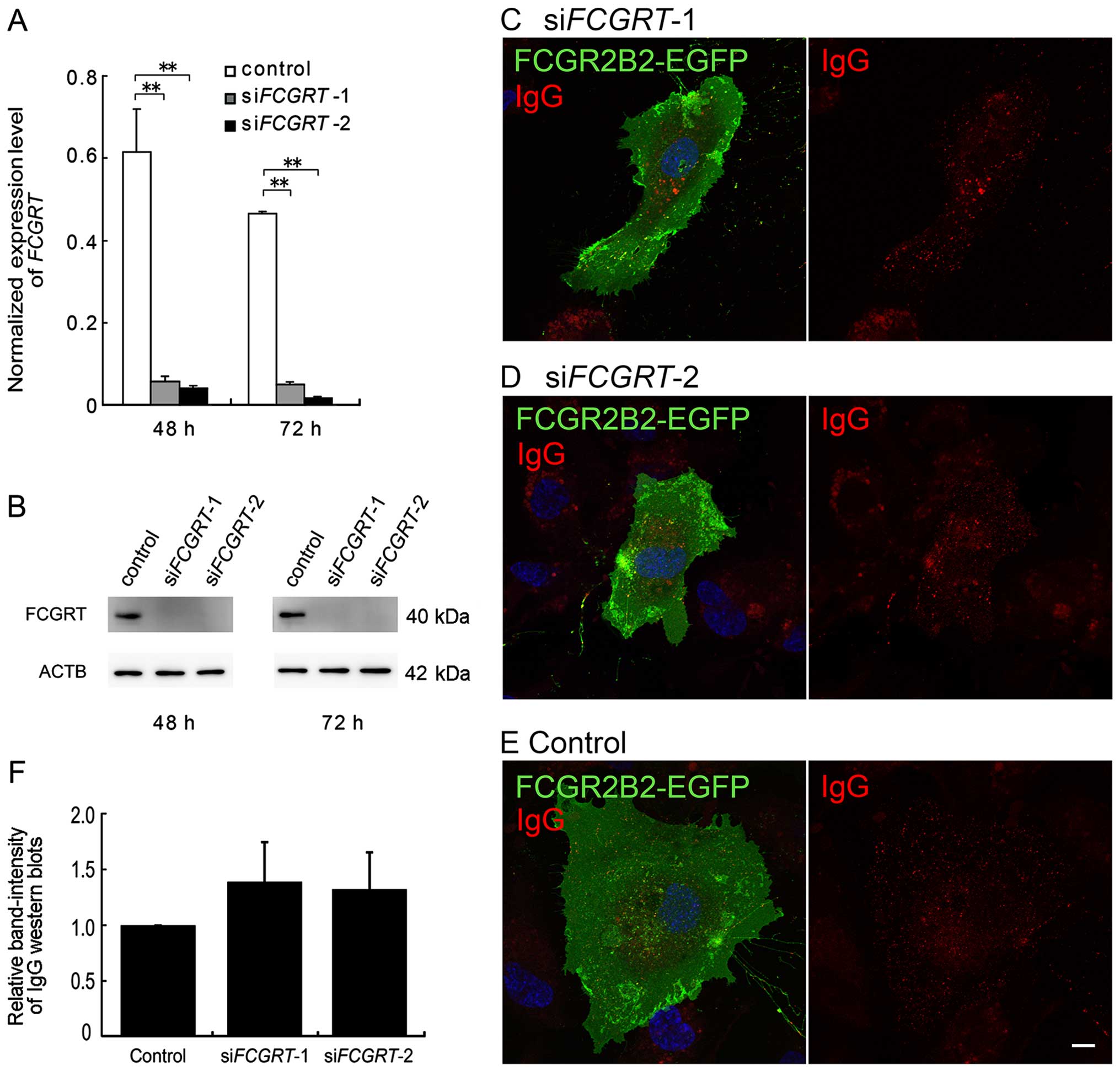
HUVECs also express FCGRT; thus, we examined the
effects of endogenous FCGRT on IgG incorporation by siRNA-mediated
knockdown. We constructed 2 types of siRNA duplexes against
FCGRT (designated as siFCGRT-1 and siFCGRT-2).
First, we evaluated the efficiency of the siRNA-mediated knockdown
using RT-qPCR and western blot analysis. After 48 h of siRNA
transfection, the mRNA expression level of FCGRT was 9.2 and
6.6% of the control cell levels with siFCGRT-1 and
siFCGRT-2, respectively. After 72 h, the expression level
was 10.6% (siFCGRT-1) and 3.4% (siFCGRT-2) of the
control (Fig. 5A). Furthermore,
the protein expression of FCGRT was markedly decreased by
siFCGRT-1 and siFCGRT-2 at both 48 and 72 h, as
determined by western blot analysis (Fig. 5B). As these siRNAs efficiently
suppressed the expression of both the FCGRT mRNA and FCGRT
protein, they can be used for subsequent experiments to investigate
the effect of endogenous FCGRT on IgG incorporation in
FCGR2B2-EGFP-expressing HUVECs.
After 18-24 h of transfection with
pFCGR2B2-EGFP, the HUVECs were transfected with siRNAs and
incubated for a further 18 h. The transfection of siFCGRT-1
and siFCGRT-2 did not affect the expression and distribution
of FCGR2B2-EGFP (Fig. 5C–E). The
internalization of Alexa 555-IgG was not altered in the cells in
which FCGRT had been knocked down (Fig. 5C and D). Furthermore, we
investigated the ability of the cells in which FCGRT had
been knocked down to tran-scytose IgG using the above-mentioned
biotin-labeled human IgG. No significant differences in the band
intensities of the heavy chain of the transcytosed, biotin-labeled
IgG were identified between the cells in which FCGRT had
been knocked down and the mock cells (Fig. 5F). Thus, our results suggested
that the IgG internalization and transcytosis were mediated
primarily by the enforced expression of FCGR2B2 rather than that of
endogenous FCGRT in the HUVECs.
Identification of FCGR2B2
compartment-associated proteins from the human placenta
We performed a proteomic analysis to identify
FCGR2B2 compartment-associated proteins, some of which may be
involved in the regulation of the trafficking of FCGR2B2
compartments. FCGR2B2 compartments were isolated from human
placental terminal villus-rich fractions by immunoprecipitation
using magnetic beads bound to a FCGR2B-specific antibody as
described in the Materials and methods. Imaging by electron
microscopy revealed that FCGR2B2 compartments were round, oval and
tubular membranous components (Fig.
6A and B). These morphological characteristics were similar to
those observed by the immunoelectron microscopy of FCGR2B2
compartments in placental endothelial cells (11). Western blot analysis revealed that
the FCGR2B2 compartments were specifically captured on the beads
coated with the anti-FCGR2B antibody (Fig. 6C).
Proteomic analysis of the FCGR2B fraction was
performed by 2D-DIGE. Twenty protein spots showed a >2-fold
higher volume ratio in the FCGR2B2 compartment fraction compared
with the control fraction. However, 4 of these spots were part of a
horizontal streaking tail; thus, they were not subjected to further
analyses. Of the 16 spots, 2 minor spots could not be separated or
detected on gels for protein identification. The remaining 14 spots
were isolated and analyzed by LC-MS/MS. The FCGR2B2
compartment-associated proteins identified by LC-MS/MS are
summarized in Table I. Of note,
LC-MS/MS revealed that these proteins contained a number of RAB
family proteins.
 | Table IFCGR2B2 compartment-associated
proteins identified by LC-MS/MS. |
Table I
FCGR2B2 compartment-associated
proteins identified by LC-MS/MS.
| GI no. | Gene symbol | Protein name | Fold change | Western blot
analysis of RAB proteins |
|---|
| 4504517 | HSPB1 | Heat shock 27 kDa
protein 1 | 3.03 | |
| 20147713 | RALA | Ras-related protein
Ral-A (precursor) | 3.03 | (−) |
| 4758984 | RAB11 A | Ras-related protein
Rab-11A | 3.03 | (+/−)a |
| 181178 | CTSB | Lysosomal
proteinase cathepsin B | 2.48, 2.02 | |
| 182518 | FTL | Ferritin light
subunit | 2.48 | |
| 4504747 | ITGA3 | Integrin alpha 3
isoform a precursor | 2.22 | |
| 119610452 | MYH10 | Myosin, heavy
polypeptide 10, non-muscle, isoform CRA_a | 2.22 | |
| 32490572 | EPB41L3 | Erythrocyte
membrane protein band 4.1-like 3 | 2.22 | |
| 558436 | DLG1 | Homolog of
Drosophila discs large protein, isoform 2 | 2.22 | |
| 115298659 | SPTA 1 | Spectrin, alpha,
erythrocytic 1 | 2.22 | |
| 5729877 | HSPA 8 | Heat shock 70 kDa
protein 8 isoform 1 | 2.22, 2.05 | |
| 71773329 | ANXA6 | Annexin VI isoform
1 | 2.22 | |
| 14585873 | DYNC1I2 | Cytoplasmic dynein
intermediate chain | 2.22 | |
| 2352945 | | Smooth muscle
myosin heavy chain SM2 | 2.22 | |
| |2851393 | POR | NADPH-cytochrome
P450 reductase | 2.22 | |
| 7706706 | SNX9 | Sorting nexin
9 | 2.22, 2.05 | |
| 4506467 | RDX | Radixin | 2.22 | |
| 340217 | VIL2 | Cytovillin 2 | 2.22 | |
| 13569962 | RAB1B | RAB1B, member RAS
oncogene family | 2.19, 2.14 | (+)b |
| 229451 | | Placental
lactogen | 2.19, 2.14 | |
| 4758988 | RAB1A | RAB1A, member RAS
oncogene family | 2.19, 2.14 | (+)b |
| 5803135 | RAB35 | RAB35, member RAS
oncogene family | 2.19, 2.14,
2.12 | ND |
| 38371739 | RAB15 | RAB15, member RAS
onocogene family | 2.19, 2.12 | ND |
| 13786129 | RAB33B | RAB33B, member RAS
oncogene family | 2.19, 2.12 | (+/−) |
| 1491714 | RAB39A | Rab-related
GTP-binding protein, Rab39A | 2.19, 2.12 | ND |
| 1710248 | ERP5 | Protein disulfde
isomerase-related protein 5 | 2.17 | |
| 550062 | RAB2 | GTP-binding
protein, RAB2A | 2.14 | ND |
| 10880989 | RAB18 | RAB18, member RAS
oncogene family | 2.14 | ND |
| 27734452 | RAB15 | Ras-related protein
Rab-15 | 2.14 | ND |
| 4758988 | RAB1A | RAB1A, member RAS
oncogene family | 2.12 | (+)b |
| 4506365 | RAB2A | RAB2A, member RAS
oncogene family | 2.12 | ND |
| 4759000 | RAB3D | RAB3D, member RAS
oncogene family | 2.12, 2.02 | (+)c |
| 1060888 | PSMD2 | Human 26S
proteasome subunit p97 | 2.06 | |
| 179468 | HSD3B |
3-beta-Hydroxysteroid dehydrogenase | 2.05 | |
| 4505257 | MSN | Moesin | 2.05 | |
| 4758304 | PDIA4 | Protein disulfde
isomerase-associated 4 | 2.05 | |
| 6470150 | HSPA 5 | BiP protein, heat
shock 70 kDa protein 5 | 2.03 | |
| 19923750 | RAB3B | RAB3B, member RAS
oncogene family | 2.02 | (+)c |
| 4506367 | RAB3A | RAB3A, member RAS
oncogene family | 2.02 | (+)c |
| 20178293 | KRT7 | Cytokeratin-7 | 2.02 | |
| 4759140 |
SLC9A3R1 | Solute carrier
family 9, isoform 3 regulator 1 | 2.02 | |
| 178027 | ACTA 3 | Alpha-actin | 2.02 | |
Subsequently, we examined the expression of some of
the RAB family proteins identified by LC-MS/MS in the isolated
FCGR2B2 compartments (Table I).
Western blot analysis revealed that both RAB3 and RAB1 were
detectable in the FCGR2B2 compartments (Fig. 6D). However, specific antibodies
against the other RABs identified by LC-MS/MS were not commercially
available for use in western blot analysis. In order to examine
which isoforms may be associated with the FCGR2B2 compartments and
which isoforms may be highly expressed in placental endothelial
cells, we investigated the mRNA expression levels of each isoform
(i.e., RAB1A, RAB1B, RAB2A, RAB3A,
RAB3B, RAB3D, RAB15, RAB18,
RAB35 and RAB39A) in placental tissues
(first-trimester and full-term placentas) and isolated endothelial
cells (placental endothelial cells and HUVECs). At first, we
examined 3 RAB3 isoforms, RAB3A, RAB3B and
RAB3D, and another highly homologous RAB3 isoform,
RAB3C. RT-qPCR revealed that RAB3A and RAB3C were
absent in the full-term placentas (Fig. 7A). The mRNA expression levels of
RAB3B, RAB3D, and other RABs were subsequently
compared among first-trimester placentas, full-term placentas,
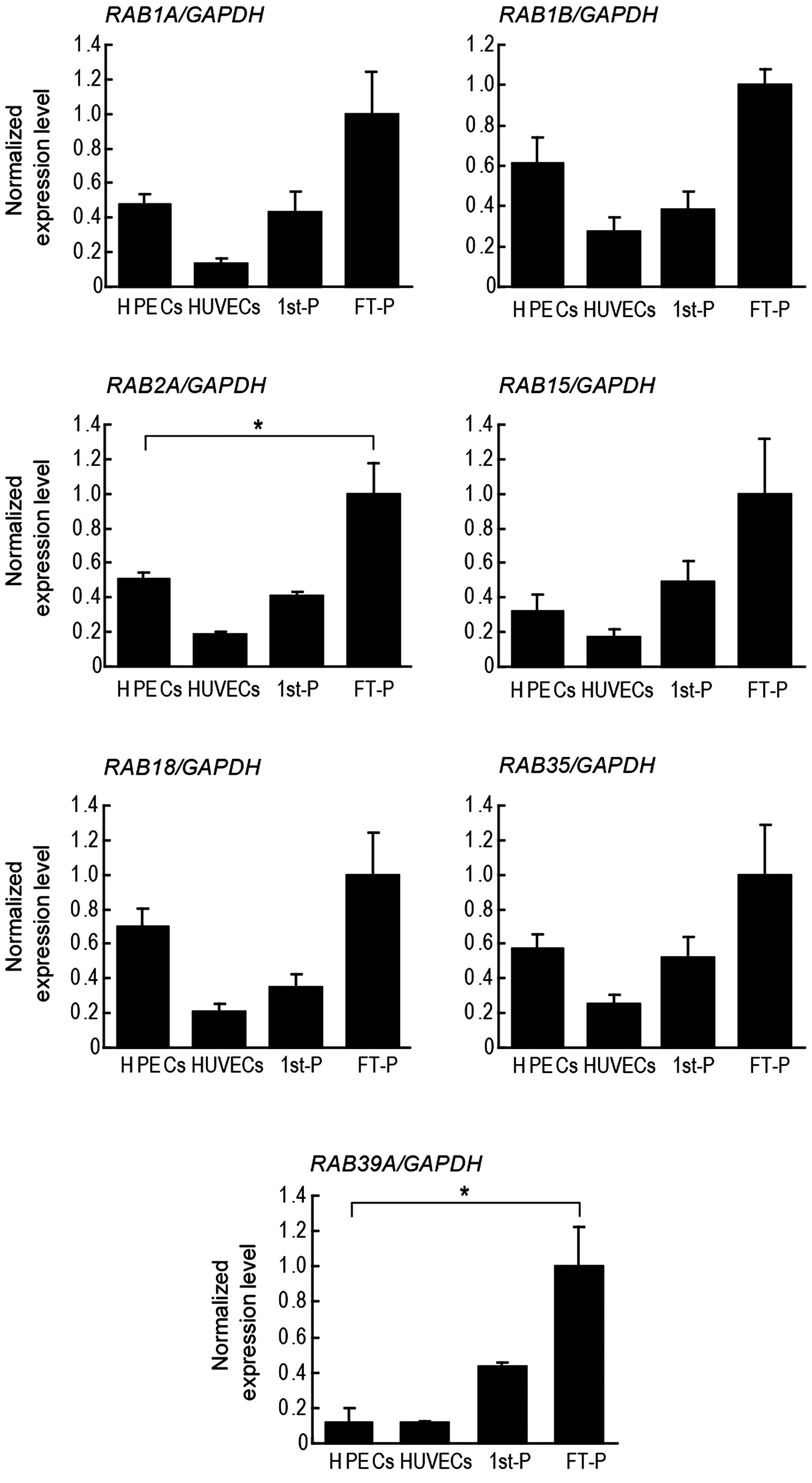
placental endothelial cells and HUVECs by RT-qPCR. RAB1A,
RAB1B, RAB2A, RAB3D, RAB15,
RAB18, RAB35 and RAB39A were expressed in all
the samples examined; RAB3B was detected in the placental
tissues, but not in the endothelial cells (Figs. 7B and 8). Among these RAB mRNAs, only
RAB3D was expressed predominantly in the placental
endothelial cells (Fig. 7B).
Therefore, we focused on RAB3D as a candidate FCGR2B2
compartment-associated protein to investigate its involvement in
the formation and/or intracellular dynamics of FCGR2B2
compartments.
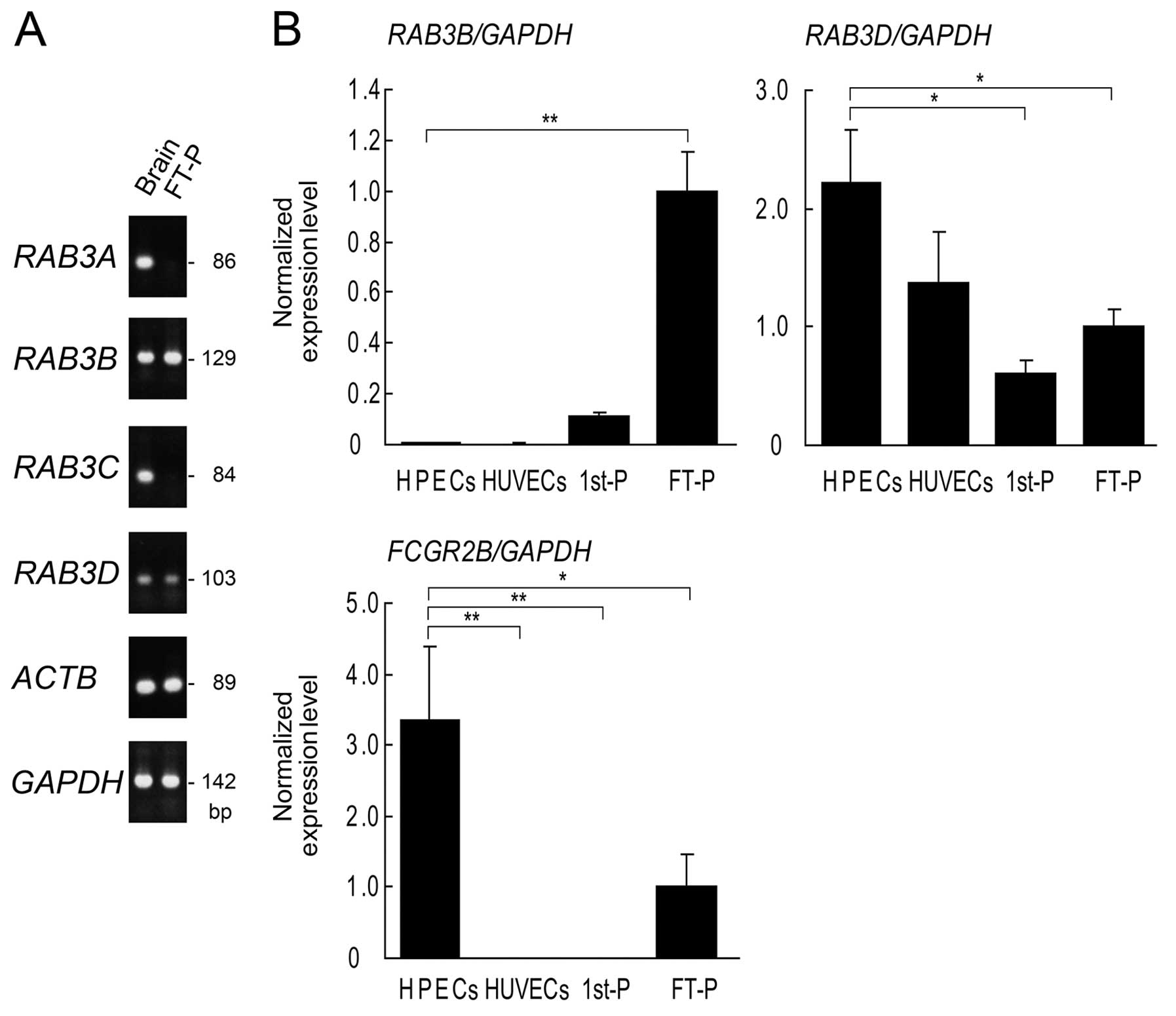 | Figure 7RT-qPCR of RAB3 isoforms in
human placental tissues and isolated endothelial cells. (A) RT-qPCR
of full-term placentas (FT-P) and human brain (Brain) as a positive
control. RAB3A and RAB3C are absent in FT-P. Other
isoforms of RAB3, RAB3B and RAB3D, are
expressed in FT-P. (B) RT-qPCR of human placental endothelial cells
(HPECs), human umbilical vein endothelial cells (HUVECs),
first-trimester placentas (1st-P) and FT-P. HPECs and HUVECs were
isolated simultaneously from FT-P and umbilical cords,
respectively. FCGR2B is highly expressed in HPECs, but is
not expressed in HUVECs. RAB3D is highly expressed in HPECs
compared to 1st-P, FT-P, and HUVEC. Expression levels were
normalized to GAPDH. Relative expression levels in FT-P were
assigned a value of 1. Data are indicated as the mean from the
results using 3 pregnant women. Error bars represent the means ±
SD. *p<0.05 and **p<0.01, one-way ANOVA
with Dunnett’s post test. FCGR2B2, low-affinity immunoglobulin
gamma Fc region receptor IIb2. |
Association of the downregulation of
RAB3D with FCGR2B2 compartments using gene specific siRNAs
We observed FCGR2B2-EGFP expression in the cells in
which RAB3D expression was downregulated by siRNA-mediated
knock down. We constructed 2 types of siRNA duplexes against
RAB3D (designated as siRAB3D-1 and siRAB3D-2).
First, we evaluated the efficiency of the siRNA-mediated knockdown
by RT-qPCR. In the HUVECs, siRAB3D-1 and siRAB3D-2
inhibited the expression of each mRNA by 9.8 and 14.2% after 24 h
and by 9.5 and 10.7% after 48 h, respectively (Fig. 9A). The protein level of RAB3D was
also reduced by siRNA-mediated knockdown (Fig. 9B).
We cultured the pFCGR2B2-EGFP-transfected HUVECs
until an EGFP signal could be observed and then treated them with
the siRAB3Ds. Following RAB3D knockdown, we observed
a redistribution of the FCGR2B2-EGFP signals in the transfected
cells; the FCGR2B2-EGFP signals were markedly reduced at the cell
periphery and accumulated as large vesicular compartments in the
juxtanuclear area (Fig. 9C and
D). These results suggest that RAB3D participates in the
formation of FCGR2B2 compartments in the transfected cells.
Discussion
In this study, we performed bio-imaging analysis of
IgG trafficking in FCGR2B2-transfected HUVECs and demonstrated that
FCGR2B2 compartments were involved in IgG transport following
basolateral IgG internalization in the transfected cells. Maternal
IgG is transferred across two cell layers of the human placenta,
the syncytiotrophoblast and the fetal placental endothelium. In the
syncytiotrophoblast, the maternal IgG incorporated by non-specific
pinocytosis is protected by FCGRT in endosomes and is subsequently
transcytosed to the basolateral surface of the cell, i.e., the
placental villous stroma. Therefore, the villous stroma contains
large amounts of maternal IgG. It is important to understand the
mechanisms through which the maternal IgG accumulated in the
villous stroma is transported across the fetal endothelium, the
second placental barrier. FCGR2B2 is abundantly and exclusively
expressed in the placental endothelium (10,11). We have previously suggested that a
distinct FCGR2B2-containing organelle in the placental endothelium
mediates the transcytosis of maternal IgG (11). However, it is unclear as to how
IgG negotiates the placental endothelial cells. We can envision at
least three possible mechanisms: passive movement along a
constitutive endocytic pathway, movement down an IgG concentration
gradient, or transfer of IgG mediated by FCGR2B2. Thus, we
developed an in vitro model system for the analysis of IgG
transport in the placental endothelium. We transfected
pFCGR2B2-EGFP vectors into HUVECs and generated
FCGR2B2-EGFP-expressing fetal endothelial cells as mimics of
placental endothelial cells. Indeed, EGFP signals indicating
FCGR2B2 were distributed in vesicular and tubular compartments that
were distinct from caveolae and secretory granules. The majority of
the internalized IgG was associated with FCGR2B2-EGFP-positive
compartments. Our in vitro findings are consistent with our
previous in vivo results showing that the FCGR2B2
compartments are intracellular organelles of the placental
endothelium, not associated with caveolae or secretory granules
(11).
FCGR2B is thought to bind preferentially to IgG in
the form of immune complexes (24,25). However, crystallographic studies
of FCGR2A and FCGR2B have suggested that the receptor dimers can
also bind to monomeric IgG (26,27). Moreover, functional mapping using
synthetic peptides corresponding to stretches of the IgG Fc
peptides has also indicated that the IgG1 CH2 domain binds to both
soluble and cell surface-expressed forms of FCGR2B (14). FCGR2B2 mediates endocytosis and
transcytosis, while its companion isoform, FCGR2B1, fails to enter
endocytic compartments (28,29). Of note, FCGR2B is typically
undetectable in endothelial cells in adult human tissues apart from
the placental endothelium and the hepatic sinusoid, and both organs
express FCGR2B2 and not FCGR2B1. Mousavi et al demonstrated
that FCGR2B2 expression in rat hepatic sinusoidal endothelial cells
serves as a recycling receptor (30); however, their study focused on the
receptor-mediating endocytosis of immune complexes. Taken together
with previous observations, the present study lends support to the
idea that FCGR2B2 plays an important role in the intracellular
trafficking of IgG in the human placental endothelium.
Based on our data, it can also be concluded that the
internalization and transcytosis of IgG is accelerated by FCGR2B2
rather than FCGRT in our in vitro model of the human
placental endothelium. Although FCGRT transports both IgG and
albumin (31), albumin cannot
easily be transported like maternal IgG across the human placenta
(32,33). One possible explanation of the
selective transfer of IgG across the placental barrier for our
findings may be that FCGRT of the syncytiotrophoblast transports
both ligands but FCGR2B2 of the placental endothelium allows only
IgG to pass. However, we have not illustrated the whole picture of
Fc receptors and their associated regulatory factors involved in
IgG transcytosis in the human placental endothelium. FCGRT is
expressed not only in the syncytiotrophoblast, but also in adult
endothelial cells; it regulates the serum levels of IgG (34,35). There is still limited knowledge
concerning intracellular trafficking in endothelial cells. It would
be of interest to investigate whether FCGRT cooperates with FCGR2B2
to transcytose IgG in the human placental endothelium by imaging
analysis. However, the anti-FCGRT antibody generated in this study
could not work well in immunocytochemistry. The involvement of
FCGRT in IgG transcytosis in the placental endothelium remains an
issue.
The mouse placenta does not transport IgG; rather,
the yolk sac is the organ responsible for maternal-fetal IgG
transport in the mouse. Maternal IgG is transported across the
mouse yolk sac endoderm by FCGRT (36). The yolk sac also expresses
FCGR2B2. However, FCGR2B2 is not present in the yolk sac in
endothelial cells; non-endothelial FCGR2B2 is not required for the
transport of IgG to the fetus (37,38). The vascular architecture of the
human placenta is quite distinct from that of the mouse yolk sac,
and FCGR2B2 is expressed in different cell types along the IgG
transport pathway between the two species. In addition, the
carboxy-terminal sequence involved in phagocytosis by murine
FCGR2B2 is not conserved in human FCGR2B2 (39). This may also be a difference in
the behavior of human and mouse FCGR2B2 which could impinge on the
intracellular trafficking. Therefore, it is unlikely that the yolk
sac is suitable for study as a model for human IgG transport across
placental endothelial cells. In vivo studies using Fc
receptor gene-knockout nonhuman primate models are important future
directions.
To identify molecules that regulate FCGR2B2
compartment trafficking in the human placenta, proteomic analysis
of FCGR2B2 compartments isolated from human placental tissues was
performed. A large number of FCGR2B2 compartment-associated
proteins was identified, including RAB family proteins, which have
also been identified in several previous proteomic studies on
synaptic vesicles (40), insulin
secretory granules (41),
endocytic vesicles (42),
clathrin-coated vesicles (43)
and clathrin-independent carriers (44). RAB proteins are small GTPases and
members of the Ras superfamily of monomeric G proteins. RAB
proteins regulate each step in the membrane trafficking process:
vesicle or tubule formation by budding from donor membranes,
intracellular vesicle delivery, vesicle tethering and fusion to
specific acceptor membranes with several specific RAB effectors
(45). The localization and novel
functional aspects of RAB proteins have been revealed (46). In this study, we focused on RAB3D
as only RAB3D was expressed predominantly in placental
endothelial cells. Using siRNA analysis, we found that RAB3D was
involved in the intracellular dynamics of FCGR2B2 compartment
formation. RAB3 proteins, known as secretory RABs, are primarily
local-ized in secretory vesicles and intracellular granules
together with several other RABs and are involved in the regulation
of each step of exocytosis with their effectors (47). RAB3D plays an important role in
the maturation and/or maintenance of secretory granules (48-51). RAB3D also participates in the
regulation of the apically directed transcytosis in rat
hepato-cytes (52). In
endothelial cells, RAB3D regulates the secretion of von Willebrand
factor that is stored in Weibel-Palade bodies, endothelial-specific
secretory granules (53). While
FCGR2B2 compartments were distinct from Weibel-Palade bodies in
FCGR2B2-EGFP-expressing HUVECs (Fig.
2D), RAB3D may serve as an important regulator for
intracellular trafficking of IgG, as well as secretory proteins in
placental endothelial cells. Although we focused on RAB3D in this
study, other RAB proteins were also expressed in placental
endothelial cells. This study did not fully explore the regulation
of intracellular trafficking and dynamics of FCGR2B2 compartments
by RAB proteins. Further functional studies of the FCGR2B2
compartment-associated RAB proteins are required to elucidate the
detailed mechanisms of maternal-fetal IgG transport in the human
placental endothelium.
In conclusion, in this study, we investigated the
dynamics and properties of FCGR2B2 compartments for IgG trafficking
using an in vitro model of placental endothelial cells. Our
data suggest the involvement of FCGR2B2 in IgG transcytosis of the
human placental endothelium, thus providing new insight into the
mechanism through which IgG crosses the human placenta.
Acknowledgments
We would like to thank Akira Katayama for assisting
in LC-MS/MS and Takuji Kosuge for providing technical assistance.
This study was supported in part by Grants-in-Aid for the
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology/Japan Society for the Promotion of
Science (MEXT), Japan and by a grant from the MEXT-Supported
Program for the Strategic Research Foundation at Private
Universities (2013–2017).
References
|
1
|
Simister NE: Placental transport of
immunoglobulin. G Vaccine. 21:3365–3369. 2003. View Article : Google Scholar
|
|
2
|
Fuchs R and Ellinger I: Endocytic and
transcytotic processes in villous syncytiotrophoblast: Role in
nutrient transport to the human fetus. Traffic. 5:725–738. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leach L, Bhasin Y, Clark P and Firth JA:
Isolation of endothelial cells from human term placental villi
using immunomagnetic beads. Placenta. 15:355–364. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ghetie V and Ward ES: Multiple roles for
the major histocompatibility complex class I-related receptor FcRn.
Annu Rev Immunol. 18:739–766. 2000. View Article : Google Scholar
|
|
5
|
Brooks DG, Qiu WQ, Luster AD and Ravetch
JV: Structure and expression of human IgG FcRII(CD32). Functional
heterogeneity is encoded by the alternatively spliced products of
multiple genes. J Exp Med. 170:1369–1385. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Callanan MB, Le Baccon P, Mossuz P, Duley
S, Bastard C, Hamoudi R, Dyer MJ, Klobeck G, Rimokh R, Sotto JJ, et
al: The IgG Fc receptor, FcgammaRIIB, is a target for deregulation
by chromosomal translocation in malignant lymphoma. Proc Natl Acad
Sci USA. 97:309–314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tridandapani S, Siefker K, Teillaud JL,
Carter JE, Wewers MD and Anderson CL: Regulated expression and
inhibitory function of Fcgamma RIIb in human monocytic cells. J
Biol Chem. 277:5082–5089. 2002. View Article : Google Scholar
|
|
8
|
Sedmak DD, Davis DH, Singh U, van de
Winkel JG and Anderson CL: Expression of IgG Fc receptor antigens
in placenta and on endothelial cells in humans. An
immunohistochemical study. Am J Pathol. 138:175–181.
1991.PubMed/NCBI
|
|
9
|
Pulford K, Ralfkiaer E, MacDonald SM,
Erber WN, Falini B, Gatter KC and Mason DY: A new monoclonal
antibody (KB61) recognizing a novel antigen which is selectively
expressed on a subpopulation of human B lymphocytes. Immunology.
57:71–76. 1986.PubMed/NCBI
|
|
10
|
Lyden TW, Robinson JM, Tridandapani S,
Teillaud JL, Garber SA, Osborne JM, Frey J, Budde P and Anderson
CL: The Fc receptor for IgG expressed in the villus endothelium of
human placenta is Fc gamma RIIb2. J Immunol. 166:3882–3889. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takizawa T, Anderson CL and Robinson JM: A
novel Fc gamma R-defined, IgG-containing organelle in placental
endothelium. J Immunol. 175:2331–2339. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mishima T, Kurasawa G, Ishikawa G, Mori M,
Kawahigashi Y, Ishikawa T, Luo SS, Takizawa T, Goto T, Matsubara S,
et al: Endothelial expression of Fc gamma receptor IIb in the
full-term human placenta. Placenta. 28:170–174. 2007. View Article : Google Scholar
|
|
13
|
Sohn HW, Krueger PD, Davis RS and Pierce
SK: FcRL4 acts as an adaptive to innate molecular switch dampening
BCR signaling and enhancing TLR signaling. Blood. 118:6332–6341.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Medgyesi D, Uray K, Sallai K, Hudecz F,
Koncz G, Abramson J, Pecht I, Sármay G and Gergely J: Functional
mapping of the Fc gamma RII binding site on human IgG1 by synthetic
peptides. Eur J Immunol. 34:1127–1135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung ST, Reddy ST, Kang TH, Borrok MJ,
Sandlie I, Tucker PW and Georgiou G: Aglycosylated IgG variants
expressed in bacteria that selectively bind FcgammaRI potentiate
tumor cell killing by monocyte-dendritic cells. Proc Natl Acad Sci
USA. 107:604–609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Claypool SM, Dickinson BL, Wagner JS,
Johansen FE, Venu N, Borawski JA, Lencer WI and Blumberg RS:
Bidirectional transepithelial IgG transport by a strongly polarized
basolateral membrane Fcgamma-receptor. Mol Biol Cell. 15:1746–1759.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tzaban S, Massol RH, Yen E, Hamman W,
Frank SR, Lapierre LA, Hansen SH, Goldenring JR, Blumberg RS and
Lencer WI: The recycling and transcytotic pathways for IgG
transport by FcRn are distinct and display an inherent polarity. J
Cell Biol. 185:673–684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dickinson BL, Badizadegan K, Wu Z, Ahouse
JC, Zhu X, Simister NE, Blumberg RS and Lencer WI: Bidirectional
FcRn-dependent IgG transport in a polarized human intestinal
epithelial cell line. J Clin Invest. 104:903–911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dickinson BL, Claypool SM, D’Angelo JA,
Aiken ML, Venu N, Yen EH, Wagner JS, Borawski JA, Pierce AT,
Hershberg R, et al: Ca2+-dependent calmodulin binding to
FcRn affects immuno-globulin G transport in the transcytotic
pathway. Mol Biol Cell. 19:414–423. 2008. View Article : Google Scholar :
|
|
20
|
Ui-Tei K, Naito Y, Takahashi F, Haraguchi
T, Ohki-Hamazaki H, Juni A, Ueda R and Saigo K: Guidelines for the
selection of highly effective siRNA sequences for mammalian and
chick RNA interference. Nucleic Acids Res. 32:936–948. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo SS, Ishibashi O, Ishikawa G, Ishikawa
T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A,
et al: Human villous trophoblasts express and secrete
placenta-specific microRNAs into maternal circulation via exosomes.
Biol Reprod. 81:717–729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akagi I, Okayama H, Schetter AJ, Robles
AI, Kohno T, Bowman ED, Kazandjian D, Welsh JA, Oue N, Saito M, et
al: Combination of protein coding and noncoding gene expression as
a robust prognostic classifier in stage I lung adenocarcinoma.
Cancer Res. 73:3821–3832. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schermelleh L, Heintzmann R and Leonhardt
H: A guide to super-resolution fluorescence microscopy. J Cell
Biol. 190:165–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takai T: Fc receptors and their role in
immune regulation and autoimmunity. J Clin Immunol. 25:1–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmidt RE and Gessner JE: Fc receptors
and their interaction with complement in autoimmunity. Immunol
Lett. 100:56–67. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maxwell KF, Powell MS, Hulett MD, Barton
PA, McKenzie IF, Garrett TP and Hogarth PM: Crystal structure of
the human leukocyte Fc receptor, Fc gammaRIIa. Nat Struct Biol.
6:437–442. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sondermann P, Huber R and Jacob U: Crystal
structure of the soluble form of the human fcgamma-receptor IIb: A
new member of the immunoglobulin superfamily at 1.7 A resolution.
EMBO J. 18:1095–1103. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hunziker W and Mellman I: Expression of
macrophage-lymphocyte Fc receptors in Madin-Darby canine kidney
cells: Polarity and transcytosis differ for isoforms with or
without coated pit localization domains. J Cell Biol.
109:3291–3302. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miettinen HM, Matter K, Hunziker W, Rose
JK and Mellman I: Fc receptor endocytosis is controlled by a
cytoplasmic domain determinant that actively prevents coated pit
localization. J Cell Biol. 116:875–888. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mousavi SA, Sporstøl M, Fladeby C, Kjeken
R, Barois N and Berg T: Receptor-mediated endocytosis of immune
complexes in rat liver sinusoidal endothelial cells is mediated by
FcgammaRIIb2. Hepatology. 46:871–884. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaudhury C, Mehnaz S, Robinson JM, Hayton
WL, Pearl DK, Roopenian DC and Anderson CL: The major
histocompatibility complex-related Fc receptor for IgG (FcRn) binds
albumin and prolongs its lifespan. J Exp Med. 197:315–322. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dancis J, Lind J, Oratz M, Smolens J and
Vara P: Placental transfer of proteins in human gestation. Am J
Obstet Gynecol. 82:167–171. 1961.PubMed/NCBI
|
|
33
|
Gitlin D, Kumate J, Urrusti J and Morales
C: Τhe selectivity of the human placenta in the transfer of plasma
proteins from mother to fetus. J Clin Invest. 43:1938–1951. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Junghans RP and Anderson CL: The
protection receptor for IgG catabolism is the
beta2-microglobulin-containing neonatal intestinal transport
receptor. Proc Natl Acad Sci USA. 93. pp. 5512–5516. 1996,
View Article : Google Scholar
|
|
35
|
Roopenian DC, Christianson GJ, Sproule TJ,
Brown AC, Akilesh S, Jung N, Petkova S, Avanessian L, Choi EY,
Shaffer DJ, et al: The MHC class I-like IgG receptor controls
perinatal IgG transport, IgG homeostasis, and fate of
IgG-Fc-coupled drugs. J Immunol. 170:3528–3533. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim J, Mohanty S, Ganesan LP, Hua K,
Jarjoura D, Hayton WL, Robinson JM and Anderson CL: FcRn in the
yolk sac endoderm of mouse is required for IgG transport to fetus.
J Immunol. 182:2583–2589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mohanty S, Kim J, Ganesan LP, Phillips GS,
Hua K, Jarjoura D, Hayton WL, Robinson JM and Anderson CL: IgG is
transported across the mouse yolk sac independently of FcgammaRIIb.
J Reprod Immunol. 84:133–144. 2010. View Article : Google Scholar
|
|
38
|
Mohanty S, Anderson CL and Robinson JM:
The expression of caveolin-1 and the distribution of caveolae in
the murine placenta and yolk sac: Parallels to the human placenta.
Placenta. 31:144–150. 2010. View Article : Google Scholar
|
|
39
|
Van den Herik-Oudijk IE, Capel PJ, van der
Bruggen T and Van de Winkel JG: Identification of signaling motifs
within human Fc gamma RIIa and Fc gamma RIIb isoforms. Blood.
85:2202–2211. 1995.PubMed/NCBI
|
|
40
|
Takamori S, Holt M, Stenius K, Lemke EA,
Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, et
al: Molecular anatomy of a trafficking organelle. Cell.
127:831–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brunner Y, Couté Y, Iezzi M, Foti M,
Fukuda M, Hochstrasser DF, Wollheim CB and Sanchez JC: Proteomics
analysis of insulin secretory granules. Mol Cell Proteomics.
6:1007–1017. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mukhopadhyay A, Nieves E, Che FY, Wang J,
Jin L, Murray JW, Gordon K, Angeletti RH and Wolkoff AW: Proteomic
analysis of endocytic vesicles: Rab1a regulates motility of early
endocytic vesicles. J Cell Sci. 124:765–775. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McPherson PS: Proteomic analysis of
clathrin-coated vesicles. Proteomics. 10:4025–4039. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Howes MT, Kirkham M, Riches J, Cortese K,
Walser PJ, Simpson F, Hill MM, Jones A, Lundmark R, Lindsay MR, et
al: Clathrin-independent carriers form a high capacity endocytic
sorting system at the leading edge of migrating cells. J Cell Biol.
190:675–691. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Grosshans BL, Ortiz D and Novick P: Rabs
and their effectors: Achieving specificity in membrane traffic.
Proc Natl Acad Sci USA. 103:11821–11827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stenmark H: Rab GTPases as coordinators of
vesicle traffic. Nat Rev Mol Cell Biol. 10:513–525. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fukuda M: Regulation of secretory vesicle
traffic by Rab small GTPases. Cell Mol Life Sci. 65:2801–2813.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Millar AL, Pavios NJ, Xu J and Zheng MH:
Rab3D: A regulator of exocytosis in non-neuronal cells. Histol
Histopathol. 17:929–936. 2002.PubMed/NCBI
|
|
49
|
Riedel D, Antonin W, Fernandez-Chacon R,
Alvarez de Toledo G, Jo T, Geppert M, Valentijn JA, Valentijn K,
Jamieson JD, Südhof TC, et al: Rab3D is not required for exocrine
exocytosis but for maintenance of normally sized secretory
granules. Mol Cell Biol. 22:6487–6497. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Evans E, Zhang W, Jerdeva G, Chen CY, Chen
X, Hamm-Alvarez SF and Okamoto CT: Direct interaction between Rab3D
and the polymeric immunoglobulin receptor and trafficking through
regulated secretory vesicles in lacrimal gland acinar cells. Am J
Physiol Cell Physiol. 294:C662–C674. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tian X, Jin RU, Bredemeyer AJ, Oates EJ,
Błazewska KM, McKenna CE and Mills JC: RAB26 and RAB3D are direct
transcriptional targets of MIST1 that regulate exocrine granule
maturation. Mol Cell Biol. 30:1269–1284. 2010. View Article : Google Scholar :
|
|
52
|
Larkin JM, Woo B, Balan V, Marks DL,
Oswald BJ, LaRusso NF and McNiven MA: Rab3D, a small GTP-binding
protein implicated in regulated secretion, is associated with the
trans-cytotic pathway in rat hepatocytes. Hepatology. 32:348–356.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Knop M, Aareskjold E, Bode G and Gerke V:
Rab3D and annexin A2 play a role in regulated secretion of vWF, but
not tPA, from endothelial cells. EMBO J. 23:2982–2992. 2004.
View Article : Google Scholar : PubMed/NCBI
|