Introduction
In developed countries, a major cause of hospital
admissions for patients with diabetes is chronic diabetic foot
ulcers, which are a common symptom of diabetes and often result in
pain and a lower quality of life (1). It has been reported that 15% of all
patients with diabetes develop chronic diabetic foot ulcers, and in
84% of cases, this leads to amputation (2,3).
There is, as yet, no widely available effective treatment strategy
for diabetic foot ulcers. Consequently, novel effective treatment
strategies for this chronic complication of diabetes are urgently
required (4,5).
Relative hypoxia is a critical stimulus for normal
wound healing, and the major cause of impaired wound healing in
patients with diabetes may be an impaired response to hypoxia
(6). Hypoxia-inducible factor
(HIF)-1, a heterodimer, includes two subunits, a hypoxia-stabilized
α-subunit (HIF-1α) and a constitutively expressed β-subunit
(HIF-1β), which plays an important role as a master regulator of
oxygen homeostasis. Under hypoxic conditions, HIF-1α is stabilized
and moves towards the nucleus, dimerizing with HIF-1β.
Subsequently, the dimer binds to a hypoxia response element (HRE),
which appears on several genes that are responsible for cell
survival during hypoxia (7).
HIF-1α mediates the expression of numerous pro-angiogenic growth
factors, such as vascular endothelial growth factor (VEGF), as well
as the recruitment of endothelial progenitor cells to sites of
vascularization through endothelial nitric oxide synthase (eNOS)
and cell motility (8–10).
Diabetic foot ulcers heal slowly due to impaired
neovascularization in response to tissue ischemia (11). Multiple growth factors and
cytokines are involved in the formation of new blood vessels during
wound healing. Of all the known pro-angiogenic molecules, VEGF is
the most important mediator that promotes angiogenesis (12–14). It has been previously reported
that the expression of VEGF is attenuated in diabetes (15). VEGF exerts major biological
effects by binding with and stimulating its receptors. Among these,
VEGF receptor 2 (VEGFR2) is an important receptor that transduces
VEGF-activated signaling in endothelial cells. The activation of
VEGFR2 leads to the phosphorylation of specific downstream signal
transduction mediators, including protein tyrosine kinase,
extracellular signal-regulated kinase (ERK) and focal adhesion
kinase (FAK). As previously demonstrated, VEGFR2 signaling is
necessary for the implementation of VEGF-induced vascular
permeability, proliferation, migration and the sprouting of
endothelial cells in vitro and neovascularization in wound
healing (16–19).
Although some therapeutic methods, such as gene
therapy and treatment with recombinant growth factors have been
used in an aim to promote angiogenesis, these methods are impeded
by limitations, such as safety issues and high costs (20). A pharmaceutical method may thus be
the most effective and advantageous method, particularly in terms
of convenience, cost and safety. Zicao is a traditional herbal
medicine used to promote wound healing that has been applied for
hundreds of years in China (21).
A survey of the published studies revealed that arnebin-1, a
naphthoquinone derivative, plays a crucial role in the
wound-healing properties of Zicao. A previous study demonstrated
that arnebin-1 significantly accelerated both normal and
hydrocortisone-impaired wound healing compared with the controls
(22). In a recent study of ours,
we also reported that arnebin-1 promoted the wound healing process
in diabetic rats (23). Thus,
arnebin-1 significantly accelerates the wound healing process;
however, the specific mechanisms involved remain unknown,
particularly those in relation to diabetic wounds.
Therefore, the aim of the present study was to
investigate the mechanisms responsible for the pro-angiogenic
effects of arnebin-1 on human umbilical vein endothelial cells
(HUVECs), as well as those responsible for its healing effects on
wounds of rats with alloxan-induced diabetes mellitus (DM). The
effects of arnebin-1 on the phosphoinositide 3-kinase
(PI3K)-dependent signaling pathway, VEGFR2 signaling and on the
expression levels of eNOS, VEGF and HIF-1α in vitro were
also determined. Moreover, to confirm the promoting effects of
arnebin-1 on neovascularization in diabetic wounds, the protein
expression levels of HIF-1α, eNOS, VEGF and CD31 were also
determined in wound tissues from non-diabetic and diabetic
rats.
Materials and methods
Materials
Arnebin-1 was purchased from Tokyo Chemical Industry
Co. Ltd. (Tokyo, Japan), and recombinant human VEGF was from
PeproTech Inc. (Rocky Hill, NJ, USA). Growth factor-reduced
Matrigel basement membrane matrix was obtained from BD Biosciences
(Bedford, MA, USA). Medium 199 (M199) and fetal bovine serum (FBS)
were purchased from Gibco (Carlsbad, CA, USA). LY294002, a PI3K
inhibitor, was purchased from Wako Pure Chemical Industries, Ltd.
(Osaka, Japan). All other reagents utilized were purchased from
Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise
specified.
Cell culture
The isolation and culture of the HUVECs was carried
out as previously described (21). Briefly, following digestion with
0.125% trypsin, the HUVECs were removed from human umbilical veins
which were obtained following delivery. The HUVECs were removed
from the umbilical veins following digestion with 0.125% trypsin
and then cultured in M199 containing 20% FBS, 100 U/ml penicillin,
100 U/ml streptomycin and 50 U/ml heparin, supplemented with 2 mM
L-glutamine, 1 mM sodium pyruvate and 5 ng/ml β-endothelial cell
growth factor (β-ECGF) at 37°C in 5% CO2 in
gelatin-coated culture flasks. Endothelial cells were identified by
their morphology (cobblestone or mosaic-like appearance) after
reaching confluence and by the presence of von Willebrand factor
(data not shown). Passage 3–6 HUVECs were used in all the
experiments. This study was approved by the Ethics Committee of Sun
Yan-sen University (Guangzhou, China).
Cell proliferation assay
Cell proliferation was examined by mitochondrial MTT
tetrazolium assay. The HUVECs were plated at 3×103
cells/well in 96-well plates. Overnight, the HUVECs were
pre-treated with or without LY294002 (2 µM), and the medium
was then replaced with the test medium supplemented with the
vehicle [dimethyl sulfoxide (DMSO)] and arnebin-1 (10−1
µM) with or without 1 ng/ml VEGF. After 24 h of incubation,
the number of viable cells was detected using MTT reagent according
to the manufacturer's instructions. In brief, 10 µl MTT (5
mg/ml) was added to 100 µl medium, and cultivated at 37°C
for 4 h. After removing the supernatant, the formazan crystals were
solubilized by the addition of DMSO. The absorbance (570 nm) of the
medium was determined using a Biotek Elx-800 plate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
Cell migration assay
Cell migration assay was performed using Transwell
chambers as previously described (21). The bottom chamber of the device
contained 600 µl of the test medium. The HUVECs
(5×104 cells/well) were added to the upper chamber and
cultured in M199 medium with 2% FBS. After 24 h of incubation, the
non-migrated cells that were above the faces of the membranes were
removed. The migrating cells were fixed with methanol for 15 min,
and then stained with 0.1% crystal violet for 20 min. The membranes
were then rinsed with 30% glacial acetic acid. Finally, the washing
solution was examined at 540 nm for the counting of the number of
HUVECs.
Tube formation assay
To examine the pro-angiogenic effect Arnebin-1, we
used the experimental in vitro Matrigel system, as
previously described (23).
Growth factor-reduced Matrigel basement membrane matrix was thawed
on ice at 4°C overnight, and all pipettes and 96-well flat bottom
plates were pre-cooled before use. The 96-well plates were coated
with 50 µl Matrigel per well for 30 min at 37°C. The HUVECs
were seeded at 4×104 cells per well in 100 µl
assay medium. After 16 h of incubation, tube-like structures were
photographed using an inverted microscope (IX71; Olympus Corp.,
Tokyo, Japan). The total tube length was quantified using ImageJ
software (NIH, Bethesda, MD, USA).
Western blot analysis
The HUVECs were lysed using protein lysis buffer and
protease inhibitor cocktail. The protein concentrations of the cell
lysates were quantified using a bicinchoninic acid assay (BCA) kit,
and equal amounts of protein were separated by SDS-PAGE and then
transferred onto polyvinylidene fluoride (PVDF) membranes
(Millipore, Billerica, MA, USA). The membranes were blocked in 5%
non-fat dried milk diluted with Tris-Buffered Saline Tween-20
(TBST; in mmol/l: Tris-HCl 20, NaCl 150, pH 7.5 nd 0.1% Tween-20)
at room temperature for 1 h and probed overnight at 4°C with a
polyclonal rabbit anti-phosphorylated (p-)VEGFR2 (#2478), a
polyclonal rabbit anti-VEGFR2 (#9698), a polyclonal rabbit
anti-p-Erk1/2, a polyclonal rabbit anti-Erk1/2, a polyclonal rabbit
anti-p-FAK (#8556), a polyclonal rabbit anti-FAK (#13009), a
polyclonal rabbit anti-p-Src (#5473), a polyclonal rabbit anti-Src
(#2109), a polyclonal rabbit anti-PI3K (#4257), a polyclonal rabbit
anti-p-PI3K (#3821), a polyclonal rabbit anti-Akt (#4691), a
polyclonal rabbit anti-p-Akt (#4060), a polyclonal rabbit
anti-p-mammalian target of rapamycin (mTOR; #2983), a polyclonal
rabbit anti-p-mTOR (#2971; all from Cell Signaling Technology,
Beverly, MA, USA), a polyclonal rabbit anti-proliferating cell
nuclear antigen (PCNA; sc-7907), a polyclonal rabbit anti-eNOS
(sc-654), a monoclonal rabbit anti-VEGF (sc-152; both from Santa
Cruz Biotechnology Inc., Santa Cruz, CA, USA) or a monoclonal
rabbit anti-HIF-1α antibody (NB-100-479; Novus Biologicals,
Littleton, CO, USA), and then incubated for 2 h with anti-rabbit
IgG (Santa Cruz Biotechnology Inc.). Incubation with polyclonal
mouse β-actin antibody (#3700; 1:3,000 dilution; Cell Signaling
Technology) or monoclonal mouse α-tubulin antibody (T5168; 1:1,000
dilution; Sigma) was used as the internal standard control. The
proteins were visualized using ECL™ western blotting detection
reagents (Amersham Biosciences Corp., Piscataway, NJ, USA). The
densitometry of the bands was quantified using ImageJ 1.38X
software.
VEGF enzyme-linked immunosorbent assay
(ELISA)
A Quantikine human VEGF ELISA kit (R&D Systems,
Minneapolis, MN, USA) was used according to the manufacturer's
instructions. Briefly, VEGF standards and the conditioned medium
from the HUVECs were placed into wells overlaid with antibody
specific for human VEGF. After binding with an VEGF enzyme-linked
polyclonal antibody specific for VEGF, the absorbance was measured
at 450 nm using a microplate reader. The VEGF concentration was
evaluated (in pg/ml) with the standard curve and adjusted for
protein concentrations.
In vivo experiments
Animals and induction of diabetes
All animal procedures were approved by the
Laboratory Animal Center of Sun Yat-sen University. As previously
described (23), male
Sprague-Dawley (SD) rats (weighing 250–300 g) were kept in
stainless steel cages under pathogen-free conditions. The rats were
housed in a controlled environment with a constant temperature of
18–22°C and a 12-h light-dark cycle; the rats were allowed access
to food and water ad libitum. The rats were allowed to
acclimatize for 4 weeks before the experimental procedures
commenced. The rats were fasted for 12 h and were injected
intraperitoneally with alloxan monohydrate dissolved in normal
saline at a double dose of 100 mg/kg every other day to induce
diabetes. Following the administration of alloxan for 3 days, the
fasting blood glucose (FBG) levels of the rats were measured using
a glucometer. The rats exhibiting FBG levels >16.7 mmol/l were
confirmed as diabetic rats for the purposes of our research. The
FBG levels were monitored before and after the experiments. The
animals were randomly divided into 4 groups (1 non-diabetic and 3
diabetic groups; n=6) as follows: i) the non-diabetic group: rats
were administered distilled water for 7 days (non-diabetic group);
ii) the first diabetic group: the diabetic animals received
distilled water (diabetic group); iii) the second diabetic group:
the diabetic animals received the vehicle (ointment without
arnebin-1; DM-vehicle; D + V group); and iv) the third diabetic
group: the diabetic animals were treated with arnebin-1 ointment
(DM-arnebin-1; D + A group) for 7 days.
Preparation of the ointment
As described in a previous study of ours (23), ointment containing siritch (1.5
g), beeswax (5 g) and lard oil (0.15 g) was heated at 70–75°C to
become solubilized, and 6.65 mg arnebin-1 (0.1%) was then added and
mixed in. Finally, the mixture was stirred until it cooled to room
temperature. This ointment was used as the test compound.
Experimental wounding
As previously described (23), SD rats were anesthetized with
sodium pentothal (35 mg/kg, by intraperitoneal injection). The hair
on the dorsal side of each rat was shaved, and the skin was
sterilized with 70% ethanol. Full thickness cutaneous wounds were
made with an 8-mm skin biopsy punch (World Precision Instruments,
Sarasota, FL, USA) under aseptic conditions. Three wounds were made
on the dorsal surface of the diabetic rats, and one wound was made
on the non-diabetic rats. The diameters of the wounds ranged
between 7.5 and 9 mm. Thereafter, the animals were individually
caged.
Drug administration
As stated above, each diabetic rat had 3 wounds on
the dorsal surface and the non-diabetic rats had 1 wound. In the D
+ V group, only wounds on the top of the dorsal side were treated
with only the vehicle base (without the test compound). In the
diabetic group, only wounds near the tail were treated with
distilled water. In the D + A group, only wounds in the middle were
treated with arnebin-1 (0.1% ointment). Thus, in each group of
rats, a different wound area was treated. The wounds at the top
served as the vehicle controls for the treated wounds. The test
compound ointment and the vehicle were applied every other day, in
quantities sufficient to cover the wounds with a thin layer. All
the treatments were continued until the day of sacrifice. The rats
were sacrificed with the use of an intraperitoneal injection of an
overdose of barbiturate.
Tissue collection
The rats were anesthetized with an overdose of
pentobarbital (200 mg/kg, injected intraperitoneally) on day 7
post-wounding. The wound and a margin of approximately 5 mm of
unwounded skin was excised. These wound tissues were snap-frozen in
liquid nitrogen until they were processed for protein
isolation.
Western blot analysis
In order to measure the levels of PCNA, CD31,
HIF-1α, VEGF and eNOS in the tissue, wounds treated with arnebin-1
or the vehicle were harvested on day 7 post-wounding. Following
excision, the tissues were homogenized in lysis buffer. The VEGF,
eNOS and HIF-1α expression levels were determined by western blot
analysis as described above.
Immunofluorescence staining for
CD31
Wound samples, taken on day 7, were embedded in
paraffin and frozen in liquid nitrogen immediately for
immunofluorescence. To assess new blood vessel formation, vessel
density was estimated after staining for CD31. Serial 6-µm
frozen sections were incubated with primary antibodies for CD31
(1:100; 550274; BD Biosciences) at 4°C overnight. Subsequently, the
sections were washed with phosphate-buffered saline (PBS) 3 times
and incubated with goat anti-rat IgG-Cy3 (1:200; A0507; Beyotime
Institute of Biotechnology, Shanghai, China) for 1 h at 37°C.
Hoechst 33342 dye (C1026; Beyotime Institute of Biotechnology) was
used to stain the nuclei for 3–5 min at room temperature. The
sections were examined and photographed under a fluorescence
microscope (IX71; Olympus Corp.). The number of CD31-positive
vessels was determined across 5 non-consecutive tissue sections for
each wound.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5.0 (GraphPad Software, Inc., USA). Data for each
study parameter from each group are presented as the means ±
standard error of the mean (SEM). Data from each group were
statistically analyzed by one-way analysis of variance (ANOVA).
Differences were considered statistically significant at
P<0.05.
Results
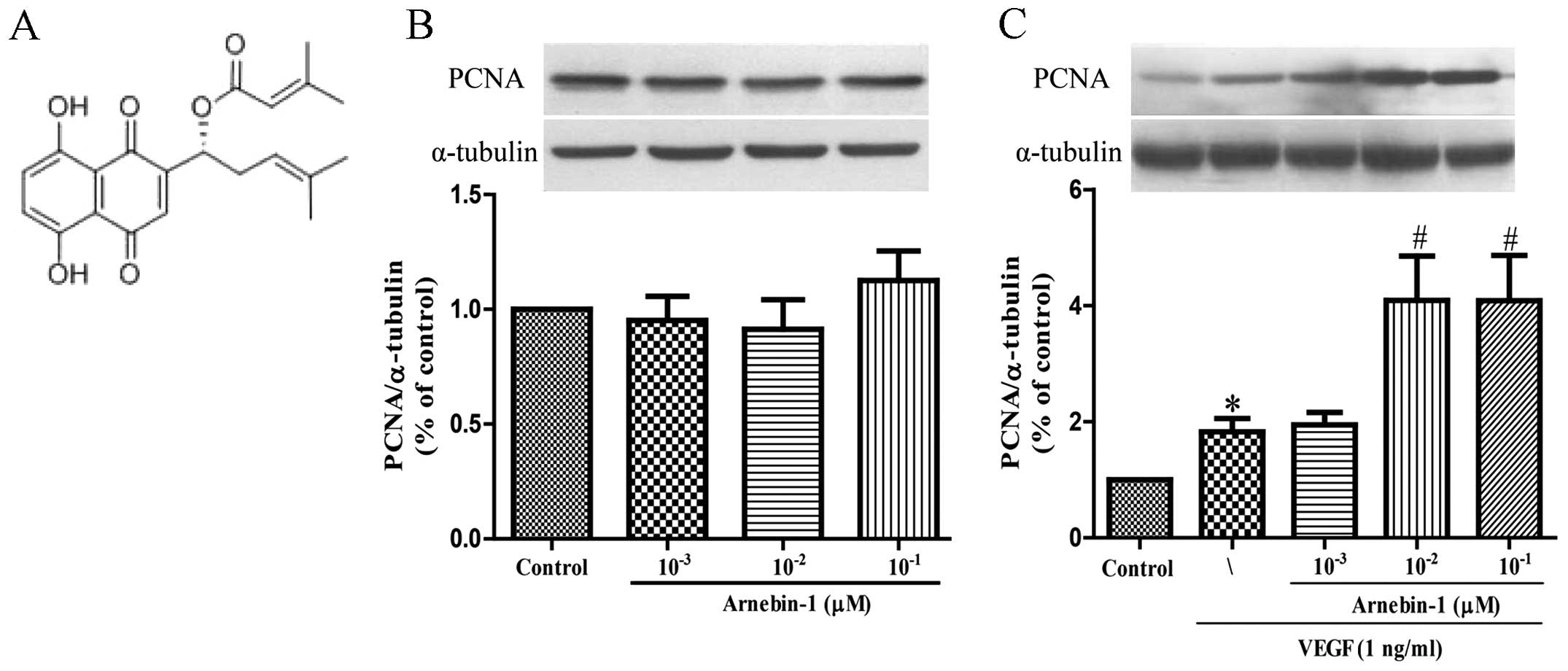
Effect of arnebin-1 on the proliferation
of HUVECs
PCNA is a nuclear cell proliferation marker. To
determine whether arnebin-1 promotes the proliferation of HUVECs,
the PCNA levels were measured by western blot analysis. At
concentrations ranging from 1×10−3 µM to
10−1 µM, arnebin-1 alone had no significant
effect on the PCNA levels (Fig.
1B). However, in the presence of VEGF (1 ng/ml), arnebin-1
significantly increased the expression of PCNA in a
concentration-dependent manner (Fig.
1C). Consistent with the results of our previous study
(23), we found that arnebin-1
had no noticeable effect on cell viability and proliferation (as no
changes were observed in PCNA expression), as evaluated by MTT
assay in the test range, but had a synergistic effect with VEGF in
that it promoted HUVEC proliferation (Fig. 5A).
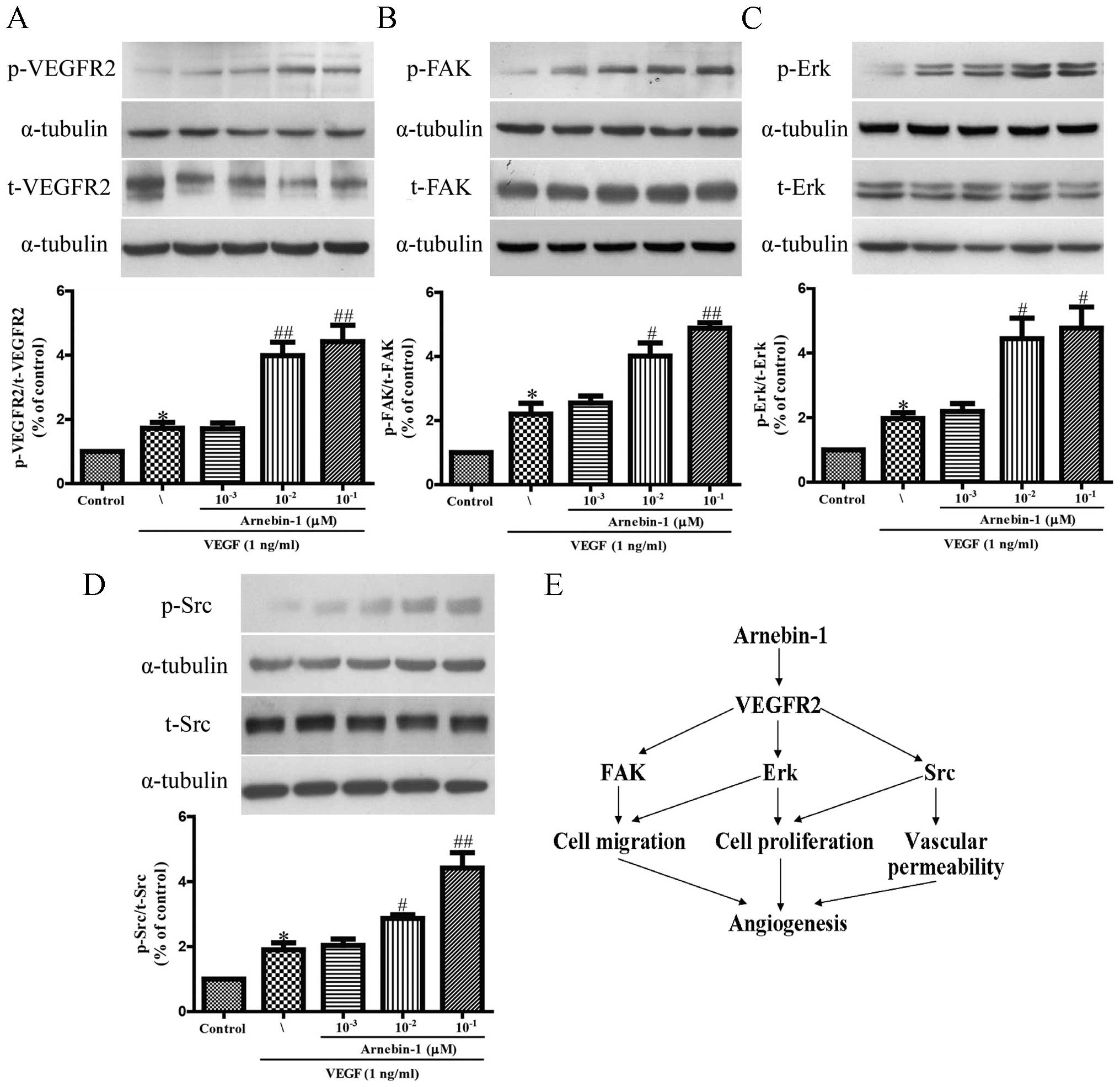
Arnebin-1 activates the VEGFR2 signaling
pathway
It has been reported that VEGFR2 phosphorylation
activates extensive downstream signaling substrates that are
closely related to endothelial cell proliferation, migration and
tube formation (30). To
investigate whether arnebin-1 activates VEGFR2 and its downstream
signaling molecules, we screened some elementary kinases related to
the VEGFR2 signaling pathway. As shown in Fig. 2, arnebin-1 significantly increased
the phosphorylation of VEGFR2, FAK, ERK and Src, induced by VEGF (1
ng/ml), in a concentration-dependent manner, which suggested that
arnebin-1 exerted its pro-angiogenic effect by directly targeting
VEGFR2 and subsequently activating the VEGFR2-induced downstream
signaling cascade. These results are consistent with those of our
previous study, which demonstrated that arnebin-1 promoted the
proliferation, migration and tube formation of HUVECs in a
concentration-dependent manner in the presence of VEGF (23).
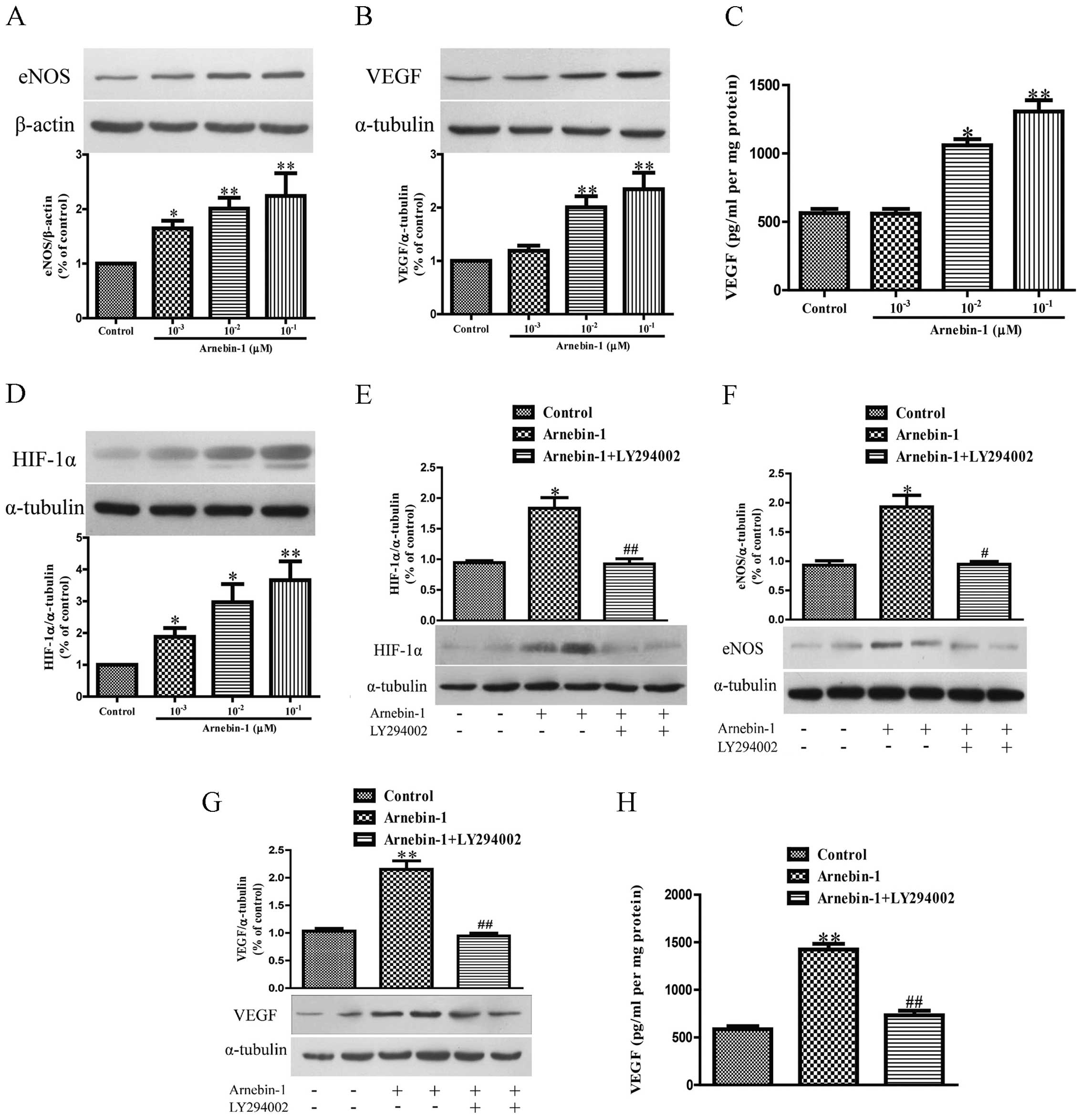
Arnebin-1 upregulates the expression
levels of eNOS, VEGF and HIF-1α in HUVECs in a PI3K-dependent
manner
Subsequently, we investigated the effects of
arnebin-1 on the expression levels of eNOS and VEGF in HUVECs. At
concentrations ranging from 1×10−3 µM to
10−1 µM, arnebin-1 significantly increased the
protein expression of eNOS in the HUVECs in a
concentration-dependent manner compared to the vehicle-treated
(control) cells (Fig. 3A).
Moreover, arnebin-1 at 10−2 and 10−1
µM also markedly increased the expression and secretion of
VEGF protein compared with the control group (Fig. 3B and C). Similarly, the expression
of HIF-1α was also markedly upregulated by arnebin-1 (Fig. 3D). We further examined whether the
upregulation of eNOS, VEGF and HIF-1α by arnebin-1 in HUVECs is
mediated by its effect on the PI3K pathway. The protein expression
of HIF-1α was markedly reduced by treatment with 2 µM
LY294002 1 h prior to stimulation with 10−1 µM
arnebin-1 (Fig. 3E). Similarly,
the protein expression of eNOS, and the expression and secretion of
VEGF protein, were also significantly decreased following
pretreatment with 2 µM LY294002 (Fig. 3F–H).
We also investigated whether arnebin-1 has any
effect on the PI3K/Akt/mTOR pathway, which functions upstream of
eNOS, VEGF and HIF-1α. Following treatment for 24 h, arnebin-1
induced a marked increase in the protein expression levels of PI3K,
Akt and mTOR in the HUVECs in a concentration-dependent manner
(Fig. 4A–C). Moreover, in the
HUVECs treated with arnebin-1 at various concentrations
(10−3, 10−2 and 10−1 µM)
for 2 h, the phosphorylation levels of these 3 proteins were
significantly increased in a concentration-dependent manner
(Fig. 4D–F). Taken together,
these results demonstrate that arnebin-1 regulates the expression
of eNOS, VEGF and HIF-1α in HUVECs in a PI3K-dependent manner.
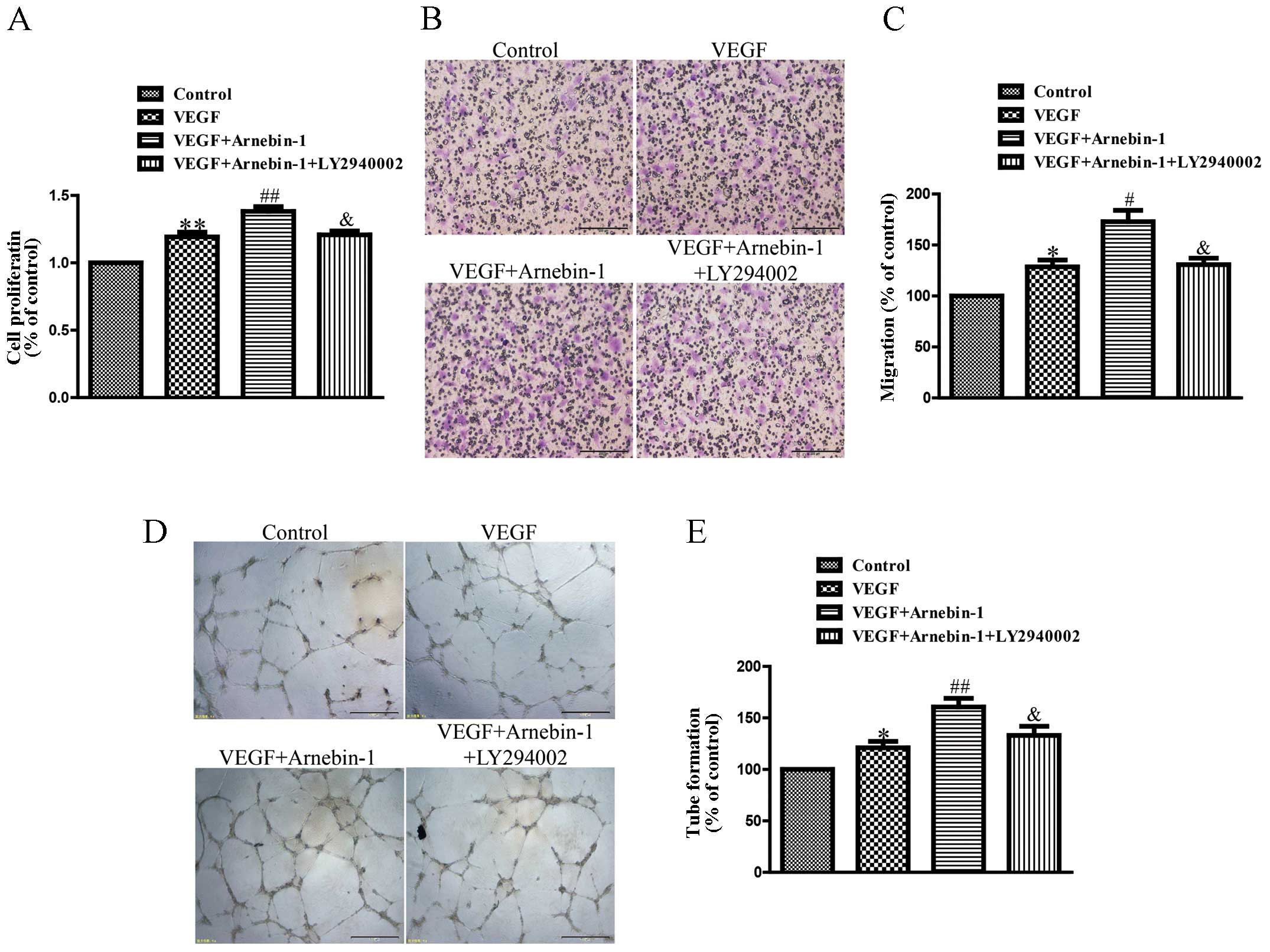
Arnebin-1 promotes the proliferation,
migration and tube formation of HUVECs through the PI3K-dependent
pathway
In a previous study (23), we confirmed that arnebin-1
significantly promoted the proliferation, migration and tube
formation of HUVECs in the presence of VEGF (1 ng/ml) in a
concentration-dependent manner, with a maximal effect at
10−1 µM. In the present study, we investigated
the mechanisms responsible for these effects of arnebin-1. As shown
in Fig. 5, the proliferation,
migration and tube formation of the HUVECs were enhanced by
stimulation with a low concentration of VEGF (1 ng/ml) compared
with the controls. Moreover, arnebin-1 at 10−1 µM
and VEGF had a synergistic effect and markedly increased these
processes compared with the cells treated with VEGF alone. However,
when the HUVECs were pre-treated with LY294002, a PI3K inhibitor,
the synergistic effects of arnebin-1 and VEGF on cell
proliferation, migration and tube formation were abolished
(Fig. 5). As was also shown,
pre-treatment with LY294002 attenuated the increase in the
expression levels of eNOS, VEGF and HIF-1α induced by arnebin-1
(Fig. 3). Collectively, these
results suggest that arnebin-1 promotes the processes of
endothelial cell proliferation, migration and tube formation which
are associated with angiogenesis through the upregulation of eNOS,
VEGF and HIF-1α in a PI3K-dependent manner.
In vivo wound healing experiments
Induction of diabetes
The mean FBG levels and body weight of the animals
are presented in Table I. The
body weights and FBG levels of the non-diabetic rats and diabetic
rats were determined before and 3 days after the alloxan injection.
The rats exhibited a 3-4-fold increase in FBG levels compared to
the normal levels after the alloxan injection and a concurrent
decrease in body weight, indicating that DM was successfully
induced in the rats. On the 7th day post-wounding, the FBG levels
remained >16.7 mmol/l. Topical therapy applied to the wounds of
the rats did not have any effect on the FBG level over the course
of study.
 | Table IEffects of arnebin-1 on body weight
and blood glucose. |
Table I
Effects of arnebin-1 on body weight
and blood glucose.
| Factors | Non-diabetic rats
(n=6) | Diabetic rats 3
days after injection (n=6) | Non-diabetic rats 7
days post-wounding (n=6) | Diabetic rats 7
days post-wounding (n=6) |
|---|
| Body weight
(g) | 284.1±4.0 | 249.5±4.6a | 310.8±7.4a | 220.0±7.7a |
| Blood glucose
(mmol/l) | 6.0±0.2 | 23.9±0.9a | 6.4±0.2 | 23.0±1.2a |
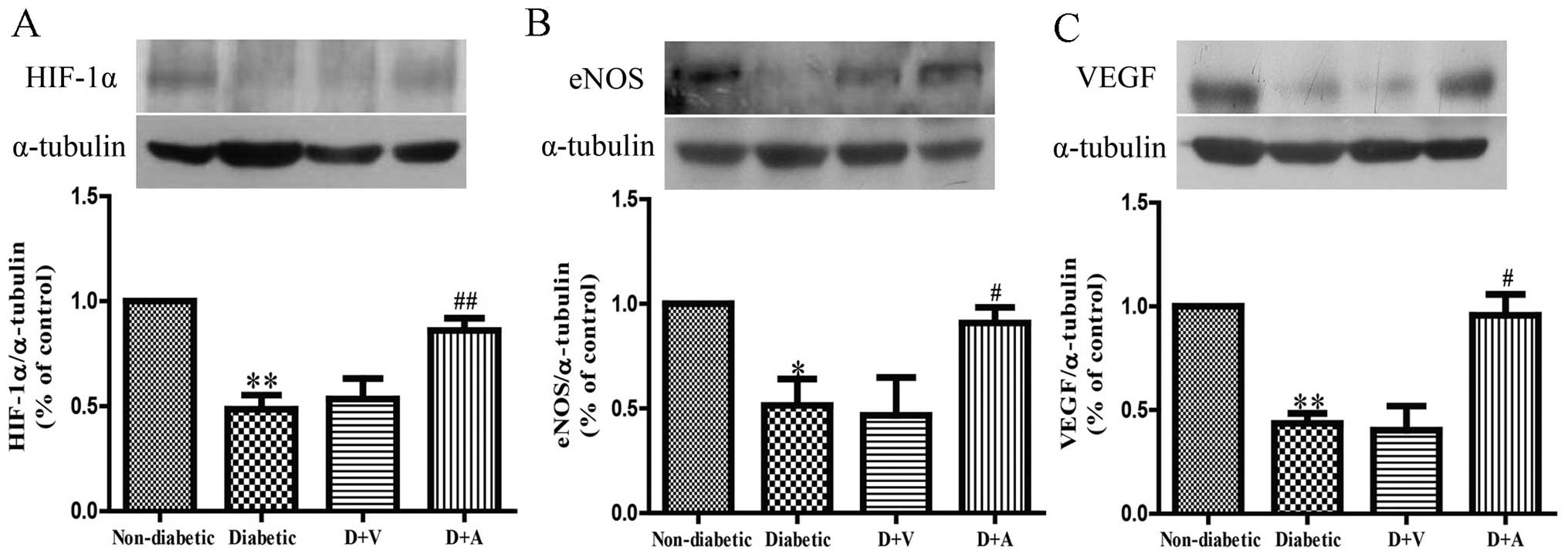
Effect of arnebin-1 on the expression
of HIF-1α, eNOS and VEGF in diabetic wounds
To investigate the mechanisms through which
neovascularization is promoted, following treatment with arnebin-1,
we measured the in vivo expression level of HIF-1α and its
target genes, VEGF and eNOS. Western blot analysis revealed that
the protein expression levels of HIF-1α, eNOS and VEGF were
markedly decreased in the diabetic wounds compared with the
non-diabetic wounds (Fig. 6). No
significant difference was observed in the levels of HIF-1α, eNOS
and VEGF between the diabetic and vehicle-treated groups. However,
the expression of HIF-1α was markedly increased in the diabetic
wounds following treatment with arnebin-1 (Fig. 6A). Compared with the diabetic and
vehicle-treated groups, a higher level of eNOS expression was
observed in the arnebin-1-treated group (Fig. 6B). Similarly, arnebin-1
significantly increased the protein expression of VEGF on the 7th
day (Fig. 6C). Taken together,
these results indicate that arnebin-1 promotes neovascularization
in the wounds of diabetic rats by upregulating the expression
levels of HIF-1α, eNOS and VEGF.
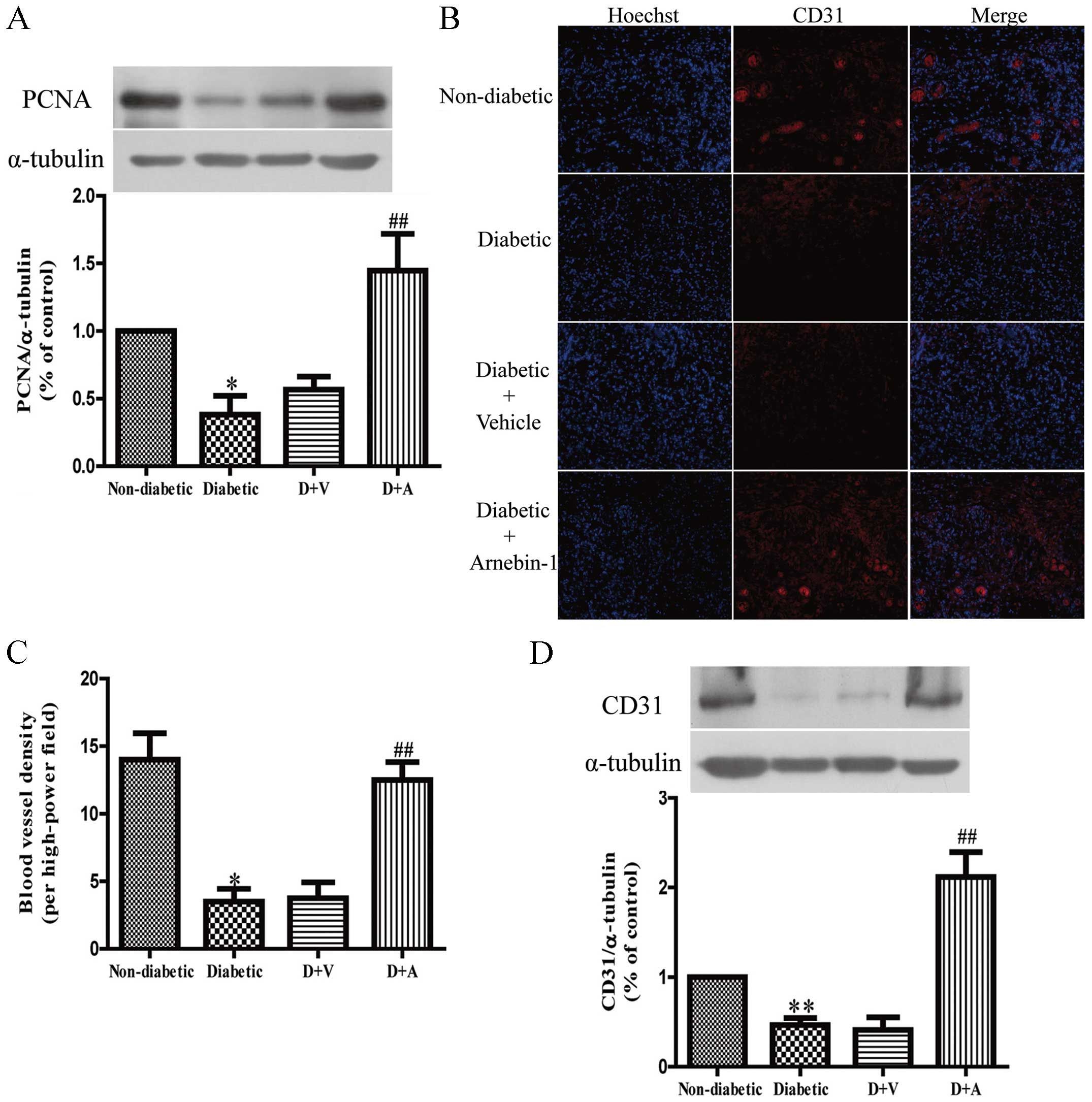
Effect of arnebin-1 on
neovascularization and diabetic wound healing
In in vitro experiments, we demonstrated that
arnebin-1 and a low concentration of VEGF significantly increased
the expression of PCNA, and that the administration of arnebin-1
without VEGF did not achieve the same result. In vivo, there
was still a low level of VEGF in the diabetic wound tissues, and
the localized application of arnebin-1 ointment to the wounds
upregulated the expression of PCNA compared with the diabetic group
the and vehicle-treated group (Fig.
7A), which was in accordance with our in vitro results.
To determine the role of arnebin-1 in neovascularization in
diabetic wounds, the expression of CD31, a biochemical marker of
angiogenesis, was examined to analyze the effects of arnebin-1. In
our previous study, using histological analysis, we demonstrated
that diabetic wounds treated with arnebin-1 exhibited an increased
capillary density on days 4 and 7 post-wounding (23). In the present study, following
immunofluorescence staining with an anti-CD31 antibody for
endothelial cells, positive staining was present in the wounds of
the non-diabetic rats (Fig. 7B).
This staining appeared to be markedly reduced in the wounds of the
diabetic control animals and the vehicle-treated diabetic animals.
We found that the number of CD31-positive blood vessels around the
granulation-formation region was increased on the 7th day following
treatment with arnebin-1. The results from quantitative analysis
revealed that capillary density was significantly greater in the
arnebin-1-treated group than the diabetic group (Fig. 7C). Moreover, the results of
western blot analysis indicated that the protein level of CD31 was
significantly increased following treatment with arnebin-1 compared
with the other diabetic groups not treated with arnebin-1 (Fig. 7D).
Discussion
At present, diabetic wounds remain a considerable
challenge in clinical practice, and current treatments are
inadequate. Only 66% of diabetic wounds ultimately heal, and up to
28% result in amputation (24–26). Deficient angiogenesis has been
noted in abnormal wound healing that leads to diabetic foot ulcers
(27). The diabetic wound
environment is characterized by a marked decrease in the
pro-angiogenic and angiogenic growth factors which regulate
angiogenesis (28). In a previous
study, we examined the effects of arnebin-1 on wound closure in
diabetic rats and confirmed that treatment with arnebin-1
significantly accelerated diabetic wound closure compared with the
rats which received the vehicle or no treatment (23). In this study, we demonstrate that
arnebin-1 is an effective inducer of neovascularization, as it
upregulates HIF-1α expression. Arnebin-1 promoted angiogenesis by
increasing the expression of VEGF, eNOS and HIF-1α in a
PI3K-dependent manner. By employing a wound model of type I
diabetes, we demonstrated that the topical application of arnebin-1
significantly accelerated the wound healing process by promoting
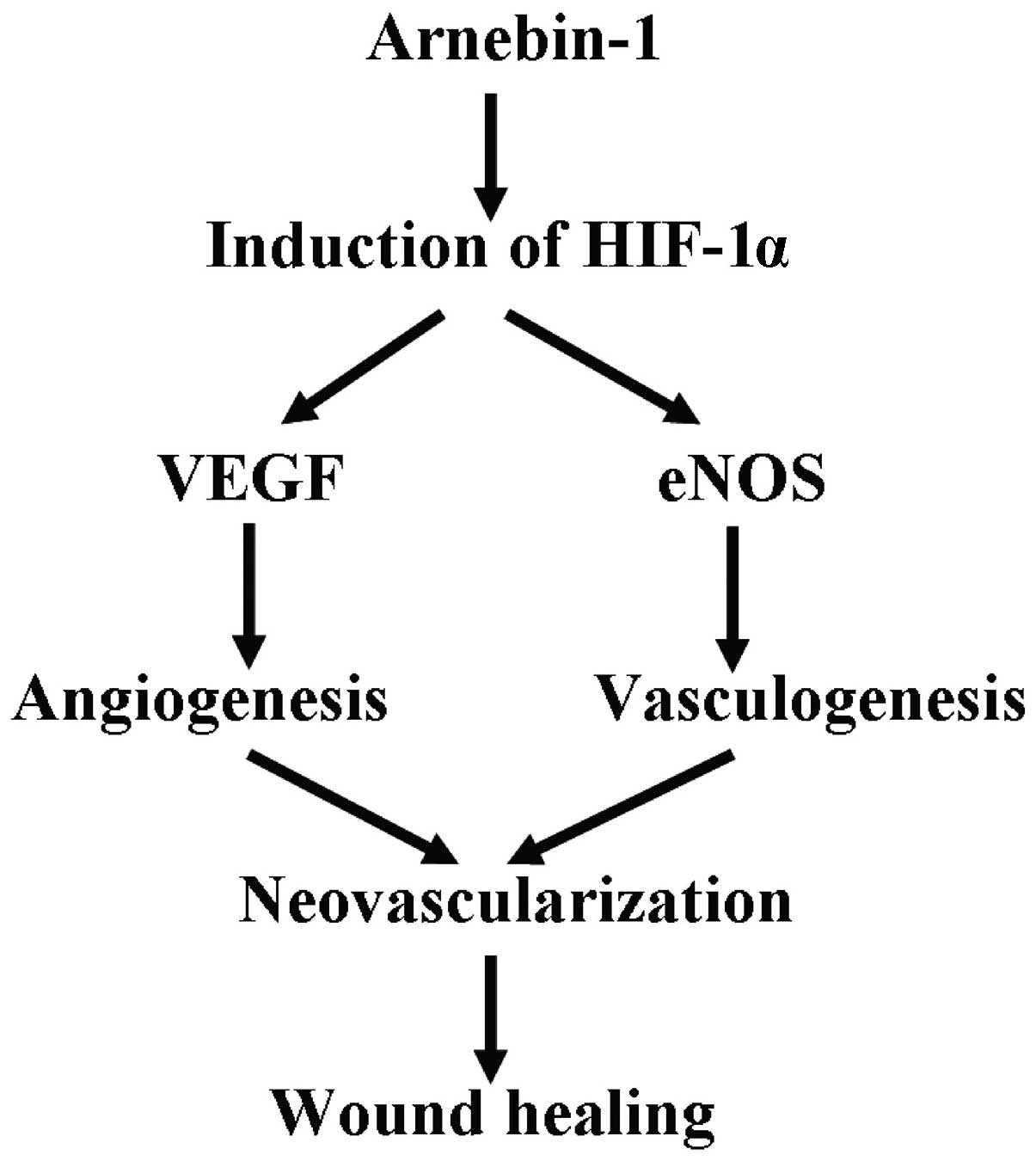
the angiogenic response. Based on these discoveries, we suggest
that the upregulation of HIF-1α is an important procedure in
arnebin-1-induced neovascularization, which contributes to wound
healing (Fig. 8).
At present, there is no effective topical drugs
which can be applied in routine clinical practice to treat diabetic
foot ulcers (29). Since the
pathogenesis of diabetic foot ulcers is complex, the application of
a growth factor, such as VEGF or PDGF is an ineffective approach to
promoting the closure of diabetic wounds. Taking into consideration
the significance of the formation of new blood vessels in tissue
repair and regeneration, previous researchers have attempted to
apply various growth factors to accelerate angiogenesis in damaged
wounds (30,31). If one substance can continuously
release several growth factors to simulate the natural
micro-environment, it could potentially be an optimal treatment for
wound healing. However, this is difficult to achieve due to
technological limitations and the high cost involved, and as a
result, the application of this treatment method is limited. New
approaches to effectively accelerate the wound healing process of
diabetic wounds are urgently required.
HIF-1α is necessary for the wound healing process,
and the entire process of normal wound healing is dependent on its
expression (32). HIF-1α plays a
critical role in cell motility and regulates the expression of
numerous pro-angiogenic growth factors, such as VEGF and also plays
a role in the recruitment of endothelial progenitor cells (33). In diabetics, insufficient
angiogenesis in wound healing could be caused by the reduction in
the expression of HIF-1α and its target molecules, such as VEGF
(11,34,32). Therefore, upregulating HIF-1α
expression is possibly a more effective treatment strategy than
applying a single growth factor. Moreover, taking into
consideration the convenience, inexpensive nature and safety of
application, the most functional and useful way to increase HIF-1α
expression is to adopt a pharmaceutical approach.
Arnebin-1 has previously been shown to promote
angiogenesis both in vitro and in vivo (23); however, its exact mechanisms of
action and functions remain to be elucidated. In this study, by
applying an excisional wound-healing model of diabetes, we
demonstrated in this study that arnebin-1 upregulates HIF-1α
expression and activates many endogenous target molecules necessary
for wound healing, such as eNOS and VEGF.
It has long been recognized that blood supply is an
important factor in wound healing. VEGF plays an essential role in
promoting the growth of new blood vessels in certain organ systems
(35). Thus, it acts as a
critical stimulus in the promotion of angiogenesis, which assists
in the healing of diabetic foot ulcers. In our previous study,
compared to the vehicle-treated wounds, significantly enhanced
vascularization was observed in the arnebin-1-treated wounds
(23). VEGF is well known as a
major regulator of neovascularization in wound healing. However,
increasing the concentration of VEGF does not consistently promote
wound healing (36). This means
that there is a limitation to using only VEGF, and indicates that
sufficient wound repair requires a variety of factors. HIF-1α is a
major transcription factor, which induces VEGF expression, as well
as the epxression of multiple molecules that are essential for
wound healing (37). In the
present study, we noted that arnebin-1 significantly increased the
protein expression of HIF-1α as well as that of eNOS and VEGF in
diabetic wounds. eNOS functions as a homing signal to mobilize
vascular endothelial progenitor cells, which are responsible for
vasculogenesis, from distant locations and recruit them to the
location of the injury (38). As
a result, we suggest that treatment with arnebin-1 induces the
expression of HIF-1α-target molecules and enhances vascularization
by promoting angiogenesis and vasculogenesis.
In our in vitro experiments, we identified
that treatment with arnebin-1 resulted in the upregulation of
HIF-1α expression in the HUVECs in a concentration-dependent
manner. Arnebin-1 did not increase the protein expression levels of
eNOS, VEGF and HIF-1α in the HUVECs when the cells were pre-tretaed
with LY294002, a PI3K inhibitor. We also noted that arnebin-1
induced the expression of angiogenic factors through HIF-1α.
According to our previous data (23), treatment with arnebin-1 with low
levels of VEGF stimulated endothelial cell function in vitro
and markedly induced cell proliferation, migration and tube
formation. In the present study, we observed that arnebin-1
promoted tube formation in HUVECs in a concentration-dependent
manner. We also discovered that the arnebin-1-induced cell
proliferation, migration and tube formation were PI3K-dependent.
The effects induced by arnebin-1 were significantly inhibited by
the suppression of the PI3K pathway which also inhibited HIF-1α
expression.
VEGFR2 signaling is necessary for vascular
endothelial cells to function. The main autophosphorylation site of
VEGFR2 is tyrosine (Tyr)1175, and its phosphorylation initiates the
downstream signaling events in endothelial cells (39). The mitogen-activated protein
kinase (MAPK)/ERK cascade and the proliferation of endothelial
cells are activated by the phosphorylation of Tyr1175 of VEGFR2,
which also mediates the VEGF-induced activation of Src-mediated
vascular permeability and cell migration (40,41). However, the activation of FAK
through VEGFR2 has also been shown to be involved in VEGF-induced
migration (42–44). In the present study, by directly
increasing VEGFR2 phosphorylation, arnebin-1 subsequently promoted
the activation of the ERK, FAK and Src signaling pathways and
increased cellular activity, which were closely related to the
upregulation of VEGF in HUVECs. These results suggest that
arnebin-1 promotes angiogenesis through autocrine mechanisms.
eNOS activity has two important functions: it
mobilizes endothelial progenitor cells from bone marrow to
peripheral blood, and induces ischemia-induced vascularization
(45). Since the PI3K/Akt/eNOS
pathway plays a key role in the process of endothelial progenitor
cell mobilization and homing (46), the results of the present study
provide further insight into the effects of arnebin-1 on this
pathway in HUVECs. Treatment with arnebin-1 markedly increased the
protein expression levels of PI3K, Akt and eNOS, indicating the
activation of the PI3K/Akt/eNOS pathway. Moreover, we noted that
this pathway was stimulated by arnebin-1, and that the expression
of HIF-1α was inhibited by LY294002. These results indicate that
the PI3K/Akt/eNOS pathway mediates arnebin-1-induced HUVEC
angiogenesis in a PI3K-dependent manner.
In conclusion, based on the outcomes of the present
study, and in conjunction with our previous data (23), we confirmed that arnebin-1
markedly promotes the angiogenesis of HUVECs in vitro and
that the topical application of arnebin-1 ointment accelerates the
wound healing process in type I diabetic rats by inducing the
expression levels of eNOS, VEGF and HIF-1α through the
PI3K-dependent signaling pathway. Topical treatment with arnebin-1
ointment may thus be considered a novel therapeutic stratety for
diabetic foot ulcers. Clinical tests are warranted to determine
whether treatment with arnebin-1 can promote wound healing in
patients with diabetes. The exact effects of arnebin-1 on
fibroblasts and keratinocytes remain also to be investigated.
Acknowledgments
This study was supported by grants from the Joint
Project of National Education Ministry and Guangdong Province (no.
2007B090400089) and (no. 2007A032702001).
References
|
1
|
Boulton AJ: The diabetic foot: Grand
overview, epidemiology and pathogenesis. Diabetes Metab Res Rev.
24(Suppl 1): S3–S6. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boulton AJ, Vileikyte L,
Ragnarson-Tennvall G and Apelqvist J: The global burden of diabetic
foot disease. Lancet. 366:1719–1724. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartus CL and Margolis DJ: Reducing the
incidence of foot ulceration and amputation in diabetes. Curr Diab
Rep. 4:413–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macfarlane RM and Jeffcoate WJ: Factors
contributing to the presentation of diabetic foot ulcers. Diabet
Med. 14:867–870. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruffieux P, Hommel L and Saurat JH:
Long-term assessment of chronic leg ulcer treatment by autologous
skin grafts. Dermatology. 195:77–80. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tandara AA and Mustoe TA: Oxygen in wound
healing - more than a nutrient. World J Surg. 28:294–300. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Covello KL and Simon MC: HIFs, hypoxia,
and vascular development. Curr Top Dev Biol. 62:37–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ceradini DJ, Kulkarni AR, Callaghan MJ,
Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP
and Gurtner GC: Progenitor cell trafficking is regulated by hypoxic
gradients through HIF-1 induction of SDF-1. Nat Med. 10:858–864.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kelly BD, Hackett SF, Hirota K, Oshima Y,
Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA and Semenza GL:
Cell type-specific regulation of angiogenic growth factor gene
expression and induction of angiogenesis in nonischemic tissue by a
constitutively active form of hypoxia-inducible factor 1. Circ Res.
93:1074–1081. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li W, Li Y, Guan S, Fan J, Cheng CF,
Bright AM, Chinn C, Chen M and Woodley DT: Extracellular heat shock
protein-90alpha: Linking hypoxia to skin cell motility and wound
healing. EMBO J. 26:1221–1233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thangarajah H, Yao D, Chang EI, Shi Y,
Jazayeri L, Vial IN, Galiano RD, Du XL, Grogan R, Galvez MG, et al:
The molecular basis for impaired hypoxia-induced VEGF expression in
diabetic tissues. Proc Natl Acad Sci USA. 106:13505–13510. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69(Suppl 3): 4–10. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999.PubMed/NCBI
|
|
15
|
Frank S, Hübner G, Breier G, Longaker MT,
Greenhalgh DG and Werner S: Regulation of vascular endothelial
growth factor expression in cultured keratinocytes. Implications
for normal and impaired wound healing. J Biol Chem.
270:12607–12613. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holmes K, Roberts OL, Thomas AM and Cross
MJ: Vascular endothelial growth factor receptor-2: Structure,
function, intracellular signalling and therapeutic inhibition. Cell
Signal. 19:2003–2012. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Meadows KN, Bryant P and Pumiglia K:
Vascular endothelial growth factor induction of the angiogenic
phenotype requires Ras activation. J Biol Chem. 276:49289–49298.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takahashi T, Ueno H and Shibuya M: VEGF
activates protein kinase C-dependent, but Ras-independent
Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial
cells. Oncogene. 18:2221–2230. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ko SH, Nauta A, Morrison SD, Zhou H,
Zimmermann A, Gurtner GC, Ding S and Longaker MT: Antimycotic
ciclopirox olamine in the diabetic environment promotes
angiogenesis and enhances wound healing. PLoS One. 6:e278442011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papageorgiou VP, Assimopoulou AN and
Ballis AC: Alkannins and shikonins: a new class of wound healing
agents. Curr Med Chem. 15:3248–3267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sidhu GS, Singh AK, Banaudha KK, Gaddipati
JP, Patnaik GK and Maheshwari RK: Arnebin-1 accelerates normal and
hydrocortisone-induced impaired wound healing. J Invest Dermatol.
113:773–781. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng Z and Zhu BH: Arnebin-1 promotes the
angiogenesis of human umbilical vein endothelial cells and
accelerates the wound healing process in diabetic rats. J
Ethnopharmacol. 154:653–662. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oyibo SO, Jude EB, Tarawneh I, Nguyen HC,
Armstrong DG, Harkless LB and Boulton AJ: The effects of ulcer size
and site, patient's age, sex and type and duration of diabetes on
the outcome of diabetic foot ulcers. Diabet Med. 18:133–138. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Armstrong DG, Lavery LA and Harkless LB:
Validation of a diabetic wound classification system. The
contribution of depth, infection, and ischemia to risk of
amputation. Diabetes Care. 21:855–859. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeffcoate WJ, Chipchase SY, Ince P and
Game FL: Assessing the outcome of the management of diabetic foot
ulcers using ulcer-related and person-related measures. Diabetes
Care. 29:1784–1787. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martin A, Komada MR and Sane DC: Abnormal
angiogenesis in diabetes mellitus. Med Res Rev. 23:117–145. 2003.
View Article : Google Scholar
|
|
28
|
Falanga V: Wound healing and its
impairment in the diabetic foot. Lancet. 366:1736–1743. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hinchliffe RJ, Valk GD, Apelqvist J,
Armstrong DG, Bakker K, Game FL, Hartemann-Heurtier A, Löndahl M,
Price PE, van Houtum WH and Jeffcoate WJ: A systematic review of
the effectiveness of interventions to enhance the healing of
chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev.
24(Suppl 1): S119–S144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bao P, Kodra A, Tomic-Canic M, Golinko MS,
Ehrlich HP and Brem H: The role of vascular endothelial growth
factor in wound healing. J Surg Res. 153:347–358. 2009. View Article : Google Scholar :
|
|
31
|
Xie L, Zhang M, Dong B, Guan M, Lu M,
Huang Z, Gao H and Li X: Improved refractory wound healing with
administration of acidic fibroblast growth factor in diabetic rats.
Diabetes Res Clin Pract. 93:396–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Botusan IR, Sunkari VG, Savu O, Catrina
AI, Grünler J, Lindberg S, Pereira T, Ylä-Herttuala S, Poellinger
L, Brismar K, et al: Stabilization of HIF-1α is critical to improve
wound healing in diabetic mice. Proc Natl Acad Sci USA.
105:19426–19431. 2008. View Article : Google Scholar
|
|
33
|
Stroka DM, Burkhardt T, Desbaillets I,
Wenger RH, Neil DA, Bauer C, Gassmann M and Candinas D: HIF-1 is
expressed in normoxic tissue and displays an organ-specific
regulation under systemic hypoxia. FASEB J. 15:2445–2453.
2001.PubMed/NCBI
|
|
34
|
Galiano RD, Tepper OM, Pelo CR, Bhatt KA,
Callaghan M, Bastidas N, Bunting S, Steinmetz HG and Gurtner GC:
Topical vascular endothelial growth factor accelerates diabetic
wound healing through increased angiogenesis and by mobilizing and
recruiting bone marrow-derived cells. Am J Pathol. 164:1935–1947.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vranckx JJ, Yao F, Petrie N, Augustinova
H, Hoeller D, Visovatti S, Slama J and Eriksson E: In vivo gene
delivery of Ad-VEGF121 to full-thickness wounds in aged pigs
results in high levels of VEGF expression but not in accelerated
healing. Wound Repair Regen. 13:51–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rey S and Semenza GL: Hypoxia-inducible
factor-1-dependent mechanisms of vascularization and vascular
remodelling. Cardiovasc Res. 86:236–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bitto A, Irrera N, Minutoli L, Calò M, Lo
Cascio P, Caccia P, Pizzino G, Pallio G, Micali A, Vaccaro M, et
al: Relaxin improves multiple markers of wound healing and
ameliorates the disturbed healing pattern of genetically diabetic
mice. Clin Sci (Lond). 125:575–585. 2013. View Article : Google Scholar
|
|
39
|
Liu J and Agarwal S: Mechanical signals
activate vascular endothelial growth factor receptor-2 to
upregulate endothelial cell proliferation during inflammation. J
Immunol. 185:1215–1221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takahashi T, Yamaguchi S, Chida K and
Shibuya M: A single autophosphorylation site on KDR/Flk-1 is
essential for VEGF-A-dependent activation of PLC-gamma and DNA
synthesis in vascular endothelial cells. EMBO J. 20:2768–2778.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eliceiri BP, Puente XS, Hood JD, Stupack
DG, Schlaepfer DD, Huang XZ, Sheppard D and Cheresh DA:
Src-mediated coupling of focal adhesion kinase to integrin
alpha(v)beta5 in vascular endothelial growth factor signaling. J
Cell Biol. 157:149–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Holmqvist K, Cross MJ, Rolny C, Hägerkvist
R, Rahimi N, Matsumoto T, Claesson-Welsh L and Welsh M: The adaptor
protein shb binds to tyrosine 1175 in vascular endothelial growth
factor (VEGF) receptor-2 and regulates VEGF-dependent cellular
migration. J Biol Chem. 279:22267–22275. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abedi H and Zachary I: Vascular
endothelial growth factor stimulates tyrosine phosphorylation and
recruitment to new focal adhesions of focal adhesion kinase and
paxillin in endothelial cells. J Biol Chem. 272:15442–15451. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Avraham HK, Lee TH, Koh Y, Kim TA, Jiang
S, Sussman M, Samarel AM and Avraham S: Vascular endothelial growth
factor regulates focal adhesion assembly in human brain
microvascular endothelial cells through activation of the focal
adhesion kinase and related adhesion focal tyrosine kinase. J Biol
Chem. 278:36661–36668. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Aicher A, Heeschen C, Mildner-Rihm C,
Urbich C, Ihling C, Technau-Ihling K, Zeiher AM and Dimmeler S:
Essential role of endothelial nitric oxide synthase for
mobilization of stem and progenitor cells. Nat Med. 9:1370–1376.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Everaert BR, Van Craenenbroeck EM, Hoymans
VY, Haine SE, Van Nassauw L, Conraads VM, Timmermans JP and Vrints
CJ: Current perspective of pathophysiological and interventional
effects on endothelial progenitor cell biology: focus on
PI3K/AKT/eNOS pathway. Int J Cardiol. 144:350–366. 2010. View Article : Google Scholar : PubMed/NCBI
|