Introduction
The parathyroid hormone (PTH) family, including
PTH-related peptide (PTHrP), regulates calcium and bone homeostasis
and a multitude of developmental processes through its receptors
(the PTHRs). Two PTHRs have been identified as PTH family ligands
in mammals, designated as parathyroid hormone 1 receptor (PTH1R)
and parathyroid hormone 2 receptor (PTH2R), and three in teleostei
fish or other non-mammalian vertebrates, including zebrafish,
seabream and chicken (1,2). PTH1R mediates the actions of PTH and
PTHrP in mammals. Human PTH2R, which is found in the brain,
pancreas, heart and kidneys, is activated by PTH and
tuberoinfundibular peptide of 39 residues (1,3,4).
PTH1R is a G protein-coupled member of the secretin receptor family
that interacts with the NH2-terminal 34 amino acids of
the ligand (5). PTH1R signaling,
which is mediated through the α-subunit of the stimulatory
G-protein promotes cyclic adenosine monophosphate production and
subsequently activates protein kinase A or protein kinase C
(6,7).
Thus far, the opinion of the majority of researchers
is that osteoclasts do not express PTHR; it is widely accepted that
PTH has an indirect effect on osteoclastogenesis by activating
PTH1R in osteoblasts or osteocytes (8–10).
Only a few researchers have considered the possibility that PTH1R
has a direct effect on osteoclasts (11,12). PTH increases osteoclast formation
and bone resorption through the regulation of receptor activator of
nuclear factor-kappa B ligand (RANKL)/osteoprotegerin expressed by
osteoblasts (13).
Vacuolar-type H+ adenosine triphosphatase
(V-ATPase), a type of proton pump, is widely present in eukaryotic
cells, and participates in various physiological processes,
particularly in the control of intracellular pH (14). V-ATPase is highly expressed in
osteoclasts and plays an important role in bone resorption
(15). The core structure of
V-ATPase has been defined: it consists of V1 and
V0 domains and an auxiliary subunit AC45, M8-9 (16). The V1 domain is an
approximately 640-kDa peripheral complex on the cytoplasmic side of
the membrane (17). This domain
is organized into several subunits (A, B1,
B2, C1, C2, D, E1,
E2, F, G1, G2, G3 and
H) and is responsible for ATP hydrolysis (16). The V0 domain is
approximately 260 kDa and is membrane-embedded. V0
consists of 10 subunits (a1, a2,
a3, a4, c, c′, c″, d1,
d2 and e) and mediates proton transport across the
membrane. In mammals, the V0 domain contains one of four
isoforms (a1, a2, a3 and
a4) of the a-subunit, which is a large integral protein
and contributes to the proton pore (18). The a1 isoform is most
highly expressed in brain and myocardial cells; a2 is
most highly expressed in the acrosomal membrane in sperm; the
a3 isoform is highly expressed by osteoclasts, microglia
and pancreatic β cells, whereas the a4 isoform is highly
expressed by renal intercalated cells (19). Disruption of the mouse
a3 encoding gene has been noted to result in severe
osteopetrosis and mutations of the a3 isoform in humans
and also leads to a condition known as auto-somal recessive
osteopetrosis including infantile malignant osteopetrosis. This
compelling genetic evidence suggests that the a3 isoform
is essential for, and specific to, osteoclastic bone resorption
(15,17,20). In addition, the d1
isoform is ubiquitously expressed, whereas the d2
isoform is expressed predominantly in the kidneys and osteoclasts.
The d2 isoform is an essential component of the
osteoclast-specific proton pump that mediates the extracellular
acidification in bone resorption (21). In d2 gene knockout
mice, failure of pre-osteoclast fusion into mature multinucleated
cells was noted, as was the osteopetrosis phenotype (22). Mutations in the
d2-subunit of V-ATPase result in reduced
tartrate-resistant acid phosphatase (TRAP) expression and decreased
fusion of osteoclast precursors (23). These data suggest that the
d2-subunit has dual functions in relation to the
regulation of osteoclast maturation and osteoclast extracellular
acidification. Thus, our study focuses on the expression of the
a3-subunit and d2-subunit, where they serve
as a component of V-ATPase in mature osteoclasts. Furthermore, we
wished to determine whether PTH has a direct effect on osteoclasts
and whether this effect is linked to V-ATPase.
Materials and methods
Reagents
C57BL/6 mice were provided by the Laboratory Animal
Center of Sun Yat-Sen University (Guangzhou, China). Six
6–10-week-old and three 7-day-old female mice were used (type
C57BL/6), typically weighting 25 g and 6.0 g, respectively. All
animal experiments were approved by the Institutional Animal Care
and Use Committee at the School of Life Sciences at Sun Yat-Sen
University. All experimental procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals and the Institutional Ethical Guidelines for Animal
Experiments. C57BL/6 mice were employed for cell cultures.
Calvarial osteoblasts from postnatal and bone marrow (BM) cells
from adult C57BL/6 mice were collected.
All primers were designed and synthesized by
Invitrogen Life Technologies (Carlsbad, CA, USA). The reverse
transcription reagent kit and the real-time polymerase chain
reaction (PCR) reagents were purchased from Takara Bio (Dalian,
China). A BCA Protein Assay kit and BCECF-AM solution were acquired
from Beyotime Institute of Biotechnology (Nanjing, China). The TRAP
staining kit was purchased from Sigma-Aldrich (St. Louis, MO, USA).
The AKP staining kit was purchased from Jiancheng Bioengineering
Institute (Jiancheng, Nanjing, China). Bovine PTH [bPTH-(1–34)]
was purchased from Bachem Bioscience Inc. (King of Prussia, PA,
USA). Soluble murine RANKL and macrophage colony-stimulating factor
(M-CSF) were purchased from R&D Systems (Minneapolis, MN, USA).
Antibodies to the V-ATPase a3-subunit (Cat. no.
H00010312-K) and d2-subunit (Cat. no. H00245972-M01)
were purchased from Abnova, Inc. (Taipei, Taiwan). Anti-rabbit
IgG-FITC antibody (Cat. no. sc-2012), and PTH1R (Cat. no. sc-20749)
and PTH2R (Cat. no. sc-30005) antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Administration of bPTH
The amino acid sequences of murine and bovine PTH
are highly conserved, as has been previously observed (24). bPTH is frequently used in research
to intervene in cell multiplication and differentiation processes
(25–27). In the present study,
bPTH-(1–34) powder was dissolved in 0.1% bovine
serum albumin (BSA) and stored at −20°C. The control cultures were
treated with the same concentration of BSA. In our experiments, the
treated groups were incubated with bPTH from day 5 to 6. The final
concentration of bPTH in the culture medium was 0.1, 1.0, 10 and
100 ng/ml.
Culture of primary mouse monocytes and
differentiation of osteoclasts
BM cells were isolated from the femurs of
6–10-week-old C57BL/6 mice by flushing the shaft with
phosphate-buffered saline (PBS) using needles, and the cells were
further dispersed several times by gentle, repeated pipetting with
a sterile pipette. The isolated cells were cultured for 24 h in
α-MEM containing 100 U/ml penicillin, 100 µg/ml streptomycin
and 10% FCS. Non-adherent BM-derived monocytes were collected and
cultured for 48 h in α-MEM (Invitrogen, Shanghai, China) containing
100 U/ml penicillin (Gibco, Shanghai, China), 100 µg/m
streptomycin (Gibco), 10% FCS (Biological Industries, Kibbutz
Beit-Haemek, Israel) and 25 ng/ml recombinant M-CSF as previously
described (28). After the
BM-derived monocytes were induced to differentiate into mononuclear
macrophages, the stromal cells and lymphocytes which cannot adhere
to the suspension culture dish were removed and the adherent cells
were collected, pelleted and counted before being seeded at
104 cells/cm2 in the presence of recombinant
RANKL. Fresh medium containing 35 ng/ml RANKL was added every 2
days until multinucleated osteoclasts formed (approximately 5
days). After the mononuclear macrophages were cultured for 5 days,
the cells were fixed and stained using the TRAP kit based on the
manufacturer's instructions (Sigma-Aldrich). TRAP-positive
multinucleated cells containing 3 or more nuclei were identified as
osteoclasts.
RNA isolation and RT-qPCR
The osteoclasts were induced in 6-well plates as
mentioned above and cultured with bPTH from day 5 to 6. Fresh
medium containing a different concentration of bPTH was replaced
every 2 days. Following culture for 6 days, total RNA was isolated
from the osteoclasts using TRIzol reagent. For RT-qPCR, cDNA was
synthesized from 1 µg total RNA using reverse transcriptase
and oligo(dT) primers in a volume of 10 µl, and the reaction
mixture was finally adjusted to 50 µl with TE buffer for
PCR. The cDNA amplification reaction mixture was initially
incubated at 95°C for 30 sec to denature DNA. Amplification was
performed for 40 cycles of 95°C for 5 sec and 60°C for 34 sec,
respectively. qPCR was performed under the following conditions:
95°C for 2 min, then 40 cycles of 95°C, 30 sec; 60°C, 1 min; 72°C,
2 min. The specificity of the PCR products was verified by melting
curve analysis. Data were normalized using the GAPDH housekeeping
gene as an endogenous control. All primers were derived against
murine sequences. The following primer sets were used: GAPDH
forward, CCATGTTTGTGATGGGTGTGAACC and reverse,
TGTGAGGGAGATGCTCAGTGTTGG; V-ATPase a3-subunit (Atp6v0a3)
forward, GAGA CCTCAACGAATCCGTGA and reverse, CGATCCGTTTCCTCCTGGA;
V-ATPase d2-subunit (Atp6v0d2) forward,
CTGGTTCGAGGATGCAAAGC and reverse, GTTGCCATAGTCCGTGGTCTG; PTH1R
forward, GCACACAGCAGCCAACATAA and reverse, CGCAGCATAAACGACAGGAA;
and PTH2R forward, GGCTGATTCTCAGTAGCTGTCT and reverse,
GGGCCAACAAATGATCCCATC.
Western blot analysis
The osteoclasts were induced in 12-cm petri dishes.
Following 48 h of co-culture with bPTH (0.1, 1.0, 10 and 100
ng/ml), protein lysates of osteoclasts were prepared in RIPA buffer
(Beyotime Institute of Biotechnology). Cell lysates (30 µg)
were electrophoresed on 12% polyacrylamide-SDS gels. Proteins were
then transferred onto a nitrocellulose membrane and incubated with
the following rabbit anti-mouse antibodies (0.2 µg/ml):
anti-V-ATPase a3-subunit, anti-V-ATPase
d2-subunit, anti-PTH1R, anti-PTH2R and anti-GAPDH as an
internal control, followed by 1 µg/ml anti-rabbit
immunoglobulin G-horseradish peroxide conjugate. The bands were
scanned, and the intensity was measured using a digital gel
electrophoresis image processing and analysis system (Tanon,
Shanghai, China).
Determination of intracellular pH
The osteoclasts were induced in 24-well plates and
treated with 0.1, 1, 10 and 100 ng/ml bPTH from day 5 to 6.
Following culture for 6 days, the cells were harvested and treated
with 20 mM ammonium chloride for 15 min and washed twice with α-MEM
free of phenol red and then placed into a 24-well plate with cover
slips. The cells were then stained with the dyeing agent, BCECF-AM,
a fluorescent probe that binds specifically to hydrogen ions, and
incubated at 37°C for 30 min. A total of 10 osteoclasts from each
well were selected randomly to determine the fluorescence intensity
(excitation at 488 nm, emission detected at 535 nm) under a laser
scanning confocal microscope (Leica SP5-FCS; Leica Microsystems
GmbH, Wetzlar, Germany), with the help of the image analyzing
software Image-Pro Plus 6.0 (Media Cybernetics, Inc., Houston, TX,
USA).
V-ATPase activity assay
To investigate V-ATPase activity, protein samples
were extracted from 5 groups with the method described in the study
by Koizumi et al (29).
The protein concentration was determined using the BCA Protein
Assay kit. V-ATPase activity assay was performed using the c
(Genmed Scientifics, Inc., Arlington, MA, USA). In the presence or
absence of bafilomycin A1 (APExBIO Technology, Houston,
TX, USA), which is a sensitive inhibitor of V-ATPase, V-ATPase
hydrolyzed the substrate ATP in a pyruvate kinase and lactate
dehydrogenase (LDH) system. In this reaction, NADH was converted to
NAD, and the activity of V-ATPase was calculated according to the
change of absorbance at 340 nm. The unit of measurement is
µmol ATP/min/mg. These assay protocols were executed
according to the manufacturer's instructions.
Bone resorption assay
The osteoclasts were induced using the foregoing
method and grown in each well of Osteo Assay Surface multiple-well
plates (Corning, NY, USA) for 6 days. Various concentrations of
bPTH were added to the culture from day 5 to 6; 10 nM bafilomycin
A1 was used to block V-ATPase. Following culture for 6
days, the plates were washed with saline and a solution of 5%
sodium hypochlorite was added for 5 min in order to detach the
cells. The cells were then stained with 1% toluidine blue. The
resorption area was examined under a microscope and each group was
compared using ImageJ software (National Institutes of Health,
Bethesda, MD, USA).
Detection of PTHRs in osteoclasts by
immunofluorescence staining
The primary antibodies used were polyclonal rabbit
anti-mouse PTH1R (Cat. no. sc-20749) and PTH2R (200 µg/ml;
Cat. no. sc-30005) (both from Santa Cruz Biotechnology, Inc.)
diluted at 1/50. The secondary antibodies were FITC-conjugated goat
anti-rabbit (Cat. no. sc-2012; Santa Cruz Biotechnology, Inc.).
DAPI (1 mg/ml) was used to identify the nuclei. The cells were
fixed in 4% PFA prior to antibody staining. After being washed with
PBS (0.01 M, pH 7.4) solution 3 times, glass cover slips were
saturated with washing solution (5% BSA) for 30 min. The cells were
incubated with the primary antibody diluted in washing solution for
30 min and then rinsed in the same solution for 15 min prior to
incubation with the secondary conjugated antibodies (1:100
dilution) for 30 min at room temperature. The cells were then
washed for 15 min and incubated with DAPI (1:1,000 dilution) for 5
min. Finally, the cells were washed for 15 min. Primary mouse
calvarial osteoblasts were isolated from 7-day-old C57BL/6 mice
using the method described by Wang et al (30). In brief, osteoblasts were isolated
from the calvaria of 7-day-old C57BL/6 mice by 4 sequential 15-min
enzyme digestions at 37°C in solution containing 0.05% trypsin-EDTA
and 0.1% collagenase P (Gibco). The cells released from the second
to fourth digestions were pooled, centrifuged, resuspended and
plated at 1.5×104/cm2 in 25cm2
culture plates in DMEM (Invitrogen, Shanghai, China) containing 10%
(v/v) FCS (Biological Industries), 100 U/m penicillin (Gibco), 100
µg/mL streptomycin (Gibco) and non-essential amino acids
(100 µM). The plated cells became confluent around days 5–7.
Then the culture medium was changed to differentiation medium. The
osteoblasts were then cultured in plastic dishes or plates
containing α-MEM supplemented with 10% (v/v) FCS, 100 U/ml
penicillin (Gibco), 100 µg/ml streptomycin (Gibco) and 2 mM
glutamine (Amresco, Solon, OH, USA). Following 48 h of culture, the
cells were stained as positive controls. Immunofluorescence and
confocal microscopy were performed immediately. No specific
staining was observed if the primary antibodies were omitted.
Statistical analysis
The results are represented as the means ± the
standard deviation (SD). Data were statistically analyzed by
one-way analysis of variance (ANOVA) using SPSS 13.0 software (SPSS
Inc., Chicago, IL, USA). All statistical tests were two-sided; a
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Impact of bPTH on osteoclast
differentiation
After the osteoclasts were induced in 96-well plates
and cultured with various concentrations of bPTH for 5 days, the
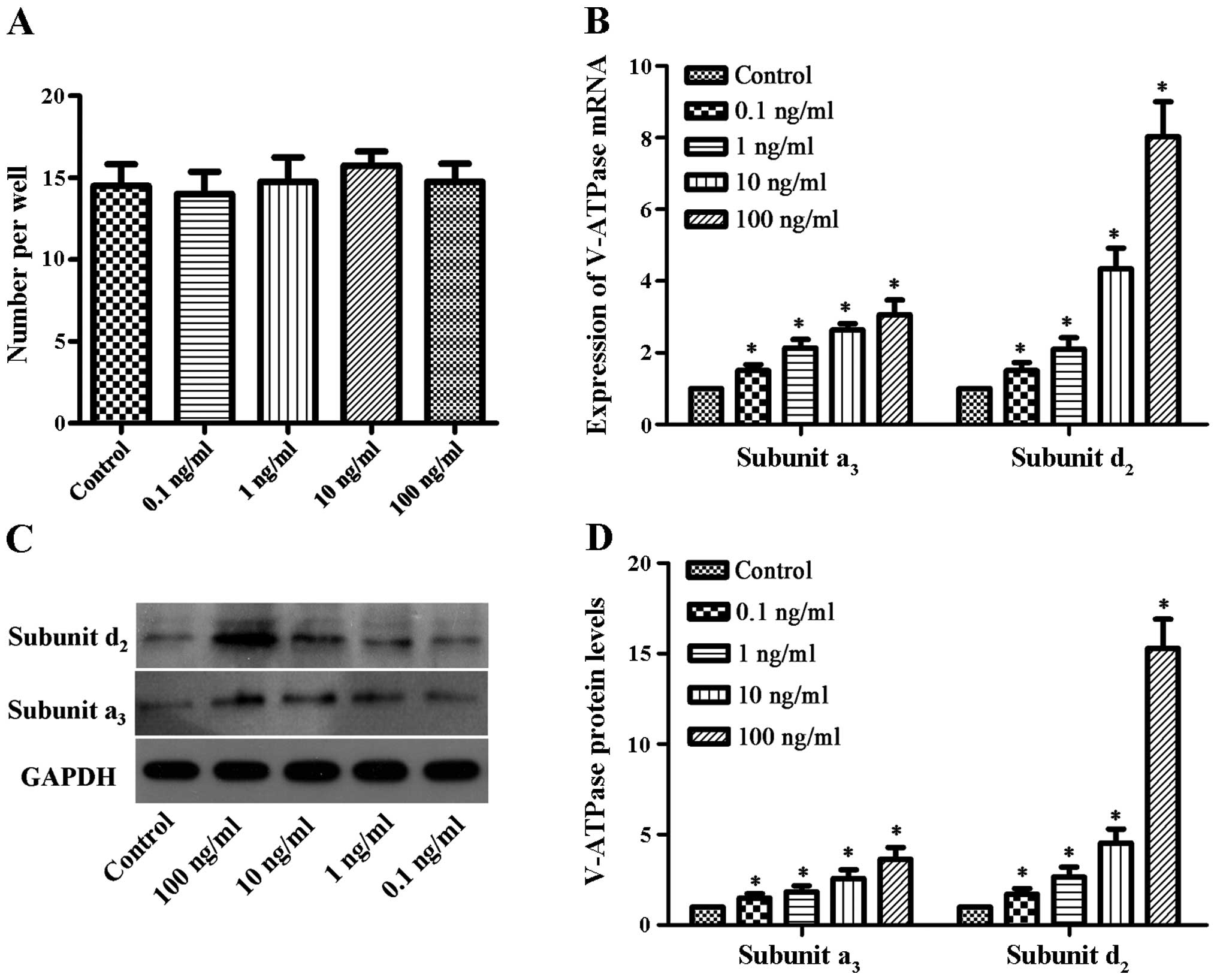
number of mature osteoclasts was determined (Fig. 1A). The number of mature
osteoclasts in the control group and bPTH-treated groups (0.1, 1,
10 and 100 ng/ml) was 14.5±2.6, 14.0±2.7, 14.8±3.0, 15.8±1.7 and
14.7±2.2, respectively. There were no significant differences
observed between the different groups. These results suggest that
bPTH does not have a great impact on osteoclast
differentiation.
Effect of bPTH on V-ATPase
expression
RT-qPCR was used to measure the mRNA expression
levels of the V-ATPase a3-subunit and
d2-subunit. Compared to the control group, the mRNA
expression of V-ATPase was found to be 1.5-3-fold higher for the
a3-subunit and 1.5-8-fold higher for the
d2-subunit (Fig. 1B).
The protein expression levels increased by 1.4-3.6-fold and
1.7-15-fold for the V-ATPase a3-subunit and
d2-subunit, respectively, in the bPTH-treated groups
compared to the control (Fig. 1C and
D). The mRNA and protein expression levels of V-ATPase differed
significantly between the bPTH-treated groups and the control
(P<0.05). These results indicated that the mRNA and protein
expression of the V-ATPase a3-subunit and the
d2-subunit were elevated in a dose-dependent manner
(with increasing concentrations of bPTH).
Effect of bPTH on intracellular pH in
osteoclasts
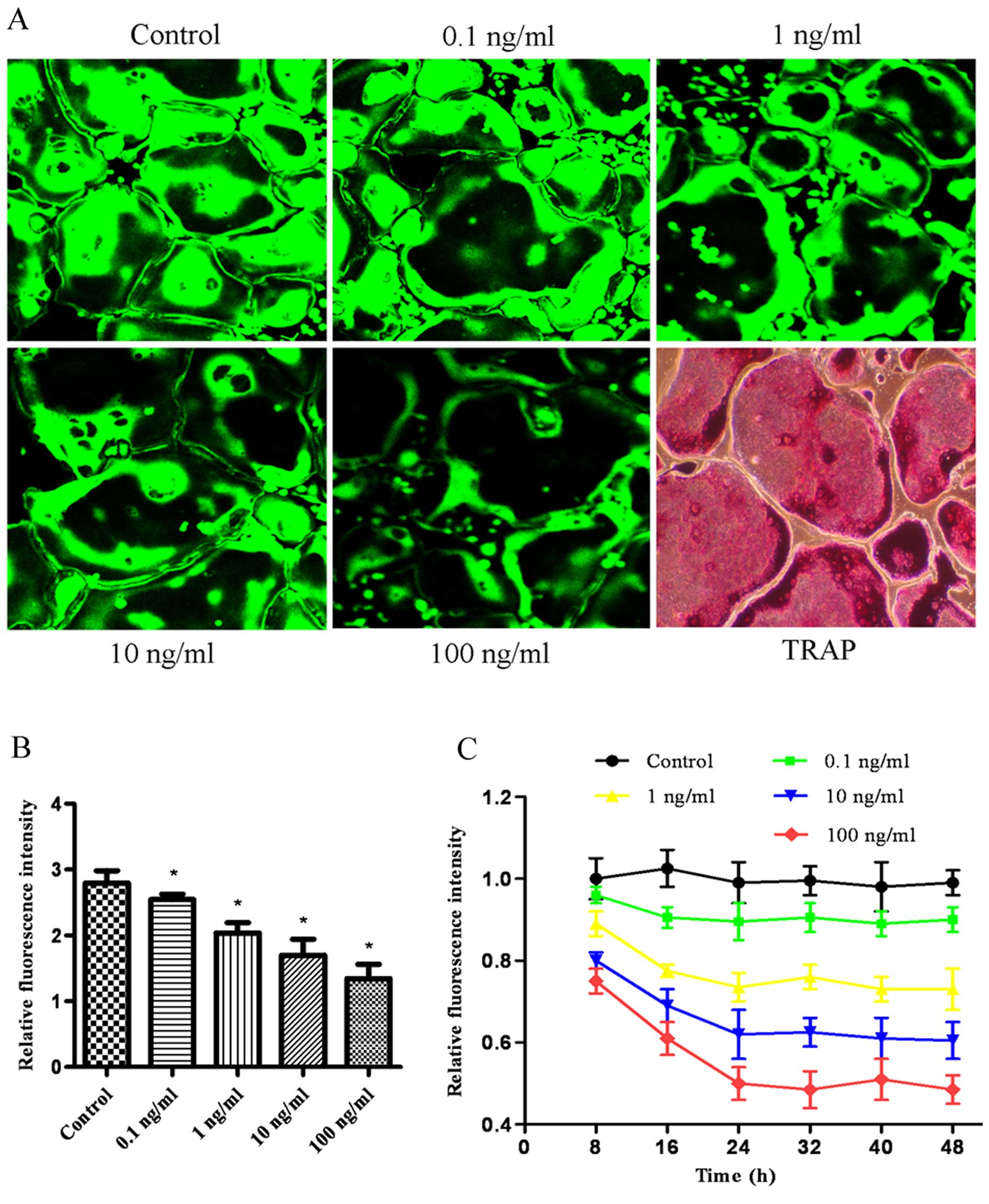
BCECF-AM is capable of sensing intracellular pH in
living cells, with the fluorescence intensity decreasing as the pH
value decreases from 9.5 to 6.2. The effect of bPTH on the
intracellular pH value in osteoclasts was assessed by laser
scanning confocal microscopy. Treatment with increasing
concentrations of bPTH, from 0.1 to 100 ng/ml, decreased the
fluorescence intensity (Fig. 2).
The fluorescence intensity decreased with time in the first 24 h
and reached a platform thereafter.
Effect of bPTH on V-ATPase activity
In this study, a significant increase in V-ATPase
activity was observed in the bPTH-treated groups compared with the
control group. The increase in V-ATPase activity indicated a
positive association with the concentrations of bPTH (Fig. 3C). V-ATPase activity was
42.59±9.97, 20.58±5.17, 6.20±2.46, 2.41±0.91 and 0.42±0.12
(µmol ATP/min/mg) in the treated groups (100, 10, 1.0 and
0.1 ng/ml) and the control, respectively.
Effect of bPTH on bone resorption
As a culture dish with its bottom coated with
calcium-phosphate thin film that would become a substrate for
osteoclasts, the Osteo Assay Surface multiple-well plate (Corning,
NY, USA) is designed to measure the bone resorption activity of
osteoclasts. The bone resorption activity of the osteoclasts can be
measured by direct observation under a phase contrast microscope
without using a scanning electron microscope (SEM). The osteoclasts
were induced and cultured on plates covered with hydroxyapatite
crystals. Following culture for 6 days, the bone resorption area of
osteoclasts in the bPTH-treated groups increased by 2–6-fold, in
parallel with the increasing concentration of bPTH in the culture
medium (Fig. 3). The bone
resorption area of the control group and bPTH-treated groups was
0.050±0.007, 0.103±0.026, 0.155±0.011, 0.213±0.027 and 0.290±0.035
mm2, respectively. In the osteoclast groups treated with
bPTH plus bafilomycin A1, bone resorption was completely
inhibited (Fig. 3A). This implies
that exposure to bPTH enhances the bone resorption capability of
osteoclasts.
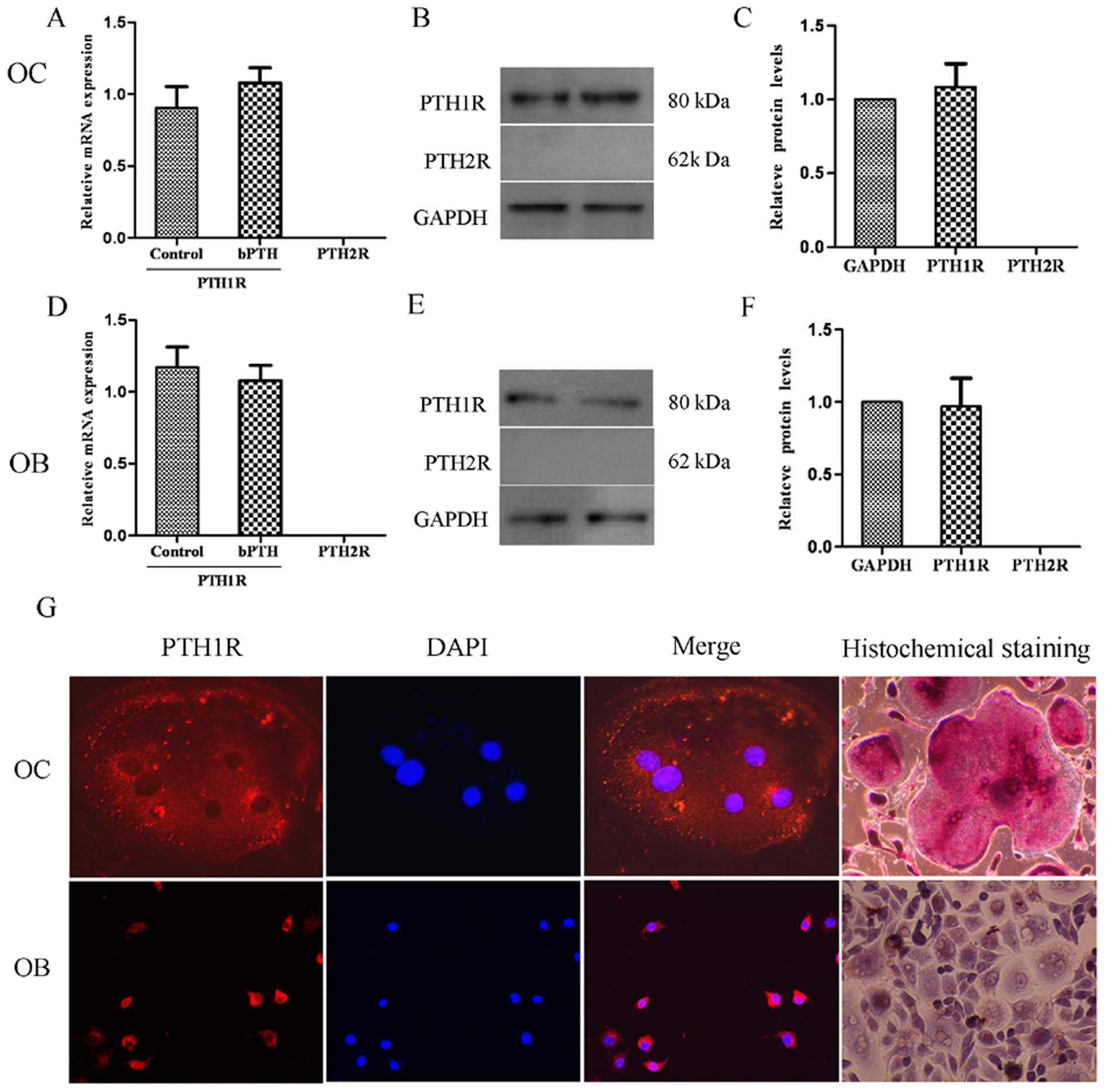
Expression of PTHRs in osteoclasts
The expression of PTH1R and PTH2R was analyzed using
RT-qPCR, western blot analysis and immunofluorescence staining
(Fig. 4). The results revealed
that the osteoclasts expressed PTH1R mRNA both in the control group
and the bPTH-treated group (1 ng/ml), but PTH2R mRNA was not
detectable. It seemed that the mRNA expression of PTH1R was not
markedly affected by the low dose of bPTH, and neither was PTH1R
protein expression, as was also detected by western blot analysis.
Moreover, positive staining for PTH1R protein was observed in both
osteoblasts and osteoclasts by laser scanning confocal microscopy.
These results suggest that PTH exerts its effects on both
osteoblasts and osteoclasts by binding to PTH1R and that downstream
signaling pathways are initiated to maintain the balance of
osteogenesis and osteolysis. However, this balance would be broken
by a high serum level of PTH, which exists in pathalogical states,
such as hyperparathyroidism.
Discussion
Osteoclasts are multinucleated cells fused from
BM-derived monocytes-macrophages, which are specialized for and
involved in bone resorption (31). In patients with end-stage renal
disease, the excessive secretion of PTH has been shown to increase
osteoclast activity, stimulate bone resorption, mobilize bone
calcium and disrupt the balance between osteoclasts and osteoblasts
(32). Generalized bone loss from
increased osteoclast activity also contributes to renal
osteodystrophy and a significant reduction in the quality of life
(33). Generally, the unique
ability of osteoclasts to degrade skeletal tissue depends on the
formation of a resorptive microenvironment between osteoclasts and
the bone surface (34). V-ATPase
plays a crucial role in this skeleton remodeling process;
nevertheless, the underlying mechanisms remain poorly understood.
The findings of the present study revealed that the exposure of
osteoclasts to bPTH induced the high expression of the V-ATPase
a3-subunit and d2-subunit, promoted V-ATPase
activity, accelerated intracellular acidification and increased the
bone-resorption capability of osteoclasts. We also found that PTH1R
exists in BM-derived osteoclasts. Based on these results, we
hypothesized that bPTH binds to PTH1R and increases the bone
resorption capability of osteoclasts by increasing the expression
of the V-ATPase a3-subunit and d2-subunit.
PTH not only promotes the formation of osteoblasts, but also
accelerates bone resorption by osteoclasts by combining with PTH1R
(Fig. 5). Therefore, V-ATPase and
PTH1R may be novel treatment targets in bone disease (35).
The formation of hydrogen ions and bicarbonate by
osteoclasts is catalyzed by carbonic anhydrase II from carbon
dioxide (36). The
chloride-bicarbonate (Cl−/HCO3−)
exchange also plays a role in the intracellular acidification of
osteoclasts (37). Previous
studies have confirmed that PTH directly stimulates the
acidification of osteoclasts (38,39). It has also been verified that PTH
promotes the expression of carbonic anhydrase II in murine marrow
cells (40). In this study, bPTH
induced a reduction in osteoclast intracellular pH, which
correlated with a decrease in fluorescence intensity. We
hypothesized that the decrease in intracellular pH in osteoclasts
was related to the carbonic anhydrase II or
Cl−/HCO3− exchange, but not
V-ATPase. Intracellular acidification may provide adequate hydrogen
ions for V-ATPase to transfer to the extracellular
microenvironment. We suggest that this change contributes to the
bone resorption capability of osteoclasts once osteoclasts are
activated by attaching to the bone surface.
The effect of PTH on osteoclasts has seldom been
studied before, as the opinion that osteoclasts do not express
PTHRs is widely accepted. In fact, PTHR expression in osteoclasts
has yet to be confirmed (41–43). Langub et al (42) detected PTH1R mRNA by in
situ hybridization in osteoclasts in sections of iliac crest
biopsies. mRNA levels of PTH1R in patients with secondary
hyperparathyroidism were higher than those in normal individuals
(42). Immunostaining also
revealed PTH1R protein in osteoclasts from diseased tissue
(42). This suggests that PTH1R
expression may be weak in normal situations and increased in
pathological states. Conversely, due to its short half-life of
approximately 4 min, PTH can be rapidly cleared in the blood
(44). Indeed, as also previously
demonstrated, cell-surface bound PTH was rapidly cleared in
approximately 20 min when fluorescent-tagged PTH was introduced to
cultures of osteoclasts (45).
Rapid clearance contributes to the difficulty of localizing the
receptor using conventional detection methods. In a previous study,
multinucleated osteoclasts were obtained from longitudinally split
animal long bones. However, despite using milder procedures, the
isolated osteoclasts were damaged, as evidenced by their failure to
exude trypan blue (46). With
fragile cells, significant cellular disintegration prevents us from
detecting membrane receptors. However, osteoclasts induced from
BM-derived monocytes are generated from a different source and
cultured under relatively stable conditions and can be stained in a
very short time. This may improve the positive rate of the
detection of PTHRs.
It should be noted that the results of this study
were based on BM-derived osteoclasts, not primary osteoclasts
derived from long bones of mice. Notwithstanding this limitation,
this study suggests a dual regulatory mechanism, whereby PTH acts
both directly on osteoblasts and also via osteoclasts. The
identification of a new mode of cell-cell communication mediated by
PTH and PTH1R has added another level of information to our
knowledge of the regulatory balance between bone formation and
resorption.
Acknowledgments
We would like to thank the physicians in the
Department of Nephrology for their helpful comments.
References
|
1
|
Guerreiro PM, Renfro JL, Power DM and
Canario AV: The parathyroid hormone family of peptides: structure,
tissue distribution, regulation, and potential functional roles in
calcium and phosphate balance in fish. Am J Physiol Regul Integr
Comp Physiol. 292:R679–R696. 2007. View Article : Google Scholar
|
|
2
|
On JS, Chow BK and Lee LT: Evolution of
parathyroid hormone receptor family and their ligands in
vertebrate. Front Endocrinol (Lausanne). 6:282015.
|
|
3
|
Dobolyi A, Palkovits M and Usdin TB: The
TIP39-PTH2 receptor system: unique peptidergic cell groups in the
brainstem and their interactions with central regulatory
mechanisms. Prog Neurobiol. 90:29–59. 2010. View Article : Google Scholar :
|
|
4
|
Potthoff SA, Janus A, Hoch H, Frahnert M,
Tossios P, Reber D, Giessing M, Klein HM, Schwertfeger E, Quack I,
et al: PTH-receptors regulate norepinephrine release in human heart
and kidney. Regul Pept. 171:35–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pioszak AA and Xu HE: Molecular
recognition of parathyroid hormone by its G protein-coupled
receptor. Proc Natl Acad Sci USA. 105:5034–5039. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abou-Samra AB, Jüppner H, Force T, Freeman
MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV and
Potts JT Jr: Expression cloning of a common receptor for
parathyroid hormone and parathyroid hormone-related peptide from
rat osteoblast-like cells: a single receptor stimulates
intracellular accumulation of both cAMP and inositol trisphosphates
and increases intracellular free calcium. Proc Natl Acad Sci USA.
89:2732–2736. 1992. View Article : Google Scholar
|
|
7
|
Silva BC and Bilezikian JP: Parathyroid
hormone: anabolic and catabolic actions on the skeleton. Curr Opin
Pharmacol. 22:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lupp A, Klenk C, Röcken C, Evert M, Mawrin
C and Schulz S: Immunohistochemical identification of the PTHR1
parathyroid hormone receptor in normal and neoplastic human
tissues. Eur J Endocrinol. 162:979–986. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Romero G, Sneddon WB, Yang Y, Wheeler D,
Blair HC and Friedman PA: Parathyroid hormone receptor directly
interacts with dishevelled to regulate beta-Catenin signaling and
osteoclastogenesis. J Biol Chem. 285:14756–14763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tohmonda T, Yoda M, Mizuochi H, Morioka H,
Matsumoto M, Urano F, Toyama Y and Horiuchi K: The IRE1α-XBP1
pathway positively regulates parathyroid hormone (PTH)/PTH-related
peptide receptor expression and is involved in pth-induced
osteoclastogenesis. J Biol Chem. 288:1691–1695. 2013. View Article : Google Scholar :
|
|
11
|
Dempster DW, Hughes-Begos CE,
Plavetic-Chee K, Brandao-Burch A, Cosman F, Nieves J, Neubort S, Lu
SS, Iida-Klein A, Arnett T and Lindsay R: Normal human osteoclasts
formed from peripheral blood monocytes express PTH type 1 receptors
and are stimulated by PTH in the absence of osteoblasts. J Cell
Biochem. 95:139–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jilka RL: Are osteoblastic cells required
for the control of osteoclast activity by parathyroid hormone? Bone
Miner. 1:261–266. 1986.PubMed/NCBI
|
|
13
|
Del Fattore A, Teti A and Rucci N:
Osteoclast receptors and signaling. Arch Biochem Biophys.
473:147–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ludwig J, Kerscher S, Brandt U, Pfeiffer
K, Getlawi F, Apps DK and Schägger H: Identification and
characterization of a novel 9.2-kDa membrane sector-associated
protein of vacuolar proton-ATPase from chromaffin granules. J Biol
Chem. 273:10939–10947. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Su Y, Zhou A, Al-Lamki RS and Karet FE:
The a-subunit of the V-type H+-ATPase interacts with
phosphofructokinase-1 in humans. J Biol Chem. 278:20013–20018.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin A, Cheng TS, Pavlos NJ, Lin Z, Dai KR
and Zheng MH: V-ATPases in osteoclasts: structure, function and
potential inhibitors of bone resorption. Int J Biochem Cell Biol.
44:1422–1435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Toro EJ, Ostrov DA, Wronski TJ and
Holliday LS: Rational identification of enoxacin as a novel
V-ATPase-directed osteoclast inhibitor. Curr Protein Pept Sci.
13:180–191. 2012. View Article : Google Scholar :
|
|
18
|
Drory O and Nelson N: The emerging
structure of vacuolar ATPases. Physiology (Bethesda). 21:317–325.
2006. View Article : Google Scholar
|
|
19
|
Manolson MF, Yu H, Chen W, Yao Y, Li K,
Lees RL and Heersche JN: The a3 isoform of the 100-kDa V-ATPase
subunit is highly but differentially expressed in large (≥10
nuclei) and small (≤nuclei) osteoclasts. J Biol Chem.
278:49271–49278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niikura K: Vacuolar ATPase as a drug
discovery target. Drug News Perspect. 19:139–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishi T, Kawasaki-Nishi S and Forgac M:
Expression and function of the mouse V-ATPase d subunit isoforms. J
Biol Chem. 278:46396–46402. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu H, Xu G and Li YP: Atp6v0d2 is an
essential component of the osteoclast-specific proton pump that
mediates extracellular acidification in bone resorption. J Bone
Miner Res. 24:871–885. 2009. View Article : Google Scholar :
|
|
23
|
Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim
N, Kang JS, Miyamoto T, Suda T, Lee SK, et al: v-ATPase
V0 subunit d2-deficient mice exhibit impaired osteoclast
fusion and increased bone formation. Nat Med. 12:1403–1409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bell O, Silver J and Naveh-Many T:
Parathyroid hormone, from gene to protein. Molecular Biology of the
Parathyroid. Naveh-Many T: Landes Bioscience and Kluwer Academic;
New York: pp. 8–28. 2005, View Article : Google Scholar
|
|
25
|
Huang JC, Sakata T, Pfleger LL, Bencsik M,
Halloran BP, Bikle DD and Nissenson RA: PTH differentially
regulates expression of RANKL and OPG. J Bone Miner Res.
19:235–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SK and Lorenzo JA: Parathyroid hormone
stimulates TRANCE and inhibits osteoprotegerin messenger
ribonucleic acid expression in murine bone marrow cultures:
correlation with osteoclast-like cell formation. Endocrinology.
140:3552–3561. 1999.PubMed/NCBI
|
|
27
|
Rhee Y, Bivi N, Farrow E, Lezcano V,
Plotkin LI, White KE and Bellido T: Parathyroid hormone receptor
signaling in osteocytes increases the expression of fibroblast
growth factor-23 in vitro and in vivo. Bone. 49:636–643. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamothe B, Lai Y, Xie M, Schneider MD and
Darnay BG: TAK1 is essential for osteoclast differentiation and is
an important modulator of cell death by apoptosis and necroptosis.
Mol Cell Biol. 33:582–595. 2013. View Article : Google Scholar :
|
|
29
|
Koizumi K, Ito Y, Kojima K and Fujii T:
Isolation and characterization of the plasma membranes from rat
ascites hepatomas and from normal rat livers, including newborn,
regenerating, and adult livers. J Biochem. 79:739–748.
1976.PubMed/NCBI
|
|
30
|
Wang YH, Liu Y, Buhl K and Rowe DW:
Comparison of the action of transient and continuous PTH on primary
osteoblast cultures expressing differentiation stage-specific GFP.
J Bone Miner Res. 20:5–14. 2005. View Article : Google Scholar
|
|
31
|
Udagawa N, Takahashi N, Akatsu T, Tanaka
H, Sasaki T, Nishihara T, Koga T, Martin TJ and Suda T: Origin of
osteoclasts: mature monocytes and macrophages are capable of
differentiating into osteoclasts under a suitable microenvironment
prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci
USA. 87:7260–7264. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Blair HC and Athanasou NA: Recent advances
in osteoclast biology and pathological bone resorption. Histol
Histopathol. 19:189–199. 2004.PubMed/NCBI
|
|
33
|
Cannata-Andía JB, Rodriguez García M and
Gómez Alonso C: Osteoporosis and adynamic bone in chronic kidney
disease. J Nephrol. 26:73–80. 2013. View Article : Google Scholar
|
|
34
|
Zou W and Teitelbaum SL: Integrins, growth
factors, and the osteoclast cytoskeleton. Ann N Y Acad Sci.
1192:27–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yuan FL, Li X, Lu WG, Li CW, Li JP and
Wang Y: The vacuolar ATPase in bone cells: a potential therapeutic
target in osteoporosis. Mol Biol Rep. 37:3561–3566. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oksala N, Levula M, Pelto-Huikko M,
Kytömäki L, Soini JT, Salenius J, Kähönen M, Karhunen PJ, Laaksonen
R, Parkkila S and Lehtimäki T: Carbonic anhydrases II and XII are
up-regulated in osteoclast-like cells in advanced human
atherosclerotic plaques-Tampere Vascular Study. Ann Med.
42:360–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Josephsen K, Praetorius J, Frische S,
Gawenis LR, Kwon TH, Agre P, Nielsen S and Fejerskov O: Targeted
disruption of the Cl−/HCO3−
exchanger Ae2 results in osteopetrosis in mice. Proc Natl Acad Sci
USA. 106:1638–1641. 2009. View Article : Google Scholar
|
|
38
|
Hunter SJ, Schraer H and Gay CV:
Characterization of isolated and cultured chick osteoclasts: the
effects of acetazolamide, calcitonin, and parathyroid hormone on
acid production. J Bone Miner Res. 3:297–303. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gay CV, Kief NL and Bekker PJ: Effect of
estrogen on acidification in osteoclasts. Biochem Biophys Res
Commun. 192:1251–1259. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang BL, Liang H, Zheng F, Li XX, Liu YB
and Dai CL: Recombinant soluble receptor activator of nuclear
factor-kappaB inhibits parathyroid hormone-induced
osteoclastogenesis in vitro. Sheng Li Xue Bao. 59:169–174.
2007.PubMed/NCBI
|
|
41
|
Gay CV, Zheng B and Gilman VR:
Co-detection of PTH/PTHrP receptor and tartrate resistant acid
phosphatase in osteoclasts. J Cell Biochem. 89:902–908. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Langub MC, Monier-Faugere MC, Qi Q, Geng
Z, Koszewski NJ and Malluche HH: Parathyroid hormone/parathyroid
hormone-related peptide type 1 receptor in human bone. J Bone Miner
Res. 16:448–456. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Faucheux C, Horton MA and Price JS:
Nuclear localization of type I parathyroid hormone/parathyroid
hormone-related protein receptors in deer antler osteoclasts:
evidence for parathyroid hormone-related protein and receptor
activator of NF-kappaB-dependent effects on osteoclast formation in
regenerating mammalian bone. J Bone Miner Res. 17:455–464. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Libutti SK, Alexander HR, Bartlett DL,
Sampson ML, Ruddel ME, Skarulis M, Marx SJ, Spiegel AM, Simmonds W
and Remaley AT: Kinetic analysis of the rapid intraoperative
parathyroid hormone assay in patients during operation for
hyperparathyroidism. Surgery. 126:1145–1150; discussion 1150–1151.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Niendorf A, Dietel M, Arps H and Childs
GV: A novel method to demonstrate parathyroid hormone binding on
unfixed living target cells in culture. J Histochem Cytochem.
36:307–309. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hefley TJ and Stern PH: Isolation of
osteoclasts from fetal rat long bones. Calcif Tissue Int.
34:480–487. 1982. View Article : Google Scholar : PubMed/NCBI
|