Introduction
Periodontitis is an inflammatory disease, which
manifests clinically as a loss of connective periodontal tissues,
including periodontal ligament (PDL) and alveolar bone, resulting
in loosening of the teeth, loss of chewing function and,
ultimately, tooth loss (1,2).
To achieve successful periodontal regeneration, the formation of
periodontal fibers, the insertion of these fibers into newly formed
cementum and the restoration of alveolar bone height are required
(3). To date, the restoration of
damaged or diseased periodontal tissues is considered to be
biologically possible, but clinically unpredictable, resulting in
only partial regeneration at best (4).
The PDL is a highly fibrous and vascular tissue,
which anchors the tooth root to the tooth socket bone and is
important in tooth anchorage, sensation, facilitating nutrient
delivery to surrounding cells and repairing damaged tissue in
response to periodontal disease or mechanical trauma (5,6).
It has been demonstrated that human the PDL contains a
heterogeneous population of cells, including cementoblasts,
osteoblasts, fibroblasts, myofibroblasts, endothelial cells, nerve
cells and epithelial cells (1,5–7).
However, the presence of paravascular progenitor stem cells within
the PDL has long been hypothesized (2,3,5,8),
Seo et al (9) isolated PDL
stem cells (PDLSCs) in the human PDL; PDLSCs exhibit mesenchymal
stem cells characteristics, including clonogenicity and high
proliferation, when transplanted into immunocompromised rodents.
The PDLSCs demonstrated the capacity to generate a
cementum/PDL-like structure and contribute to periodontal tissue
repair. Therefore the application of PDL stem cells may hold
promise as a therapeutic approach for the reconstruction of tissues
destroyed by periodontal diseases (10).
To investigate this potential, a thorough
understanding of PDLSCs is required. Whether inflammatory changes
in the periodontium can affect stem cells remains to be elucidated.
A full understanding of the properties of stem cells from
periodontitis-affected teeth is required prior to them being used
to treat periodontal disease. In the present study, the effects of
periodontitis on the proliferation and differentiation of PDLSCs
were investigated through in vivo and in vitro
experiments. The aim of the present study was to provide a better
understanding of the mechanism of periodontitis, and offer insight
into the selection of candidate cells for periodontal regeneration
between healthy and periodontitis-affected PDLSCs.
Materials and methods
Patients
Disease free premolars and third molars were
collected from 10 patients, following extraction for orthodontic
treatment purposes, aged between 30 and 40 years old. Diseased
teeth were obtained from 10 patients with generalized chronic
periodontitis, undergoing tooth extraction, also aged between 30
and 40 years of age. The clinical diagnosis of chronic periodontal
disease was based on visual and radiographic assessment of the
periodontal tissues, and on measurements of the space between the
tooth and gum. These spaces are normally 1–3 mm in depth, and
deepen as supporting connective tissue and bone are lost (11). All selected patients presented
with generalized chronic periodontitis with more than one pocket
depth ≥5 mm. All subjects were free of any clinical evidence of
recent infection. The subjects included in the study had never
smoked, had no history of systemic disease, had received no
periodontal treatment, had not received antibiotics,
anti-inflammatory drugs or any other drugs in the pervious 6
months, and were not alcohol or antioxidant vitamin consumers.
Individuals living in this region consume a diet rich in fish,
vegetables and fruit due to the conditions in the region and
traditional lifestyles. This study was approved by the Ethics
Committee of the Tianjin Stomatological Hospital (Tianjin, China).
Written informed consent was provided by all participants prior to
enrollment.
Cell culture
Primary cultures from the two groups were obtained
by culturing explants of the healthy and periodontitis-affected
periodontal tissues from patients undergoing extractions. The PDL
tissue was gently scraped from the surface of the middle part of
the root, minced into 1-mm3 cubes and placed into 6-well
culture dishes (Costar, Cambridge, MA, USA). The explants were
grown in a-minimal essential medium (MEM) supplemented with 10%
fetal bovine serum (FBS), 0.292 mg/ml glutamine, 100 U/ml
penicillin/streptomycin and 100 µM/l ascorbic acid. The
cultures were incubated at 37°C in a humidified atmosphere of 5%
CO2.
Flow cytometric analysis
Cell-surface antigen expression was performed using
flow cytometric analysis; 1×106 PDLCs were washed in
PBS, and then incubated with mouse anti-human monoclonal STRO-1
(dilution 1:100; R&D Systems, Inc., Minneapolis, MN, USA)
antibodies for 30 min at 4°C. The cells were washed twice with cold
phosphate-buffered saline (PBS) containing 2% FBS, and incubated
with 1 µg fluorescein isothiocyanate (FITC)-conjugated goat
anti-mouse IgM antibodies (Santa Cruz Biotecgnology, Inc., Santa
Cruz, CA, USA) for 30 min at 4°C. Mouse isotype antibodies (BD
Biosciences, Franklin Lakes, NJ, USA) served as a control. The
labeled cells were analyzed using a flow cytometer (Cell Lab
Quanta; Beckman Coulter, Fullerton, CA, USA).
Isolation of PDLSCs
To obtain STRO-1+ stem cells, the two
cell populations were sorted using immunomagnetic beads (Dynabeads;
Dynal Biotech, Oslo, Norway), according to the manufacturer's
instructions. The cells (~4×106) were incubated with
mouse anti-human STRO-1 supernatant (R&D Systems) for 30 min at
4°C. These cells were then washed with PBS/5% FBS and re-suspended
with rat anti-mouse IgM-conjugated Dynabeads (four beads per cell)
for 60 min using a rotary mixer. Following washing, the
bead-positive cells were segregated using a magnetic particle
separator and subsequently seeded into 75-cm2 culture
flasks (Costar) at 37°C in 5% CO2.
Colony-forming unit-fibroblast assay
Colony-forming assays were performed using the
PDLSCs, which were plated at a density of between 0.25 and
1×104/well in a 6-well plate. Following 10 days of
culture, the two cultures were fixed with 4% buffered formaldehyde
and then stained with 0.1% toluidine blue (Sigma-Aldrich, St.
Louis, MO, USA). The cells were visualized using a Cambridge 360
Scanning Electron Microscope (Carl Zeiss SMT, Thornwood, NY, USA).
Aggregates of >50 cells were scored as colonies.
Osteogenic and adipogenic
differentiation
For osteogenesis, the PDLSCs were plated at a
density of 5×103 cm2 in 24-well plates and
cultured for 3 days. The cells were then incubated with a-MEM,
supplemented with 10% FBS, 50 µM L-ascorbic acid 2-phosphate
(Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich) and 100
nM dexamethasone (Sigma-Aldrich), for 2 weeks to induce mineral
formation. The cells were fixed with 70% ethanol for 15 min and
stained with 2% alizarin red (pH 4.0; Sigma-Aldrich) for 15 min.
The nodule area was measured quantitatively using an image analysis
system (Image-Pro Plus 5.0; Media Cybernetics, Inc., Baltimore, MD,
USA).
For adipogenesis, the PDLSCs were plated at a
density of 5×103 cm2 in 24-well plates and
were cultured for 3 days. The cells were then incubated with a-MEM,
supplemented with 10% FBS, 0.5 mM methylisobutylxantine, 0.5
µM hydrocortisone and 60 µM indomethacin
(Sigma-Aldrich). The cells were cultured for an additional 21 days.
The adipogenic cultures were fixed in 70% ethanol for 15 min and
stained with 2% fresh Oil Red O solution (Sigma-Aldrich) for 15
min. The lipid area was measured quantitatively using an image
analysis system (Image-Pro Plus 5.0; Media Cybernetics, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
PDLSCs at 90% confluence were harvested. The total
celluar RNA was isolated from the maintenance cell cultures using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA).
RT-PCR was performed with 1 mg RNA using a PrimeScript RT reagent
kit (Takara, Dalian, China). The RNA concentration was calculated
using a Nanodrop ND2000 spectrophotometer (Nano-Drop Technologies,
Wilmington, DE, USA). qPCR was performed using 1 µg cDNA
(12). First-strand cDNA
syntheses were performed, as reported previously (12). For qPCR a QuantiTect SYBR-Green
kit (Toyobo, Osaka, Japan) and ABI Prism 7700 Sequence Detection
System (Applied Biosystems, Foster City, CA, USA) were used. Primer
sequences for Runx2 (GenBank accession no. NM 004348), collagen
type I (GenBank accession no. NM 000088), osteocalcin (GenBank
accession no. NM 000711), were as follows: Runx2, sense
5′-CCCGTGGCCTTCAAGGT-3′ and anti-sense 5′-CGTTACCCGCCATGACAGTA-3′;
collagen type I, sense 5′-CCAGAAGAACTGGTACATCAGCAA-3′ and
anti-sense 5′-CGCCATACTCGAACTGGAATC-3′; and osteocalcin, sense
5′-AGCAAAGGTGCAGCCTTTGT-3′ and anti-sense
5′-GCGCCTGGGTCTCTTCACT-3′. The gene expression of GAPDH was used as
a reference gene in all applications. The qPCR eactions were
performed using the following cycling conditions: 95°C for 10 min,
then 45 cycles of 95°C for 15 sec followed by 60°C for 1 min.
Alkaline phosphatase (ALP) activity
assay
At defined time-points, the activity of ALP in the
PDLSCs was detected using an ALP assay kit (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). In brief, the cells
(1×103/well) were seeded into a 96-well plate. Following
culture, cells were washed three times with 0.01 M PBS and 50 ml
cold 10 mM Tris/HCl buffer (pH 7.4; Sigma-Aldrich) containing 0.1%
Triton X-100 (Sigma-Aldrich) added prior to incubation at 4°C
overnight. Subsequently, 100 µl ALP substrate solution (2 mM
MgCl2 and 16 mM p-nitrophenyl phosphate;
Sigma-Aldrich) was mixed with each sample. Following incubation at
37°C for 30 min, the reaction was terminated by the addition of 50
ml 0.2 M NaOH. The optical density values were measured at 405 nm
in a spectrophotometer using a microplate reader (DU 640).
In vivo differentiation assay
For a single in vivo transplantation,
2×106 PDLSCs were mixed with 20 mg ceramic bovine bone
(CBB; Research and Development Center for Tissue Engineering,
Fourth Military Medical University, Xi'an, China), powders and the
mixture was centrifuged briefly at 1,300 rpm at room temperature
for 3 min and the supernatant was discarded. The pelleted powder
with adherent cells was mixed with 15µl Matrigel (Beixiu,
Guangzhou, China) to form a clot, which was implanted into
subcutaneous pockets on the dorsal surface of 4-week-old NOD/SCID
mice (weighing 20 g). The animals were divided into 2 groups, 15
rats in each group. The animals were housed in filter top cages (at
26°C) and were given a standard diet and water with antibiotics and
antimycotics ad libitum. All animal use protocols were
reviewed and approved by the Animal Care Committee of Fourth
Military Medical University. The implants were recovered after 8
weeks, fixed in 4% paraformaldehyde for 2 days, and then
decalcified for a further 10 days in 10% EDTA (Sigma-Aldrich) prior
to embedding in paraffin. For histological analysis, 5 µm
sections of the implants were prepared and stained using
hematoxylin and eosin (H&E).
Statistical analysis
Statistical significance was assessed using a
χ2 test and an independent samples t-test with SPSS
v.12.0 software (SPSS, Inc., San Rafael, CA, USA). P<0.05 was
considered to indicate a statistically significant difference. All
data acquisition and analyses were performed in a
blinded-manner.
Results
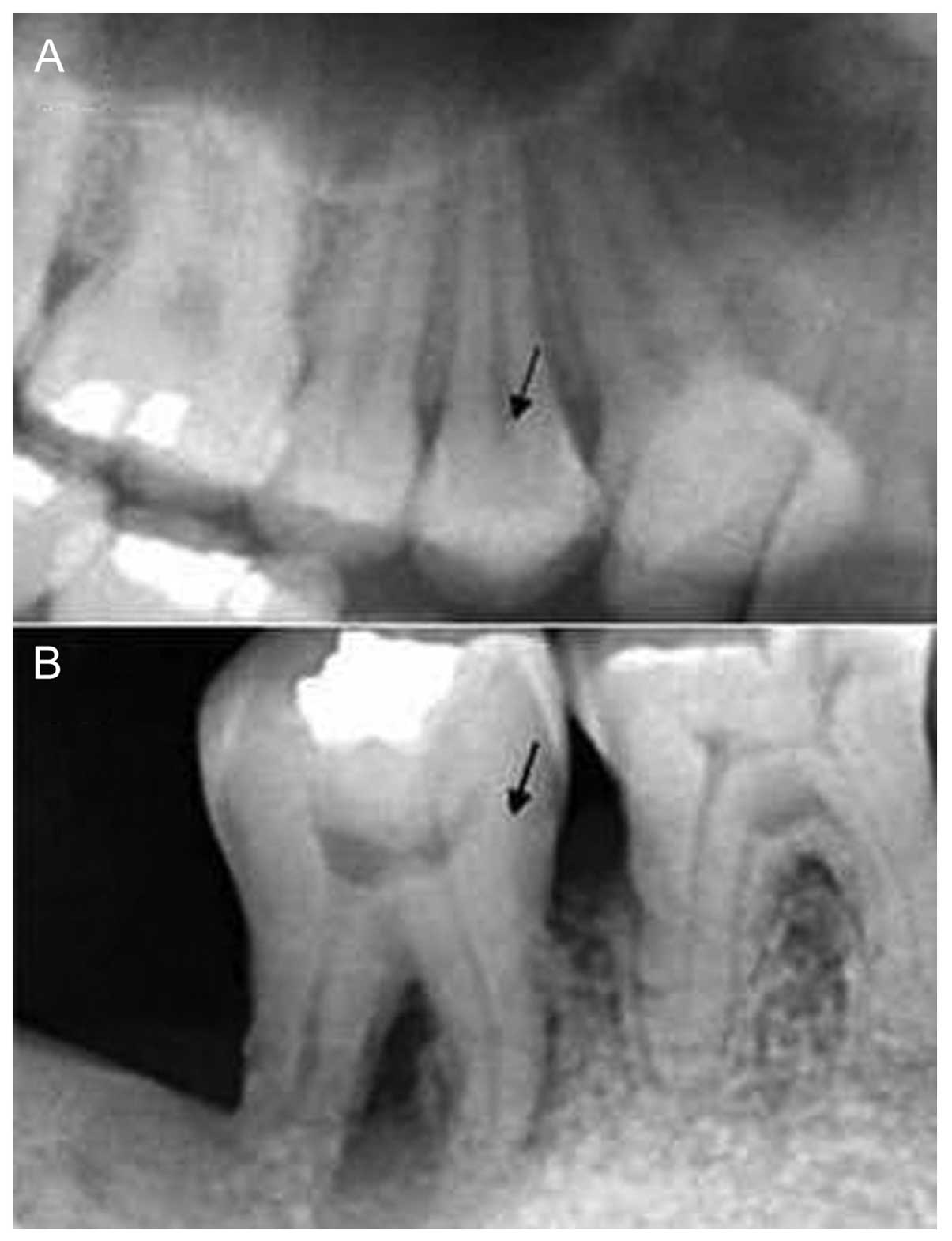
Radiographic features of periodontal
tissues
The clinical diagnosis of the periodontal disease
was based on radiographic assessment of the periodontal tissues, as
shown in Fig. 1.
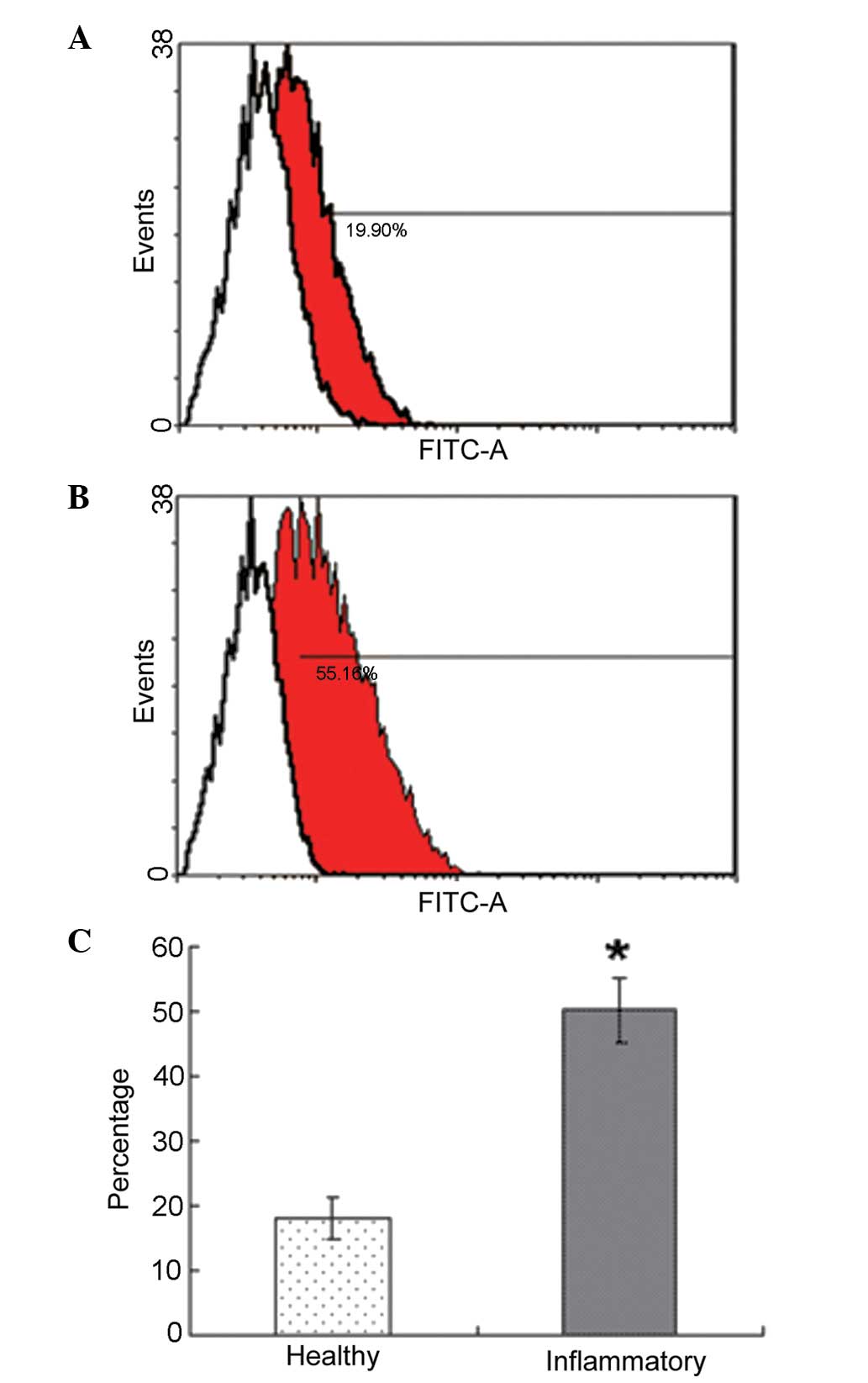
Effect of periodontitis on the number of
PDLSCs
FACS analysis of the PDL cells demonstrated that the
percentage positivity for STRO-1 in the periodontitis-affected PDL
cells was significantly higher than those in the healthy PDL cells.
The rate of STRO-1+ cells was 18.2±3.2% in the healthy
donors and 50.3±5.1% in the periodontitis-affected donors
(P<0.05; Fig. 2).
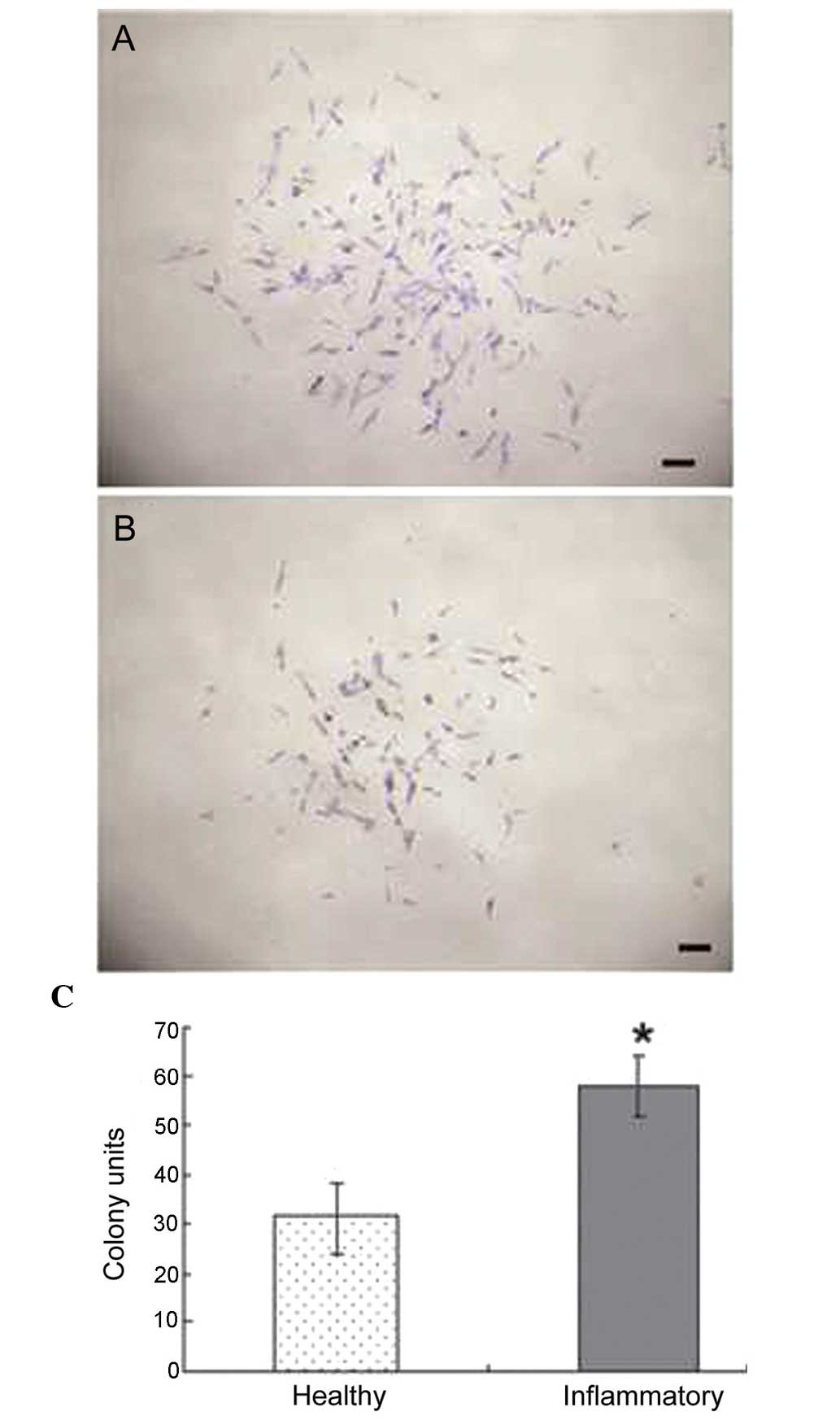
Effect of periodontitis on the
proliferation of PDLSCs
The ability of colony forming was compared between
healthy PDLSCs and periodontitis-affected PDLSCs. The cells in the
two groups demonstrated the ability to form multi-cell clusters
(Fig. 3A and B). In the PDLSCs
from the healthy donors, the formation of ~30 colonies, generated
from single cells cultured at low density for 10 days, was
observed; whereas the PDLSCs from the periodontitis-affected donors
generated 50 colonies. These results revealed a statistically
significant difference, with the PDLSCs from the
periodontitis-affected donors having increased colony forming
abilities, compared with the healthy PDLSCs (Fig. 3C).
Effect of periodontitis on the in vitro
pluripotential capacity of PDLSCs
During osteogenic stimulation, the appearance of
undifferentiated PDLSCs change from their characteristic
spindle-shaped morphology to a cuboidal appearance. The deposition
of calcified matrix on the culture dishes was assessed by the
quantity of alizarin red staining. A decline in the quantity of
mineralized matrix formed was observed in the diseased PDLSC
culture (Fig. 4A and B).
As adipogenesis progressed, the accumulation of
large cytoplasmatic vacuoles containing lipids was detected using
Oil Red staining, and were visualized under phase-contrast
microscopy. The adipocytes, which developed in the cultures derived
from healthy donors accumulated more fat than the adipocytes, which
developed in the cultures derived from periodontitis-affected
donors (Fig. 4C and D). A
significant difference was observed in the in vitro A
significant difference was observed in the in vitro
osteogenic activity (P<0.05; Fig.
4E).and adipogenic activity in the two cell groups (P<0.05;
Fig. 4F).
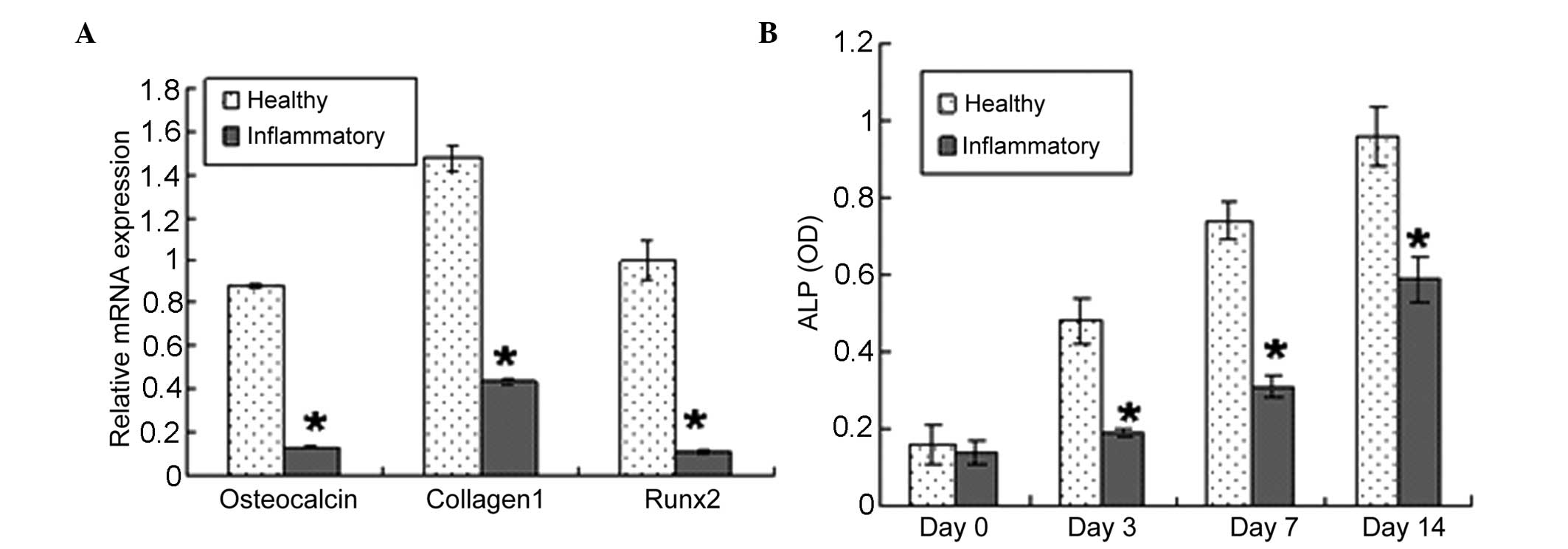
Effect of periodontitis on gene
expression and ALP activity in PDLSCs
The present study used RT-qPCR to examine whether
changes in the differentiation potential of the PDLSCs were
accompanied by changes in the expression of phenotype-specific gene
markers and ALP activity. In basal, non-differentiating conditions,
the PDLSCs derived from periodontitis-affected donors expressed
lower mRNA levels of the osteoblast markers, Runx2, collagen 1 and
osteocalcin, compared with the PDLSCs isolated from healthy donors
(Fig. 5A). The PDLSCs from
healthy donors exhibited higher ALP reactivity, compared with the
PDLSCs from periodontitis-affected donors between days 3 and 14 of
the observation period (Fig.
5B).
Effect of periodontitis on the in vivo
differentiation of PDLSCs
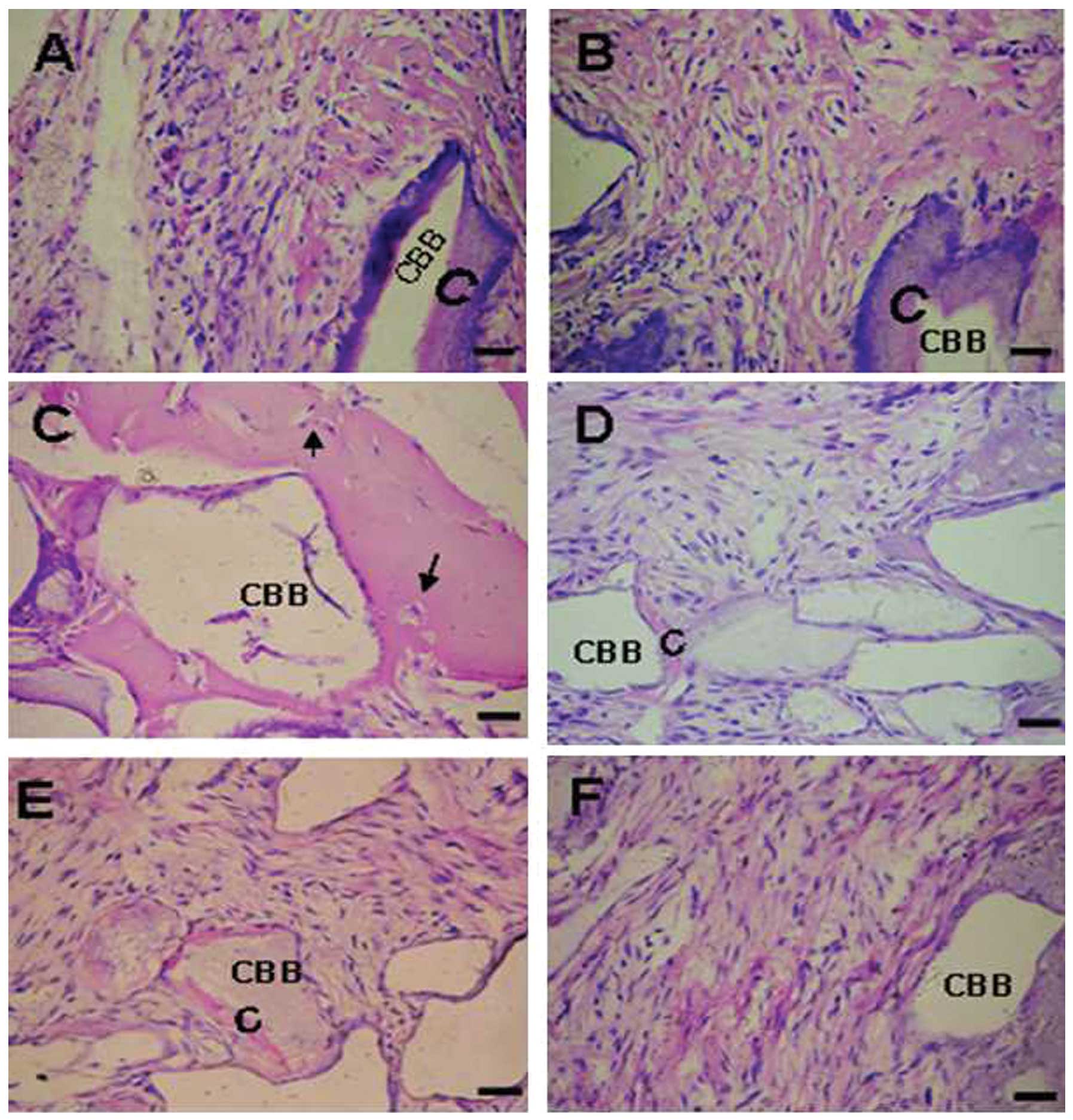
The PDLSC transplants from the healthy donors were
observed to posses the tissue-regenerative capacity to generate
cementum-like mineralized tissue on the border of the CBB, with
PDL-like fibrous tissue interfacing these areas (Fig. 6A and B) and cementocyte-like cells
embedded in the mineralized structure (Fig. 6C; arrows). By contrast, the
implants containing periodontitis-affected PDLSCs formed small
quantities of mineralized tissue lining the CBB surfaces (Fig. 6D and E); and fibrous tissues were
observed within the CBB only (Fig.
6F).
Discussion
For decades periodontists have investigated ways to
repair the damage that occurs during periodontitis. This has
included the use of a range of surgical procedures and a variety of
grafting materials. Due to issues of safety, effectiveness and
stability, their use for periodontal regeneration has been
questioned (2,4,13).
Biological approaches based on the principles of tissue-engineering
have emerged as prospective alternatives. Therefore, PDLSCs offer
potential for the development of novel cell-based therapies to
treat the damage caused by trauma or periodontal disease (2,6,14).
Whether PDLSCs from periodontitis-affected teeth can be used to
regenerate periodontal tissues remains to be elucidated. Previous
investigation of the in vitro and in vivo features of
stem cells from periodontitis-affected PDL has been limited, and
investigation of the changes in the proliferation and
differentiation potentials of PDLSCs when extracted from healthy
and periodontitis-affected donors is of significant interest.
Therefore, the present study investigated for the first time, to
the best of our knowledge, the association between periodontitis
and PDL stem cells.
In the present study, PDLSCs were successfully
isolated and characterized from periodontitis-affected donors.
Previous studies have demonstrated that the putative stem cell
marker, STRO-1, which is used to isolate and purify bone marrow
stromal stem cells, is also expressed by human PDLSCs. Thus, its
expression can be used to isolate and characterize human PDLSCs
(3,6). The present study demonstrated that
the percentage of positivity for STRO-1 from periodontitis-affected
PDL cells was significantly higher than that in the healthy PDL
cells. The effect of periodontitis on the number of stem cells
revealed in the present study was also in agreement with previous
data that wider distributions of stem cells are detected in
sections of diseased ligaments (15). The increase in stem cell
populations may be caused by the early recruitment and migration of
paravascular cells, as has been observed in the wounded mice PDL
(15,16). In the present study, the
clonogenic capabilities of PDLSCs from the periodontitis-affected
donors were higher, compared with those from the healthy donors.
Digirolamo et al (17)
reported that the replicative potential of cells in culture is
determined best using a simple colony-forming assay, and the
samples with the high colony forming efficiency exhibit the highest
replicative potential. In the present study, marked differences in
the growth pattern of PDLSCs obtained from the healthy and
periodontitis-affected donors were observed. This suggested that
periodontitis had a significant effect on PDLSCs, in terms of
proliferative activity. One possibility may be that
periodontitis-affected tissue-derived stem cells exhibit higher
mitotic properties, compared with cells derived from healthy
donors.
In addition to the effect of periodontitis on PDLSC
numbers and proliferation, the present study investigated its
effects on function, in which the PDLSCs were characterized by
their capacity to differentiate into various stromal cell lineages
in vitro and in vivo. In the presence of osteogenic
differentiation medium, the PDLSCs from healthy donors exhibited a
higher alizarin-red-positive area, compared with the PDLSCs from
periodontitis-affected donors. This result indicated increased
osteogenic characteristics in the healthy PDLSCs. These results
were supported by the gene expression profiles, determined using
RT-qPCR analyses. The RT-qPCR results revealed
periodontitis-associated PDLSC declines in the expression of Runx2,
the earliest differentiation marker for committed osteogenetic
precursor cells (18), and the
expression of collagen and osteocalcin osteoblast markers (19). Analysis of these osteoblast
markers revealed that the healthy PDLSCs had higher inherent
differentiation, compared with the diseased PDLSCs. ALP is vital in
calcified tissue formation by regulating phosphate transfer
(20). In the present study, the
higher ALP activity and expression of cementoblast phenotype
suggested that PDLSCs from healthy donors may be more likely to
differentiate into osteoblast/cementoblast-like cells. In addition
to their osteogenic differentiation capacity, PDLSCs can
differentiate into adipocytes, and thus, adipocyte differentiation
has been used as a marker for the multipotential nature of the
cells (21). In the present
study, the periodontitis-affected PDLSCs formed fewer adipocytes
than the healthy PDLSCs. These findings suggested that
periodontitis reduced the pluripotential capacity of the PDLSCs.
The present study performed an in vivo tissue regeneration
assay, based on subcutaneous implantation of PDLSCs into a mouse
transplantation model. The findings demonstrated that periodontitis
decreased the ability of the PDLSCs to form periodontal tissues
in vivo, which was in accordance with the previous
suggestion that proliferation and differentiation are inversely
correlated with each other due to the existence of dual-function
regulators involved in controlling the two processes (22,23). Periodontitis-affected individuals
generally have an impaired ability to regenerate tissues (11). The present findings suggested that
this impairment may be due to the decrease in stem cell
differentiation ability. Periodontitis-associated defects in PDL
function may be due to quantitative stem cell defects and, whether
the cellular mechanism may be associated with the accumulation of
growth factors and cytokines, including fibroblast growth factor,
platelet-derived growth factor, insulin-like growth factor,
transforming growth factor, tumor necrosis factor-α, interleukin
(IL)-lα, IL-1β, IL-6 and IL-12, merit further investigation.
Previous studies have demonstrated that the excessive and/or
continuous production of cytokines in inflamed periodontal tissues
may be responsible for the progression of periodontitis and
periodontal tissue destruction (24,25). As previously reported, a complex
network of growth factors and cytokines guides cellular
differentiation and regeneration in all organs and tissues
(26). Therefore, the results of
the present study demonstrated that certain components of
inflammatory cytokines act, not only as promoters for cell
proliferation, but also as inhibitors for PDLSC differentiation.
The impaired regenerative potential of inflamed PDLSCs may be
improved by a decrease or dilution of inflammatory cytokines.
As the development of novel medical therapies that
use adult stem cell transplants to cure, repair or even grow a new
organ (19) progresses, it is of
important clinical significance to clarify the biological
characteristics and functional changes of PDLSCs in
periodontitis-affected individuals, to guide the treatment of
periodontal disease. In conclusion, the present study demonstrated
that, during periodontitis, the status of PDLSCs change with
respect to their proliferation and intrinsic differentiation
potential. The present study revealed that the decrease in the
differentiation capacity of PDLSCs from periodontitis-affected
donors may result in the decrease of periodontal tissue forming
ability. In addition, the findings suggested that treatment, which
increases the differentiation potential of PDLSCs is an important
aim in developing novel therapeutic interventions to treat
periodontal disease. If PDLSCs emerge as a candidate cell source
for periodontal engineering, healthy PDL is a preferable source. If
they are from diseased teeth, PDLSCs may commit to periodontal
tissues by modulating cytokines and/or other differentiation
strategies. Therefore, the therapeutic potential of healthy and
inflammatory PDLSCs differs, which is important to consider when
these cells are considered for therapy.
Acknowledgments
The present study was supported by grants from the
Nature Science Foundation of China (grant. nos. 31030033 and
81171001). The authors would like to thank Dr Lei Wang and Dr Huan
Shen (both from the School of Stomatology, Fourth Military Medical
University) for their assistance with animal experiments.
References
|
1
|
Gronthos S, Mrozik K, Shi S and Bartold
PM: Ovine periodontal ligament stem cells: isolation,
characterization, and differentiation potential. Calcif Tissue Int.
79:310–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin NH, Gronthos S and Bartold PMS: Stem
cells and periodontal regeneration 2000. Aust Dent J. 53:108–121.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ivanovski S, Gronthos S, Shi S and Bartold
PM: Stem cells in the periodontal ligament. Oral Dis. 12:358–363.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Benatti BB, Silvério KG, Casati MZ, Sallum
EA and Nociti FH Jr: Physiological features of periodontal
regeneration and approaches for periodontal tissue engineering
utilizing periodontal ligament cells. J Biosci Bioeng. 103:1–6.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ibi M, Ishisaki A, Yamamoto M, et al:
Establishment of cell lines that exhibit pluripotency from
miniature swine periodontal ligaments. Arch Oral Biol.
52:1002–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartold PM, Xiao Y, Lyngstaadas SP, Paine
ML and Snead ML: Stem cells and periodontal regeneration.
Periodontol. 40:164–172. 2006. View Article : Google Scholar
|
|
7
|
Tomokiyo A, Maeda H, Fujii S, Wada N,
Shima K and Akamine A: Development of a multipotent clonal human
periodontal ligament cell line. Differentiation. 76:337–347. 2008.
View Article : Google Scholar
|
|
8
|
Nagatomo K, Komaki M, Sekiya I, Sakaguchi
Y, Noguchi K, Oda S, Muneta T and Ishikawa I: Stem cell properties
of human periodontal ligament cells. J Periodont Res. 41:303–310.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seo BM, Miura M, Gronthos S, Bartold PM,
Batouli S, Brahim J, Young M, Robey PG, Wang CY and Shi S:
Investigation of multi-potent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Zheng Y, Ding G, Fang D, Zhang C,
Bartold PM, Gronthos S, Shi S and Wang S: Periodontal ligament stem
cell-mediated treatment for periodontitis in miniature swine. Stem
Cells. 26:1065–1073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pihlstrom BL, Michalowicz BS and Johnson
NW: Periodontal diseases. Lancet. 366:1809–1820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lindroos B, Mäenpää K, Ylikomi T, Oja H,
Suuronen R and Miettinen S: Characterisation of human dental stem
cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun.
368:329–335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartold PM, Xiao Y, Lyngstaadas SP, Paine
ML and Snead ML: Principles and applications of cell delivery
systems for periodontal regeneration. Periodontol. 41:123–135.
2006. View Article : Google Scholar
|
|
14
|
Sonoyama W, Liu Y, Fang D, et al:
Mesenchymal stem cell-mediated functional tooth regeneration in
swine. PLoS One. 1:e792006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SC, Marino V, Gronthos S and Bartold
PM: Location of putative stem cells in human periodontal ligament.
J Periodont Res. 41:547–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCulloch CA and Melcher AH: Cell density
and cell generation in the periodontal ligament of mice. Am J Anat.
167:43–58. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Digirolamo CM, Stokes D and Colter D:
Propagation and senescence of human marrow stromal cells in
culture: a simple colony-forming assay identifies samples. with the
greatest potential to propagate and differentiate. Br J Haematol.
107:275–281. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fakhry A, Ratisoontorn C, Vedhachalam C,
et al: Effects of FGF-2/-9 in calvarial bone cell cultures:
differentiation stage-dependent mitogenic effect, inverse
regulation of BMP-2 and noggin, and enhancement of osteogenic
potential. Bone. 36:254–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moerman EJ, Τeng K, Lipschitz DA and
Lecka-Czernik B: Aging activates adipogenic and suppresses
osteogenic programs in mesenchymal marrow stroma/stem cells: the
role of PPAR-γ transcription factor and TGF-β/BMP signaling
pathways. Aging Cell. 3:379–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Beck GR Jr, Zerler B and Moran E:
Phosphate is a specific signal for induction of osteopontin gene
expression. Proc Natl Acad Sci USA. 97:8352–8357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu J, Jin F, Tang L, et al: Dentin
non-collagenous proteins (dNCPs) can stimulate dental follicle
cells to differentiate into cementoblast lineages. Biol Cell.
100:291–302. 2008. View Article : Google Scholar
|
|
23
|
Zhu L and Skoultchi AI: Coordinating cell
proliferation and differentiation. Curr Opin Genet Dev. 11:91–95.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okada H and Murakami S: Cytokine
expression in periodontal health and disease. Crit Rev Oral Biol
Med. 9:248–266. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roberts FA, McCaffery KA and Michalek SM:
Profile of cytokine mRNA expression in chronic adult periodontitis.
J Dent Res. 76:1833–1839. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ioannidou E: Therapeutic modulation of
growth factors and cytokines in regenerative medicine. Curr Pharm
Des. 12:2397–2408. 2006. View Article : Google Scholar : PubMed/NCBI
|