Introduction
Allergic diseases, such as allergic asthma, rhinitis
and atopic dermatitis are a major health concern in the modern
world, and are often caused by environmental pollution (1). Allergies develop from complex
interactions between genes and the environment. In developed
countries, the prevalence and risk of allergic disorders have both
steadily increased for decades (2). Mast cells are known to be closely
associated with immediate-type hypersensitivity through the release
of allergic mediators and cytokines following activation by FcεRI.
Allergen cross-linking with specific immunoglobulin E (IgE) bound
to FcεRI triggers mast cell activation, inducing the rapid
secretion of preformed allergic mediators and de novo
synthesized mediators, such as histamine, cytokines, proteases and
derivatives of arachidonic acid (3). Histamine, one of the major allergic
mediators, plays a key role in various physiological and
pathological responses, particularly allergic reactions (4).
Calcium, which acts as a secondary messenger in mast
cells, is associated with the increasing degranulation of mast
cells (3). Signal transducing
enzymes, including phospholipase C and phosphoinositide 3-kinase,
stimulate calcium influx following the activation of FcεRI
(5). Activated mast cells can
produce histamine, as well as a wide variety of inflammatory
mediators, such as proteoglycans, eicosanoids, proteases,
transforming growth factor-β, chemokines and cytokines, such as
tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-4 and
IL-13 (3,6). The activation of mitogen-activated
protein kinases (MAPKs) and nuclear factor (NF)-κB is also
accompanied by the binding of allergens with IgE. MAPKs and NF-κB
are important mediators of cellular responses from extracellular
signals and are thought to regulate the expression of
pro-inflammatory molecules, particularly TNF-α, IL-6 and IL-8
(7–9).
Pogostemon cablin (Blanco) Benth (P.
cablin) is a widely used traditional medicine in Korea. P.
cablin contains various biologically active compounds, such as
patchouli alcohol, pogostone, eugenol, α-bulnesene, patchoulene and
rosmarinic acid (10,11). The pharmacological activity of
P. cablin is due to essential oils, which constitute
approximately 1.5% of P. cablin and patchouli alcohol, which
constitutes >50% of P. cablin. The anti-fungal,
anticancer and anti-emetic properties of P. cablin have been
demonstrated in a number of studies over the years (12–16). In Korea, the aqueous extract of
P. cablin (AEPC) has long been used in the treatment of
gastrointestinal disorders, such as indigestion, vomiting and
diarrhea (17). Although various
studies have been published on the biological effects of P.
cablin (12–16), to the best of our knowledge, there
is no study available to date on the anti-allergic and inflammatory
effects of P. cablin. Thus, the aim of the present study was
to examine the inhibitory effects of AEPC on allergic inflammation
and to define the underlying mechanisms of these effects using
animal models and mast cells.
Materials and methods
Animals
Imprinting control region (ICR) mice (n=150, male,
aged 6 weeks) and Sprague-Dawley (SD) rats (n=10, male, aged 10
weeks) were purchased from Dae Han Biolink (Daejeon, Korea). The
animals were housed 5 per cage in a laminar air flow room
maintained at a temperature of 22±2°C and a relative humidity of
55±5% throughout the study. The care and treatment of the mice and
rats were in accordance with the guidelines established by the
Public Health Service Policy on the Humane Care and Use of
Laboratory Animals and were approved by the Institutional Animal
Care and Use Committee of Kyungpook National University (Daegu,
Korea).
Reagents and cell culture
Compound 48/80, anti-dinitrophenol (DNP) IgE,
DNP-human serum albumin (HSA), pyrrolidine dithiocarbamate (PDTC),
phorbol 12-myristate 13-acetate (PMA), calcium ionophore A23187,
and o-phthaldialdehyde (OPA) were all purchased from Sigma
Aldrich (St. Louis, MO, USA). Forward and reverse primers for human
TNF-α, IL-6 and IL-8 were purchased from Genotech (Daejeon, Korea)
and ELISA kits were purchased from BD Biosciences (San Diego, CA,
USA). Human mast cells (HMC-1; kind gift from Professor D.K. Kim,
Department of Immunology, School of Medicine, Chonbuk National
University, Jeonju, Korea) and rat peritoneal mast cells (RPMCs)
were grown in Iscove's modified Dulbecco's medium (IMDM) and
α-minimum essential medium (Gibco, Grand Island, NY, USA),
respectively, supplemented with 10% heat-inactivated fetal bovine
serum (HyClone, Logan, UT, USA), 100 U/ml penicillin G (HyClone),
100 µg/ml streptomycin (HyClone) and 250 ng/ml amphotericin
B (HyClone) at 37°C in 5% CO2. HMC-1 cells at passages
4–8 were used throughout the study.
Preparation of RPMCs
The peritoneal cells were isolated from SD rats as
previously described (18). In
brief, the rats were anesthetized with CO2 and injected
with 20 ml of Tyrode's buffer A (10 mM HEPES, 130 mM NaCl, 5 mM
KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose
and 0.1% bovine serum albumin) into the peritoneal cavity, and the
abdomen was gently massaged for approximately 90 sec. The
peritoneal cavity was carefully opened, and the fluid containing
peritoneal cells was aspirated using a Pasteur pipette. The
peritoneal cells were sedimented at 150 g for 10 min at room
temperature and resuspended in Tyrode's buffer A. Mast cells were
separated from the major components of rat peritoneal cells, i.e.,
macrophages and small lymphocytes. Peritoneal cells were suspended
in 1 ml of Tyrode buffer A, layered on 2 ml of Histodenz
(Sigma-Aldrich) solution, and centrifuged at 400 × g for 10 min at
4°C. The cells remaining at the buffer-Histodenz interface were
aspirated and discarded, and the cells in the pellet were washed
and resuspended. Mast cell preparations were approximately 95% pure
based on toluidine blue staining. More than 95% of the cells were
viable based on trypan blue exclusion (data not shown).
Preparation of AEPC
P. cablin used in the present study was
purchased from the oriental drug store, Bohwa Dang (Jeonju, Korea)
and identified by Dr D.K. Kim at the College of Pharmacy, Woosuk
University (Jeonju, Korea). A voucher specimen was deposited at the
Herbarium of the College of Pharmacy, Woosuk University. The sample
was extracted with purified water at 70°C for 5 h (2 times) in a
water bath. The extract was then filtered, lyophilized and stored
at 4°C. The yield of dried extract from the starting crude
materials was approximately 15.3%. The dried extract of AEPC was
dissolved in saline or Tyrode's buffer A prior to use.
Compound 48/80-induced systemic
anaphylaxis
The mice (n=10/group) were administered an
intraperitoneal injection of 8 mg/kg body weight (BW) of the mast
cell degranulation compound 48/80, as previously described
(18). The mice were divided into
the following groups: group A, compound 48/80 + saline; group B,
compound 48/80 + AEPC 10 mg/kg; group C, compound 48/80 + AEPC 50
mg/kg; group D, compound 48/80 + AEPC 100 mg/kg; group E, compound
48/80 + AEPC 500 mg/kg; group F, compound 48/80 + AEPC 1,000 mg/kg;
group G, AEPC 1,000 mg/kg only. AEPC was intraperitoneally
administered at doses of 10–1,000 mg/kg BW 1 h prior to the
injection of compound 48/80. For the time-dependent experiments,
AEPC (1,000 mg/kg) was intra- peritoneally administered at 5, 10,
20 and 30 min after the injection of compound 48/80 (n=10/group).
Mortality was monitored for 1 h after the induction of anaphylactic
shock.
IgE-mediated passive cutaneous
anaphylaxis (PCA)
IgE-mediated PCA was induced as previously described
(19). To induce the PCA
reaction, the skin on the ears of the mice (n=5/group) was
sensitized with an intradermal injection of anti-DNP IgE (0.5
µg/site) for 48 h. The mice were divided into the following
groups: group A, saline only; group B, IgE/DNP-HSA; group C,
IgE/DNP-HSA + AEPC 1 mg/kg, group D: IgE/DNP-HSA + AEPC 10 mg/kg,
group E: IgE/DNP-HSA + AEPC 100 mg/kg, group F: IgE/DNP-HSA + AEPC
1,000 mg/kg AEPC was intraperitoneally administered at doses of
1–1,000 mg/kg BW 1 h prior to the intravenous injection of DNP-HSA
(1 mg/mouse) and 4% Evans blue (1:1) mixture. Thirty minutes after
the challenge, the mice were euthanized using carbon dioxide, and
the skin of both ears was collected for measurements with pigment
dye. The amount of dye was determined colorimetrically following
extraction with a mixture of 1 ml of 1 M KOH and 9 ml of acetone
and phosphoric acid (5:13). The absorbance intensity of the extract
was detected using a spectrophotometer (Molecular Devices,
Sunnyvale, CA, USA) at 620 nm.
Histamine and β-hexosaminidase assay
To assess mast cell degranulation, the release of
histamine from mast cells was detected using the
o-phthaldialdehyde spectrofluorometric procedure and a
fluorescent plate reader (Molecular Devices) at an excitation
wavelength of 360 nm and an emission wavelength of 440 nm, as
previously described, and the level of β-hexosaminidase was read
using the spectrophotometer at 405 nm, as previously described
(20,21). The RPMCs (2×104
cells/well in 24-well plates) were pre-treated with or without AEPC
for 30 min and then stimulated with compound 48/80 (5 µg/ml)
for 10 min. The cells were separated from the released histamine by
centrifugation at 400 × g for 5 min at 4°C. β-hexosaminidase
substrate buffer (100 mM sodium citrate, 1 mM 4-nitrophenyl
N-acetyl-β-D-galactosaminide, pH 4.5) was added following by
incubation for 1 h at 37°C. The reaction was terminated using stop
solution (0.1 M Na2CO3 and NaHCO3;
Sigma Aldrich).
Measurement of intracellular calcium
levels
Intracellular calcium levels were measured with the
use of the fluorescence indicator, Fluo-3/AM (Invitrogen, Carlsbad,
CA, USA), as previously described (20). The RPMCs (1×104
cells/well in 96-well plates) were pre-incubated with Fluo-3/AM for
1 h at 37°C. After washing the dye from the cell surface with
Tyrode's buffer B (137 mM NaCl, 5.5 mM glucose, 12 mM
NaHCO3, 2.7 mM KCl, 0.2 mM
NaH2PO4, 1 mM MgCl2 and 1.8 mM
CaCl2), the cells were pre-treated with or without AEPC
for 30 min and then stimulated with compound 48/80 (5
µg/ml). The fluorescence intensity was detected using a
fluorescent plate reader at an excitation wavelength of 485 nm and
an emission wavelength of 520 nm. The intracellular calcium level
was calculated using relative absorbance as the control value of
1.
Cell viability
Cell viability was assayed using an XTT assay kit
(Welgene Inc., Seoul, Korea) according to the manufacturer's
instructions and as previously described (22). The HMC-1 cells (1×105
cells/well in 96-well plates) were pre-treated with various
concentrations of AEPC (1–1,000 µg/ml) for 24 h and
incubated with XTT plus phenazine methosulfate reagent for 2 h at
37°C. The absorbance intensity was detected using a
spectrophotometer at 450 nm. Cell viability was calculated using
relative absorbance as the control value of 100%.
RNA extraction and
reverse-transcription-quantitative polymerase chain reaction
(RT-qPCR)
Prior to the isolation of total cellular RNA, the
HMC-1 cells (1×106 cells/well in 24-well plates) were
pre-treated with or without AEPC for 30 min and stimulated with PMA
(40 nM) plus calcium ionophore A23187 (1 µM) (PMACI) for 2
h. RNAiso Plus reagent (Takara Bio Inc., Shiga, Japan) was used to
extract the RNA, according to the manufacturer's instructions.
Complementary DNA (cDNA) was synthesized from 2 µg of total
RNA using the Maxime RT PreMix kit (iNtRON Biotechnology, Daejeon,
Korea). To measure the expression levels of TNF-α, IL-6 and IL-8,
RT-qPCR was carried out using the Maxime PCR PreMix kit (iNtRON
Biotechnology) and the Thermal Cycler Dice TP850 (Takara Bio)
according to the manufacturer's instructions. For RT-PCR, the total
reaction mixture (20 µl) was composed of the following: 1
µl of cDNA (100 ng), 1 µl of each of forward and
reverse primers (0.4 µM) and 17 µl of
dH2O. For quantitative (real-time) PCR (qPCR), the total
reaction mixture (25 µl) was composed of the following: 1.5
µl of cDNA (200 ng), 1 µl of each of forward and
reverse primers (0.4 µM), 12.5 µl of SYBR Premix Ex
Taq (Takara Bio) and 9 µl of dH2O. The primer
sequences used were as follows: TNF-α forward, 5′-CCT ACC AGA CCA
AGG TCA AC-3′ and reverse, 5′-AGG GGG TAA TAA AGG GAT TG-3′; IL-6
forward, 5′-AAA GAG GCA CTG GCA GAA AA-3′ and reverse, 5′-ATC TGA
GGT GCC CAT GCT AC-3′; IL-8 forward, 5′-ACA GCA GAG CAC ACA AGC
TT-3′ and reverse, 5′-CTG GCA ACC CTA CAA CAG AC-3′; β-actin
forward, 5′-GGA CTT CGA GCA AGA GAT GG-3′ and reverse, 5′-AGC ACT
GTG TTG GCG TAC AG-3′. The conditions for the qPCR steps were
similar to those described in a previous study (22).
Enzyme-linked immunosorbent assay
(ELISA)
The secretion of pro-inflammatory cytokines was
measured by ELISA, as previously described (8). The HMC-1 cells (1×106
cells/well in 24-well plates) were pre-treated with or without AEPC
for 30 min and stimulated with PMACI for 8 h. ELISA was performed
using an ELISA kit (BD Biosciences) on a 96-well Nunc-Immuno plate
(Thermo Fisher Scientific, Waltham, MA, USA) according to the
manufacturer's instructions. After terminating the reaction with a
substrate, the absorbance intensity was detected using a
spectrophotometer at 450 nm.
Protein extraction and western blot
analysis
Nuclear and cytosolic proteins were both extracted
as previously described (23).
Prior to protein extraction, the HMC-1 cells (2×106
cells/well in 6-well plates) were pre-treated with or without AEPC
for 30 min and stimulated with PMACI for 2 h. Followikng suspension
in 100 µl of cell lysis buffer A (0.5% Triton X-100, 150 mM
NaCl, 10 mM HEPES, 1 mM EDTA/Na3VO4, 0.5 mM
PMSF/DTT and 5 µg/ml leupeptin/aprotinin), the cells were
vortexed, incubated for 5 min on ice and centrifuged at 400 × g for
5 min at 4°C, and the supernatant was then gathered as the
cytosolic protein extract. The pellets were washed 3 times with 1
ml of PBS and suspended in 25 µl of cell lysis buffer B (25%
glycerol, 420 mM NaCl, 20 mM HEPES, 1.2 mM MgCl2, 0.2 mM
EDTA, 1 mM Na3VO4, 0.5 mM PMSF/DTT and 5
µg/ml leupeptin/aprotinin), vortexed, sonicated for 30 sec,
incubated for 20 min on ice and centrifuged at 15,000 × g for 15
min at 4°C, and the supernatant was then gathered as the nuclear
protein extract. Samples of protein were electrophoresed using
8–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred onto nitrocellulose membranes. NF-κB, IκBα, actin and
p38 MAPK were assayed using the following antibodies purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-NF-κB
(sc-109), anti-IκBα (sc-371), anti-actin (sc-8432; mouse
monoclonal; 1:1,000), and Cell Signaling Technology Inc. (Danvers,
MA, USA); anti-phospho-p38 (#9211) and anti-p38 (#9212).
Immunodetection was carried out using a chemiluminescent substrate
(Thermo Fisher Scientific).
Transient transfection and luciferase
activity assay
The HMC-1 cells (2×106 cells/well in
6-well plates) were seeded in serum/antibiotics-free IMDM 1 day
prior to transient transfection. The expression vectors containing
the NF-κB luciferase reporter construct (pNF-κB-LUC, plasmid
containing the NF-κB binding site; Stantagen, Grand Island, NY,
USA) or empty vectors were transfected using 8 µl of
Lipofectamine 2000 reagent (Invitrogen). Following incubation for 5
h, the medium was replaced with IMDM containing 10% FBS and
antibiotics. The cells were allowed to recover at 37°C for 20 h and
subsequently were stimulated as indicated. The cell lysate was
prepared and assayed for luciferase activity using a Luciferase
assay system (Promega, Madison, WI, USA) according to the
manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using Prism5
(GraphPad Software, San Diego, CA, USA), and the effects of
treatment were analyzed using one-way ANOVA followed by Dunnett's
test. A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Effects of AEPC on systemic and cutaneous
anaphylaxis
To determine the effectS of AEPC on allergic
reaction in vivo, compound 48/80-induced systemic
anaphylaxis and IgE-mediated PCA were induced in mice for
immediate-type hypersensitivity. The intraperitoneal injection of
compound 48/80 (8 mg/kg BW), a mast cell degranulator, induces
lethal systemic anaphylaxis (18). The mice (n=10/group) were
intraperitoneally administered 200 µl saline or various
doses of AEPC (10–1,000 mg/kg BW) 1 h prior to the intraperitoneal
injection of compound 48/80. Following the administration of
compound 48/80, we monitored the mice for 1 h and the mortality
rate was recorded. All mice injected with compound 48/80 and saline
suffered fatal anaphylactic shock, whereas the mortality rate was
reduced in the mice administered AEPC in a dose-dependent manner
(Table I). In addition, the
mortality rate of the mice administered AEPC (1,000 mg/kg BW) at 5,
10, 20 and 30 min after the injection of compound 48/80 was
increased in a time-dependent manner (Table II).
 | Table IDose-dependent effects of AEPC on
compound 48/80- induced systemic anaphylaxis. |
Table I
Dose-dependent effects of AEPC on
compound 48/80- induced systemic anaphylaxis.
| AEPC treatment
(mg/kg BW) | Compound 48/80 (8
mg/kg BW) | Mortality rate
(%) |
|---|
| None (saline) | + | 100 |
| 10 | + | 100 |
| 50 | + | 80 |
| 100 | + | 60 |
| 500 | + | 20 |
| 1,000 | + | 0 |
| 1,000 | – | 0 |
 | Table IITime-dependent effects of AEPC on
compound 48/80- induced systemic anaphylaxis. |
Table II
Time-dependent effects of AEPC on
compound 48/80- induced systemic anaphylaxis.
| AEPC treatment
(mg/kg) | Time (min) | Compound 48/80 (8
mg/kg BW) | Mortality rate
(%) |
|---|
| None (saline) | 0 | + | 100 |
| 1,000 | 5 | + | 0 |
| 1,000 | 10 | + | 40 |
| 1,000 | 20 | + | 80 |
| 1,000 | 30 | + | 100 |
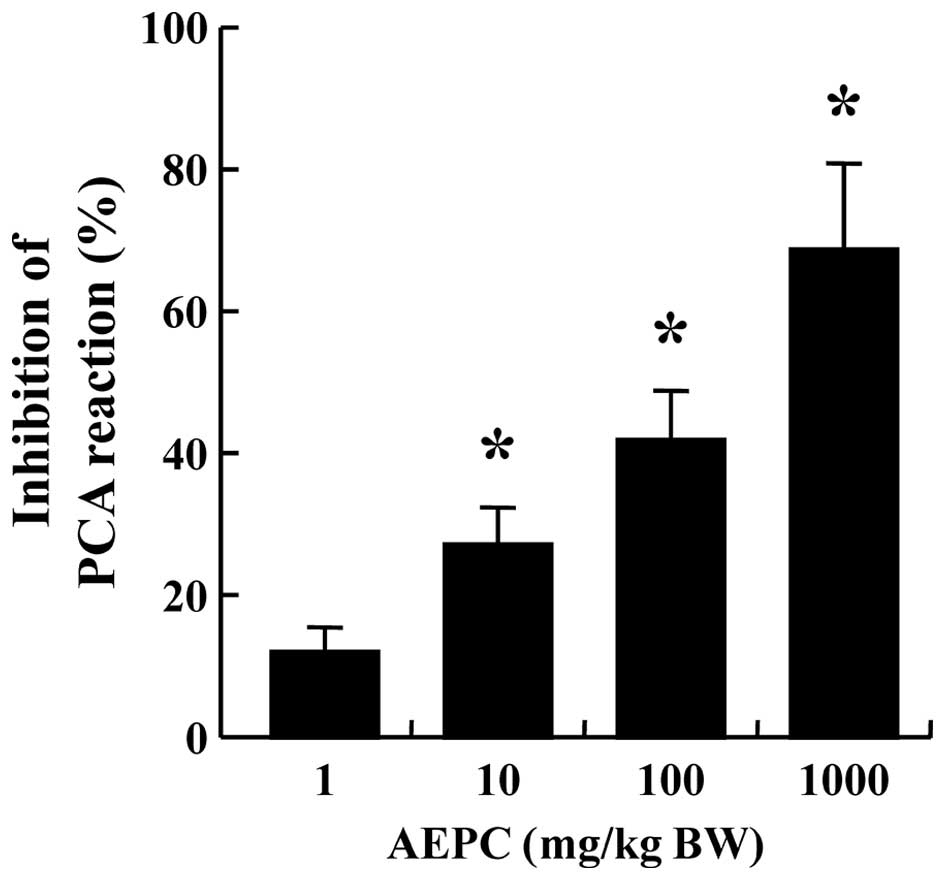
Another animal model, of IgE-mediated PCA, was used
to confirm the anti-allergic effects of AEPC (19). Both ears of each mouse were
sensitized by an intradermal injection of anti-DNP IgE (0.5
µg/site) prior to the intravenous injection of DNP-HSA (1
mg/mouse) and a 4% Evans blue (1:1) mixture to induce the PCA
reaction. After the antigen challenge, a blue spot was visible at
the sensitized site due to the increased vascular permeability
caused by the release of histamine from mast cells. AEPC was
administered intraperitoneally 1 h prior to the challenge with
antigen. The administration of AEPC reduced the size and blue color
of the spot, thus inhibiting the PCA reaction in a dose-dependent
manner (Fig. 1).
Effect of AEPC on compound 48/80-induced
degranulation and calcium influx
Histamine and β-hexosaminidase released from mast
cells are important allergic mediators, and the inhibition of mast
cell degranulation is the proper therapeutic target for allergic
disorders (24). Thus, in this
study, we evaluated the effects of AEPC on the release of histamine
and β-hexosaminidase induced by compound 48/80. Treatment with
compound 48/80 resulted in the increased release of histamine and
β-hexsosaminidase from the RPMCs (data not shown). By contrast,
AEPC suppressed the release of histamine and β-hexsosaminidase
induced by compound 48/80 in a dose-dependent manner (Fig. 2A and B). These results support the
hypothesis that AEPC inhibits compound 48/80-induced anaphylaxis by
blocking degranulation of mast cells.
To investigate the mechanisms responsible for the
inhibitory effects of AEPC on the release of histamine, we measured
the intracellular calcium levels. Calcium influx in mast cells is
critical to the release of histamine (25). Antigen cross-linking of IgE bound
to FcεRI causes the calcium influx, which stimulates granule
fusion-to-cell membranes consequentially through the binding of
synaptotagmin to the soluble NSF attachment protein receptor
(SNARE) complex (26). Compound
48/80 induced an increase in the intracellular calcium levels in
the RPMCs; however, AEPC counteracted this increase (Fig. 2C). Therefore, our findings suggest
that AEPC inhibits the release of histamine by blocking calcium
movement into mast cells. The concentration of AEPC used in this
study was not cytotoxic to the HMC-1 cells (Fig. 2D).
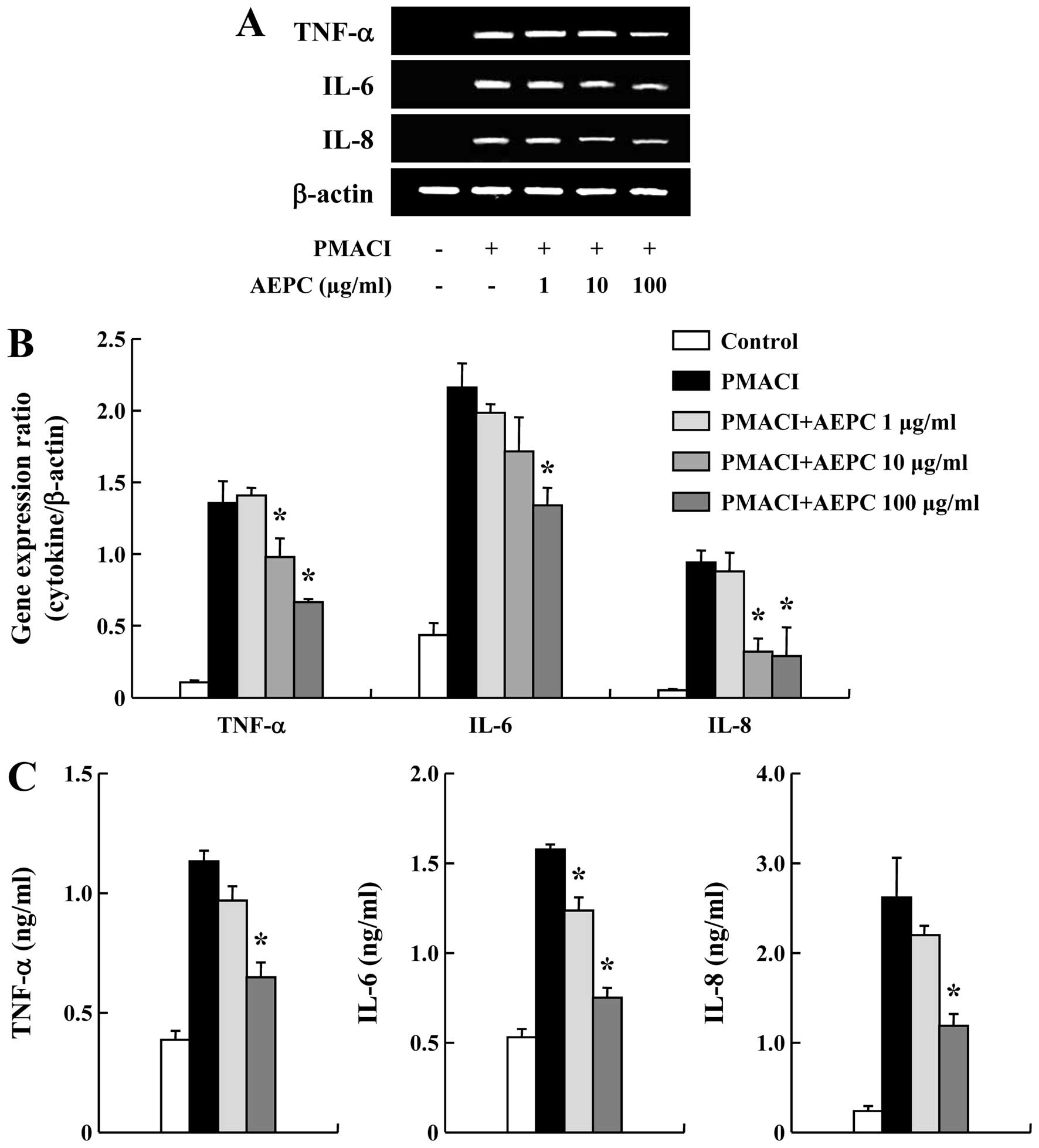
Effect of AEPC on the expression of
pro-inflammatory cytokines
Pro-inflammatory cytokines are important to the
progression of chronic allergic inflammation (27). Thus, we examined the effects of
AEPC on the gene expression of pro-inflammatory cytokines in HMC-1
cells, such as TNF-α, IL-6 and IL-8. AEPC attenuated the
PMACI-induced increase in the mRNA expression of TNF-α, IL-6 and
IL-8 in a dose-dependent manner (Fig.
3A and B). To confirm the effects of AEPC on the mRNA
expression of pro-inflammatory cytokines, cultured media were used
in the ELISA for assaying the secretion of TNF-α, IL-6 and IL-8.
Stimulation of the cells with PMACI for 8 h induced the secretion
of cytokines. On the contrary, AEPC inhibited the secretion of
TNF-α, IL-6 and IL-8 from the PMACI-stimulated HMC-1 cells
(Fig. 3C).
Effects of AEPC on the activation of
NF-κB and p38 MAPK
To elucidate the mechanisms responsible for the
inhibitory effects of AEPC on cytokine expression, we investigated
the activation of the transcription factors, NF-κB and p38 MAPK,
which have previously been reported to play important roles in
immune and inflammatory responses (23). Stimulation of the HMC-1 cells with
PMACI caused the degradation of IκBα and the translocation of p65
NF-κB into the nucleus. AEPC reduced the PMACI-induced activation
of NF-κB by blocking the degradation of IκBα (Fig. 4A). To confirm the suppressive
effects of AEPC on the activation of NF-κB, we performed an
NF-κB-dependent gene reporter assay. PDTC, an inhibitor of NF-κB,
was used as a positive control. The HMC-1 cells were transiently
transfected with an NF-κB-luciferase reporter construct or an empty
vector. Stimulation of the cells with PMACI increased the
luciferase activity in the cells transfected with the
NF-κB-luciferase reporter construct. AEPC significantly diminished
the increased luciferase activity induced by PMACI (Fig. 4B). The MAPK pathway is also known
to play a major role in the regulation of pro-inflammatory
mediators (28,29). Thus, we evaluated the effects of
AEPC on the activation of MAPKs. It is clear that AEPC attenuated
PMACI-induced p38 MAPK phosphorylation (Fig. 4C).
Discussion
Currently, there are many allergic diseases for
which no cure has been found. In particular, anaphylaxis, which can
be lethal, is induced by the systemic release of allergic
mediators, such as histamine, chemokines and cytokines from mast
cells in only a few minutes (30). The first aim of the present study
was to examine whether AEPC has anti-allergic properties. As shown
by our results, AEPC inhibited compound 48/80-induced systemic
anaphylaxis and IgE-mediated PCA. Numerous reports have previously
mentioned that stimulation with compound 48/80 or antigen-IgE
initiates the signaling pathway, which results in histamine being
released from mast cells (3,9,24).
Histamine is considered a crucial mediator in acute inflammation
and immediate-type hypersensitivity. It has previously been
demonstrated that histamine affects the development of the
antigen-specific immune response, the maturation of dendritic
cells, alters T cell-polarizing capacity, and leads to chronic
allergies by selectively recruiting major effector cells into the
allergic region and via the maturation, activation and polarization
of immune cells (4,31). We observed in the present study
that the rapidly elevated degranulation of histamine and
β-hexosaminidase in RPMCs stimulated with compound 48/80 was
reduced by AEPC. Therefore, we posit that AEPC regulates
anaphylaxis by inhibiting the degranulation of mast cells.
Intracellular calcium levels are critical to the release of
allergic mediators from mast cells (8). In addition, blocking calcium
movement across the membranes of mast cells is a useful strategy
for anti-allergic drugs to hinder the secretion of mast cells
(25). In the present study, we
noted that AEPC decreased calcium influx in a dose-dependent
manner. Thus, we propose that AEPC exerts a suppressive effect on
immediate-type hypersensitivity by reducing the degranulation of
mast cells caused and blocking calcium influx.
HMC-1 cells are appropriate tools for the in
vitro examination of the expression of pro-inflammatory
cytokines (32). Various
cytokines, including TNF-α, IL-6 and IL-8, which are produced in
HMC-1 cells by stimulation with PMACI, are well-recognized to
trigger and sustain allergic inflammatory reactions. Mast cells are
major donors of cytokines in the human dermis. TNF-α, a well-known
pro-inflammatory factor from mast cells, is important for the
development of mast cells despite the fact that it is not a growth
factor and that it promotes the interaction of endothelial
leukocytes by inducing the expression of adhesion molecules
(33–35). Local accumulation of IL-6 mediates
the PCA reaction as well as promoting type 2 T helper (Th2)
modulation by assisting T cell survival (36,37). The IL-8-dependent recruitment of
neutrophils enhances inflammation in chronic allergic diseases
(38). These reports suggest that
the inhibition of pro-inflammatory cytokines is a most important
aspect of reducing allergic inflammation. In the present study, we
noted that AEPC inhibited the gene expression and secretion of
TNF-α, IL-6 and IL-8 in HMC-1 cells stimulated with PMACI. As a
result, we suggest that the anti-allergic and anti-inflammatory
effects of AEPC are caused by the inhibition of TNF-α, IL-6 and
IL-8 in mast cells.
In order to elucidate the mechanisms responsible for
the anti-allergic and anti-inflammatory effects of AEPC on mast
cells, we examined the activation of NF-κB. The activation of
NF-κB, a transcription factor which is of significance for
inflammatory mediators, plays a critical role in chronic
inflammatory diseases as it regulates the expression of various
inflammatory and immune genes, including TNF-α and IL-1β (39). The phosphorylation and proteolytic
degradation of IκBα are required for the tranlocation of NF-κB into
the nucleus (7). In our study,
PMACI stimulated the nuclear trans-location of NF-κB, which was
regulated by AEPC, which then blocked the degradation of IκBα in
mast cells. Moreover, the transcription caused by NF-κB was also
obstructed. Thus, it is possible that AEPC attenuates the
expression of downstream cytokines by inhibiting the activation of
NF-κB. It is well known that the MAPK signaling cascade is also
involved in inflammation (28).
There are three types of MAPKs, namely p38, ERK and JNK. We
examined the effects of AEPC on the activation of p38 MAPK, as
previous studies noted that the phosphorylation of p38 MAPK is
essential for the expression of the pro-inflammatory cytokines
(40,41). In the present study, we
demonstrated that AEPC inhibited the activation of p38 MAPK, as was
expected. According to our results, AEPC reduced the expression of
allergic inflammatory mediators by suppressing the activation of
transcription factors, particularly NF-κB and p38.
In the present study, we provide evidence that AEPC
inhibits the allergic inflammatory reaction. We suggest that AEPC
inhibits the degranulation of mast cells and the expression of
pro-inflammatory cytokines via the reduction of calcium influx, and
the activation of NF-κB and p38 MAPK. As we used whole aqueous
extract of P. cablin (AEPC), not a purified single compound,
the biological effects of the individual active components are not
clear at this time. Efforts to identify the active components from
AEPC in allergic inflammatory symptoms are ongoing in our
laboratory. However, the results presented herein provide insight
into the mechanisms responsible for the anti-allergic and
anti-inflammatory effects of AEPC, as well as evidence that AEPC
contributes to the prevention or treatment of mast cell-mediated
allergic inflammatory diseases.
Acknowledgments
The present study was supported by the National
Research Foundation of Korea grant funded by the Korean government
(2012M3A9B6055416, 2014R1A5A2009242 and NRF-2013R1A1A4A01006557)
and the High Value-added Food Technology Development Program,
Ministry of Agriculture, Food and Rural Affairs.
References
|
1
|
Takizawa H: Impact of air pollution on
allergic diseases. Korean J Intern Med. 26:262–273. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nauta AJ, Engels F, Knippels LM, Garssen
J, Nijkamp FP and Redegeld FA: Mechanisms of allergy and asthma.
Eur J Pharmacol. 585:354–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galli SJ, Kalesnikoff J, Grimbaldeston MA,
Piliponsky AM, Williams CM and Tsai M: Mast cells as 'tunable'
effector and immunoregulatory cells: Recent advances. Annu Rev
Immunol. 23:749–786. 2005. View Article : Google Scholar
|
|
4
|
Jutel M, Blaser K and Akdis CA: Histamine
in allergic inflammation and immune modulation. Int Arch Allergy
Immunol. 137:82–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pandey V, Mihara S, Fensome-Green A,
Bolsover S and Cockcroft S: Monomeric IgE stimulates NFAT
translocation into the nucleus, a rise in cytosol Ca2+,
degranulation, and membrane ruffling in the cultured rat basophilic
leukemia-2H3 mast cell line. J Immunol. 172:4048–4058. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bradding P, Feather IH, Wilson S, Bardin
PG, Heusser CH, Holgate ST and Howarth PH: Immunolocalization of
cytokines in the nasal mucosa of normal and perennial rhinitic
subjects. The mast cell as a source of IL-4, IL-5, and IL-6 in
human allergic mucosal inflammation. J Immunol. 151:3853–3865.
1993.PubMed/NCBI
|
|
7
|
Azzolina A, Bongiovanni A and Lampiasi N:
Substance P induces TNF-alpha and IL-6 production through NF kappa
B in peritoneal mast cells. Biochim Biophys Acta. 1643:75–83. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim SH, Jun CD, Suk K, Choi BJ, Lim H,
Park S, Lee SH, Shin HY, Kim DK and Shin TY: Gallic acid inhibits
histamine release and pro-inflammatory cytokine production in mast
cells. Toxicol Sci. 91:123–131. 2006. View Article : Google Scholar
|
|
9
|
Shin TY, Kim SH, Suk K, Ha JH, Kim I, Lee
MG, Jun CD, Kim SY, Lim JP and Eun JS: Anti-allergic effects of
Lycopus lucidus on mast cell-mediated allergy model. Toxicol Appl
Pharmacol. 209:255–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hsu HC, Yang WC, Tsai WJ, Chen CC, Huang
HY and Tsai YC: Alpha-bulnesene, a novel PAF receptor antagonist
isolated from Pogostemon cablin. Biochem Biophys Res Commun.
345:1033–1038. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu YP: Chinese Materia Medica. Harwood
Academic Publishers; The Netherlands: pp. 307–308. 1998
|
|
12
|
Kocevski D, Du M, Kan J, Jing C, Lačanin I
and Pavlović H: Antifungal effect of Allium tuberosum, Cinnamomum
cassia, and Pogostemon cablin essential oils and their components
against population of Aspergillus species. J Food Sci.
78:M731–M737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jeong JB, Choi J, Lou Z, Jiang X and Lee
SH: Patchouli alcohol, an essential oil of Pogostemon cablin,
exhibits anti-tumorigenic activity in human colorectal cancer
cells. Int Immunopharmacol. 16:184–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Kinoshita K, Koyama K, Takahashi
K, Tai T, Nunoura Y and Watanabe K: Anti-emetic principles of
Pogostemon cablin (Blanco) Benth. Phytomedicine. 6:89–93. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kiuchi F, Matsuo K, Ito M, Qui TK and
Honda G: New sesquiterpene hydroperoxides with trypanocidal
activity from Pogostemon cablin. Chem Pharm Bull (Tokyo).
52:1495–1496. 2004. View Article : Google Scholar
|
|
16
|
Ichikawa K, Kinoshita T and Sankawa U: The
screening of Chinese crude drugs for Ca2+ antagonist
activity: identification of active principles from the aerial part
of Pogostemon cablin and the fruits of Prunus mume. Chem Pharm Bull
(Tokyo). 37:345–348. 1989. View Article : Google Scholar
|
|
17
|
Guo JX, Kimura T, But PPH and Sung CK:
International Collation of Traditional and Folk Medicine. World
Scientific Publishing Co. Pte. Ltd; Singapore: pp. 99–100. 2001
|
|
18
|
Kim HH, Park SB, Lee S, Kwon TK, Shin TY,
Park PH, Lee SH and Kim SH: Inhibitory effect of putranjivain A on
allergic inflammation through suppression of mast cell activation.
Toxicol Appl Pharmacol. 274:455–461. 2014. View Article : Google Scholar
|
|
19
|
Kim HH, Kim DS, Kim SW, Lim SH, Kim DK,
Shin TY and Kim SH: Inhibitory effects of Diospyros kaki in a model
of allergic inflammation: role of cAMP, calcium and nuclear
factor-κB. Int J Mol Med. 32:945–951. 2013.PubMed/NCBI
|
|
20
|
Kim HH, Bae Y and Kim SH: Galangin
attenuates mast cell-mediated allergic inflammation. Food Chem
Toxicol. 57:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Itoh T, Tsukane M, Koike M, Nakamura C,
Ohguchi K, Ito M, Akao Y, Koshimizu S, Nozawa Y, Wakimoto T, et al:
Inhibitory effects of whisky congeners on IgE-mediated
degranulation in rat basophilic leukemia RBL-2H3 cells and passive
cutaneous anaphylaxis reaction in mice. J Agric Food Chem.
58:7149–7157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bae Y, Lee S and Kim SH: Chrysin
suppresses mast cell-mediated allergic inflammation: involvement of
calcium, caspase-1 and nuclear factor-κB. Toxicol Appl Pharmacol.
254:56–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JK, Oh HM, Lee S, Park JW, Khang D,
Lee SW, Lee WS, Rho MC and Kim SH: Oleanolic acid acetate inhibits
atopic dermatitis and allergic contact dermatitis in a murine
model. Toxicol Appl Pharmacol. 269:72–80. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galli SJ and Tsai M: IgE and mast cells in
allergic disease. Nat Med. 18:693–704. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalesnikoff J and Galli SJ: New
developments in mast cell biology. Nat Immunol. 9:1215–1223. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma HT and Beaven MA: Regulators of
Ca2+ signaling in mast cells: potential targets for
treatment of mast cell-related diseases? Adv Exp Med Biol.
716:62–90. 2011. View Article : Google Scholar
|
|
27
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arbabi S and Maier RV: Mitogen-activated
protein kinases. Crit Care Med. 30(Suppl 1): S74–S79. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beyaert R, Cuenda A, Vanden Berghe W,
Plaisance S, Lee JC, Haegeman G, Cohen P and Fiers W: The p38/RK
mitogen-activated protein kinase pathway regulates interleukin-6
synthesis response to tumor necrosis factor. EMBO J. 15:1914–1923.
1996.PubMed/NCBI
|
|
30
|
Boden SR and Wesley Burks A: Anaphylaxis:
a history with emphasis on food allergy. Immunol Rev. 242:247–257.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jutel M, Watanabe T, Akdis M, Blaser K and
Akdis CA: Immune regulation by histamine. Curr Opin Immunol.
14:735–740. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Möller A, Henz BM, Grützkau A, Lippert U,
Aragane Y, Schwarz T and Krüger-Krasagakes S: Comparative cytokine
gene expression: regulation and release by human mast cells.
Immunology. 93:289–295. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sillaber C, Bevec D, Butterfield JH,
Heppner C, Valenta R, Scheiner O, Kraft D, Lechner K, Bettelheim P
and Valent P: Tumor necrosis factor alpha and interleukin-1 beta
mRNA expression in HMC-1 cells: differential regulation of gene
product expression by recombinant interleukin-4. Exp Hematol.
21:1271–1275. 1993.PubMed/NCBI
|
|
34
|
Hu ZQ, Kobayashi K, Zenda N and Shimamura
T: Tumor necrosis factor-alpha- and interleukin-6-triggered mast
cell development from mouse spleen cells. Blood. 89:526–533.
1997.PubMed/NCBI
|
|
35
|
Walsh LJ, Trinchieri G, Waldorf HA,
Whitaker D and Murphy GF: Human dermal mast cells contain and
release tumor necrosis factor alpha, which induces endothelial
leukocyte adhesion molecule 1. Proc Natl Acad Sci USA.
88:4220–4224. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mican JA, Arora N, Burd PR and Metcalfe
DD: Passive cutaneous anaphylaxis in mouse skin is associated with
local accumulation of interleukin-6 mRNA and immunoreactive
interleukin-6 protein. J Allergy Clin Immunol. 90:815–824. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dienz O and Rincon M: The effects of IL-6
on CD4 T cell responses. Clin Immunol. 130:27–33. 2009. View Article : Google Scholar :
|
|
38
|
Silvestri M, Bontempelli M, Giacomelli M,
Malerba M, Rossi GA, Di Stefano A, Rossi A and Ricciardolo FL: High
serum levels of tumour necrosis factor-alpha and interleukin-8 in
severe asthma: Markers of systemic inflammation? Clin Exp Allergy.
36:1373–1381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Karin M: NF-kappaB as a critical link
between inflammation and cancer. Cold Spring Harb Perspect Biol.
1:a0001412009. View Article : Google Scholar
|
|
40
|
Manthey CL, Wang SW, Kinney SD and Yao Z:
SB202190, a selective inhibitor of p38 mitogen-activated protein
kinase, is a powerful regulator of LPS-induced mRNAs in monocytes.
J Leukoc Biol. 64:409–417. 1998.PubMed/NCBI
|
|
41
|
Shapiro L and Dinarello CA: Osmotic
regulation of cytokine synthesis in vitro. Proc Natl Acad Sci USA.
92:12230–12234. 1995. View Article : Google Scholar : PubMed/NCBI
|