Introduction
In animals, different types of stress such as heat,
transportation and chemical factors contribute to lethal
pathological symptoms related to cardiovascular diseases, such as
cardiac arrhythmia, seizure or hypovolemic shock with tachycardia
and, eventually, circulatory collapse (1–3).
In the clinical diagnosis and treatment of heat stroke,
approximately 25% of patients experience failure of ≥1 organ
systems. In mammals, sudden death may occur as a result of
stress-induced damage to cardiac tissue and myocardial cells
(4,5).
Heat shock proteins (HSPs) are ubiquitously
expressed and highly conserved in prokaryotes and eukaryotes
(6). HSP family members are
molecular chaperones that are important for the regulation of
several fundamental cellular processes under normal conditions
(7). However, they also play a
protective role during pathological processes (8,9).
HSPs play an important role in intracellular protein transport,
cytoskeletal architecture, mutation masking, regulation of
translation, intracellular redox homeostasis and protection against
spontaneous or induced programmed cell death (10).
HSP70, a member of the HSP70 family, is associated
with enhanced post-ischemic myocardial recovery in adult rat hearts
and with the reduction of infarct size (11). It interacts with other proteins
and maintains or alters their conformational states (12). Under normal conditions, heat shock
factor 1 (HSF1) presents as an HSF-HSP70 heterodimer; however, HSF1
has been shown to interact with HSP70 under stress conditions
(13).
HSP90, which belongs to the HSP90 family, and as
previously demonstrated, does not act generally in nascent protein
folding (14). At the molecular
level, HSP90 binds to substrate proteins, which are in a
near-native state and thus at a late stage of folding (15), poised for activation by ligand
binding or interaction with other factors (16). Defects in cell physiology caused
by HSP90 disruption lead to tissue- and organism-level defects.
HSP90 is essential for various cellular processes, such as protein
folding, protein degradation, signal transduction cascades and
morphological evolution. HSP90 affinity chromatography experiments
have indicated that HSP90 interacts with HSF1 in human cells
(17).
HSP60, which belongs to the HSP60 family, is
anti-apoptotic and provides protection against cell death by
maintaining mitochondrial oxidative phosphorylation (18–20). HSP60 is typically located in the
mitochondria of eukaryotic cells (21). It assists in the protection
against protein aggregation (22)
and in transporting proteins from the cytoplasm to organelles
(23).
Crystallin, alpha B (CRYAB, also known as HSP
beta-5) is a member of the small HSP family (24) that has chaperone-like properties,
including the ability to prevent the accumulation of denatured
proteins and increase cell tolerance to stress. Following its
induction by cellular stresses, including heat and reactive oxygen
species, CRYAB promotes cell survival and inhibits apoptosis
(25). HSP induction in the
myocardium may be a cardioprotective cellular response (26).
Previous studies have indicated that the dramatic
increase in HSP expression is a key part of the heat shock
response, which is primarily controlled by HSFs (27,28). HSF1 is a major transcriptional
regulator of HSPs, existing as a trimer with constitutive DNA
binding activity (29). In the
absence of cellular stress, HSF1 is repressed through its
association with HSP. However, in response to stress, HSF1 binds to
specific sequences in HSP promoters and stimulates HSP expression
(30). The question as to whether
HSF1 can trigger all HSPs remains unanswered. In order to address
this intriguing question, in this study, we detected the levels of
HSPs and HSF1 in heat-stressed rat myocardial cells in vitro
and analyzed and compared the data using STRING (version 9.1) to
determine the association between HSF1 and HSPs.
Materials and methods
Cell culture and exposure to heat
Primary neonatal rat myocardial cells were provided
by Shanghai Fu Meng Gene Biotechnology Co., Ltd. (Shanghai, China).
The cells were cultured in Dulbecco's modified Eagle's medium
(DMEM, No. 11965-084) 10%, supplemented with 10% fetal calf serum
(No. 10270-098) were purchased from Gibco, Thermo Scientific,
Shanghai, China, at 37°C in 5% CO2 for 3 days; the
viability was >85%. The cells were divided into different
experimental groups, each consisting of 9 cell culture plates.
Heat-stressed cells were exposed to heat at 42°C, whereas the
control cells were exposed to a normal temperature of 37°C. One
plate from each group was removed from the incubator at the start
of the experiment (0 min) and after 10, 20, 40, 60, 120, 240, 360
and 480 min.
Semi-quantitative detection of HSP and
HSF1 expression levels by western blot analysis
The heat-stressed cells were washed with
phosphate-buffered saline (PBS) 3 times, and proteins were
extracted by lysis in sodium dodecyl sulfate (SDS)-polyacrylamide
gel Laemmli sample buffer. The protein extracts were boiled for 5
min prior to loading equal amounts of protein (10 µg) for
10% SDS-polyacrylamide gel electrophoresis. Proteins were
transferred onto nitrocellulose membranes by electrotransfer and
the membranes were blocked with 5% skimmed milk in Tris-buffered
saline [20 mM Tris-HCl (pH 7.6), 137 mM NaCl] containing 0.1%
Tween-20 (TBST) for 1 h at room temperature. The membranes were
incubated with anti-rat HSF1 monoclonal antibody [1:1,000; ab61382;
Abcam Trading (UK) Company Ltd.], anti-rat HSP90 monoclonal
antibody [ab79849; Abcam Trading (UK) Company Ltd.], anti-rat HSP70
monoclonal antibody [1:1,000; ab5442; Abcam Trading (UK) Company
Ltd.], anti-rat CRYAB monoclonal antibody [1:1000; ab13496; Abcam
Trading (UK) Company Ltd.], or anti-rat β-actin (ACTB) monoclonal
antibody [1:1,000, ab8224; Abcam Trading (UK) Company Ltd.] for 16
h at 4°C. After washing with TBST, the membranes were incubated
with peroxidase-conjugated goat anti-mouse immunoglobulin G at room
temperature for 1 h, and the antibody-antigen complexes were
detected using Western Blotting Luminol Reagent (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Bands on the developed
film were quantified using Quantity One software version 4.6.2
(Bio-Rad, Hercules, CA, USA). The intensity of each band was
normalized to that of β-actin.
Total RNA isolation and reverse
transcription-PCR
Total RNA was isolated from the cells in the
experimental and control groups using TRIzol reagent according to
the manufacturer's instructions (Trizol-RNAiso Plus reagent,
D9108A; Takara, China). The RNA concentrations were measured at 260
nm using a spectrophotometer (M200PRO; Tecan, Austria). Serial
dilutions of RNA were prepared with ribonuclease-free water; 2
µg of each sample were reverse transcribed using a
Transcript Moloney murine leukemia virus (M-MLV) kit (Invitrogen,
Shanghai, China) according to the manufacturer's instructions and
stored at −80°C until use. Random decamers and oligo(dT) were
obtained from a RETROscript kit (AM1710; Ambion, Austin, TX,
USA).
Primers
Primers were designed to anneal specifically to each
target mRNA. HSF1, HSP90, HSP70, CRYAB
and β-actin mRNA sequences were obtained from the National
Center for Biotechnology Information (Bethesda, MD, USA) GenBank
database (accession nos: NC_005108.2, NP_077369.1 and NC_005111.2).
The primers were designed using Primer Premier 5.0 software for
conventional and reverse transcription-PCR amplification. The
sequences were as follows: HSF1 sense,
5′-ACCCCAGCCTCTGCCTGCT-3′ and antisense,
5′-TTCCCACTCGGGCTCCAGCA-3′; HSP90 sense, 5′-CCC
GGTGCGGTTAGTCACGT-3′ and antisense, 5′-TCCAGAGC GTCTGAGGAGTTGGA-3′;
HSP70 sense, 5′-GTCCCTCAAGAGCCCAACCCCAT-3′ and antisense,
5′-ACGTGGTCTAGTGGAAGCCACCA-3′; CRYAB sence,
5′-CGTCGGCTGGGATCCGGTACT-3′ and antisence,
5′-CACGAAGAGCGCCAGGACGA-3′; β-actin sense, 5′-CCCATCTATGAGG
GTTCA-3′ and antisense, 5′-TCACGCACGATTTCC-3′. The expected lengths
of the HSF1, HSP90, HSP70, CRYAB and
β-actin PCR products were 153, 214, 124, 153 and 128 bp,
respectively. Primers were synthesized by Invitrogen.
Quantitative (real-time) PCR (qPCR)
Each DNA sample (2 µl, 25X dilution) was
suspended in 2X SYBR Premix Ex TaqT™ (DRR041S; Takara, China) with
25 pmol of each sense and antisense primer, and double distilled
water was added to a total volume of 25 µl. qPCR was
performed using an ABI 7300 Real-Time PCR system (Applied
Biosystems Foster City, CA, USA). The thermal profile was
established according to the manufacturer's instructions. Briefly,
this protocol consisted of enzyme activation at 95°C for 3 min,
followed by 45 cycles of denaturation at 95°C for 5 sec, and
annealing and elongation at 52°C for 30 sec. For each run, a
negative control tube without DNA was run along with the
experimental samples. A 2-fold dilution series of the template was
used in the qPCR assays. The HSPs and HSF1 mRNA
expression levels of all samples were normalized using the
following formula: relative quantity of HSF1/HSP mRNA =
2−∆∆Ct, where ∆∆Ct = [(Cthsf/hsps mRNA −
Ctβ-actin mRNA)test group −
(Cthsf/hsps mRNA − Ctβ-actin
mRNA)control group].
Analysis of HSF1 and HSP interaction
We used the STRING (version 9.1) database
(http://string-db.org/), which aims to provide a
global perspective for as many organisms as feasible. The database
scores and integrates known and predicted associations, resulting
in comprehensive protein networks covering >1,100 organisms
(31).
Statistical analysis
Statistical analysis of the differences between the
experimental group and control group values was performed using
one-way analysis of variance followed by the Duncan's multiple
comparison test with SPSS version 20.0 software (IBM, Armonk, NY,
USA). A value of P<0.05 was considered to indicate a
statistically significant difference when the experimental groups
were compared with the controls. The values reported are the means
± SD. Three replicates were used for all experiments (n=3).
Results
HSP and HSF1 expression under heat stress
conditions
We detected and measured the expression levels of
HSPs (HSP90, HSP70, CRYAB) and HSF1 by western blot analysis
(Fig. 1). The results reaveled
that HSF1 expression was significantly decreased (P<0.01) in
response to heat stress for up to 120 min (Fig. 1). Following exposure to heat
stress for 240 min or longer, the HSF1 expression levels gradually
and signifi-cantly increased, and remained elevated following 480
min of exposure to heat stress (Fig.
1). Compared with the control group, HSP90 expression decreased
following exposure of the cells to heat stress for 10 to 40 min,
and then increased from 60 to 480 min of exposure to heat stress,
demonstrating an expression trend similar to that of HSF1 (Fig. 1). However, the epxression of HSP70
in the experimental group was decreased at 10 and 40 min of
exposure to heat stress (P<0.05), and then further decreased
significantly after 120 min of exposure to heat stress (P<0.01)
until the time point of 480 min of exposure to heat stress
(Fig. 1). The expression of HSP70
followed an opposite trend to that of HSF1. In contrast to the
other HSPs, the CRYAB levels did not show any significant changes
during the 480 min of exposure to heat stress (Fig. 1).
 | Figure 1Heat shock proteins (HSPs) and heat
shock factor (HSF1) protein expression in heat-stressed rat
myocardial cells in vitro. HSPs and HSF1 levels were
normalized to those of β-actin during 480 min of exposure to heat
stress. HSF1 levels increased gradually and significantly from 240
min of exposure and onwards, and remained elevated after 480 min of
heat stress. Compared with the control group, HSP90 expression
decreased from 10 to 40 min of exposure to heat, and then increased
from 60 to 480 min of heat stress. From 10 to 60 min, the overall
expression levels of HSP70 decreased compared with those of the
control group, but not at 20 and 60 min of exposure. After 120 min
of exposure, the level of HSP70 expression remained low until 480
min. There was no significant change in the crystallin, alpha B
(CRYAB) levels during the 480 min of exposure to heat stress. The
different letters each indicate a different factor as follows: Aa,
HSF1; Bb, HSP90; Cc, SP70; Dd, CRYAB. Upper case letters (A, B and
C) represent P<0.01; lower case letter (c) represents P<0.05.
All the P-values were compared with the control group. |
HSF1 and HSP mRNA mRNA levels under heat
stress conditions
The mRNA levels of HSF1 and HSPs are
shown in Fig. 2. The mRNA
expression of β-actin was not altered in response to heat
stress (data not shown). The HSF1 mRNA levels increased
rapidly and in a stepwise manner throughout the time course of
exposure to heat stress and reached maximal levels (3-fold
induction) after 480 min of exposure to heat stress (Fig. 2). The mRNA expression levels of
HSP90 increased after 10 min of exposure to heat stress and
reached maximal levels after 240 min of exposure to heat stress
(P<0.01; Fig. 2). All other
time periods of exposure to heat stress resulted in significantly
higher mRNA levels of HSP90 compared to the controls
(P<0.01; Fig. 2). The
HSP70 mRNA levels increased significantly following 10 min
of exposure to heat stress and continued to increase thereafter
(P<0.01), reaching maximal levels at 360 min of exposure
(Fig. 2). All the heat
stress-associated genes were present at higher levels in the
heat-stressed cells compared to the control group. The mRNA levels
of CRYAB were also markedly increased in the heat-stressed
myocardial cells (P<0.01; Fig.
2).
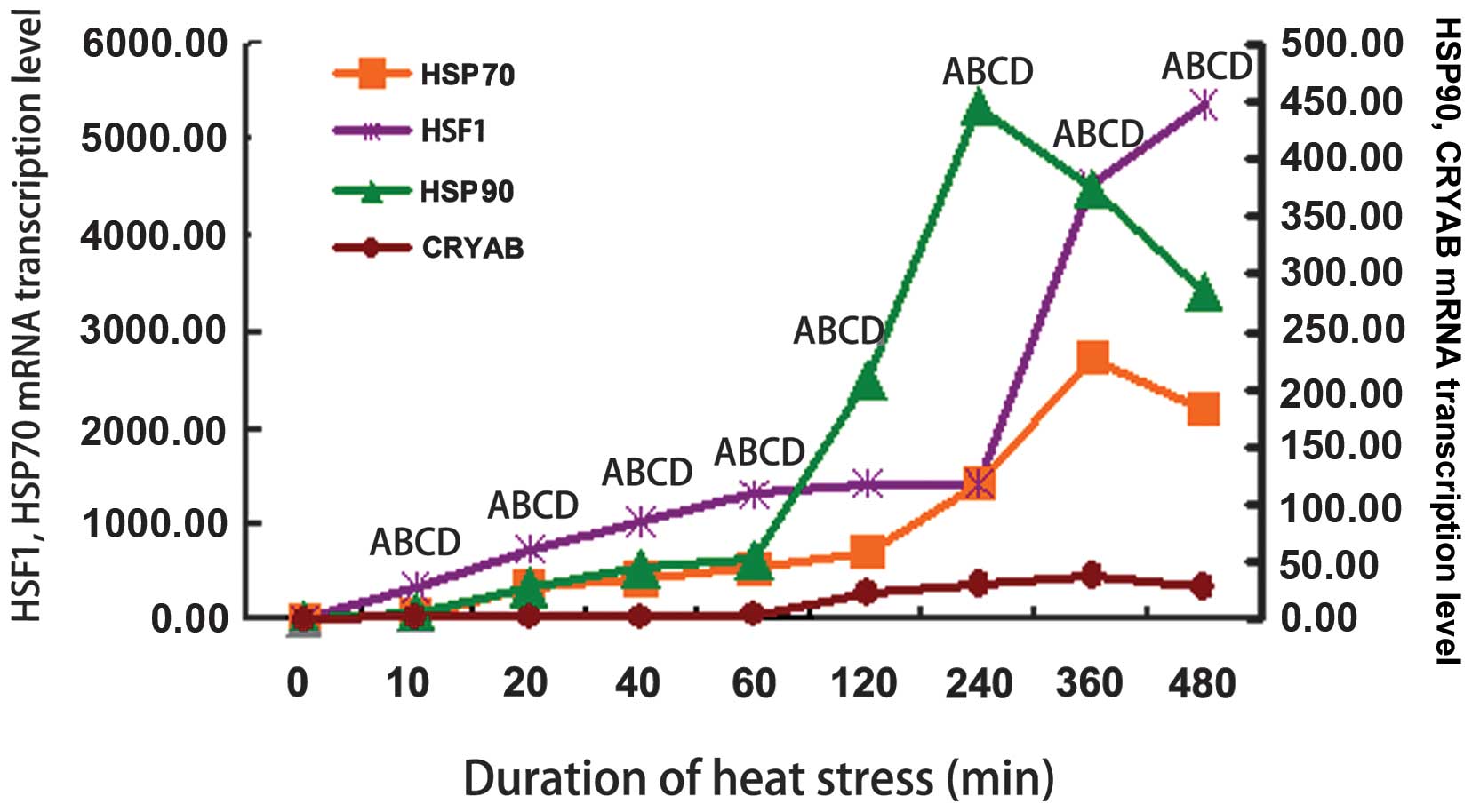 | Figure 2mRNA expression of heat shock
proteins (HSPs) and heat shock factor (HSF1) in
primary rat myocardial cells in vitro before and after
exposure to heat stress. HSPs and HSF1 mRNA levels
were normalized to those of β-actin. HSF1 mRNA levels
increased rapidly and in a stepwise manner throughout the time
course and reached maximal levels (3-fold induction) after 360 min
of exposure to heat stress. The mRNA level of HSP90
increased after 10 min of exposure to heat stress, reaching the
maximal level after 240 min of exposure. All other periods of
exposure to heat stress resulted in significantly higher levels of
HSP90 mRNA than the controls. HSP70 mRNA increased
significantly from 10 to 360 min of heat stress compared with the
control group. Only the level at 480 min was slightly lower than
360 min of heat stress. The transcription levels of crystallin,
alpha B (CRYAB) markedly increased in the heat-stressed
myocardial cells. (Aa, HSF1; Bb, HSP90; Cc, HSP70; Dd, CRYAB. ABCD,
P<0.01). The different letters each indicate a different factor
as follows: A, HSF1; B, HSP90; C, HSP70; D, CRYAB. Upper case
letters (A, B, C and D) represent P<0.01. P-values were compared
with the control group. |
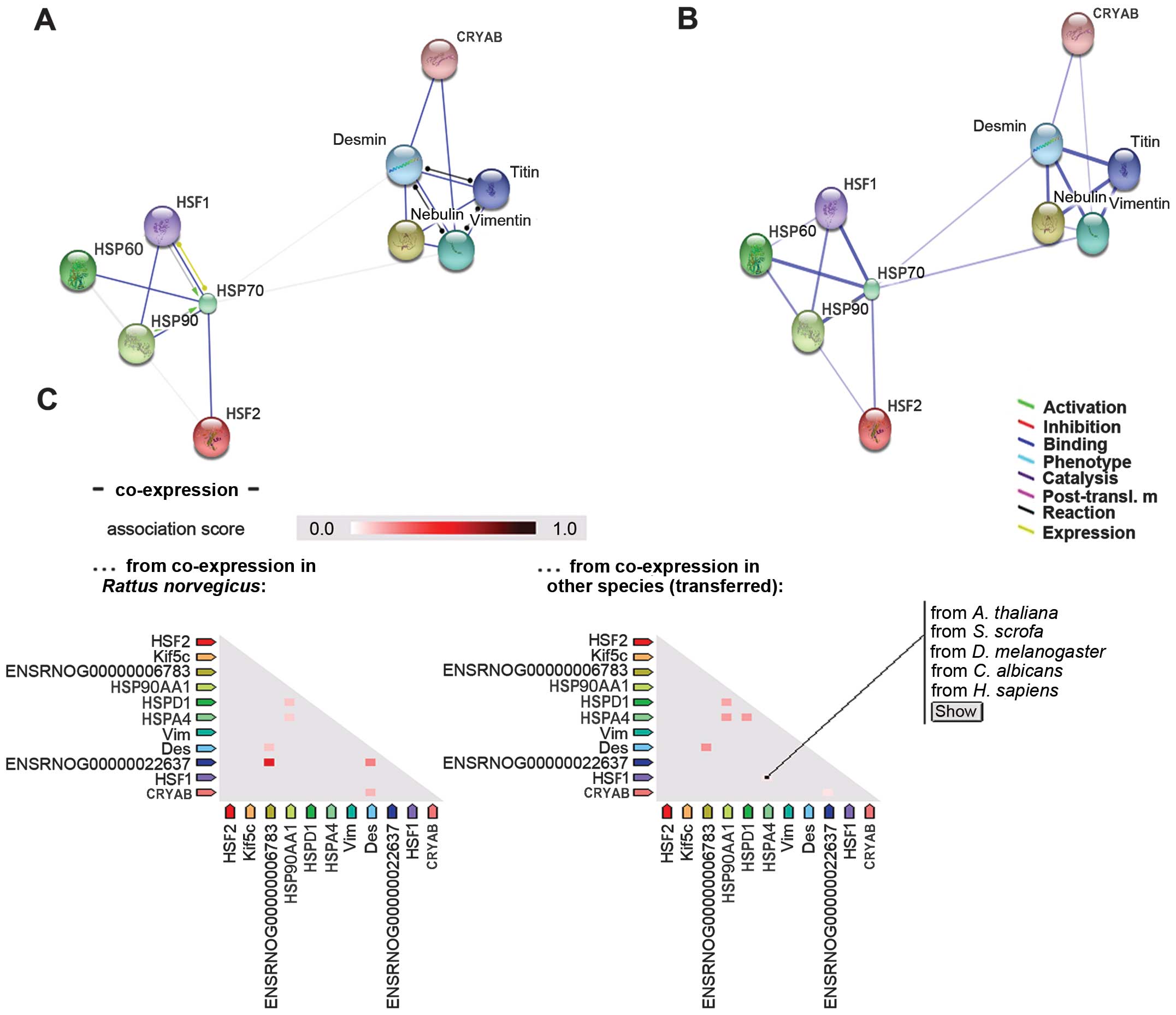
Association between HSF1 and HSPs
According to the rat data on the STRING database
(version 9.1), HSF1 interacted with HSP70 and HSP90 (Fig. 3A). In the confidence view on the
STRING database (Fig. 3B), a
thicker line represented the association between HSF1 and HSP70
(indicating a stronger interacion) than that between HSF1 and
HSP90. The interaction of HSF1 with HSP60 was not as strong as that
between HSP70 and HSP90. However, there appeared to be no
interaction between HSF1 and CRYAB. Under normal conditions in
rats, HSF1 is not co-expressed with any of the HSPs (Fig. 3C). However, there is evidence in
other species that HSF1 is co-expressed with HSP70 (Homo
sapiens). Of note, western blot analysis revealed that the
protein expression levels of HSF1 followed a similar trend to those
of HSP90 following exposure to heat stress, although the expression
of HSP70 followed an opposite trend (Fig. 1). It has been suggested that HSP70
represses HSF1 during heat stress (12). Furthermore, a previous study
indicated that HSF1 is not the sole factor for HSP60 in rats
[Buriro et al (36)].
Furthermore, data analysis confirmed that the expression levels of
CRYAB were inconsistent with those of HSF1.
Discussion
The 'heat shock response' was first described in
1964 in Drosophila by Ritossa (32) and is characterized by the
increased expression of a particular protein superfamily, HSPs.
HSPs account for up to 2–3% of the total cellular proteins at their
baseline level, and their expression is strongly induced upon
cellular stress (33,34). This induction is mediated by a
specific family of transcription factors, the HSFs. According to
our results, the mRNA levels of HSF1 and HSPs
markedly increased from the onset of heat stress (10 min) compared
with the control group. It has been demonstrated that heat stress
induces HSP gene folding (35), and our results are consistent with
this finding. However, the HSF1 protein expression levels were not
simultaneously increased as were the HSF1 mRNA levels during
heat stress. These findings may be associated with the fact that a
single HSF1 phosphorylation event has been shown to result in the
rapid aggregation of HSF1 into a trimer that can bind to HSE and
initiate the transcription process (36–39). The expression levels of HSP90 were
also not consistent with its mRNA levels. The lack of a correlation
between the mRNA levels of HSP90 and the protein levels of
HSP90 may be related to the complex post-transcriptional mechanisms
that are involved in turning mRNAs into proteins (40). In the present study, the HSP70
protein levels decreased from 10 to 480 min of exposure to heat
stress, except after 20 min of exposure to heat stress, which was
also not consistent with its mRNA level. It has been reported that
HSP70 reduces the infarct size in an in vivo transgenic
mouse model of myocardial ischemia and reperfusion (41). HSP70 may interact with damaged
proteins to play a protective role in rat primary myocardial cells.
In this study, in contrast to its mRNA levels, the CRYAB protein
expression levels were not significantly altered during 480 min of
exposure to heat stress, which suggests that the protein expression
was delayed or overtaxed due to the rapid consumption of CRYAB at
the onset of heat stress. CRYAB is required for myocardial cell
balance in response to stress (42). In our study, the mRNA levels of
HSF1, HSP90, HSP70 and CRYAB increased
significantly in primary neonatal rat myocardial cells in
vitro following exposure to heat stress, and these factors may
act as important markers in response to adverse environmental
conditions in the heart (4).
Heat stress can contribute to protein misfolding,
which in turn can trigger the stress response, leading to HSP
expression (43). The
transcription factor HSF1 induces HSP expression when the
environmental temperature rises above the physiological range
(44). HSF1 is inactive under
normal conditions and is present in a heterocomplex with HSPs
(45). Other studies, as well as
ours, have observed different HSPs in normal and heat-stressed rat
myocardial cells (46,47). In the present study, HSF1 levels
were increased after 120 min from 240 min of heat stress onwards.
However, HSP70 expression tended to decrease following exposure to
heat stress, particularly after 120 min of exposure to heat. STRING
analysis determined that HSP70 was more closely connected with HSF1
than the other HSPs. Evidently, our findings indicate that HSF1
does not induce HSP70 expression in primary neonatal rat myocardial
cells in vitro. It has been suggested that HSP70 is a major
HSF1 repressor in human cells (48). Non-native proteins that accumulate
under stress conditions may compete with HSF1 to bind to the
chaperone heat shock (cognate) protein 70 (HSP/c70), and unbound
HSF1 may homotrimerize and acquire transcriptional competence
(12,13). HSP70 prevents the in vitro
conversion of HSF1 from a non-DNA-binding form to a DNA-binding
form. It has also been suggested that HSP70 may play a regulatory
role in HSF activation (12). The
results of the present study indicated that HSP70 plays a
repressive role, acting with HSF1 in rat heart cells in
vitro. As shown in Fig. 3C,
HSP70 is not co-expressed with other proteins in rats; however, the
opposite is true in other species. In addition, it was observed
that HSF2 interacts with HSP70 (Fig.
4A). Previous studies have confirmed that paternal HSF2
modifies endogenous HSP70.1 expression (49). However, the specific mechanisms
through which HSF2 interacts with HSP70 in rat myocardial cells
remain to be investigated.
HSP90 is a major soluble cellular protein and is
most commonly located in the cytoplasm. It is considered a key
factor at the crossroads between genetics and epigenetics, and it
has been postulated that it is a capacitor for phenotypic variation
and morphological evolution (50). The interaction between HSF1 and
HSP90 appears to be dynamic (48). In the present study, HSP90
expression followed a similar trend to that of HSF1, suggesting
that HSF1 plays a role in regulating HSP90. In human cells, a
HSP90-containing HSF1 complex is formed during stress (48). Therefore, HSF1 may bind with HSP90
under stress conditions to play a protective role. In the present
study (Fig. 4A), the interaction
between HSF2 and HSP90 was very weak. Therefore, further
confirmation of the mechanisms involved in this interaction is
required.
In the highly crowded cellular environment,
different chaperones follow distinct strategies to achieve the
general goal of preventing protein misfolding and aggregation. As a
member of the small HSP family, CRYAB acts as a molecular chaperone
involved in increasing cellular tolerance to stress. In
vertebrates, CRYAB expression has been detected in the heart, eyes,
lungs, liver and several other tissues under normal conditions
(51,52). CRYAB appears to be constitutively
expressed in unstressed cells and is essential for maintaining cell
homeostasis. It acts as a molecular chaperone to facilitate
polypeptide transport, folding and assembly (51). In a previous study, the
adenovirus-mediated transgenic overexpression of CRYAB revealed
that in myocardial cells, the protein protects microtubules from
acute ischemic damage (53).
Whether HSFs can induce CRYAB expression in rat cardiac cells under
stress conditions was one of the questions addressed in the present
study. We found that the trend for CRYAB expression was not
consistent with that of HSF1. Additionally, STRING analysis
determined that CRYAB did not interact with either HSF1 or HSF2
(Figs. 3 and 4). It may interact with intermediate
filaments to protect cytoskeletal organization in cardiomyocytes
(24). In cardiomyocytes, CRYAB
is localized within the I-band and M-line region of myofibrils, and
appears to be involved in the organization of cytoskeletal
structures (54). As shown in
Fig. 4B, CRYAB binds with desmin
and vimentin in rat cells in vitro, and may be co-expressed
with desmin under certain conditions (Fig. 4C), suggesting that CRYAB may
combine with desmin to play a protective role under stress
conditions.
In conclusion, HSF1 is not the key HSF for all HSPs,
particularly CRYAB, in rat myocardial cells in vitro.
Moreover, HSF1 may play different roles in its interaction with
HSPs in different species or cell types. STRING analysis confirmed
our previous finding that HSF1 is not the sole factor for HSP60
(36).
Acknowledgments
This study was supported by grants from the National
Key Basic Research Program of China (973 Program) (2014CB138502),
the National Natural Science Foundation of China (31372403), the
National Department Public Benefit Research Foundation
(Agriculture) (201003060-11), the Priority Academic Program
Development of Jiangsu Higher Education Institutions (PAPD),
Graduate research and innovation projects in Jiangsu Province and
the Sino-German Agricultural Cooperation Project of the Federal
Ministry of Food, the Agriculture and Consumer Production, Berlin,
Germany.
References
|
1
|
Gisolfi CV, Matthes RD, Kregel KC and
Oppliger R: Splanchnic sympathetic nerve activity and circulating
catecholamines in the hyperthermic rat. J Appl Physiol (1985).
70:1821–1826. 1991.
|
|
2
|
Mirchandani HG, McDonald G, Hood IC and
Fonseca C: Heat-related deaths in Philadelphia - 1993. Am J
Forensic Med Pathol. 17:106–108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scheers-Masters JR, Schootman M and Thach
BT: Heat stress and sudden infant death syndrome incidence: A
United States population epidemiologic study. Pediatrics.
113:e586–e592. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu J, Bao E, Yan J and Lei L: Expression
and localization of Hsps in the heart and blood vessel of
heat-stressed broilers. Cell Stress Chaperones. 13:327–335. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Lv Y, Yue Z, Islam A, Rehana B,
Bao E and Hartung J: Effects of transportation on expression of
Hsp90, Hsp70, Hsp27 and αB-crystallin in the pig stomach. Vet Rec.
169:3122011. View
Article : Google Scholar
|
|
6
|
Li Z and Srivastava P: Heat-shock
proteins. Curr Protoc Immunol (Appendix 1). 58:A.1T.1–A.1T.6.
2004.
|
|
7
|
Adhikari AS, Sridhar Rao K, Rangaraj N,
Parnaik VK and Mohan Rao Ch: Heat stress-induced localization of
small heat shock proteins in mouse myoblasts: Intranuclear lamin
A/C speckles as target for alphaB-crystallin and Hsp25. Exp Cell
Res. 299:393–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borges JC and Ramos CH: Protein folding
assisted by chaperones. Protein Pept Lett. 12:257–261. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walter S and Buchner J: Molecular
chaperones - cellular machines for protein folding. Angew Chem Int
Ed Engl. 41:1098–1113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guisbert E, Yura T, Rhodius VA and Gross
CA: Convergence of molecular, modeling, and systems approaches for
an understanding of the Escherichia coli heat shock response.
Microbiol Mol Biol Rev. 72:545–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Locke M and Tanguay RM: Diminished heat
shock response in the aged myocardium. Cell Stress Chaperones.
1:251–260. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abravaya K, Myers MP, Murphy SP and
Morimoto RI: The human heat shock protein hsp70 interacts with HSF,
the transcription factor that regulates heat shock gene expression.
Genes Dev. 6:1153–1164. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baler R, Zou J and Voellmy R: Evidence for
a role of Hsp70 in the regulation of the heat shock response in
mammalian cells. Cell Stress Chaperones. 1:33–39. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nathan DF, Vos MH and Lindquist S: In vivo
functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc
Natl Acad Sci USA. 94:12949–12956. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jakob U, Meyer I, Bügl H, André S,
Bardwell JC and Buchner J: Structural organization of procaryotic
and eucaryotic Hsp90. Influence of divalent cations on structure
and function. J Biol Chem. 270:14412–14419. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Young JC, Moarefi I and Hartl FU: Hsp90: A
specialized but essential protein-folding tool. J Cell Biol.
154:267–273. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nadeau K, Das A and Walsh CT: Hsp90
chaperonins possess ATPase activity and bind heat shock
transcription factors and peptidyl prolyl isomerases. J Biol Chem.
268:1479–1487. 1993.PubMed/NCBI
|
|
18
|
Arya R, Mallik M and Lakhotia SC: Heat
shock genes - integrating cell survival and death. J Biosci.
32:595–610. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirchhoff SR, Gupta S and Knowlton AA:
Cytosolic heat shock protein 60, apoptosis, and myocardial injury.
Circulation. 105:2899–2904. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Veereshwarayya V, Kumar P, Rosen KM,
Mestril R and Querfurth HW: Differential effects of mitochondrial
heat shock protein 60 and related molecular chaperones to prevent
intracellular β-amyloid-induced inhibition of complex IV and limit
apoptosis. J Biol Chem. 281:29468–29478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malik ZA, Kott KS, Poe AJ, Kuo T, Chen L,
Ferrara KW and Knowlton AA: Cardiac myocyte exosomes: Stability,
HSP60, and proteomics. Am J Physiol Heart Circ Physiol.
304:H954–H965. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campanella C, Cappello F, Bucchieri F, et
al: Hsp60 secretion and migration from cancer cells: A proposal for
a multistage pathway. FASEB J. 26:521–526. 2012.
|
|
23
|
Fink AL: Chaperone-mediated protein
folding. Physiol Rev. 79:425–449. 1999.PubMed/NCBI
|
|
24
|
Singh BN, Rao KS, Ramakrishna T, Rangaraj
N and Rao ChM: Association of αB-crystallin, a small heat shock
protein, with actin: Role in modulating actin filament dynamics in
vivo. J Mol Biol. 366:756–767. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ashby RS, Megaw PL and Morgan IG: Changes
in retinal alphaB-crystallin (cryab) RNA transcript levels during
periods of altered ocular growth in chickens. Exp Eye Res.
90:238–243. 2010. View Article : Google Scholar
|
|
26
|
McCully JD, Lotz MM, Krukenkamp IB and
Levitsky S: A brief period of retrograde hyperthermic perfusion
enhances myocardial protection from global ischemia: Association
with accumulation of Hsp 70 mRNA and protein. J Mol Cell Cardiol.
28:231–241. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morimoto RI, Sarge KD and Abravaya K:
Transcriptional regulation of heat shock genes. A paradigm for
inducible genomic responses. J Biol Chem. 267:21987–21990.
1992.PubMed/NCBI
|
|
28
|
Wu C: Heat shock transcription factors:
Structure and regulation. Annu Rev Cell Dev Biol. 11:441–469. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Westerheide SD, Raynes R, Powell C, Xue B
and Uversky VN: HSF transcription factor family, heat shock
response, and protein intrinsic disorder. Curr Protein Pept Sci.
13:86–103. 2012. View Article : Google Scholar
|
|
30
|
Wu AM, Amdams LG and Pugh R:
Immunochemical and partial chemical characterization of fractions
of membrane-bound smooth lipopolysaccharide-protein complex from
Brucella abortus. Mol Cell Biochem. 75:93–102. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franceschini A, Szklarczyk D, Frankild S,
et al: STRING v9.1: Protein-protein interaction networks, with
increased coverage and integration. Nucleic Acids Res.
41:D808–D815. 2013. View Article : Google Scholar :
|
|
32
|
Ritossa FM: Experimental activation of
specific loci in polytene chromosomes of Drosophila. Exp Cell Res.
35:601–607. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ashburner M and Bonner JJ: The induction
of gene activity in drosophilia by heat shock. Cell. 17:241–254.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Spradling A, Pardue ML and Penman S:
Messenger RNA in heat-shocked Drosophila cells. J Mol Biol.
109:559–587. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Trinklein ND, Chen WC, Kingston RE and
Myers RM: Transcriptional regulation and binding of heat shock
factor 1 and heat shock factor 2 to 32 human heat shock genes
during thermal stress and differentiation. Cell Stress Chaperones.
9:21–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Buriro R, Lv YJ, Ali I, Tang S, Liu ZJ,
Zhang M, Adem A, Hartung J and Bao ED: Temporal variations of Hsp60
and HSF-1 in primary rat myocardial cells in vitro under heat
stress. Genet Mol Res. 12:3003–3016. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colett MS, Larson R, Gold C, Strick D,
Anderson DK and Purchio AF: Molecular cloning and nucleotide
sequence of the pestivirus bovine viral diarrhea virus. Virology.
165:191–199. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cotto C, Berille J, Souquet PJ, Riou R,
Croisile B, Turjman F, Giroux B, Brune J and Trillet-Lenoir V: A
phase II trial of fote-mustine and cisplatin in central nervous
system metastases from non-small cell lung cancer. Eur J Cancer.
32A:69–71. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sorger PK: Heat shock factor and the heat
shock response. Cell. 65:363–366. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Islam A, Lv YJ, Abdelnasir A, et al: The
role of Hsp90α in heat-induced apoptosis and cell damage in primary
myocardial cell cultures of neonatal rats. Genet Mol Res.
12:6080–6091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hutter JJ, Mestril R, Tam EK, Sievers RE,
Dillmann WH and Wolfe CL: Overexpression of heat shock protein 72
in transgenic mice decreases infarct size in vivo. Circulation.
94:1408–1411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang S, Buriro R, Liu Z, Zhang M, Ali I,
Adam A, Hartung J and Bao E: Localization and expression of Hsp27
and αB-crystallin in rat primary myocardial cells during heat
stress in vitro. PLoS One. 8:e690662013. View Article : Google Scholar
|
|
43
|
Ananthan J, Goldberg AL and Voellmy R:
Abnormal proteins serve as eukaryotic stress signals and trigger
the activation of heat shock genes. Science. 232:522–524. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tulapurkar ME, Asiegbu BE, Singh IS and
Hasday JD: Hyperthermia in the febrile range induces HSP72
expression proportional to exposure temperature but not to HSF-1
DNA-binding activity in human lung epithelial A549 cells. Cell
Stress Chaperones. 14:499–508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Santoro MG: Heat shock factors and the
control of the stress response. Biochem Pharmacol. 59:55–63. 2000.
View Article : Google Scholar
|
|
46
|
Madrigano J, Mittleman MA, Baccarelli A,
Goldberg R, Melly S, von Klot S and Schwartz J: Temperature,
myocardial infarction, and mortality: effect modification by
individual and area-level characteristics. Epidemiology.
24:439–446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Locke M, Noble EG, Tanguay RM, Feild MR,
Ianuzzo SE and Ianuzzo CD: Activation of heat-shock transcription
factor in rat heart after heat shock and exercise. Am J Physiol.
268:C1387–C1394. 1995.PubMed/NCBI
|
|
48
|
Zou J, Guo Y, Guettouche T, Smith DF and
Voellmy R: Repression of heat shock transcription factor HSF1
activation by HSP90 (HSP90 complex) that forms a stress-sensitive
complex with HSF1. Cell. 94:471–480. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Le Masson F and Christians E: HSFs and
regulation of Hsp70.1 (Hspa1b) in oocytes and preimplantation
embryos: New insights brought by transgenic and knockout mouse
models. Cell Stress Chaperones. 16:275–285. 2011. View Article : Google Scholar :
|
|
50
|
Erlejman AG, Lagadari M, Toneatto J,
Piwien-Pilipuk G and Galigniana MD: Regulatory role of the
90-kDa-heat-shock protein (Hsp90) and associated factors on gene
expression. Biochim Biophys Acta. 1839:71–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Acunzo J, Katsogiannou M and Rocchi P:
Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and
HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol.
44:1622–1631. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Garrido C, Paul C, Seigneuric R and
Kampinga HH: The small heat shock proteins family: The long
forgotten chaperones. Int J Biochem Cell Biol. 44:1588–1592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ray PS, Martin JL, Swanson EA, Otani H,
Dillmann WH and Das DK: Transgene overexpression of alphaB
crystallin confers simultaneous protection against cardiomyocyte
apoptosis and necrosis during myocardial ischemia and reperfusion.
FASEB J. 15:393–402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Haskin CL, Athanasiou KA, Klebe R and
Cameron IL: A heat-shock-like response with cytoskeletal disruption
occurs following hydrostatic pressure in MG-63 osteosarcoma cells.
Biochem Cell Biol. 71:361–371. 1993. View Article : Google Scholar : PubMed/NCBI
|