Introduction
Stroke is the leading cause of adult disability, as
well as the second most common cause of mortality worldwide
(1). Ischemic stroke accounts for
approximately 80% of all strokes (1,2).
Over the past decade, thrombolysis has been established as an
effective treatment in the most acute phase of ischemic stroke.
However, many patients develop lifelong disabilities following
ischemic stroke as they do not receive the necessary treatment
within the therapeutic time window. Therefore, increasing the
window of therapeutic efficacy of established treatments or
identifying other therapies with alternative targets is necessary
to improve the neurological outcomes in ischemic stroke victims.
Targeting key cellular survival/proliferation mechanisms may
improve prognosis. Recently, it was reported that the signaling
molecule, β-catenin, is degraded in the peri-infarct area of the
brain following focal cerebral ischemia (3–7).
In a previous study, it was demonstrated that in doubleridge mice,
which have a reduced expression of Dkk-1, an antagonist of
Wnt/β-catenin signaling, the reduction of β-catenin was attenuated
and the infarct volume was reduced following middle cerebral artery
occlusion (MCAO) (5). This
suggests that prevenint the decrease in Wnt/β-catenin signaling in
cerebral ischemia may prove to be a potential novel therapeutic
modality.
In addition, the conditional expression of
stabilized β-catenin in neural progenitor cells (NPCs) enlarges the
cortical surface area through the expansion of the progenitor cell
population (8). The
overexpression of Wnt3 has been shown to increase neurogenesis in
adult hippocampal precursor cells, while the blockade of Wnt
signaling reduces neurogen-esis both in vitro and in
vivo (9). These studies also
implicate the canonical Wnt/β-catenin pathway in the proliferation
and self-renewal of NPCs (3,8,9).
Traditional Chinese medicine (TCM) has long been an
important component of complementary and alternative medicine in
several Asian countries, and recently in Western society. Modern
research has revealed the potential therapeutic effects of TCM in
the treatment of various diseases, including cerebrovascular
diseases and cancer (10,11). Its unique functions in gene
therapy have also been discussed (12,13). Electro-acupuncture (EA) is a
traditional therapeutic method used in China, widely used for both
the prevention and treatment/rehabilitation of cerebral ischemia.
Nevertheless, the mechanisms responsible for its effects are not
yet fully understood. Previous studies have indicated that EA
significantly attenuates neurological deficits, and reduces infarct
volume and mortality in both animal models of stroke and in
patients suffering from stroke when administered at appropriate
acupoints with suitable stimulation parameters (14–19). Two specific acupoints, Quchi
(LI11) and Zusanli (ST36), are one of the most effective
prescriptions commonly used in EA treatment of ischemic stroke
(17,20). Preliminary data have demonstrated
that EA at these two acupoints significantly promotes NPC
proliferation following cerebral ischemia in the subventricular
zone (SVZ) of the lateral ventricle, and in the subgranular zone
(SGZ) of the hippocampus (17,20). A growing body of evidence suggests
that cortex-derived neural stem/progenitor cells may contribute to
the repair of ischemic lesions of the cerebral cortex (21–23). Based on these and other findings,
the elucidation of the Wnt signaling mechanisms underlying the
promoting effects of EA on NPC proliferation in the cortical
peri-infarct area after stroke, is an important step toward
validating the clinical application and benefits of this treatment
modality in the treatment of ischemic stroke.
Materials and methods
Materials and reagents
TRIzol reagent was purchased from Life Technologies
(Carlsbad, CA, USA). The RevertAid™ First Strand cDNA Synthesis kit
and Taq DNA Polymerase were purchased from Fermentas (Hanover, MD,
USA). Primary antibodies against glial fibrillary acidic protein
(GFAP, a marker for reactive astrocytes; #3670), glycogen synthase
kinase-3 (GSK3; #5676) and β-actin (#4970), and horseradish
peroxidase (HRP)-conjugated secondary antibodies (anti-mouse,
#7076; anti-rabbit, #4970) were all obtained from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Anti-microtubule-associated
protein 2 (MAP2, a marker of neurons; ab32454), anti-nestin (a
marker of progenitor cells and astrocytes; 2Q178) and
anti-β-catenin (ab22656) primary antibodies were all obtained from
Abcam (Cambridge, MA, USA). All other chemicals, unless otherwise
stated, were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animals and groups
Adult male Sprague-Dawley rats (weighing 250–280 g)
were obtained from Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). All experiments were performed strictly in
accordance with the International Ethical Guidelines and the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals.
A total of 72 rats were randomly divided into 4
groups (18 rats in each group) as follows: i) sham-operated (sham)
group; ii) MCAO group; iii) MCAO + EA group: ischemic rats treated
with EA at the Quchi (LI11) and Zusanli (ST36) acupoints; and ⅳ)
sham + EA group: sham-operated rats treated with EA.
Induction of focal cerebral ischemia
A rat model of focal cerebral ischemia/reperfusion
(I/R) was utilized in this study. The left middle cerebral artery
(MCA) was occluded by the placement of an embolus at the origin of
the MCA, as previously described (24). Following anesthetization with 10%
chloral hydrate (300 mg/kg), each rat was placed in the prone
position. A midline incision was made on the dorsal surface of the
skull, and the skull was thinned with a burr hole over the left
parietal cortex (5 mm lateral and 1 mm posterior to the bregma)
without injury to the dura mater. The laser Doppler perfusion
monitor (LDF100C; Biopac Systems, Inc., Goleta, CA, USA) was
attached to the skull with dental cement. With the rat in a supine
position, MCAO was performed via ligation of the left common
carotid artery (CCA) and external carotid artery (ECA) and closure
of the internal carotid artery (ICA). The embolus was gently
advanced within the left ICA to the origin of the MCA, until a
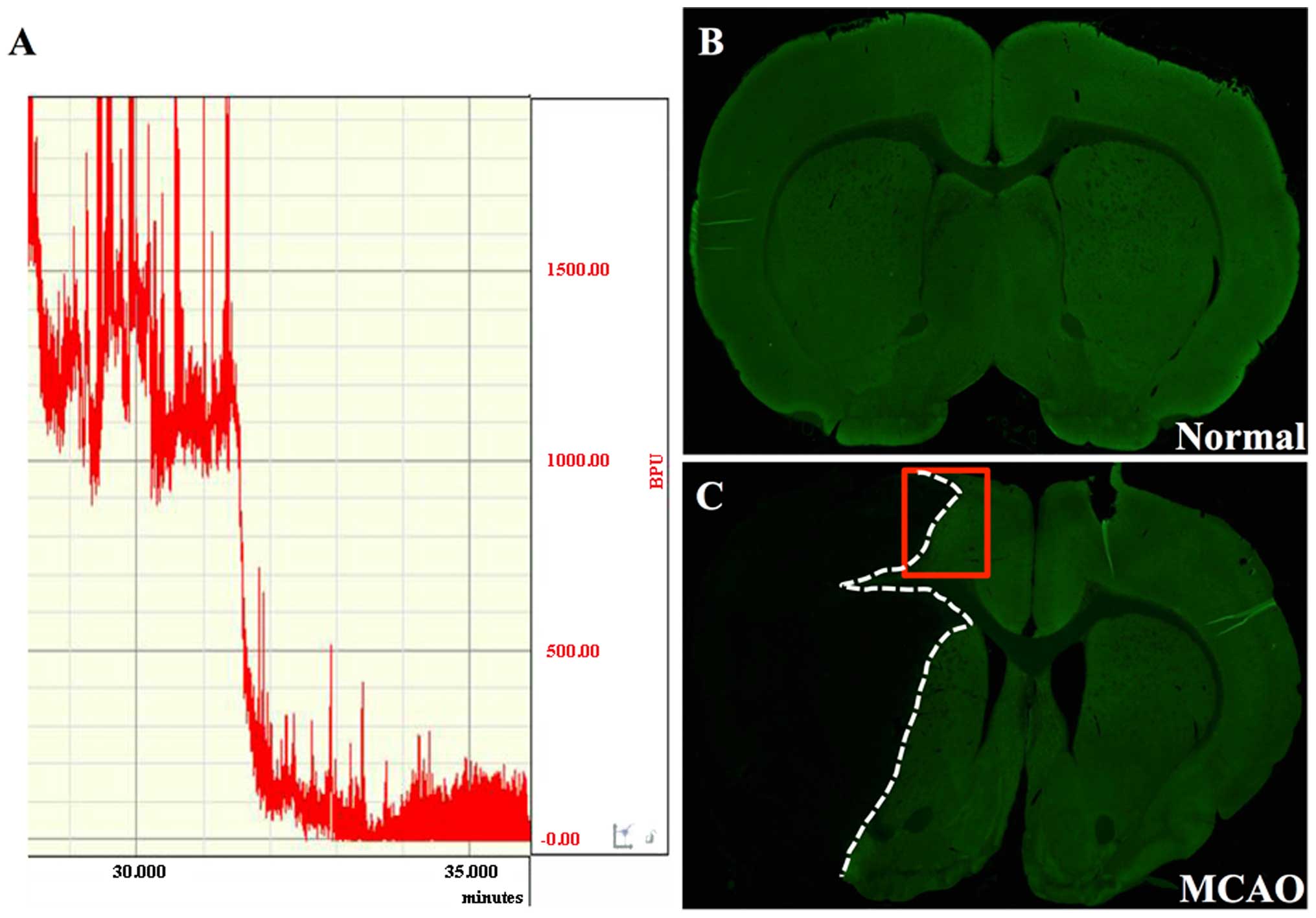
slight resistance was encountered (20±2 mm). Cerebral blood flow
was measured beginning 5 min prior to the induction of occlusion.
Ischemic rats that showed a stable drop of 80% in blood perfusion
units (BPU) compared with the baseline level (before MCAO), were
used in the subsequent experiments. Reperfusion was achieved by
removing the intraluminal occlusive embolus to restore blood supply
to the MCA area 2 h later. Animals subjected to sham operation were
treated in a similar manner, but without ligations and
occlusions.
Neurological assessment
Neurological deficits were assessed to confirm
successful MCAO. A neurological score was assigned to each animal 2
h following I/R, in a blinded manner, according to a
well-established 5-point neurological scale (24): score 0, no apparent deficits; 1,
failure to fully extend the right forepaw; 2, circling to the
right; 3, falling or leaning over to the right; 4, no spontaneous
walking and a depressed level of consciousness; and 5, dead. Rats
subjected to MCAO with neurological deficit scores of 1–3 were used
in the subsequent experiments.
Treatment wtih EA
EA was applied at the LI11 (Quchi, in the depression
lateral to the anterior aspect of the radius joint of the forelimb)
and ST36 (Zusanli, 5 mm below the head of fibula under the knee
joint and 2 mm lateral to the anterior tibial tubercle) acupoints
on the right paralyzed limb using an EA stimulation instrument
[Model G6805; Shanghai Marine Instrument General Factory (SMIF),
Shanghai, China]. Two stainless steel acupuncture needles, 0.3 mm
in diameter, connected to the output terminals of the EA
stimulation instrument, were inserted at a depth of 2–3 mm at the
LI11 and ST36 acupoints. The acupoints were stimulated with
disperse-dense waves of 1 or 20 Hz frequencies for 30 min, once a
day, and the current intensity was maintained slightly below the
level that induced visible muscle contraction. Treatment commenced
on the day following the operation and continued daily until the
animals were sacrificed.
Measurement of cerebral infarct
volume
Three days following cerebral I/R injury, the rats
were euthanized under deep anesthesia using 10% chloral hydrate and
perfused transcardiacally with 0.9% NaCl. The brains of all the
rats were rapidly removed and sliced into 5 coronal blocks at a
thickness of 2 mm per section. The fresh slices were incubated in
2% (w/v) 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich)
solution in phosphate-buffered saline (PBS; HyClone, Beijing,
China) for 20 min, at 37°C in the dark. Images of the 5 sections
were captured using a high-resolution digital camera (PowerShot
SX20 IS; Canon) and examined by a blinded observer to determine the
infarct size using computerized image analysis software (Motic Med
6.0 system; Motic China Group Co.,. Ltd., Shenzhen, China). The
infarct volume data are expressed as a percentage of the total
brain volume. Lesion volume was estimated using an indirect method
to avoid the effects of tissue swelling or shrinkage: 100×
(contralateral hemisphere volume - non-infarct ipsilateral
hemisphere volume)/contralateral hemisphere volume, as previously
described (25).
Tissue preparation
The rats were anesthetized with 10% chloral hydrate
and intracardially perfused with chilled saline followed by 0.01 M
PBS containing 4% paraformaldehyde through the left ventricular
lumen of the heart. The brains were collected and post-fixed in 4%
paraformaldehyde at 4°C overnight, and then embedded in paraffin.
Coronal sections were cut into 5-µm-thick sections, and used
for immunofluoresence staining. For western blot analysis RT-PCR,
the ischemic boundary zones were extracted from the ischemic brains
and prepared accordingly.
Immunofluorescence staining
The brain sections were processed by
immunofluorescence staining using several specific cell markers
(MAP2, neurons; GFAP, astrocytes; nestin, NPC and astrocytes).
Coronal sections (5-µm-thick) were de-paraffinized in
dimethylbenzene, hydrated successively in gradient ethanols, and
antigens were retrieved twice in 0.1 M citrate buffer (pH 6.0). The
sections were blocked in blocking buffer (10% normal goat serum,
0.3% Triton X in PBS) for 1 h at room temperature, then incubated
with primary antibodies at 4°C overnight. After washing in 0.01 M
PBS, the sections were incubated for 2 h at room temperature with a
combination of goat anti-mouse IgG H&L (FITC; ab6785; Abcam)
and goat anti-rabbit IgG H&L (TRITC; ab6718; Abcam) secondary
antibodies. The sections were stained with DAPI (Vector
Laboratories, Burlingame, CA, USA) to localize the nuclei and were
coverslipped for observation. The labelled sections were visualized
and imaged using a confocal microscope (LSM710 META NLO; Carl
Zeiss, Oberkochen, Germany).
Western blot analysis
The left cerebral tissues were dissected out and
homogenized in RIPA buffer containing Protease Inhibitor Cocktail
(Roche Applied Science, Mannheim, Germany) and PMSF. The samples
were kept on ice for 30 min and the insoluble material was removed
by centrifugation at 14,000 × g for 15 min. The protein
concentration was quantified by BCA assay (Pierce Biotechnology,
Inc., Rockford, IL, USA). Brain homogenates (50 µg) were
separated by SDS-PAGE and transferred onto PVDF membranes. The
membranes were subsequently blocked for 2 h with 5% non-fat
powdered milk in Tris-buffered saline containing 0.1% Tween-20
(TBST) and then incubated overnight at 4°C with appropriate primary
antibodies: GFAP, Wnt1 (SAB2102711; Sigma-Aldrich), GSK3, β-catenin
and β-actin (at a dilution of 1:1,000). The membranes were then
washed with TBST followed by incubation with the appropriate
HRP-conjugated secondary antibody for 1–2 h at room temperature.
Normalization of the results was ensured by running parallel
western blot analyses with β-actin antibody. The optical density
was quantified using a Bio-Image Analysis System (Bio-Rad,
Hercules, CA, USA), with the value of the sham-operated group
designated as 1.0.
RNA extraction and RT-PCR
Total RNA was extracted using TRIzol Reagent (Life
Technologies). The RNA concentrations were determined by OD260/280
readings using a GeneQuant spectrophotometer (Amersham Biosciences,
Amersham, UK). The oligo(dT)-primed RNA (3 µg) was
reverse-transcribed using the RevertAid™ First Strand cDNA
Synthesis kit (Fermentas, Chicago, IL, USA) according to the
manufacturer's instructions. Semi-quantitative PCR was performed to
measure the Wnt1, GSK3, β-catenin and β-actin mRNA expression
levels. The primer sequences used for each gene are listed in
Table I. The samples were
analyzed by gel electrophoresis (1.5% agarose). The DNA bands were
examined using a Gel Documentation System (Model Gel Doc 2000;
Bio-Rad), with the value of the sham-operated group designated as
1.0.
 | Table IPrimer sequences used for PCR. |
Table I
Primer sequences used for PCR.
| Gene names | Forward | Reverse |
|---|
| Wnt1 | 5′-CAG TGG AGC AAC
GGT ATG AG-3′ | 5′-TTC TTC CCT GCC
TTG ATG T-3′ |
| GSK3 | 5′-AGA CCA AAA TCA
TCT ACC AC-3′ | 5′-ACT CTG TGC CTG
TCT CAT-3 |
| β-catenin | 5′-CAT CCT TAT CCC
TCC TCA CGC-3′ | 5′-TTA TTG GTC TGT
CCA CGG TCT-3″ |
| β-actin | 5′-CGG GAG AAC AGG
GTA TGA-3′ | 5′-CAG GCT GGA AGG
AGA AGA T-3′ |
Cell quantification and statistical
analysis
The infarct area was defined by tissue
autofluorescence, while the peri-infarct area was defined by the
presence of MAP2-positive immunofluorescent cells. Cell
quantification in the cortical peri-infarct area was performed by
observers blinded to the sample identity using Image-Pro Plus 6.0
software. The results are expressed as the number of MAP2-positive
cells/cm2. All data were analyzed using the SPSS package
for Windows (version 16.0) and are presented as the means ±
standard error of the mean (SEM). Statistical data analysis was
performed with the unpaired Student's t-test, the Mann-Whitney U
test or ANOVA. Differences with P<0.05 were considered
statistically significant.
Results
EA alleviates neurological deficits and
reduces infarct volume after stroke
Compared with the rats in the sham-operated and sham
+ EA groups, which did not present with any signs of cerebral
injury, all rats in both the MCAO and MCAO + EA groups demonstrated
obvious manifestations of neurological deficits and cerebral
infarction (Fig. 1 and Table II). There were no statistically
significant differences observed between the MCAO and MCAO + EA
groups at 2 h after cerebral I/R injury. However, EA administered
at the Zusanli and Quchi acupoints for 3 days significantly
improved neurological deficits (MCAO, 2.08±0.23; MCAO + EA,
1.42±0.19; P<0.05) (Table
II), and decreased the cerebral infarct volume (MCAO,
35.39±1.56%; MCAO + EA, 22.39±2.50%; P<0.01) (Fig. 1), demonstrating the therapeutic
efficacy of EA against cerebral I/R injury.
 | Table IIAssessment of neurological
deficits. |
Table II
Assessment of neurological
deficits.
| Group | 2 h after I/R | 3 days after
I/R |
|---|
| Sham | 0 | 0 |
| MCAO | 2.33±0.19 | 2.08±0.23 |
| MCAO + EA | 2.42±0.19 | 1.42±0.19a |
| Sham + EA | 0 | 0 |
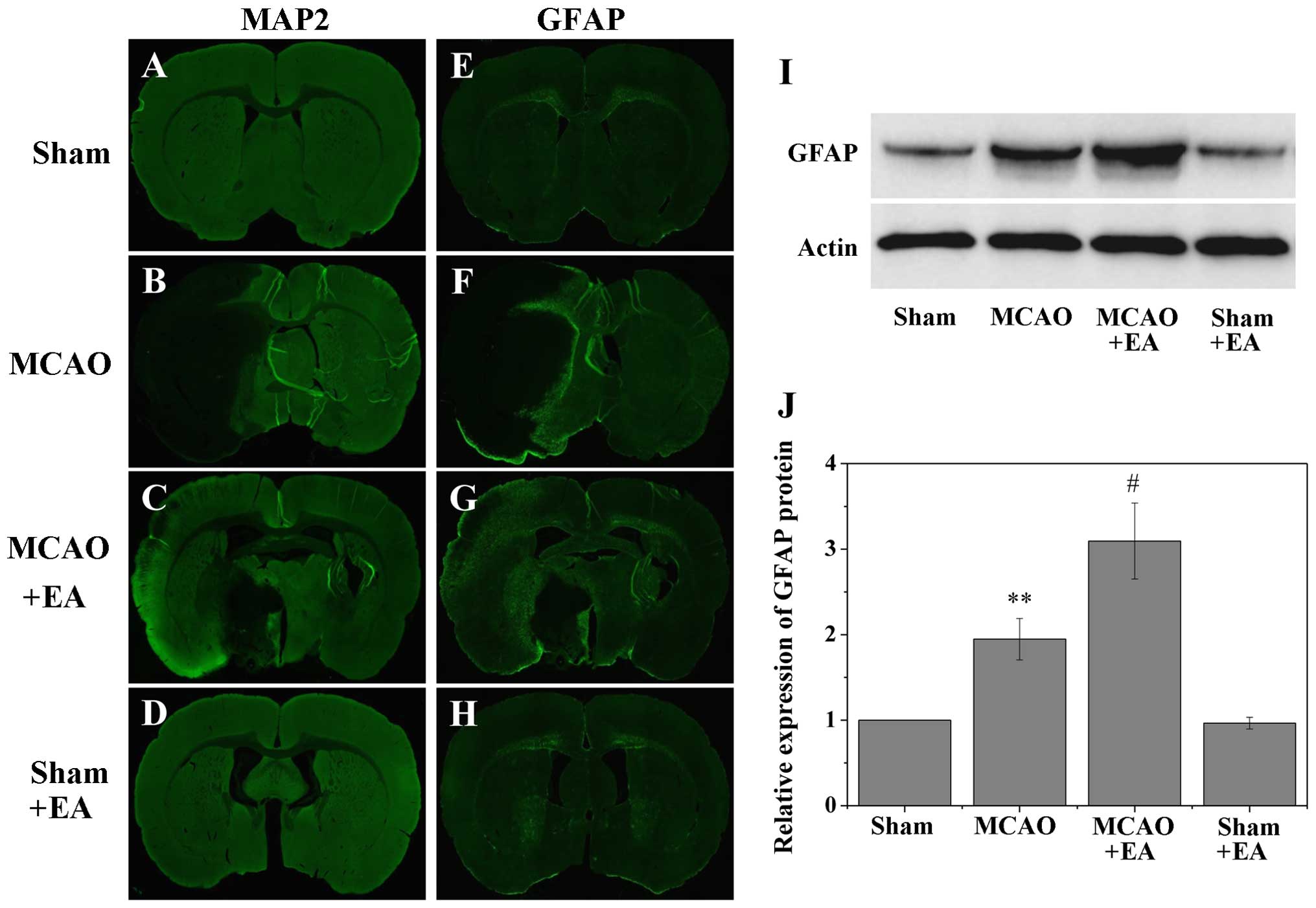
EA accelerates the proliferation of
GFAP-positive reactive astrocytes in the cortical peri-infarct area
after stroke
The border of the infarct core was defined by MAP2
staining (Fig. 2), and the
subsequent results identified a few GFAP-positive cells in the
non-ischemic cortex (Fig. 3E and
H); however, the majority of GFAP-positive cells were observed
in the post-stroke cortex, specifically in the peri-infarct area
(Fig. 3F and G). Western blot
analysis also revealed a significant increase in GFAP expression in
the MCAO and MCAO + EA groups within the post-stroke cortex,
compared with the comparable MCA area in the sham-operated (sham)
and sham + EA groups (sham, 1±0; MCAO, 1.95±0.24; MCAO + EA,
3.10±0.44; and sham + EA, 0.97±0.07; P<0.01 vs.sham and sham +
EA groups; Fig. 3I and J). In
addition, overall GFAP expression was significantly higher in the
MCAO + EA group than in the MCAO group (P<0.05), suggesting that
treatment with EA promoted the proliferation of GFAP-positive
reactive astrocytes in the cortical peri-infarct area after
stroke.
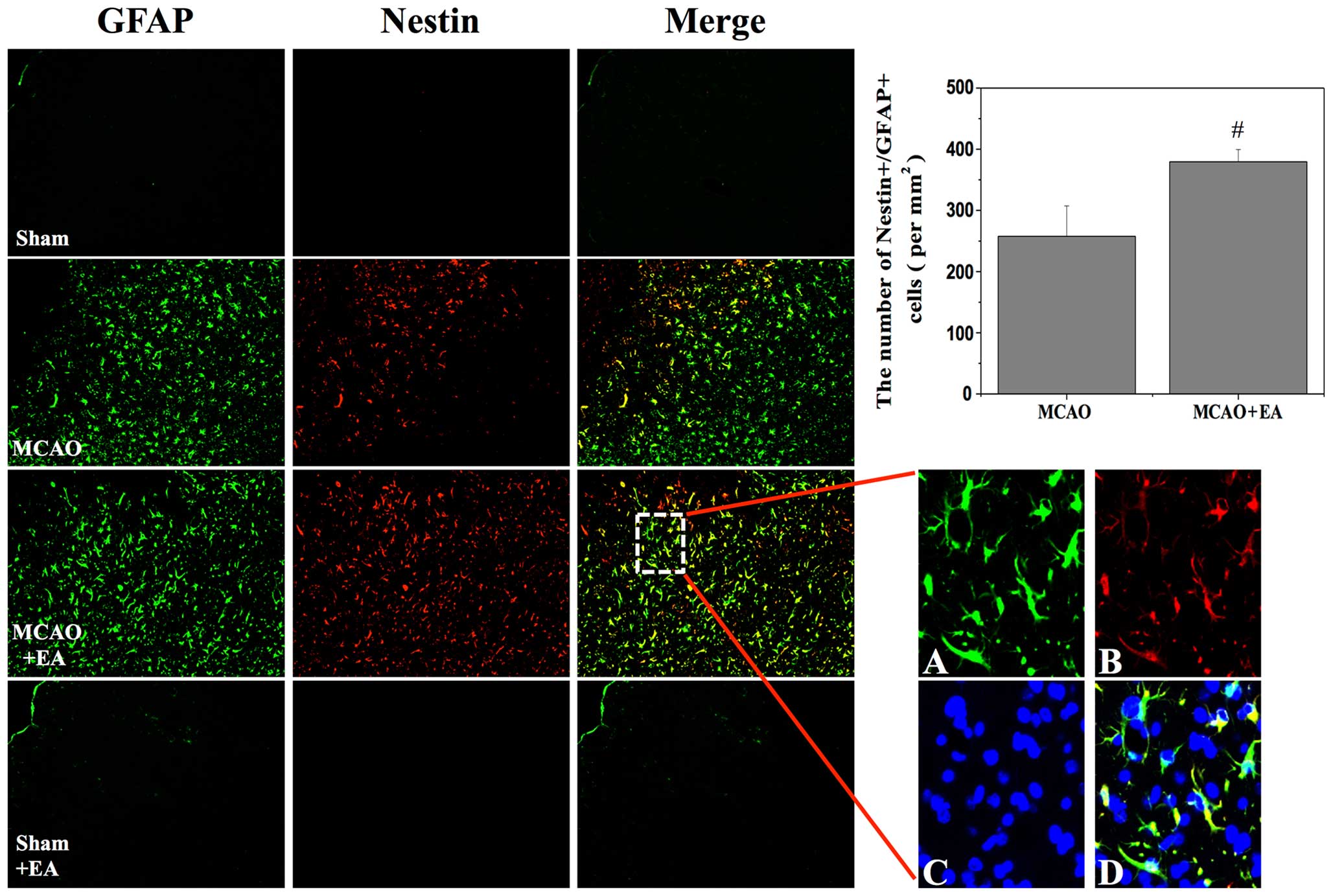
EA enhances the proliferation of NPCs in
the cortical peri-infarct area after stroke
The presence of nestin/GFAP-positive cells within
the post-stroke cortex was investigated in order to assess the
generation of injury-induced NPCs. Previous studies have reported
that nestin and GFAP-positive cells can acquire stem cell activity
in the cortical peri-infarct area after stroke (21,22). In the present study, a
nestin-positive subpopulation of NPCs formedon the ipsilateral, but
not the contralateral side of the brain after stroke. At 3 days
after stroke, the number of nestin-positive cells was significantly
higher in the MCAO + EA group than in the MCAO group. Similarly,
the number of nestin/GFAP-positive cells was significantly higher
in the MCAO + EA group (MCAO, 257.72±49.73; MCAO + EA,
379.56±20.05; P<0.05; Fig. 4),
demonstrating that EA potentially increased the proliferation of
the NPCs in the cortical peri-infarct area after stroke.
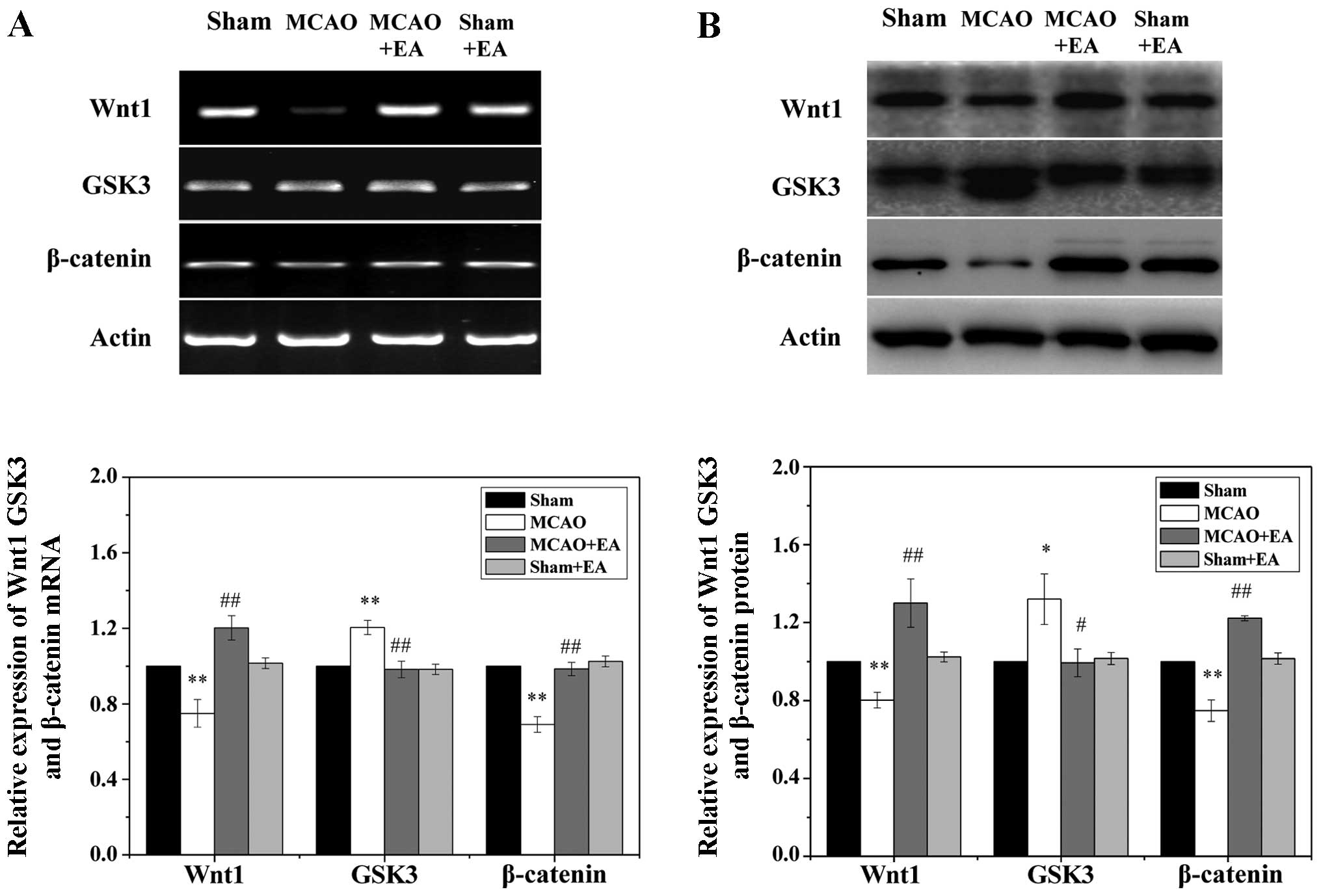
EA regulates the activation of the Wnt
pathway in the cortical peri-infarct area after stroke
To examine the effects of EA on the Wnt signaling
pathway, we measured the protein and mRNA levels of Wnt1, GSK3 and
β-catenin in the ischemic cortex by western blot analysis and
RT-PCR. As shown in Fig. 5, focal
cerebral I/R injury significantly reduced the expression of Wnt1
and β-catenin, while the transcription of GSK3 was significantly
increased in the ischemic cortex at 3 days following stroke when
compared to the sham and sham + EA groups (P<0.05). Of note, the
decrease in the expression of Wnt1 and β-catenin was circumvented
by treatment with EA, and the upregulated transcription of GSK3 was
significantly reduced following treatment with EA (P<0.05).
Taken together, these results suggest that EA applied at the Quchi
and Zusanli acupoints significantly promotes the activation of the
Wnt signaling pathway in the peri-infarct cortex.
Discussion
In response to stroke, subpopulations of cortical
reactive astrocytes proliferate and express several proteins
commonly associated with neural stem/progenitor cells, such as
GFAP, nestin and RC2 (21–23).
Shimada et al (21)
demonstrated that GFAP-expressing reactive astrocytes can be
isolated from the cortical peri-infarct area 3 days after stroke,
and de-differentiated into reactive astrocyte-derived neural
stem/progenitor cells with self-renewal and multipotent properties
when grown under neurosphere conditions. Lineage tracing identified
reactive astrocytes as a cell of origin for neural stem cells
(NSCs) derived from cortical peri-infarct tissues after stroke
(21).
In this study, in order to investigate the effects
of EA on NPC proliferation via Wnt signaling, we treated
sham-operated and rats subjected to MCAO with electric stimulation
at the Quchi (LI11) and Zusanli (ST36) acupoints on the
contralateral paralyzed limb. Our results revealed that EA applied
at these acupoints 1 day following cerebral I/R injury and once
daily for 3 consecutive days, significantly improved neurological
function (MCAO, 2.08±0.23; MCAO + EA, 1.42±0.19; P<0.05) and
attenuated the increase in the cerebral infarct volume (MCAO,
35.39±1.56%; MCAO + EA, 22.39±2.50%; P<0.01) induced by MCAO.
Our findings corroborate those of previous studies that used a
model of transient focal cerebral ischemia to demonstrate the
therapeutic efficacy of EA (14–17). Furthermore, immunofluorescence
staining was performed to observe several markers associated with
the activation of NPCs. Our results revealed that the number of
proliferating nestin/GFAP-positive NPCs was significantly increased
within the post-stroke cortex in the MCAO group (257.72±49.73)
(Fig. 4) but even more so in the
MCAO + EA group (379.56±20.05, P<0.05 vs. MCAO group),
suggesting that EA promoted the proliferation of neural
stem/progenitor cells in rats subjected to MCAO. The emergence of
NPCs in the peri-infarct area is a documented event in the brain
after stroke (21–23). However, the signaling pathway
controlling the proliferation of these NPCs is poorly defined.
Of note, previous studies have indicated that
Wnt/β-catenin signaling is critically involved in the regulation of
the proliferation and differentiation of NPCs (3,8,9).
The Wnt/β-catenin pathway is activated when a Wnt ligand binds to
its seven-trans-membrane receptors, the Frizzled proteins. The
activation of the Wnt pathway inhibits GSK-3β, which results in the
cytoplasmic accumulation of β-catenin. Stabilized β-catenin then
translocates into the nucleus and interacts with the transcription
factors TCF/Lef to activate downstream genes such as cyclin D1 and
c-myc (26,27).
Cerebral ischemia profoundly reduced the
transcription of Wnt1 and β-catenin and increased the expression of
GSK3. Treatment with EA reversed these effects (Fig. 5). Moreover, this is consistent
with the results of previous reports that the Wnt pathway is
markedly degraded after stroke (3–5).
In a previous study, when assessed at 3 days following an
endothelin-1 (Et-1) injection, treatment with lithium ions
prevented the decrease in the expression of β-catenin in the
ischemic cortex (5). In this
study, the treatment of NPCs with EA significantly increased the
expression of Wnt1 and β-catenin, while inhibiting the
transcription of GSK3. These data indicate that EA applied at the
Quchi (LI11) and Zusanli (ST36) acupoints promoted the
proliferation of NPCs in the cortical peri-infarct area via the
Wnt/β-catenin pathway.
In conclusion, the results of the present study
strongly suggest that treatment with EA provides robust protection
against transient cerebral ischemic injury and promotes the
proliferation of neural stem/progenitor cells in response to
isch-emia via the Wnt/β-catenin pathway. Our data are supported by
evidence in the current literature. These results may provide a
theoretical and experimental basis for the future clinical
application of EA and its potential use in the treatment of
cerebral ischemia.
However, even with optimal stimulation parameters,
treatment with EA targets multiple mechanisms in order to achieve
its protective effects against ischemic insults. Therefore, the
precise mechanisms of action associated with this treatment the
reparative process in the post-ischemic brain requires further
investigation.
Acknowledgments
This study was sponsored by the National Natural
Science Foundation of China (grant nos. 81273835 and 81373778). We
would like to thank Clarity Manuscript Consultants, LLC, for their
assistance in the editing of this manuscript.
Abbreviations:
|
EA
|
electro-acupuncture
|
|
NPCs
|
neural progenitor cells
|
|
MCAO
|
middle cerebral artery occlusion
|
|
SVZ
|
subventricular zone
|
|
SGZ
|
subgranular zone
|
|
TTC
|
2,3,5-triphenyltetrazolium
chloride
|
|
GFAP
|
glial fibrillary acidic protein
|
|
MAP2
|
microtubule-associated protein 2
|
|
CCA
|
common carotid artery
|
|
ECA
|
external carotid artery
|
|
ICA
|
internal carotid artery
|
References
|
1
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roger VL, Go AS, Lloyd-Jones DM, Benjamin
EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al
American Heart Association: Statistics Committee and Stroke
Statistics Subcommittee: Heart disease and stroke statistics - 2012
update: a report from the American Heart Association. Circulation.
125. pp. e2–e220. 2012, View Article : Google Scholar
|
|
3
|
Hirabayashi Y, Itoh Y, Tabata H, Nakajima
K, Akiyama T, Masuyama N and Gotoh Y: The Wnt/beta-catenin pathway
directs neuronal differentiation of cortical neural precursor
cells. Development. 131:2791–2801. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H, Ren C, Gao X, Takahashi T,
Sapolsky RM, Steinberg GK and Zhao H: Hypothermia blocks
beta-catenin degradation after focal ischemia in rats. Brain Res.
1198:182–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mastroiacovo F, Busceti CL, Biagioni F,
Moyanova SG, Meisler MH, Battaglia G, Caricasole A, Bruno V and
Nicoletti F: Induction of the Wnt antagonist, Dickkopf-1,
contributes to the development of neuronal death in models of brain
focal ischemia. J Cereb Blood Flow Metab. 29:264–276. 2009.
View Article : Google Scholar
|
|
6
|
Scott EL and Brann DW: Estrogen regulation
of Dkk1 and Wnt/β-Catenin signaling in neurodegenerative disease.
Brain Res. 1514:63–74. 2013. View Article : Google Scholar :
|
|
7
|
Sun FL, Wang W, Zuo W, Xue JL, Xu JD, Ai
HX, Zhang L, Wang XM and Ji XM: Promoting neurogenesis via
Wnt/β-catenin signaling pathway accounts for the neurorestorative
effects of morroniside against cerebral ischemia injury. Eur J
Pharmacol. 738:214–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pöschl J, Grammel D, Dorostkar MM,
Kretzschmar HA and Schüller U: Constitutive activation of β-catenin
in neural progenitors results in disrupted proliferation and
migration of neurons within the central nervous system. Dev Biol.
374:319–332. 2013. View Article : Google Scholar
|
|
9
|
Lie DC, Colamarino SA, Song HJ, Désiré L,
Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR
and Gage FH: Wnt signalling regulates adult hippocampal
neurogenesis. Nature. 437:1370–1375. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhai XF, Chen Z, Li B, Shen F, Fan J, Zhou
WP, Yang YK, Xu J, Qin X, Li LQ and Ling CQ: Traditional herbal
medicine in preventing recurrence after resection of small
hepatocellular carcinoma: a multicenter randomized controlled
trial. J Integr Med. 11:90–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang J, Li F, Wei C, Song H, Wu L, Tang Y
and Jia J: Rationale and design of a multicenter, phase 2 clinical
trial to investigate the efficacy of traditional Chinese medicine
SaiLuoTong in vascular dementia. J Stroke Cerebrovasc Dis.
23:2626–2634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang LN, Wang Y, Lu Y, Yin ZF, Zhang YH,
Aslanidi GV, Srivastava A, Ling CQ and Ling C: Pristimerin enhances
recombinant adeno-associated virus vector-mediated transgene
expression in human cell lines in vitro and murine hepatocytes in
vivo. J Integr Med. 12:20–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ling CQ, Wang LN, Wang Y, Zhang YH, Yin
ZF, Wang M and Ling C: The roles of traditional Chinese medicine in
gene therapy. J Integr Med. 12:67–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Shen X, Tang H, Li J, Xiang T and
Yu W: Using microPET imaging in quantitative verification of the
acupuncture effect in ischemia stroke treatment. Sci Rep.
3:10702013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JH, Choi KH, Jang YJ, Bae SS, Shin BC,
Choi BT and Shin HK: Electroacupuncture acutely improves cerebral
blood flow and attenuates moderate ischemic injury via an
endothelial mechanism in mice. PLoS One. 8:e567362013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin Z, Liang J, Wang J and Kolattukudy PE:
Delayed brain ischemia tolerance induced by electroacupuncture
pretreatment is mediated via MCP-induced protein 1. J
Neuroinflammation. 10:632013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tao J, Xue XH, Chen LD, Yang SL, Jiang M,
Gao YL and Wang XB: Electroacupuncture improves neurological
deficits and enhances proliferation and differentiation of
endogenous nerve stem cells in rats with focal cerebral ischemia.
Neurol Res. 32:198–204. 2010. View Article : Google Scholar
|
|
18
|
Mazighi M, Meseguer E, Labreuche J and
Amarenco P: Bridging therapy in acute ischemic stroke: a systematic
review and meta-analysis. Stroke. 43:1302–1308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu P, Mills E, Moher D and Seely D:
Acupuncture in post-stroke rehabilitation: a systematic review and
meta-analysis of randomized trials. Stroke. 41:e171–e179. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tao J, Chen B, Gao Y, Yang S, Huang J,
Jiang X, Wu Y, Peng J, Hong Z and Chen L: Electroacupuncture
enhances hippocampal NSCs proliferation in cerebral
ischemia-reperfusion injured rats via activation of notch signaling
pathway. Int J Neurosci. 124:204–212. 2014. View Article : Google Scholar
|
|
21
|
Shimada IS, LeComte MD, Granger JC,
Quinlan NJ and Spees JL: Self-renewal and differentiation of
reactive astrocyte-derived neural stem/progenitor cells isolated
from the cortical peri-infarct area after stroke. J Neurosci.
32:7926–7940. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakagomi T, Taguchi A, Fujimori Y, Saino
O, Nakano-Doi A, Kubo S, Gotoh A, Soma T, Yoshikawa H, Nishizaki T,
et al: Isolation and characterization of neural stem/progenitor
cells from post-stroke cerebral cortex in mice. Eur J Neurosci.
29:1842–1852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakagomi T, Molnár Z, Nakano-Doi A,
Taguchi A, Saino O, Kubo S, Clausen M, Yoshikawa H, Nakagomi N and
Matsuyama T: Ischemia-induced neural stem/progenitor cells in the
pia mater following cortical infarction. Stem Cells Dev.
20:2037–2051. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Swanson RA, Morton MT, Tsao-Wu G, Savalos
RA, Davidson C and Sharp FR: A semiautomated method for measuring
brain infarct volume. J Cereb Blood Flow Metab. 10:290–293. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A: The cyclin D1
gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad
Sci USA. 96:5522–5527. 1999. View Article : Google Scholar : PubMed/NCBI
|